Pairing location-based contact tracing and targeted indoor insecticide applications can effectively prevent dengue transmission.
Keywords: transmission chain, insecticide, transmission heterogeneity, human mobility, Vector control, Aedes aegypti
Abstract
The widespread transmission of dengue viruses (DENV), coupled with the alarming increase of birth defects and neurological disorders associated with Zika virus, has put the world in dire need of more efficacious tools for Aedes aegypti–borne disease mitigation. We quantitatively investigated the epidemiological value of location-based contact tracing (identifying potential out-of-home exposure locations by phone interviews) to infer transmission foci where high-quality insecticide applications can be targeted. Space-time statistical modeling of data from a large epidemic affecting Cairns, Australia, in 2008–2009 revealed a complex pattern of transmission driven primarily by human mobility (Cairns accounted for ~60% of virus transmission to and from residents of satellite towns, and 57% of all potential exposure locations were nonresidential). Targeted indoor residual spraying with insecticides in potential exposure locations reduced the probability of future DENV transmission by 86 to 96%, compared to unsprayed premises. Our findings provide strong evidence for the effectiveness of combining contact tracing with residual spraying within a developed urban center, and should be directly applicable to areas with similar characteristics (for example, southern USA, Europe, or Caribbean countries) that need to control localized Aedes-borne virus transmission or to protect pregnant women’s homes in areas with active Zika transmission. Future theoretical and empirical research should focus on evaluation of the applicability and scalability of this approach to endemic areas with variable population size and force of DENV infection.
INTRODUCTION
Current efforts to contain the global spread of dengue viruses (DENV) and the recent emergence of Chikungunya and Zika viruses are proving futile. Most tropical and subtropical countries experience variable levels of endemic dengue transmission and are vulnerable to the occurrence of large and rapidly propagating outbreaks primarily vectored by the endophilic mosquito Aedes aegypti (1–4). Limited availability and efficacy of a dengue vaccine (5, 6), coupled with the cocirculation of dengue with Chikungunya and Zika viruses, are pushing policy-makers to keep vector control as an indispensable tool for disease mitigation (7). Unfortunately, although there are a few well-documented historical examples of A. aegypti being eliminated or significantly reduced through vector control (for example, hemispheric yellow fever eradication plan and dengue control in Singapore and Cuba), implementing these large-scale vertical programs in contemporaneous urban environments is now considered unfeasible (1). Thus, achieving the World Health Organization’s goal of reducing global dengue mortality by 50% and morbidity by 25% by 2020 (8) and preventing the severe health outcomes associated with Zika virus infection will require adoption of integrated approaches combining multiple tools that can act synergistically, particularly vector control and (when they become widely available) vaccines (7). Theoretically, vaccination increases herd immunity, increasing the sustainability of vector control, whereas vector control reduces the force of infection by mosquitoes, leading to lower vaccination thresholds needed to sustain long protection.
Contemporaneous dengue vector control tends to be reactive in nature, responding to clinically apparent disease manifestations [which suffer from delays inherent to passive surveillance systems (9)], focusing on the cases’ primary residence, and depending strongly on area-wide interventions such as ultralow volume or thermal fogging of insecticides sprayed from streets; vector control is further limited by constraints in public health personnel and resources during large outbreaks (10). Unfortunately,there is little epidemiological evidence that existing methods, which have been inspired by the vertical yellow fever interventions implemented in the 1950s, are adequate to prevent or contain virus transmission (7, 10–13). A. aegypti mosquitoes predominantly rest indoors, primarily in hanging objects, inside bedrooms, and on various surface materials (14, 15), a trait that severely limits the efficacy of interventions such as truck-mounted ultralow volume insecticide sprays or thermal fogging (16, 17). Indoor space spraying with portable ultralow volume sprayers has greater efficacy at reaching resting adult mosquitoes, but its impact is transient because of the lack of residual effect (18, 19). When properly performed, indoor residual spraying of insecticides (IRS) can have a significant impact on adult A. aegypti populations and likely also on dengue prevention (15, 20–23). A recent meta-analysis concluded that there is need for more empirical evidence on the potential utility of IRS for dengue prevention (13). The fact that IRS is time-consuming and dependent on specialized human resources has also hindered its adoption by policy-makers. Scalability of highly effective but time-consuming methods such as IRS will remain a serious challenge unless novel approaches for delivery are developed.
Recent field observational studies and mathematical models outline the relevance of human movement in city-wide DENV propagation (20, 24–26). However, public health interventions respond reactively to clinically apparent cases of dengue disease by relying on the residential addresses of each confirmed (or suspected) case to trigger interventions (16, 27). Although this approach likely prevents an important fraction of all transmission events, it does not address transmission in places other than homes (24, 28). Given that human exposure to infectious mosquitoes and virus transmission by viremic individuals can be spatially disassociated (25), focusing surveillance efforts in reconstructing dengue transmission chains can be a more efficient means of proactively responding to disease outbreaks. Contact tracing is a well-established public health surveillance and control strategy that aims at following chains of infection rather than isolated cases, with the ultimate goal of performing targeted control in them (29). Theory and empiric evidence indicate that, for pathogens that do not propagate randomly across populations, contact tracing can increase both efficiency and effectiveness of interventions in comparison to blanket application (29–33). While movement-focused contact tracing is routinely performed to characterize travel history and identify dengue importations, its applicability to identification of likely places of infection within metropolitan areas has not yet been evaluated.
The goals of this study were to investigate the relevance of location-based contact tracing in reconstructing metropolitan dengue transmission chains of a large DENV-3 outbreak that occurred in Cairns, Australia, between 2008 and 2009 and to evaluate the epidemiological impact of IRS targeted at A. aegypti resting locations [targeted IRS (TIRS)] on the occurrence of dengue symptomatic disease.
