Abstract
Rationale
Hydrogen peroxide (H2O2) regulates vascular tone in the human microcirculation under physiological and pathophysiological conditions. It dilates arterioles by activating BKCa channels in subjects with coronary artery disease (CAD), but its mechanisms of action in subjects without CAD (non-CAD) as compared to those with CAD remain unknown.
Objective
We hypothesize that H2O2-elicited dilation involves different K+ channels in non-CAD versus CAD, resulting in an altered capacity for vasodilation during disease.
Methods and Results
H2O2 induced endothelium-independent vasodilation in non-CAD adipose arterioles, which was reduced by paxilline, a BKCa channel blocker, and by 4-AP, a KV channel blocker. Assays of mRNA transcripts, protein expression and subcellular localization revealed that KV1.5 is the major KV1 channel expressed in vascular smooth muscle cells (VSMCs) and is abundantly localized on the plasma membrane. The selective KV1.5 blocker DPO-1 and the KV1.3/1.5 blocker Psora-4 reduced H2O2-elicited dilation to a similar extent as 4-AP, but the selective KV1.3 blocker PAP-1 was without effect. In arterioles from CAD subjects, H2O2-induced dilation was significantly reduced and this dilation was inhibited by paxilline but not by 4-AP, DPO-1 or Psora-4. KV1.5 cell membrane localization and DPO-1-sensitive K+ currents were markedly reduced in isolated VSMCs from CAD arterioles, although mRNA or total cellular protein expression were largely unchanged.
Conclusions
In human arterioles, H2O2-induced dilation is impaired in CAD, which is associated with a transition from a combined BKCa- and KV (KV1.5)-mediated vasodilation toward a BKCa-predominant mechanism of dilation. Loss of KV1.5 vasomotor function may play an important role in microvascular dysfunction in CAD or other vascular diseases.
Keywords: Hydrogen peroxide, potassium channels, voltage-gated potassium channels, calcium-activated, vasodilation, endothelium-dependent hyperpolarization factor
Subject Terms: Vascular Biology, Ion Channels/Membrane Transport, Oxidant Stress, Coronary Artery Disease, Vascular Disease
INTRODUCTION
Hydrogen peroxide (H2O2), a diffusible reactive oxygen species (ROS), has been recognized as an important regulator of vascular tone and homeostasis under physiological and pathophysiological conditions.1–3 As an endothelium-derived hyperpolarization (EDH) factor, H2O2 induces smooth muscle cell hyperpolarization and vasodilation in human coronary and adipose arterioles from subjects with coronary artery disease (CAD).4–7 Other studies have also demonstrated H2O2-induced hyperpolarization and dilation in normal human and animal arteries.8 The mechanisms of H2O2-induced vasodilation have not been fully elucidated; however, two main types of K+ channels in vascular smooth muscle cells (VSMCs), large-conductance Ca2+-activated K+ (BKCa) channels and voltage-gated K+ (KV) channels, have been variably implicated in different vascular beds.9 For instance, BKCa channels contribute to H2O2-induced dilation in porcine coronary arteries.10,11 In contrast, 4-aminopyridine (4-AP)-sensitive KV channels mediate dilation to H2O2 in canine coronary arteries,12 rat coronary and mesenteric arteries,12, 13 and porcine coronary resistance arteries.14 Using coronary arterioles from CAD subjects, we found that H2O2 opens smooth muscle BKCa channels to elicit smooth muscle hyperpolarization and relaxation.6, 7 The mechanisms of dilation by H2O2 in subjects without CAD (non-CAD) versus those with CAD, as well as the functional consequences, remain unknown, but there is evidence that BKCa and KV constitute two major K+ currents in VSMCs isolated from non-CAD human arteries.15
In addition to H2O2-induced activation of K+ channels, excessive and/or prolonged elevation of ROS can exert differential effects on vascular K+ channel function in disease.9, 16–18 Depending on the sensitivity of individual K+ channels and the oxidative species involved, ROS can activate, inhibit, or leave unaltered K+ channel function. 9 For example, impaired functions of KV channels have been shown in various animal models of cardiovascular disease, while BKCa channels may exhibit either gain or loss of function under pathophysiological conditions.9 The disease-associated alteration of vascular K+ channel function in humans is less well understood.19 In the present study, we tested the hypothesis that H2O2-elicited dilation involves different K+ channels in non-CAD versus CAD arterioles, resulting in an altered vasodilatory response in CAD. Using an integrated approach comprising isolated vascular reactivity measurement, molecular and immunohistochemical analyses, and electrophysiology, we assessed the role of two different types of K+ channels (BKCa vs. KV) in H2O2-induced dilation of human arterioles from non-CAD and CAD subjects. We further identified specific KV1 channels, the major vascular KV channel subfamily, in VSMCs and examined their functional contribution to H2O2-induced dilation. The impact of CAD on the function of KV1 channels and potential underlying mechanisms were also determined.
METHODS
Tissue acquisition
Fresh human adipose tissues (pericardial, visceral and subcutaneous, n=24, 68 and 28, respectively.) were obtained as discarded surgical specimens from a total of 120 patients undergoing abdominal surgeries or cardiopulmonary by-pass procedures, and unused whole hearts (n=14) acquired from Donor Network. Patient demographic information is summarized in Online Table I.
Videomicroscopy
Arterioles (internal diameter, 100–250 μm) were carefully dissected from human adipose tissues and cannulated with two glass micropipettes for measurements of diameter with a video system as previously described.20 Arterioles were preconstricted with endothelin-1 to approximately 30–50% of the baseline internal diameter. Relaxation responses to cumulative addition of H2O2 (1–100 μmol/L) to the vessel bath were determined in the absence and presence of 30 min preincubation with various modulators, including BKCa and KV blockers. At the end of each experiment, papaverine (100 μmol/L) was added to determine the maximal internal diameter for normalization of dilator responses. Unless otherwise stated, experiments were performed on endothelium-intact arterioles and in the presence of L-NAME (100 μmol/L) and Indomethacin (10 μmol/L).
Enzymatic isolation of vascular cells
Vascular smooth muscle cells (VSMCs) were enzymatically dissociated from arteries as previously described.7, 20 Cells were placed on ice and used the same day.