RESULTS
Characterizing human exposure patterns using contact tracing data
Contact tracing performed on 902 laboratory-confirmed DENV cases identified 2064 premises (home and other residential or work sites, from now on named “locations”) (Fig. 1A). An average of 2.9 (SD, 1.3) contact locations (including home residences) were visited within each case’s period of virus exposure, with 32.7% of all cases reporting their home as their only mosquito contact location (fig. S1). The inferred town connectivity network shows Cairns as a potential central hub for DENV exposure and transmission in the metropolitan area (Fig. 1B). Most (92%) contact locations of Cairns residents were found within the city, and up to 60% of all contact locations in satellite towns were found within Cairns (Fig. 1B). This uneven distribution of contact locations gave rise to different movement kernels between Cairns and satellite town residents (Fig. 1, C and D). While only 10% of all movements of Cairns residents occurred more than 6 km from their home, most (73%) movements of residents of satellite towns occurred more than 6 km from their residence, primarily into Cairns. Most movements of Cairns residents were found within the city, and the occurrence of contact locations in close proximity (that is, within 200 m) of their residence was rare (only 2.3% of all movements; Fig. 1D). Finally, distance to Cairns was negatively correlated with the proportion of contact locations of each town that occurred in Cairns (Fig. 1E).
Fig. 1. Metropolitan DENV epidemic transmission evidenced through contact tracing.
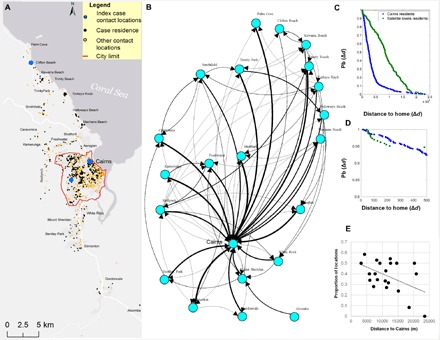
(A) Total of 902 confirmed DENV cases during the 2008–2009 outbreak and associated exposure locations reported through contact tracing. Blue dots show the contact locations of the epidemic’s index case, which initiated outbreaks both in Cairns and Clifton Beach. (B) Relative connectivity between towns, measured as the proportion of all contact locations of DENV cases residing in a given town that occurred at the other towns and thickness proportional to relative frequency of total locations (range, 0.1 to 1). For instance, an arrow going from Palm Cove (north) to Cairns shows that 50% of all contact locations occurred in Cairns (and the remaining 50% locations in Palm Cove, as no other lines connect Palm Cove to other towns). Location of towns in the network is proportional to their geographic location. (C) Probability density distribution (Pb) showing that a confirmed DENV case moved a given distance from his or her residence (Δd), calculated both for Cairns and satellite town residents. (D) Same as (C), showing detail for the distance range of 0 to 500 m. (E) Proportion of all contact locations of a given town that were found in the city of Cairns as a function of the Euclidian distance of each town to Cairns. The linear relationship was statistically significant (P = 0.03).
Space-time analyses of contact tracing data
Space-time analyses allowed reconstruction of the epidemic throughout metropolitan Cairns by statistically identifying the most likely transmission pathway of 823 of 902 (91%) symptomatic dengue cases. The Knox test yielded the same number of spatiotemporally linked contact locations and symptomatic cases (1207 and 823, respectively) when run using temporal windows of 20 and 30 days. The epidemics’ index case (a Cairns resident who introduced the virus from Indonesia) was also likely responsible for initiating a localized outbreak in the town of Clifton Beach, 22 km north of Cairns (see the Supplementary Text). From the two virus introduction points (Cairns and Clifton Beach), DENV propagated throughout the metropolitan area (Fig. 2). On average, 39.6% (SD, 27.2%; range, 0 to 81.8%) of putative virus transmission locations of residents of satellite towns were local (occurred within their respective town). The remaining putative transmission locations of residents of satellite towns could be tracked to locations found within Cairns (Fig. 2). However, for Cairns residents, putative transmission locations were found primarily (95.2%) within the city (Fig. 2). Areas such as Trinity Park/Palm Cove and Gordonvale lacked significant local transmission (all cases were linked to transmission in Cairns) (Fig. 2). This may be due to the prevalence of new housing with screened windows in the former area, and, in Gordonvale, to low vector populations resulting from source reduction campaigns during a dengue outbreak 3 years earlier (2006).
Fig. 2. Sources and sinks of metropolitan DENV transmission.
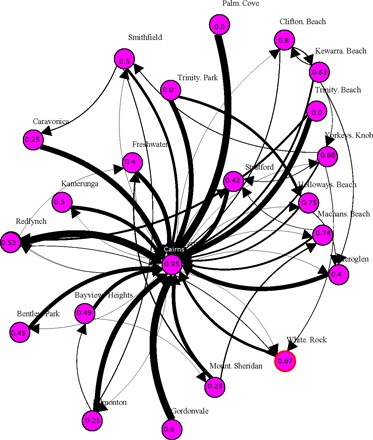
Space-time interaction tests identified putative virus transmission sites for 823 of 902 symptomatic laboratory-confirmed DENV cases during the 2008–2009 outbreak. The resulting DENV transmission network shows Cairns as the primary virus transmission hub. Direction of arrows indicates residents of a locality (origin) whose exposure occurred on a different locality (destination) and the thickness of edges is proportional to the total number of locations that occurred on a given destination locality. Numbers inside each pink circle point to the proportion of putative transmission locations that occurred within that locality. For instance, for residents of Palm Cove (north), none of the contact locations in the town were associated with local DENV transmission. Instead, all transmission locations of Palm Cove residents occurred within Cairns.
A total of 420 geographically independent space-time clusters of local DENV transmission were identified (fig. S2). Exclusion of contact locations (that is, using solely the home residence to infer transmission chains) decreased the number of space-time clusters (420 to 276) and the overall connectivity of the transmission network (fig. S3). A large proportion (273 of 420, 65%) of clusters were generated by two or more cases residing in or visiting the same location (focal transmission areas). It is worth noting a large cluster within a multifamily housing unit where more than 30 cases were spatiotemporally linked (fig. S3). The size of clusters that extended beyond isolated houses was heterogeneous and ranged from 2 to 65 locations connected in space-time (Fig. 3). The geographic progression of space-time clusters showed a rapid spread from the index cases’ neighborhood to the entire city, with a high concentration of transmission chains in the neighborhood where the index case lived (Fig. 3). Throughout metropolitan Cairns, 359 of 839 (42.8%) cases were spatiotemporally linked to their residence only, while the remaining 57.2% of cases included both their home and other out-of-home contact locations (394 of 839, 47.0%) or only to out-of-home locations (86 of 839, 10.2%) as components of their putative transmission chain (Fig. 4). This high out-of-home virus activity was consistent across towns (Fig. 4).