Patch-clamp recording of K+ currents
Whole-cell K+ currents were measured in freshly dissociated smooth muscle cells using the standard (ruptured-patch) or perforated patch-clamp method as previously described.15, 21 The pipette solution contained (in mmol/L) 90 potassium aspartate, 30 KCl, 20 NaCl, 1 MgCl2, 1 Mg-ATP, 1 EGTA, and 10 HEPES (pH 7.2 with KOH). The bath solution was composed of (in mmol/L) 140 NaCl, 5 KCl, 0.1 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES (pH 7.4 with NaOH). Paxilline (100 nmol/L) was added in the bath solution to further minimize BKCa currents and thus allow relative isolation of KV currents. Unless otherwise stated, all chemicals were applied to the bath through perfusion. Experiments were performed at room temperature.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from human adipose arteries and arterioles (200–500 μm) was extracted and cDNA was synthesized. The cDNA was amplified using a touch-down PCR protocol with gene-specific primers. Relative KVα1.5 gene expression was quantified with real-time PCR using pooled sample cDNA from each non-CAD and CAD group. For primer sequences refer to Online Table III.
Immunoblotting
Human adipose arteries and arterioles (200–500 μm) and coronary arteries (200–2,000 μm) were dissected and membrane proteins were prepared with a differential centrifugation method as described previously.22 Protein samples (20 μg) were separated by 10% SDS-PAGE. Membranes were blotted with a primary antibody against a specific KV1 α-subunit, BKCa, and Na+/K+-ATPase (1:2,000 dilution), followed by a horseradish-peroxidase conjugated secondary antibody (1:20,000 dilution). Membranes were developed using the ECL Prime reagent (Amersham).
Immunohistochemistry
Freshly dissected arteries were embedded in OTC compound, frozen on dry-ice, and cut into 10-μm sections.20 Sections were blocked with 5% normal goat serum and probed with a monoclonal or polyclonal antibody against a specific KV1 α-subunit (1:200 dilution), followed by secondary probing with an Alexa Fluor 568-conjugated goat anti-rabbit or anti-mouse IgG antibody (1:400 dilution). Sections were counterstained with DAPI and mounted in SlowFade antifade medium (Invitrogen). Images were immediately captured using a confocal fluorescence microscope (model A1-R, Nikon).
Immunocytochemistry
Freshly isolated VSMCs were fixed with 4% paraformaldehyde and permeabilized with 0.1% saponin.7 Cells were then incubated either with a monoclonal or polyclonal antibody specific to a KV1 α-subunit (1:200 dilution), followed by an appropriate goat secondary antibody conjugated with Alexa Fluor 488 (1:400 dilution). Cells were then mounted and images were captured using a confocal fluorescence microscope.
Chemicals
DPO-1, paxilline, and Psora-4 were obtained from Tocris, stromatoxin from Alomone, and TCEP from Thermo Scientific. All other chemicals were purchased from Sigma. Stock solutions were prepared in distilled water, except for the following: CP339818, DPO-1, PAP-1, paxilline, and Psora-4 (ethanol); 4-AP (HCl, pH readjusted to 7.4); and indomethacin (0.1 mol/L Na2CO3).
Statistical analysis
All data are presented as mean±SEM. Comparisons of concentration-response curves of isolated vessels were performed using 2-way repeated measures analysis of variance (ANOVA), followed by the Student-Newman-Keuls multiple-comparison test. Other comparisons were made using 1-way ANOVA or Student t-test. P values <0.05 were considered statistically significant.
RESULTS
KV channels contribute to H2O2-induced vasodilation in non-CAD but not in CAD human arterioles
Previous studies have shown impaired function of smooth muscle K+ channels in animal models of vascular disease, such as hypertension and metabolic syndrome. 9, 16–18 It remains largely unknown whether K+ channel function is similarly altered in humans. We first examined the role of BKCa and KV channels in H2O2-induced dilation using adipose arterioles from non-CAD and CAD subjects. Accumulating evidence indicates that peripheral arterioles such as those from adipose tissues, which are more readily available, can serve as a surrogate for assessing systemic and coronary arteriolar function.19
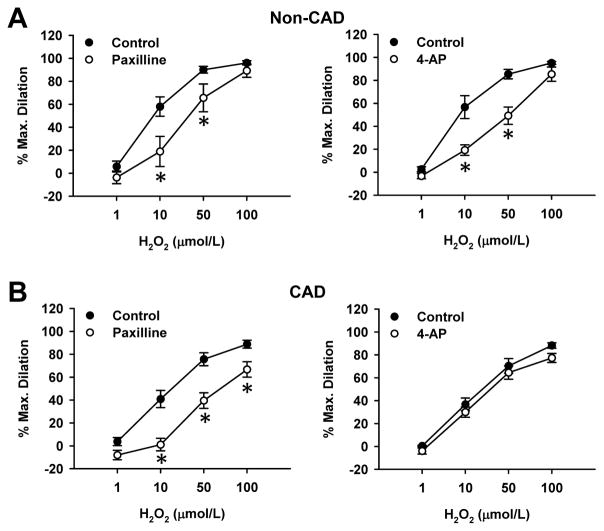
In non-CAD adipose arterioles (Figure 1A), H2O2 (1–100 μmol/L) induced vasodilation in a concentration-dependent manner (% maximal dilation, 96±2). Treatment of arterioles with paxilline (100 nmol/L), a potent BKCa channel blocker, induced a rightward shift of H2O2-induced dilation (% dilation at 10 μmol/L H2O2, 19±13 vs. 58±8 in control, and % dilation at 50 μmol/L H2O2, 66±12 vs. 90±3 in control, n=5, P<0.05). The general KV channel blocker 4-AP (10 mmol/L) caused a similar rightward shift in the response to H2O2 (% dilation at 10 μmol/L H2O2, 19±5 vs. 57±10 in control, % dilation at 50 μmol/L H2O2, 49±8 vs. 85±4 in control, n=5, P<0.05). Combined blockade of BKCa and KV channels abolished dilation up to 30 μmol/L H2O2 (Online Figure IA), indicating that these two K+ channels mediate a major portion of H2O2-induced dilation in non-CAD human adipose arterioles. The potency of H2O2 (EC50, 12±1 μmol/L; n=18, Online Figure IC) is similar to those reported in canine12 and human7 coronary arterioles.
Figure 1. Role of BKCa and KV channels in H2O2-induced dilation of human adipose arterioles from non-CAD and CAD subjects.