Fig. 3. Spatiotemporal pattern of DENV propagation within Cairns.
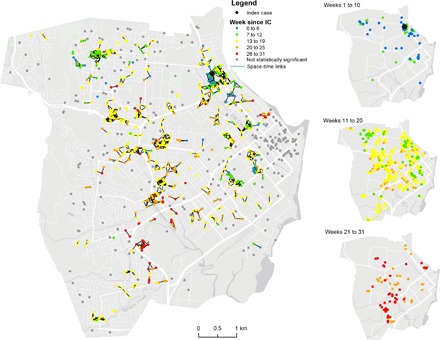
Locations that were statistically linked in space-time and where DENV transmission was likely to occur (that is, putative transmission locations). Colors show the week of onset of symptoms (measured as time since the onset of symptoms of the epidemic’s index case). Panels on the right show the same data but in temporal cuts as the epidemic progressed.
Fig. 4. Location types involved in DENV transmission chains.
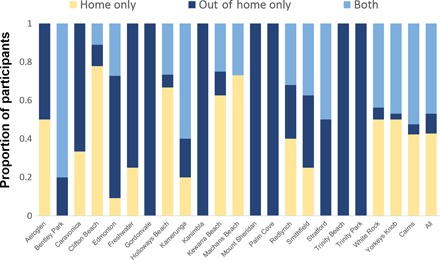
Proportion of participants whose putative transmission locations identified by space-time interaction tests included their home only, one or more out-of-home locations, or both the home and out-of-home locations.
Impact of TIRS at putative transmission sites
As the epidemic progressed, TIRS applications were scaled up to contain the spread of the virus, reaching a total of 5428 locations treated over a 31-week period (figs. S4 and S5). All DENV case contact locations and immediate neighboring premises were used to quantify the effectiveness of TIRS in preventing symptomatic DENV transmission in comparison with untreated controls. The distribution of TIRS and control houses was comparable both in time (both treatments being incorporated proportionally within the same period; fig. S6) and space (control and TIRS blocks were mixed within neighborhoods; fig. S7). The time to the next DENV case was significantly lower in houses treated with TIRS versus untreated houses (mixed-effects Cox proportional hazards model, coeff = −0.99 ± 0.17; Z = −5.8; P < 0.001; SD of random effects, 0.72; Fig. 5). While the proportion of houses without DENV cases remained high for control houses, a significant reduction in DENV was detected in TIRS houses for at least 100 days after treatment (Fig. 5). The two curves showed no significant difference within the first 10 days (mixed-effects Cox proportional hazards model, coeff = 0.35 ± 0.31; Z = 1.12; P = 0.26; Fig. 5), likely because of the occurrence of cases in treated houses whose exposure may have occurred before spraying (10 days can be considered an upper limit from infection to onset of symptoms). Most (73.3%) locations receiving TIRS had one additional case spatiotemporally linked to them, while control houses had a much larger number of additional cases (fig. S8). When all cases were considered, the calculated effectiveness of TIRS in preventing symptomatic DENV infections, compared to control houses, was 0.861, and 0.961 using conservative estimates that excluded cases occurring within 10 days after intervention (Table 1).
Fig. 5. Impact of TIRS performed on contact locations on the occurrence of future dengue cases.
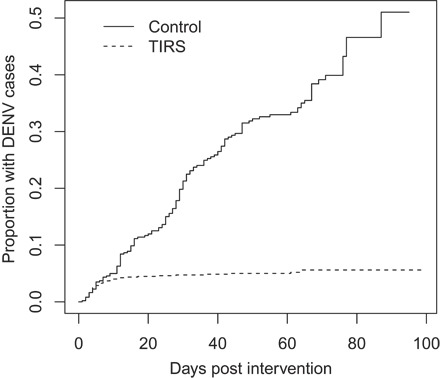
Expressed as the proportion of locations with DENV cases at a given time after intervention (treatment locations; dashed line) or onset of symptoms of the first DENV case (control locations; solid line).
Table 1. Effectiveness of TIRS applied at contact locations in preventing dengue symptomatic infections during the 2008–2009 DENV outbreak that occurred in Cairns, Australia.
| Scenario | Treatment | Total locations | No. of DENV-positive locations | Proportion infection | Effectiveness |
| All cases | IRS | 1007 | 52 | 0.052 | 0.861 |
| Control | 369 | 137 | 0.371 | ||
| Excluding first 10 days* | IRS | 817 | 11 | 0.013 | 0.961 |
| Control | 325 | 113 | 0.348 |
*This scenario excludes the first 10 days after intervention to exclude transmission events likely originated before the intervention.
DISCUSSION
When pathogen transmission occurs in a nonrandom fashion through specific contact chains, there are significant benefits to modification of surveillance and control programs to account for this transmission heterogeneity (29–33). In Cairns, enhancing DENV passive surveillance by incorporation of location-based contact tracing and TIRS provided an unprecedented level of detail for understanding and responding to a virus outbreak. Although this approach may be effective in similar settings (for example, urban centers affected by sporadic outbreaks and with strong public health infrastructure in southern USA, Europe, or the Caribbean), the potential for implementation of contact tracing and TIRS in less-developed DENV endemic areas will need to be evaluated.
Reconstructing the full DENV transmission network during an epidemic is virtually impossible because not all links are detectable by surveillance system, particularly asymptomatic infections [some of which with the ability to contribute to transmission (34)] and symptomatic individuals not seeking health care (35). By capitalizing on the additional information obtained through contact tracing, we were able to gain a higher empirical insight into the complexity inherent to the epidemic transmission of DENV compared to studies that use the home residence as the unit of analysis. Human mobility was a major mechanism responsible for long-distance virus propagation, while within close proximity of the home residence (<200 m), the probability of detecting contact locations was negligible (<5%). These findings agree with previous research from urban areas in the Peruvian Amazon and Vietnam that show highly focal transmission of DENV (24, 28). The role of humans as dispersers of infection was demonstrated by our observations of the structure of the resulting DENV metropolitan transmission networks, which presented a strong source-sink structure in which most transmission events occurred within the city of Cairns. This pattern, also described when analyzing data at coarser spatial scales (36, 37), has important public health implications because vector control activities are generally deployed on those satellite towns that are potentially serving as sinks for transmission.