H2O2 induced dose-dependent dilation in adipose arterioles. The dilation was reduced by paxilline (100 nmol/L), a BKCa channel blocker, in both non-CAD (A, left) and CAD (B, left) arterioles. In contrast, 4-AP (10 mmol/L), a general KV channel blocker, reduced the dilation in non-CAD (A, right) but not CAD (B, right) arterioles, suggesting a loss of KV channel function in disease. *P<0.05 versus control; n=5–6 vessels/group.
In CAD arterioles (Figure 1B), H2O2-induced dilation was also blocked by paxilline (% dilation at 10 μmol/L, 1±6 vs. 41±8 in CAD control, % dilation at 50 μmol/L, 40±7 vs. 76±6 in CAD control, n=5, P<0.05). However, the dilation was not affected by 4-AP. These results indicate that KV channel-dependent dilation in response to H2O2 is impaired in CAD, whereas consistent with our previous findings in coronary arterioles,7 BKCa remains functional in disease.
Incubation of arterioles with 4-AP induced significant vasoconstriction in non-CAD but not in CAD subjects (Online Table II), indicating that KV channels regulate basal vascular tone in non-CAD subjects.
H2O2-induced dilation involves smooth muscle hyperpolarization and is redox sensitive
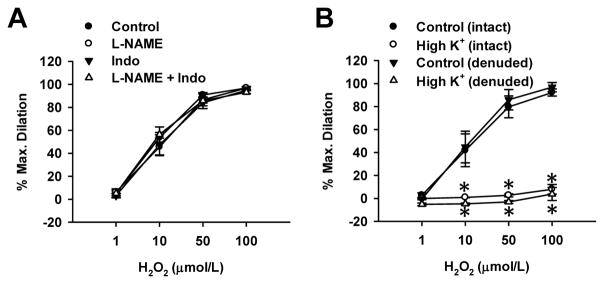
H2O2-induced dilation was not affected by L-NAME (100 μmol/L), a NOS inhibitor, indomethacin (Indo, 10 μmol/L), a COX inhibitor, alone or in combination, suggesting that endothelial NO and prostacyclin do not contribute to H2O2-induced dilation. In addition, H2O2 induced similar dilation in endothelium-intact and denuded arterioles (Figure 2B), further inferring that the dilation is mediated via an endothelium-independent, likely smooth muscle mechanism. Next, the role of smooth muscle hyperpolarization in H2O2-induced dilation was examined by using high K+ (60 mmol/L)-Krebs-PSS in the presence of L-NAME and indomethacin (Figure 2B). Dilation to H2O2 was abolished by high K+ in both endothelium-intact and -denuded arterioles (% dilation at 100 μmol/L, 8±4 and 4±6, respectively, n=3, P<0.05 vs. control), indicating H2O2-induced dilation is dependent on membrane hyperpolarization of VSMCs.
Figure 2. Effects of NOS and COX inhibition, endothelium denudation, and high K+ on H2O2-induced dilation in non-CAD human adipose arterioles.
A, The dilation was not affected by the nitric oxide synthase (NOS) inhibitor L-NAME (100 μmol/L), the cyclooxygenase inhibitor indomethacin (Indo, 10 μmol/L), alone or in combination. n=6 vessels/group. B, The dilation was not affected by removal of the endothelium, but was abolished by high K+ (60 mmol/L). n=3 vessels/group; *P<0.05 versus control.
Treatment of adipose arterioles with exogenous catalase (1000 U/mL), a H2O2 metabolizing enzyme, also completely abolished H2O2-induced dilation (Online Figure II). An important mechanism by which H2O2 elicits biological effects is through oxidizing thiol groups of its target proteins.23 Indeed, DTT (3 mmol/L), a membrane-permeant and thiol-specific reducing agent, quickly reversed H2O2-induced dilation within 3–5 min (% dilation at 100 μmol/L H2O2, 1±3 vs. 83±4 before DTT, n=3, P<0.05). However, TCEP (5 mmol/L), a membrane-impermeable thiol-reducing agent, had no effect. These data further confirm that H2O2 is a potent, specific and reversible smooth muscle relaxant, a characteristic consistent with its putative important signaling role in human arterioles.
Detection of KV1 subunit mRNA and protein expression in non-CAD human arteries
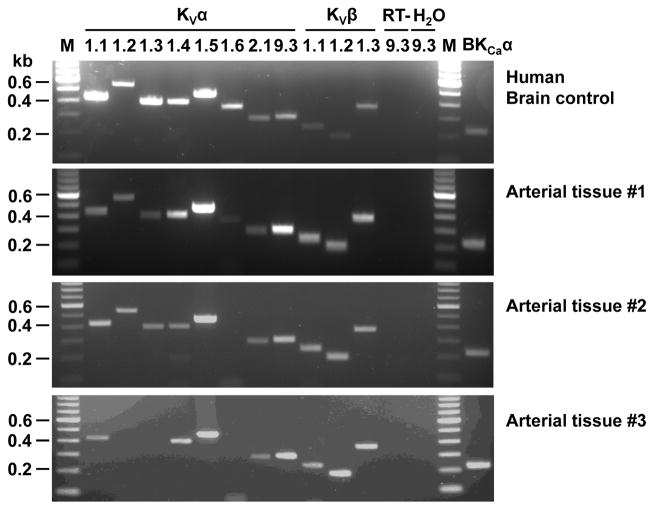
The specific type(s) of KV channels expressed in human arteries remains largely unexplored. We focused on KV1 channels (shaker-related family), which are functionally significant KV channels in most animal vascular beds studied24 and are 4-AP-sensitive. Figure 3 shows mRNA expression of different KV1 channel subunits in non-CAD adipose arterioles as assessed by RT-PCR. Several KV1 α (pore-forming)-subunits, including 1.1, 1.2, 1.4, 1.5, as well as BKCa α-subunits, were consistently found in different samples including denuded vessels (n=5), with KV1.5 being the most abundantly expressed KV1 α-subunit. The two other KV1 α-subunits (1.3 and 1.6) were variably expressed in some but not all samples. We also detected the α-subunits that form a second type of vascular KV channels (2.1, 9.3), KV1 β-(accessory)-subunits (1.1–1.3), although these subunits were not further pursued in the following immunoblotting assays.
Figure 3. The mRNA expression of KV1 and BKCa channel subunits in non-CAD human adipose arteries and arterioles.
Three representative gel images of RT-PCR amplification products from adipose arteries and arterioles are shown (lower 3 panels). KVα1.1, α1.2, α1.4, α1.5, KVα2.1, KVα9.3, KVβ1.1-β1.3, and BKCa were consistently found in different samples, whereas KVα1.3 and α1.6 subunits were variably detected. As a positive control, human brain samples from a normal subject were found to express all KV and BKCa channel subunits studied (top panel). RT-, without reverse transcription; H2O, without template; M, marker.
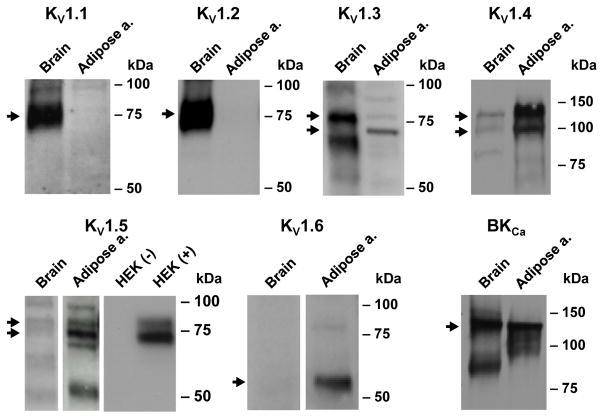
Using subunit-specific antibodies, we examined the protein expression of KV1.1–1.6 and BKCa α-subunits in the membrane fraction prepared from non-CAD adipose arteries/arterioles (n=2). Consistent with mRNA expression data, KV1.4 and 1.5, and BKCa channels were readily detected at the protein level (Figure 4). KV1.3 and 1.6 proteins were also found in non-CAD arteries/arterioles, whereas KV1.1 and 1.2 proteins were not detected. The absence of KV1.2 in human vascular samples was unexpected since it is a dominant KV1 α-subunit that forms channel complex with KV1.5 in rat cerebral arteries.25 The failure to detect KV1.1 or 1.2 in arterial samples does not seem to result from non-reactivity of the antibodies used because abundant expression of these two proteins was observed in human brain parallel controls.
Figure 4. Western blot detection of KV1 and BKCa channel α subunits.
Representative images of protein expression of KV1 and BKCa channel-forming α subunits in the membrane fraction of non-CAD human adipose arteries. Consistent with mRNA expression, KV1.5 protein was detected in adipose arteries. KV1.3, 1.4, and 1.6 were also detected in these samples. Human brain tissue (membrane fraction) was included as a positive control, which was found to abundantly express KV1.1 and 1.2, as well as BKCa. Total lysates of HEK293 cells with (+) or without (-) exogenous KV1.5 overexpression were also included as an additional control for KV1.5. Arrow indicates mature forms of KV1 or BKCa protein, with the approximate molecular mass values as follows: 75 kDa (1.1), 75 kDa (1.2), 75 kDa doublet (1.3), 110 kDa doublet (1.4), 75 kDa doublet (1.5), 60 kDa (1.6), and 130 kDa (BKCa).
KV1 channels (except KV1.6) are expressed as mature N-glycosylated proteins in native tissues such as brain. 26 As shown in Figure 4, the apparent molecular mass values of KV1 proteins in human brain or adipose arterial tissues were approximately 75 kDa (1.1 and 1.2), doublet around 75 kDa (1.3 and 1.5), doublet around 110 kDa (1.4), and 60 kDa (1.6). Whereas KV1.6 appears to be unmodified, KV1.1–1.5 channels are of higher apparent molecular weights corresponding to glycosylated or other post-translationally modified forms as previously reported in the brain or vascular tissues.25,27
Detection of KV1 subunit protein localization in non-CAD human arteries
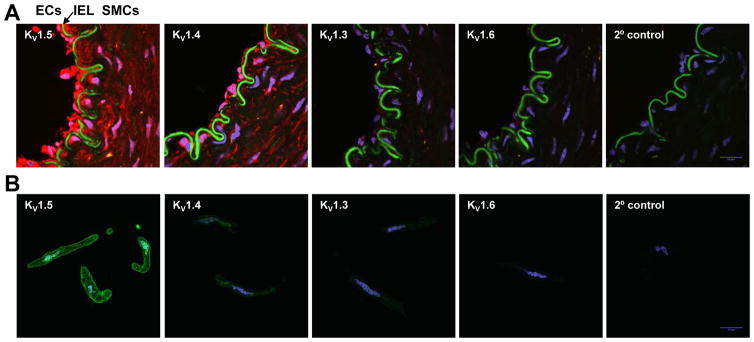
To determine the cell type-specific localization of KV1 α-subunits in human arteries, we performed frozen-section immunofluorescence staining of human adipose arterioles. KV1 proteins were labeled with red Alexa-568-conjugated secondary antibody and images were captured with a confocal fluorescence microscope. Thus, autofluorescence intrinsic to the internal elastic lamina can be detected in the green FITC channel and used to visually divide the endothelium and smooth muscle layers. As shown in Figure 5A, KV1.5 and to a less amount KV1.4 proteins were readily detected in the smooth muscle layer. Surprisingly, these two proteins were also abundantly expressed in endothelial cells (ECs). A small amount of KV1.3 and 1.6 were also detected in ECs.
Figure 5. Immunofluorescence localization of KV1 α-subunits in human adipose arterioles.
KV1.5 is the major KV1 channel protein expressed in human adipose arteriolar smooth muscle cells. A: Confocal immunofluorescence images of KV1 α-subunit proteins (red) in cross tissue sections (10 μm) of an intact human adipose arteriole. KV1.5 and 1.4 of a lower level were detected in smooth muscle cells (SMCs) and endothelial cells (ECs). KV1.3 and 1.6 subunits were also faintly visible in ECs. Cell nuclei were stained with DAPI (blue). IEL, internal elastic lamina (green auto-fluorescence). B: Confocal immunofluorescence images of corresponding KV1 α-subunit proteins (green) in freshly dissociated SMCs from a human adipose arteriole. KV1.5 protein was mainly localized on the cell membrane of SMCs. Cell nuclei was stained with DAPI (blue). Data are representative of >3 independent tissues.