The recent Ebola outbreak in West Africa has provided solid evidence for the utility of incorporating contact tracing within resource-poor countries (38) but also points to significant gaps in local public health infrastructure (39). In large urban centers (for example, megacities) or areas that experience a high force of DENV infection, tracking the mobility of every case and using this information to inform control actions may not be feasible because of the limitations in personnel and the need to simultaneously cover multiple areas with reported contact locations. Thus, there is a need to assess the scalability and cost-effectiveness of contact tracing for DENV and other Aedes-borne viruses. For sexually transmitted pathogens, network models have been elegantly used to evaluate how contact structure, population size, the presence of asymptomatic infections, and spatial heterogeneity affect the efficacy of contact-based interventions (29, 33). Given the strong spatial heterogeneity in vector distribution (40) and the potential synergistic connections between human mobility, symptoms, and mosquito exposure (41), translating findings from directly transmitted to vector-borne pathogens may not be realistic. Mathematical or simulation models that account for human movement and out-of-home human-mosquito contacts [for example, see studies by Perkins et al. and Hladish et al. (42, 43)] could be used to theoretically evaluate the context within which contact tracing and targeted control may be more efficient at preventing virus transmission. Because both mosquito distribution and human mobility define the contact structure for DENV transmission (25), theoretical explorations could address key knowledge gaps, such as the impact of contact-based control across a wide range of DENV transmission intensity scenarios, the ability to detect and prevent inapparent infections able to contribute to transmission, and the critical population size beyond which contact-based interventions are ineffective.
We acknowledge that our approach has several limitations, but nonetheless consider it valuable for understanding the spatial dimension of dengue transmission and control. Because of confidentiality issues, we were not able to analyze information on the specific attributes of each location (for example, type of premises, time spent there). This information could have provided a better description of “risky” premises. We are aware of one case where an automobile repair shop with a large number of A. aegypti containers (tires and car fenders) located east of Cairns served as an important transmission focus. A multifamily housing unit located near the index case’s home was also a large contributor to transmission, with more than 30 cases being spatiotemporally linked to it. We identified transmission chains by linking records of symptomatic cases, ignoring the occurrence of asymptomatic (or underreported) cases. An independent blood donor study performed on Cairns residents during the outbreak estimated clinical/subclinical ratios of 1:0.59. We consider this a highly conservative estimate. Longitudinal DENV cohort studies have provided estimates of clinical/subclinical ratios orders of magnitude higher than what was reported in Cairns (44). Thus, because asymptomatic individuals have the potential to infect A. aegypti (34), we consider our inferred transmission chain as a subset of the full transmission network. The lack of entomological information was another limitation. While BG-Sentinel trap data from a few Cairns neighborhoods showed high-vector abundance at the beginning of the outbreak (45), the lack of records for the entire city limited our ability to estimate the entomologic impact of interventions and to refine our space-time interaction analyses by validating our identification of transmission areas with measures of vector abundance/presence.
There is a paucity of information about the efficacy of current vector control methods in preventing DENV or other Aedes-borne viruses (7, 13). This lack of evidence has led to widespread implementation of vector control tools irrespective of the context within which they are effective (for example, use of truck-mounted ultralow volume to control indoor-resting A. aegypti mosquitoes). Likewise, although our calculations of effectiveness for TIRS show an impressive level of household-level protection, there are several challenges in widespread implementation of this methodology across endemic settings. The method is laborious and, thus, not especially suited as a reactive program to cases in large urban centers or areas that experience a large force of infection; quite simply, there is insufficient time and staff to treat all houses. TIRS may be more suitable within reactive programs in areas with an established public health structure that emphasize case detection and rapid containment. Insecticide resistance, another challenge in A. aegypti control (46), can be mitigated by implementation of TIRS using an insecticide to which mosquitoes are susceptible [like the organophosphate pirimiphos-methyl (47) or carbamates such as bendiocarb (48)].
To further enhance the potential of TIRS and to increase coverage and applicability, new means of delivery will have to be developed. During the Cairns outbreak, emergency funding used an additional 50 vector control staff (a three-person spray team treats 20 to 30 houses in a day). However, even with additional resources, it took 3 months to control DENV in the city (after local resources were overwhelmed with the amount of premises they had to spray). A yet to be evaluated alternative is proactive use of TIRS in a strategic campaign performing prophylactic control before the advent of the transmission season targeting high-risk areas (for example, neighborhoods). Evidence from Iquitos, Peru, shows that indoor space spraying early in the transmission season can significantly reduce transmission of DENV (49). Prophylactic preseason control would involve identifying high-risk areas (areas identified from passive surveillance with history of high dengue occurrence) where source reduction/cleanup campaigns can be combined with TIRS at high coverage. These interventions can be performed during the month that precedes the beginning of the transmission season. For the case of Zika virus, premises (or frequented places such as day care centers and schools) of pregnant women could also be targeted for long-term prophylactic protection using TIRS. Undoubtedly, there is a need to rethink the control of Aedes-borne pathogens within the context of an integrated vector management program that takes local eco-epidemiological conditions and available resources into full consideration.
MATERIALS AND METHODS
Study area
The city of Cairns is the port of entry for most DENV introductions in Queensland, Australia (50). Surrounding Cairns are small coastal towns (population, <10,000) that are part of the metropolitan Cairns area (total 2011 population, ~154,000). After a large outbreak of DENV-2 early in the 1990s, Queensland Health developed the Dengue Fever Management Plan (see the Supplementary Text), focused on early detection of DENV importations, characterization of likely exposure sites of locally acquired cases by performing phone interviews (location-based contact tracing), TIRS application (see below for a detailed description of the methodology) of pyrethroid insecticides in households within 50 to 100 m of suspected transmission foci (residence and high-risk contact areas), larviciding (using s-methoprene, an insect growth regulator) within a 200-m radius of these premises, the deployment of lethal ovitraps in selected premises (51), and community education.
Diagnosis of all clinically suspected DENV cases was performed at local laboratories using rapid immunochromatographic tests and enzyme-linked immunosorbent assay (ELISA) to detect dengue immunoglobulin M (IgM). All positive serum samples were forwarded to the reference laboratory, where they were screened for the presence of anti-dengue IgM and IgG using a combined pool of flavivirus antigens in capture enzyme immunoassay. Positive IgM samples were further analyzed using flavivirus-specific IgM ELISA capture assays to identify the serotype of the infecting DENV (52). Additionally, real-time TaqMan reverse transcription polymerase chain reaction was performed on samples collected early in the acute illness to detect DENV RNA (53).