The limited resolution power of immunohistochemistry did not allow individual VSMCs to be distinguished even at 600X magnification. To further examine the subcellular localization of KV1 proteins, we freshly dissociated VSMCs from adipose arterioles for immunocytochemistry. As shown in Figure 5B, KV1.5 protein was predominantly localized on the plasma membrane of dissociated SMCs. Interestingly, KV1.5 was also detected on the nuclei envelope, a cellular structure that is continuous with endoplasmic reticulum (ER) and serves as a site of initial protein synthesis for some membrane proteins.28, 29 KV1.4 protein was also detected in VSMCs, however the expression of KV1.4 protein was lower than that of KV1.5 and also seemed largely intracellular.
KV1.5 as a major functional KV1 channel in H2O2-induced dilation of non-CAD but not CAD human arterioles
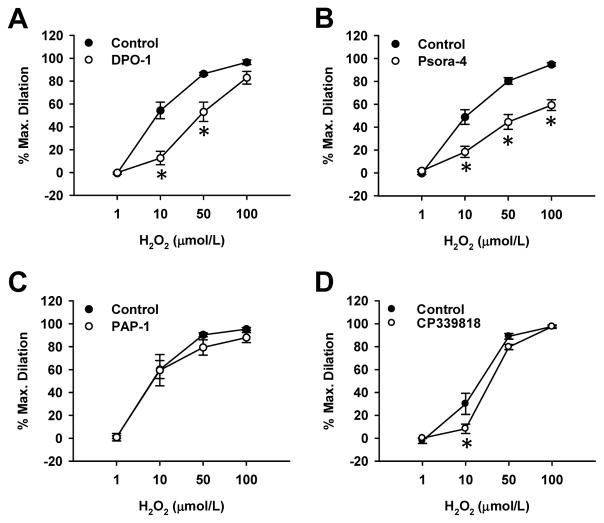
Given that KV1.5 is 4-AP-sensitive and abundantly expressed in VSMCs of human adipose arteries/arterioles, we subsequently examined whether this KV1 channel is functionally involved in human arteriolar dilation. Adipose arterioles from non-CAD subjects were pretreated with subtype-specific KV channel blockers and examined for H2O2-induced vasodilation. We found that selective KV1.5 channel blocker DPO-1 (1 μmol/L) markedly reduced H2O2-induced dilation (Figure 6A; % dilation at 10 μmol/L, 13±6 vs. 55±7 in control, % dilation at 50 μmol/L, 53±9 vs. 86±1 in control, n=5, P<0.05), to a similar extent as after 4-AP treatment (Figure 1). Selective KV1.3/1.5 blocker Psora-4 (30 nmol/L) caused a similar rightward shift in H2O2-induced dilation, but further reduced the maximal dilation to 100 μmol/L H2O2 (59±5% vs. 95±2% in control, n=5, P<0.05; Figure 6B). In contrast, selective KV1.3 blocker PAP-1 (10 nmol/L) did not affect H2O2-induced dilation (Figure 6C; n=5). The KV1.3/1.4 blocker CP-339818 (3 μmol/L) slightly attenuated the dilation only at 10 μmol/L H2O2 (Figure 6D), suggesting a minor contribution of KV1.4 to H2O2-induced dilation. In addition, H2O2-induced and DPO-1-inhibitable dilation was significantly reduced in arterioles treated with KV1.5 siRNA (Online Figure III). Together, these results suggest that KV1.5 serves as a major 4-AP-sensitive KV1 channel contributing to H2O2-induced dilation in non-CAD adipose arterioles.
Figure 6. Role of KV1.5 in H2O2-induced dilation of non-CAD human adipose arterioles.
The dilation was blocked by DPO-1 (1 μmol/L, A), a selective KV1.5 channel blocker, and Psora-4 (30 nmol/L, B), a KV1.3/1.5 blocker. However, the dilation was not affected by PAP-1 (10 nmol/L, C), a KV1.3 blocker. CP-339818 (3 μmol/L, D), a KV1.3/1.4 blocker, slightly attenuated the dilation induced by 10 μmol/L H2O2 only. *P<0.05 versus control; n=5 vessels/group.
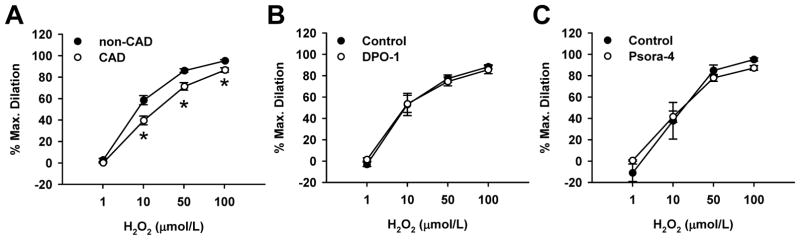
Compared to non-CAD arterioles, there was a slight but statistically significant reduction of H2O2-induced dilation in CAD (% dilation at 10 μmol/L H2O2, 40±4 vs. 59±4 in non-CAD, % dilation at 50 μmol/L, 71±3 vs. 86±2 in non-CAD, n=18/group, P<0.05; Figure 7A). In contrast to non-CAD arterioles (Figure 6), KV1.5 channel blockade by either DPO-1 or Psora-4 did not alter H2O2-induced dilation in CAD (Figure 7B and 7C), a finding consistent with ineffectiveness of 4-AP in CAD arterioles. These results indicate a loss of functional KV channels, smooth muscle KV1.5 in particular, in human arterioles during CAD.
Figure 7. Role of KV1.5 in H2O2-induced dilation of CAD adipose arterioles.
Compared to non-CAD arterioles, H2O2-induced dilation was shifted rightward in CAD arterioles (A). DPO-1 (1 μmol/L, B) and Psora-4 (30 nmol/L, C) failed to alter the dilation in CAD arterioles. n=18 (A) or 3–6 (B, C) vessels/group; *P<0.05 versus control.