Location-based contact tracing was performed on every confirmed or suspected case of dengue by public health nurses who performed phone interviews asking specific questions about the locations that accounted for most of the daytime activities during the 2 to 4 weeks before the onset of symptoms (the procedures for contact tracing are described in the Supplementary Text). Interviews were also performed with the persons each patient identified as potential primary or secondary contacts (for example, workmates, relatives, friends) to enhance the detection of secondary infections (20). All contact tracing locational data were promptly mapped in a geographic information system (GIS) (ArcGIS 9.1) and used to target vector control activities, with the goal of rapidly containing virus propagation at its source (20). The Dengue Action Response Team (DART), the branch responsible for all vector control activities, prioritizes insecticide applications on each case’s likely transmission site (home and places identified by the public health nurses as high-risk transmission areas based on contact tracing data) as information enters their decision support system, generally within a few days of notification of each DENV case.
TIRS was performed using pyrethroid insecticides (SC 2.5% lambda-cyhalothrin, Demand) applied on A. aegypti resting sites, such as on exposed low walls (<1.5 m), under furniture, inside closets, and on any dark and moist surface where mosquitoes may be found resting (27). Unlike IRS, which requires complete removal of furniture and spraying of all exposed surfaces, TIRS was selectively applied in areas known to be resting sites for mosquitoes, markedly reducing application time (an average Cairns house is treated in 30 min) and insecticide use. This study focuses on the Cairns DENV-3 outbreak that occurred between November 2008 and May 2009 (45), to date the largest in record for Northern Queensland [a total number of 902 confirmed cases in metropolitan Cairns and 1025 in the entire North Queensland for the 2008–2009 outbreak and a total capital investment of over 1.1 million U.S. dollars in vector control and associated prevention activities (9)]. The human population characteristics, entomologic conditions, virus genotype, and virulence descriptions for the 2008–2009 Cairns outbreak were presented previously (9, 45). This study focuses on the spatiotemporal dynamics of such outbreak as well as the impact of interventions on virus transmission.
Data analysis plan
The study involved four interrelated analytical goals. First, we analyzed the reported contact locations from each confirmed case to characterize the connectivity between towns and to quantify key mobility parameters. A mobility network (expressing the proportion of all contact locations occurring between pairs of towns) was calculated from the contact tracing data set and plotted using the igraph package in the R statistical software (54). We also used R to calculate the cumulative probability density of the distance of each contact location to the home residence of each case.
Second, we applied a local space-time interaction test (local Knox test) to contact tracing data (house and contact locations for each case) to identify the locations statistically identified as most likely DENV transmission sites. This method tests whether the number of location pairs found in a particular time-space window (for example, pairs separated by M meters and T days) are significantly different from the number of locations expected in the same window given the total number of locations, considering the period over which the epidemic took place and the extent of the study area (55). Significance was assessed by comparing the observed values of the test statistic [run using a spatial window of 100 m and a temporal window of 20 days as upper limits for mosquito dispersal and virus cumulative incubation periods in humans and mosquitoes, respectively (56)], with the expected values under the null hypothesis of random case occurrence (in space and time) by performing 999 Monte Carlo simulations. Contact locations of cases were spatiotemporally linked if they were found within 100 m of each other and also if the onset of symptoms of each case within that spatial window occurred within 20 days (that is, our predefined threshold between the onset of symptoms of one case and the occurrence of symptoms in secondary cases that originated by mosquitoes infected by the initial case). The Knox test was also run using a temporal window of 30 days to evaluate the sensitivity of the results to the possibility of longer exposure times to infectious mosquitoes. The Knox test was run using the software ClusterSeer (TerraSeer). The output from the space-time interaction test was then characterized. Specifically, we estimated the metropolitan DENV transmission network as the proportion of contact locations statistically linked in space-time that occurred between pairs of towns using the same methods as for the mobility network. We also mapped the location of all statistically significant space-time clusters and determined, for each confirmed DENV case, who was linked in space-time to other cases, whether his or her putative transmission locations were his or her residence, an out-of-home location, or both (given that we were analyzing transmission chains, a case can be linked to more than one location).
The fourth analytical step involved querying those locations identified with the Knox test to evaluate the impact of TIRS performed on contact locations on DENV transmission. Specifically, given the magnitude of the outbreak, not all likely transmission sites were reached by DART. The main causes of not spraying a home included the lack of sufficient personnel to perform TIRS on all locations scheduled for control on a given week, the finding of closed premises at the time of visit, home residents denying TIRS treatment because of personal concerns, or the fact that some locations were mistakenly not identified as “at risk” by DART after visually querying the information from contact tracing in the Cairns GIS. This uneven intervention coverage (normal in outbreak situations) allowed us to innovatively quantify the likelihood of future DENV cases in houses that received TIRS and in houses that did not. Specifically, we noted the number of days that elapsed between the onset of symptoms of the first case and the onset of symptoms of future cases in houses receiving TIRS (treatment) or not receiving TIRS (control). These data were then treated as a time-to-event data set and analyzed using a mixed-effects Cox proportional hazard model with the package coxme in R (57). For this analysis, we used neighborhood (fig. S7) as a random intercept of the model to account for potential nonindependence of observations within close proximity from each other. During the outbreak, DART only performed TIRS once in each premise because residual effect of the insecticide was assumed to last for at least 2 to 3 months.
We consider this not to be a fully controlled study but more an evaluation of TIRS under natural operational conditions. We thus used the results of our spatial analyses to calculate the effectiveness of TIRS, expressed as the proportional reduction in the occurrence of future DENV cases at locations receiving the intervention, and calculated as the equation described in Halloran et al. (58): effectiveness = 1 − (risk of infection in treatment group)/(risk of infection in control group), where the risk of infection was calculated as the proportion of locations that had at least one infection after treatment or the onset of symptoms of the first DENV case in control houses (that is, our evaluation was based on transmission at a location rather than seroconversions).
The protocols for storing, analyzing, and reporting the results on the 2008–2009 Cairns dengue epidemic data were approved by Queensland Health’s Human Research Ethics Committee (protocol HREC/09/QCH/52/AM01).
Supplementary Material
Acknowledgments
We would like to thank all those who assisted with the investigation and management of the 2008–2009 DENV outbreak in North Queensland. They include personnel from the Tropical Public Health Unit (including A. Richards, J. Hanna, R. Spencer, D. Brookes, R. Spark, S. Heggie, P. Endres, L. Thomson, B. McCulloch, and the members of the DART) and all those who were involved with the mosquito control responses in the field. This study is dedicated to the late R. Spark, whose faith in the Queensland dengue control program has sustained it for over 20 years. Funding: G.M.V.-P. received support from the Emory Global Health Institute and Marcus Foundation (project #00052002) and the NSF (NSF/DEB/EEID:1640698) to perform this research. S.A.R. is funded by National Health and Medical Research Council Senior Research Fellowship 1044698. Author contributions: G.M.V.-P. performed analyses and wrote the manuscript; B.L.M., P.H., and S.A.R. performed the research and wrote the manuscript; and J.A.C. performed analyses and wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Original data used in this manuscript are subject to human subjects identification and cannot be made freely available. Contact the lead author (G.M.V.-P.) if interested in signing a data sharing agreement to gain access to the data set.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/2/e1602024/DC1
Supplementary Text
fig. S1. Distribution of contact locations (including the home residence) reported by each confirmed case of DENV.
fig. S2. Results from local space-time interaction test, showing the contact locations and significant space-time links found within prespecified windows of 100 m and 20 days.
fig. S3. DENV transmission chains.
fig. S4. The cumulative number of confirmed symptomatic cases of DENV reported (blue) and the cumulative number of targeted indoor residual sprays performed (orange) during the 2008–2009 outbreak that affected the metropolitan Cairns area, Australia.
fig. S5. Distribution of interventions performed in Cairns in response to the 2009 DENV-3 epidemic: TIRS with pyrethroid insecticides, the placement of lethal A. aegypti ovitraps, and community education (State Emergency Service).
fig. S6. Temporal distribution of the number of locations analyzed in the Cox proportional hazards model evaluating the impact of TIRS on dengue transmission.
fig. S7. Spatial distribution of locations analyzed in the Cox proportional hazards model evaluating the impact of TIRS on dengue transmission.
fig. S8. Number of secondary dengue cases spatiotemporally linked to locations TIRS-sprayed or not sprayed at all (control).
fig. S9. Form used by Queensland’s medical general practitioners reporting suspected or confirmed cases to the Tropical Public Health Unit.
fig. S10. Dengue case report forms used by the Tropical Public Health Unit nurses to interview suspected or confirmed dengue cases (and their contacts) and ascertain the locations visited while viremic and, ultimately, the most likely place of transmission (called “acquired where” in the form).
REFERENCES AND NOTES
- 1.Gubler D. J., Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 10, 100–103 (2002). [DOI] [PubMed] [Google Scholar]
- 2.van Panhuis W. G., Choisy M., Xiong X., Chok N. S., Akarasewi P., Iamsirithaworn S., Lam S. K., Chong C. K., Lam F. C., Phommasak B., Vongphrachanh P., Bouaphanh K., Rekol H., Hien N. T., Thai P. Q., Duong T. N., Chuang J.-H., Liu Y.-L., Ng L.-C., Shi Y., Tayag E. A., Roque V. G. Jr, Lee Suy L. L., Jarman R. G., Gibbons R. V., Velasco J. M. S., Yoon I.-K., Burke D. S., Cummings D. A. T., Region-wide synchrony and traveling waves of dengue across eight countries in Southeast Asia. Proc. Natl. Acad. Sci. U.S.A. 112, 13069–13074 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S., Gething P. W., Brady O. J., Messina J. P., Farlow A. W., Moyes C. L., Drake J. M., Brownstein J. S., Hoen A. G., Sankoh O., Myers M. F., George D. B., Jaenisch T., Wint G. R., Simmons C. P., Scott T. W., Farrar J. J., Hay S. I., The global distribution and burden of dengue. Nature 496, 504–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musso D. J., Gubler D. J., Zika virus. Clin. Microbiol. Rev. 29, 487–524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead S. B., Dengue vaccine development: A 75% solution? Lancet 380, 1535–1536 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Vannice K. S., Roehrig J. T., Hombach J., Next generation dengue vaccines: A review of the preclinical development pipeline. Vaccine 33, 7091–7099 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Achee N. L., Gould F., Perkins T. A., Reiner R. C. Jr, Morrison A. C., Ritchie S. A., Gubler D. J., Teyssou R., Scott T. W., A critical assessment of vector control for dengue prevention. PLOS Neglected Trop. Dis. 9, e0003655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO), Global strategy for dengue prevention and control 2012-2020 (WHO, 2012); http://apps.who.int/iris/handle/10665/75303.