Potential mechanism of impaired KV1.5 function in CAD human arteries
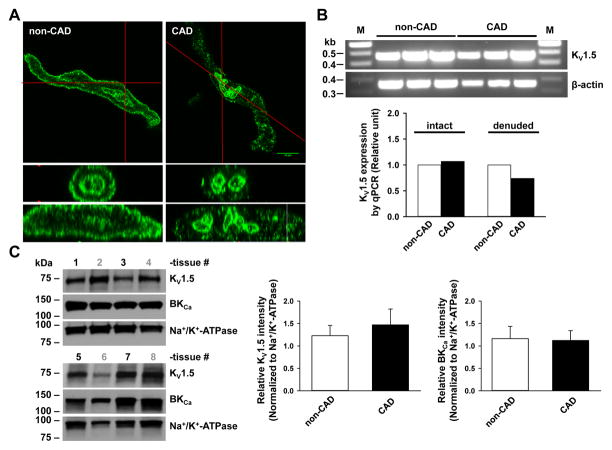
We further examined the potential mechanisms responsible for impaired KV1.5 function in CAD vessels. By immunostaining of freshly dissociated VSMCs, we found that the plasma membrane staining of KV1.5 protein was markedly reduced in CAD subjects (Figure 8A). Analysis of plasma membrane/cytoplasmic ratio of KV1.5 fluorescence further confirmed reduced plasma membrane distribution of this protein in VSMCs from CAD subjects as compared to non-CAD subjects (1.83±0.12 and 2.62±0.15, respectively; P<0.05). Intriguingly, KV1.5 immunofluorescence from the nuclear envelope was comparable in CAD vs non-CAD samples.
Figure 8. Comparison of KV1.5 subcellular localization, total mRNA and protein expression, and K+ current in non-CAD and CAD human arterioles.
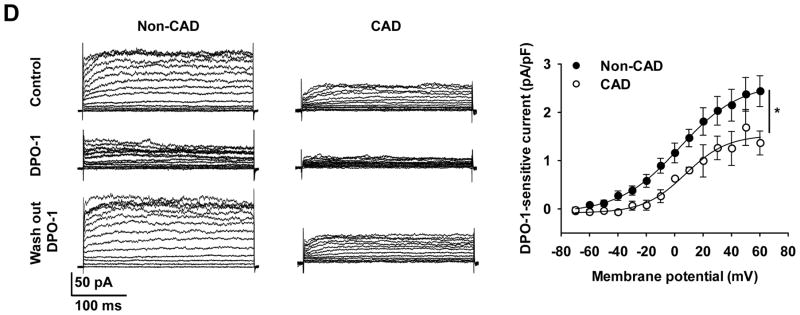
A: Immunofluorescence detection of KV1.5 subunit proteins (green) in freshly dissociated VSMCs from non-CAD and CAD human adipose arterioles. The plasma membrane expression of KV1.5 was markedly reduced in CAD as compared to non-CAD VSMCs. Lower two images represent cross-section views of top images vertically sectioned along red lines. Images are representative of results obtained from 3–4 each of non-CAD and CAD tissues. B: RT-PCR analysis of KV1.5 mRNA. Top, end-point PCR gel images of KV1.5, as well as β-actin control from non-CAD and CAD human adipose arterioles (n=3/group). Lower, relative abundance of KV1.5 mRNA in non-CAD and CAD, normalized to the mean of β-action and ATP5o by quantitative PCR. Tissues were processed individually for mRNA extraction and cDNA synthesis before an equal amount of individual cDNA samples was pooled within each group for PCR analysis (n=6, 6, 4 and 4 for non-CAD intact, CAD intact, non-CAD denuded, and CAD denuded groups, respectively). C: Western blot detection of KV1.5, BKCa and Na+/K+-ATPase proteins in non-CAD (tissue number in black) and CAD (tissue number in gray) human coronary arteries (n=4 tissues/group). Right, summarized data. D: Effect of DPO-1 on voltage-elicited whole-cell K+ currents in VSMCs freshly isolated from non-CAD and CAD human adipose arterioles. Currents were elicited by progressive 10 mV depolarizing steps from a holding potential of −70 mV to +60 mV. Left, representative traces recorded from two cells at baseline (control), 5–10 min after bath perfusion of 1 mmol/L DPO-1, and 5–10 min after DPO-1 washout, with a cell capacitance of 17 pF (non-CAD) and 18 pF (CAD), respectively. Right, averaged I-V relationships for DPO-1-sensitive K+ currents (normalized to cell capacitance) in non-CAD and CAD myocytes; n=4–8 cells from each non-CAD (n=6) and CAD (n=4) subjects. *P<0.05 versus non-CAD.
In contrast to KV1.5, BKCa protein localization on the plasma membrane was maintained in VSMCs from CAD subject as compared to non-CAD subjects (Online Figure IV-A; calculated plasma membrane/cytoplasmic ratio, 4.03±0.32 and 3.07±0.18, respectively). We also examined KV1.5 and BKCa immunofluorescence in ECs isolated from adipose arterioles of non-CAD and CAD subjects (Online Figure IV-A and B). Whole cell immunofluorescence of KV1.5 in ECs seemed comparable between CAD and non-CAD, although further analysis of plasma membrane/cytoplasmic ratio is difficult due to small cell size of isolated ECs. The immunoreactivity for BKCa channel α-subunits in ECs was minimal, which is in line with our previous findings that mRNA transcripts of BKCa were not detected in isolated ECs from human coronary arterioles.7 The specificity of KV1.5 antibodies was confirmed by using HEK293 cells with and without KV1.5-DDK transfection and double-staining technique (Online Figure V).
Using end-point RT-PCR analysis, the mRNA level of KV1.5 in CAD vessels was comparable to that of non-CAD tissues when normalized to the housekeeping gene β-actin (Figure 8B). These results were further confirmed by the quantitative analysis of mRNA expression using real-time PCR (Figure 8B, bar graph). Analysis of relative mRNA expression of KV1.5 normalized to the mean of two housekeeping genes (β-actin and ATP5o) did not show marked change in non-CAD and CAD vessels (intact n=6/group, denuded n=4/group). A slight increase (7%) in intact CAD vessels but a moderate decrease (26%) in denuded CAD vessels was observed.
We also compared the expression of KV1.5 protein in the membrane fraction of human coronary arteries (HCAs) from non-CAD and CAD patients (Figure 8C). Because vessels collected from CAD adipose tissues were usually limited and were not sufficient for membrane protein preparation, HCAs were used instead for these experiments. Our recent studies have shown that human conduit and resistance arteries express a similar profile of KV1 channels at both mRNA and protein levels.30 The average protein expression level of KV1.5 subunits normalized to Na+/K+-ATPase showed a trend toward an increase (but not statistically significant) in patients with CAD compared to those without (n=4 non-CAD and CAD subjects, respectively), although in 1 sample (out of 4) from CAD patients the level of KV1.5 channel expression was much lower than average. The protein expression of BKCa did not differ in the two groups.