- 9.Vazquez-Prokopec G. M., Chaves L. F., Ritchie S. A., Davis J., Kitron U., Unforeseen costs of cutting mosquito surveillance budgets. PLOS Neglected Trop. Dis. 4, e858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison A. C., Zielinski-Gutierrez E., Scott T. W., Rosenberg R., Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLOS Med. 5, e68 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George L., Lenhart A., Toledo J., Lazaro A., Han W. W., Velayudhan R., Runge Ranzinger S., Horstick O., Community-effectiveness of temephos for dengue vector control: A systematic literature review. PLOS Neglected Trop. Dis. 9, e0004006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horstick O., Runge-Ranzinger S., Nathan M. B., Kroeger A., Dengue vector-control services: How do they work? A systematic literature review and country case studies. Trans. R. Soc. Trop. Med. Hyg. 104, 379–386 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Bowman L. R., Donegan S., McCall P. J., Is dengue vector control deficient in effectiveness or evidence?: Systematic review and meta-analysis. PLOS Neglected Trop. Dis. 10, e0004551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perich M. J., Davila G., Turner A., Garcia A., Nelson M., Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City, Panama. J. Med. Entomol. 37, 541–546 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Chadee D. D., Resting behaviour of Aedes aegypti in Trinidad: With evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasites Vectors 6, 255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.P. Reiter, D. J. Gubler, Surveillance and control of urban dengue vectors, in Dengue and Dengue Hemorragic Fever, D. J. Gubler, G. Kuno, Eds. (CAB International, 1997). [Google Scholar]
- 17.Castle T., Amador M., Rawlins S., Figueroa J. P., Reiter P., Absence of impact of aerial malathion treatment on Aedes aegypti during a dengue outbreak in Kingston, Jamaica. Rev. Panam. Salud Publica 5, 100–105 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Koenraadt C. J., Aldstadt J., Kijchalao U., Kengluecha A., Jones J. W., Scott T. W., Spatial and temporal patterns in the recovery of Aedes aegypti (Diptera: Culicidae) populations after insecticide treatment. J. Med. Entomol. 44, 65–71 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Pant C. P., Mathis H. L., Residual effectiveness of ULV aerosols against Aedes aegypti in Bangkok: A study of sumithion and malathion applied by a portable ULV machine. Southeast Asian J. Trop. Med. Public Health 4, 231–237 (1973). [PubMed] [Google Scholar]
- 20.Vazquez-Prokopec G. M., Kitron U., Montgomery B., Horne P., Ritchie S. A., Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLOS Neglected Trop. Dis. 4, e920 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giglioli G., An investigation of the house-frequenting habits of mosquitoes of the British Guiana coastland in relation to the use of DDT. Am. J. Trop. Med. Hyg. 28, 43–70 (1948). [DOI] [PubMed] [Google Scholar]
- 22.Nathan M. B., Giglioli M. E., Eradication of Aedes aegypti on Cayman Brac and Little Cayman, West Indies, with Abate (Temephos) in 1970-1971. Bull. Pan Am. Health Organ. 16, 28–39 (1982). [PubMed] [Google Scholar]
- 23.Hanna J. N., Ritchie S. A., Phillips D. A., Serafin I. L., Hills S. L., van den Hurk A. F., Pyke A. T., McBride W. J., Amadio M. G., Spark R. L., An epidemic of dengue 3 in far North Queensland, 1997-1999. Med. J. Aust. 174, 178–182 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Stoddard S. T., Forshey B. M., Morrison A. C., Paz-Soldan V. A., Vazquez-Prokopec G. M., Astete H., Reiner R. C. Jr, Vilcarromero S., Elder J. P., Halsey E. S., Kochel T. J., Kitron U., Scott T. W., House-to-house human movement drives dengue virus transmission. Proc. Natl. Acad. Sci. U.S.A. 110, 994–999 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiner R. C. Jr, Stoddard S. T., Scott T. W., Socially structured human movement shapes dengue transmission despite the diffusive effect of mosquito dispersal. Epidemics 6, 30–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mammen M. P., Jr, Pimgate C., Koenraadt C. J. M., Rothman A. L., Aldstadt J., Nisalak A., Jarman R. G., Jones J. W., Srikiatkhachorn A., Ypil-Butac C. A., Getis A., Thammapalo S., Morrison A. C., Libraty D. H., Green S., Scott T. W., Spatial and temporal clustering of dengue virus transmission in Thai villages. PLOS Med. 5, e205 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie S. A., Hanna J. N., Hills S. L., Piispanen J. P., McBride W. J. H., Pyke A., Spark R. L., Dengue control in North Queensland, Australia: Case recognition and selective indoor residual spraying. Dengue Bull. 26, 7–13 (2002). [Google Scholar]
- 28.Anders K. L., Nga L. H., Thuy N. T. V., Ngoc T. V., Tam C. T., Tai L. T., Truong N. T., Le Duyen H. T., Trung V. T., Kien D. T. H., Wolbers M., Wills B., Chau N. V. V., Tho N. D., Simmons C. P., Households as foci for dengue transmission in highly urban Vietnam. PLOS Neglected Trop. Dis. 9, e0003528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eames K. T. D., Keeling M. J., Contact tracing and disease control. Proc. Biol. Sci. 270, 2565–2571 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd-Smith J. O., Schreiber S. J., Kopp P. E., Getz W. M., Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garnett G. P., Anderson R. M., Contact tracing and the estimation of sexual mixing patterns: The epidemiology of gonococcal infections. Sex. Transm. Dis. 20, 181–191 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Ross J. V., Black A. J., Contact tracing and antiviral prophylaxis in the early stages of a pandemic: The probability of a major outbreak. Math. Med. Biol. 32, 331–343 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Eames K. T. D., Keeling M. J., Modeling dynamic and network heterogeneities in the spread of sexually transmitted diseases. Proc. Natl. Acad. Sci. U.S.A. 99, 13330–13335 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duong V., Lambrechts L., Paul R. E., Ly S., Lay R. S., Long K. C., Huy R., Tarantola A., Scott T. W., Sakuntabhai A., Buchy P., Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 112, 14688–14693 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarti E., L’Azou M., Mercado M., Kuri P., Siqueira J. B. Jr, Solis E., Noriega F., Ochiai R. L., A comparative study on active and passive epidemiological surveillance for dengue in five countries of Latin America. Int. J. Infect. Dis. 44, 44–49 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Wesolowski A., Qureshi T., Boni M. F., Sundsøy P. R., Johansson M. A., Rasheed S. B., Engø-Monsen K., Buckee C. O., Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc. Natl. Acad. Sci. U.S.A. 112, 11887–11892 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pepin K. M., Leach C. B., Marques-Toledo C., Laass K. H., Paixao K. S., Luis A. D., Hayman D. T. S., Johnson N. G., Buhnerkempe M. G., Carver S., Grear D. A., Tsao K., Eiras A. E., Webb C. T., Utility of mosquito surveillance data for spatial prioritization of vector control against dengue viruses in three Brazilian cities. Parasites Vectors 8, 98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixon M. G., Taylor M. M., Dee J., Hakim A., Cantey P., Lim T., Bah H., Camara S. M., Ndongmo C. B., Togba M., Touré L. Y., Bilivogui P., Sylla M., Kinzer M., Coronado F., Tongren J. E., Swaminathan M., Mandigny L., Diallo B., Seyler T., Rondy M., Rodier G., Perea W. A., Dahl B., Contact tracing activities during the Ebola virus disease epidemic in Kindia and Faranah, Guinea, 2014. Emerging Infect. Dis. 21, 2022–2028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greiner A. L., Angelo K. M., McCollum A. M., Mirkovic K., Arthur R., Angulo F. J., Addressing contact tracing challenges—Critical to halting Ebola virus disease transmission. Int. J. Infect. Dis. 41, 53–55 (2015). [DOI] [PubMed] [Google Scholar]