We next examined whole-cell K+ currents in VSMCs freshly dissociated from non-CAD and CAD adipose arterioles (Figure 8D). In non-CAD VSMCs, progressive depolarizing steps from a holding potential of −70 mV elicited outward currents at membrane potentials positive to −40 mV that were subsequently reduced by DPO-1 (1 μmol/L). Compared to non-CAD, baseline K+ currents in CAD were lower. Although K+ currents were further reduced by DPO-1 in CAD, DPO-1-sensitive current density was markedly reduced as compared to non-CAD. Because K+ currents were recorded with low-Ca2+ pipette and bath solutions and in the presence of the BKCa channel blocker paxilline (100 nmol/L), the contribution of BKCa to whole-cell K+ currents was minimal. Recording of KV currents were also confirmed by the findings that 4-AP concentration-dependently decreased the outward K+ currents (Online Figure VI). Together, the above results suggest that mainly the reduced plasma membrane expression of smooth muscle KV1.5 subunits, rather than a change of mRNA or total protein, contributes to impaired vasomotor function of KV1.5 channels in human arteries with CAD.
DISCUSSION
The major new findings of this study are three-fold. First, H2O2 induces potent endothelium-independent vasodilation in adipose arterioles from non-CAD subjects that is largely mediated by BKCa and KV channels. Second, KV1.5 is the major type of smooth muscle KV1 channel responsible for KV-dependent H2O2 dilation in non-CAD arterioles. Third, the H2O2-elicited response is reduced in CAD arterioles and is accompanied by a loss of KV (especially KV1.5)- but not BKCa-dependent dilation. The impaired function of KV1.5 may result from reduced cell surface localization of the channel protein without significant change of mRNA or total protein expression. Together, these results demonstrate a transition from BKCa- and KV-mediated vasodilation toward a BKCa-predominant mechanism of dilation in the human microcirculation during CAD. While the pathophysiological significance of this K+ channel transition remains to be explored, the loss of KV1.5-mediated dilation to H2O2 or other vasodilators may represent an important mechanism contributing to microvascular dysfunction in humans with CAD or other vascular diseases.31–33
Smooth muscle K+ channels in H2O2-induced dilation
Using HCAs from subjects with CAD, we previously reported that H2O2 induces vasodilation by activating smooth muscle BKCa channels, an effect secondary to H2O2-induced dimerization of protein kinase G.7 BKCa channels have also been implicated in H2O2-induced dilation in different vascular beds such as porcine coronary arteries.10,11, However, other studies indicate that KV channels but not BKCa channels contribute to the dilatory effect of H2O2 in canine and rat coronary arteries.12 In the present study, we found that H2O2 induces high-K+-sensitive dilation in non-CAD adipose arterioles that is inhibited by blockers of both BKCa (paxilline and iberiotoxin) and KV channels (4-AP). Furthermore, a combination of BKCa and KV channel blockers almost completely abolished H2O2-induced dilation (up to 30 μmol/L H2O2), indicating that both BKCa and KV mediates H2O2-induced dilation in non-CAD human arterioles. The mechanisms by which H2O2 dilation involves distinct K+ channels in different species or vascular beds remain to be established.
There is evidence that endothelial pathways contribute to vasomotor effects of H2O2, including COX-derived prostacyclin.34 However, in human adipose arterioles, H2O2-induced dilation was not affected by inhibition of either NO and COX or endothelial denudation, confirming that the dilation is mostly smooth muscle-dependent.
Membrane-permeant thiol reducing agent DTT completely blocked H2O2-induced dilation in human adipose arterioles, whereas membrane-impermeable thiol reagent failed to reverse the dilation. These data support a H2O2-mediated redox modification of K+ channels proposed earlier12 but further suggest an intracellular site of action. Indeed, H2O2 can alter the activities of ion channels such as L-type Ca2+ channel35 and ATP-sensitive K+ channel.36 H2O2 also activates smooth muscle KV2.1 channels in rat mesenteric arteries through a similar mechanism via S-glutathionylation.13 It remains to be determined whether H2O2-induced dilation involves redox modification of KV channels, or alternatively other intermediate signaling proteins as we reported previously for protein kinase G in H2O2-induced BKCa activation.7
KV1 channel subtypes in human vasculature
The expression of specific KV subunit channel gene products and proteins varies greatly among species and vascular beds,24 and the molecular identity of KV channels, especially 4-AP-sensitive KV1 channels, in the human vasculature remains largely unknown. Using RT-PCR analysis of mRNA transcripts, we consistently detected KVα1.1, 1.2, 1.4, 1.5, 2.1, 9.3, and KVβ1.1–1.3 in both intact and denuded adipose arterioles from non-CAD subjects, while sample-to-sample variations were noted for KVα1.3 and 1.6 (Figure 3). Among six KV1 α-subunits, KV1.3–1.6 but not KV1.1–1.2 were also detected at the protein level (Figure 4). Immunofluorescence assay of vessel sections and freshly dissociated VSMCs further revealed that KV1.5 is the main subunit expressed on the plasma membrane of VSMCs. KV1.4 seems mainly intracellular in adipose VSMCs. We also found KV1.5, 1.4, and to a much less extent, KV1.3 and 1.6 in the endothelium of adipose arterioles.
Pharmacological studies further demonstrated that KV1.5 is a major functional KV1 channel responsible for H2O2-induced dilation in non-CAD adipose arterioles (Figure 6). This is based on the findings that KV1.5-selective blocker DPO-1 reduced H2O2-induce dilation by the same extent as the general KV1 blocker 4-AP. KV1.5 siRNA also significantly reduced H2O2-induced dilation in non-CAD arterioles (Online Figure III). Selective KV1.3/1.5 blocker Psora-4 caused a similar rightward shift while KV1.3 blocker failed to affect the dilation. Psora-4 further reduced the maximal dilation to 100 μmol/L H2O2, which may be because Psora-4 is >20-fold more potent for KV1.5 (IC50, 7.7 nmol/L37) than DPO-1 (IC50, 0.2–0.3 μmol/L38). Finally, KV1.3/1.4 blocker CP-339818 slightly reduced the vasodilator response to H2O2, suggesting a minor role of KV1.4.