- 40.LaCon G., Morrison A. C., Astete H., Stoddard S. T., Paz-Soldan V. A., Elder J. P., Halsey E. S., Scott T. W., Kitron U., Vazquez-Prokopec G. M., Shifting patterns of Aedes aegypti fine scale spatial clustering in Iquitos, Peru. PLOS Neglected Trop. Dis. 8, e3038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez-Prokopec G. M., Perkins T. A., Waller L. A., Lloyd A. L., Scott R. C. Jr, Reiner T. W. Jr, Kitron U., Coupled heterogeneities and their impact on parasite transmission and control. Trends Parasitol. 32, 356–367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perkins T. A., Scott T. W., Le Menach A., Smith D. L., Heterogeneity, mixing, and the spatial scales of mosquito-borne pathogen transmission. PLOS Comput. Biol. 9, e1003327 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hladish T. J., Pearson C. A. B., Chao D. L., Rojas D. P., Recchia G. L., Gómez-Dantés H., Halloran M. E., Pulliam J. R. C., Longini I. M., Projected impact of dengue vaccination in Yucatán, Mexico. PLOS Neglected Trop. Dis. 10, e0004661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.T. Endy, I. Yoon, M. P. Mammen, Prospective cohort studies of dengue viral transmission and severity of disease, in Dengue virus, A. L. Rothman Ed. (Springer, 2010), pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie S. A., Pyke A. T., Hall-Mendelin S., Day A., Mores C. N., Christofferson R. C., Gubler D. J., Bennett S. N., van den Hurk A. F., An explosive epidemic of DENV-3 in Cairns, Australia. PLOS ONE 8, e68137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranson H., Burhani J., Lumjuan N., Black W. C. IV, Insecticide resistance in dengue vectors. TropIKAnet 1, 1–12 (2010). [Google Scholar]
- 47.Oxborough R. M., Kitau J., Jones R., Feston E., Matowo J., Mosha F. W., Rowland M. W., Long-lasting control of Anopheles arabiensis by a single spray application of micro-encapsulated pirimiphos-methyl (Actellic® 300 CS). Malar. J. 13, 37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ossè R., Aikpon R., Padonou G. G., Oussou O., Yadouléton A., Akogbéto M., Evaluation of the efficacy of bendiocarb in indoor residual spraying against pyrethroid resistant malaria vectors in Benin: Results of the third campaign. Parasites Vectors 5, 163 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoddard S. T., Wearing H. J., Morrison R. C. Jr, Reiner A. C., Astete H., Vilcarromero S., Alvarez C., Ramal-Asayag C., Sihuincha M., Rocha C., Halsey E. S., Scott T. W., Kochel T. J., Forshey B. M., Long-term and seasonal dynamics of dengue in Iquitos, Peru. PLOS Neglected Trop. Dis. 8, e3003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanna J. N., Ritchie S. A., Outbreaks of dengue in North Queensland, 1990-2008. Commun. Dis. Intell. 33, 32–33 (2009). [PubMed] [Google Scholar]
- 51.Ritchie S. A., Buhagiar T. S., Townsend M., Hoffmann A., Van Den Hurk A. F., McMahon J. L., Eiras A. E., Field validation of the gravid Aedes trap (GAT) for collection of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 51, 210–219 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Taylor C., Simmons R., Smith I., Development of immunoglobulin M capture enzyme-linked immunosorbent assay to differentiate human flavivirus infections occurring in Australia. Clin. Diagn. Lab. Immunol. 12, 371–374 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warrilow D., Northill J. A., Pyke A., Smith G. A., Single rapid TaqMan fluorogenic probe based PCR assay that detects all four dengue serotypes. J. Med. Virol. 66, 524–528 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Csardi G., Nepusz T., The igraph software package for complex network research. InterJ. Compl. Syst. 1695, 1–9 (2006). [Google Scholar]
- 55.Knox E. G., Barlett M. S., The detection of space-time interactions. J. R. Stat. Soc. Ser. C. Appl. Stat. 13, 25–30 (1964). [Google Scholar]
- 56.Chan M., Johansson M. A., The incubation periods of dengue viruses. PLOS ONE 7, e50972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.T. M. Therneau, coxme: Mixed effects Cox models. R package version 2.2–5 (The Comprehensive R Archive Network, 2015); https://cran.r-project.org/web/packages/coxme/index.html.
- 58.M. E. Halloran, I. M. Longini Jr., C. J. Struchiner, Design and Analysis of Vaccine Studies (Springer, 2010), 390 pp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/2/e1602024/DC1
Supplementary Text
fig. S1. Distribution of contact locations (including the home residence) reported by each confirmed case of DENV.
fig. S2. Results from local space-time interaction test, showing the contact locations and significant space-time links found within prespecified windows of 100 m and 20 days.
fig. S3. DENV transmission chains.
fig. S4. The cumulative number of confirmed symptomatic cases of DENV reported (blue) and the cumulative number of targeted indoor residual sprays performed (orange) during the 2008–2009 outbreak that affected the metropolitan Cairns area, Australia.
fig. S5. Distribution of interventions performed in Cairns in response to the 2009 DENV-3 epidemic: TIRS with pyrethroid insecticides, the placement of lethal A. aegypti ovitraps, and community education (State Emergency Service).
fig. S6. Temporal distribution of the number of locations analyzed in the Cox proportional hazards model evaluating the impact of TIRS on dengue transmission.
fig. S7. Spatial distribution of locations analyzed in the Cox proportional hazards model evaluating the impact of TIRS on dengue transmission.
fig. S8. Number of secondary dengue cases spatiotemporally linked to locations TIRS-sprayed or not sprayed at all (control).
fig. S9. Form used by Queensland’s medical general practitioners reporting suspected or confirmed cases to the Tropical Public Health Unit.
fig. S10. Dengue case report forms used by the Tropical Public Health Unit nurses to interview suspected or confirmed dengue cases (and their contacts) and ascertain the locations visited while viremic and, ultimately, the most likely place of transmission (called “acquired where” in the form).