In non-CAD adipose arterioles, the dilation induced by the highest concentration of H2O2 (100 μmol/L) was blocked by high K+ but only slightly blocked by the combination of BKCa and KV channel blockers, indicating that potential involvement of other K+ channels. Several studies reported that KV2 α-subunits or KV2.1/9.3 heterotetramers contribute to the regulation of vascular tone in rodent arteries.39, 40 In the present study, we detected vascular KV2.1 mRNA (Figure 3) and protein expression (data not shown); however, selective KV2.1 and KV2.1/9.3 blocker stromatoxin did not alter H2O2-induced dilation in non-CAD arterioles (Online Figure I). Redox-sensitive KV7 channels represent another potential candidate, but a recent study indicate that they do not contribute to H2O2 dilation in porcine coronary arteries.41 Further investigations are needed to elucidate the role of other K+ channels in human arterioles.
Alteration of K+ channel function in CAD
Compared to non-CAD arterioles, H2O2-induced dilation was significantly reduced in CAD arterioles (Figure 7A). Furthermore, H2O2-induced dilation of CAD adipose arterioles was inhibited by only BKCa but not KV channel blockers. To the best of our knowledge, this is the first report on the recruitment of different smooth muscle K+ channels in H2O2-induced vasodilation from health to disease. The expression of BKCa mRNA and protein, as well as protein localization, was not altered by CAD, a finding consistent with that of our previous study in HCAs.7 We thus conclude that BKCa channels remain functional in CAD arterioles. In other vascular beds or species, the effect of disease on the expression and function of BKCa channels remain complex or controversial.9, 16–18 For example, BKCa channel activity is increased in human VSMCs obtained from coronary atherosclerotic lesions.42 Animal studies support that BKCa channel expression is increased in hypertension, possibly as a compensatory mechanism for the downregulation of KV channel expression.18 However, BKCa activity is reduced by exposure to high glucose or high concentrations of H2O243 and in the porcine model of metabolic syndrome.44
The reduction of H2O2-induced dilation in CAD arterioles can be mainly attributed to a loss of KV, especially KV1.5, channel function in VSMCs. In contrast to non-CAD adipose arterioles, blocking of KV1.5 or other KV1 channels did not affect H2O2-induced dilation in CAD vessels (Figure 1 and 7). Alternations in vascular KV channel function and/or expression have also been reported in other pathological conditions such as pulmonary45 and systemic46 hypertension, metabolic syndrome47 and diabetes,48 but the identities of individual KV channels involved haven’t been well established. In human atrial myocytes, chronic atrial fibrillation, which is associated with oxidative stress or elevated ROS, reduces KV1.5 (IKur) expression.49
The precise mechanisms of impaired KV function under pathological conditions remain poorly understood. By examining KV1.5 mRNA/protein expression and subcellular localization and KV1.5 currents, we provide initial evidence that a reduced plasma membrane expression of KV1.5 in VSMCs, rather than a change of mRNA and total protein synthesis, may be mainly responsible for the reduced KV1.5 channel function in CAD. There is accumulating evidence that ion channels can recycle between the cytosol and cell membrane and this dynamic recycling determines the number of functional channels present in the plasma membrane.50–52 For example, a fraction of KV1.5 channels on the cell membrane is rapidly internalized with a half time of ~10 min and some return to the surface with a half time of ~30 min.52 In a HL-1 cell line, elevated ROS induces fairly rapid (within 60 min) reduction of surface expression of KV1.5.53 It remains to be tested in future studies whether prolonged elevation of ROS during CAD modulates cellular trafficking of smooth muscle KV1.5 channels to reduce their membrane expression in human arterioles.
Potential study limitations
We found that KV1.5 and KV1.4 channels are expressed in ECs of human adipose arterioles. It is thus possible that H2O2 may activate endothelial KV channels to induce endothelial hyperpolarization and subsequent vasodilation. Although the expression and function of endothelial KV channels are poorly understood,17 there is evidence on the role of KV channels in endothelium-dependent hyperpolarization and dilation in arteries such as porcine coronary arteries41,54 and guinea-pig coronary and carotid arteries.55, 56 However, H2O2-induced dilation of adipose arterioles is largely smooth muscle-dependent, and therefore the contribution of endothelial KV channels seems unlikely, at least in the present experimental settings. Nevertheless, we have not excluded a potential role for endothelial KV channels in the dilation to H2O2 under other conditions (e.g., endogenous H2O2 generated in ECs).
The present study used arterial tissues from human subjects with a variety of conditions that can affect vasodilator responses. By necessity, the non-CAD tissue samples are often collected from subjects with diverse diseases and thus are not true normal controls. To minimize potential confounding effects of underlying disease, only subjects with no more than 1 risk factor for CAD and no evidence of CAD were classified as non-CAD for this study. We further addressed this limitation with a statistical approach, identifying and controlling the influence of individual risk factors. Analysis of risk factors for CAD did not show an impact due to hypertension, hyperlipidemia, sex, or age on H2O2-induced dilation. There is an interaction between CAD and BMI (body mass index) on EC50 of H2O2 response (Online Figure VIII); however, this interaction may require further investigation in another cohort with a larger sample size. We also used adipose arterioles from several regions of the body; however, pilot studies indicate that H2O2-induced dilation was similar among visceral, subcutaneous, or pericardial adipose arterioles within non-CAD and CAD subject groups (Online Figure VII).
A limitation of the functional studies with regard to H2O2-induced dilation is that we use a largely pharmacological approach, which may have off-target effects on other proteins such as other families of KV channels. To mitigate this possibility, we used several chemically distinct blockers of KV1 channels, and the results obtained with these blockers invariably pointed toward an important role of KV1.5 in H2O2-induced dilation of non-CAD adipose arterioles. In addition, we found that KV1.5-targeted blockers had no significant effects in CAD arterioles, suggesting that any non-specific effects should be minimal in the present study. A recent study also reported a preferential inhibition of KV1.5 by DPO-1 using VSMCs dissociated from wild-type versus KV1.5 knockout mice.57 Nevertheless, a molecular approach using KV1.5 siRNA was also included to determine the specific role of KV1.5 in H2O2-induced dilation of non-CAD arterioles.
Clinical implications
Microvascular dysfunction has been implicated in a wide variety of pathologies including obesity-associated insulin resistance, inflammation in visceral fat, and ischemic heart disease.31–33 In the absence of CAD or its risk factors, traditional vasodilator factors (i.e., NO and prostacyclin) are important for vasodilation in human coronary and adipose arterioles.19 With the onset of CAD, the dilation is switched to a new mechanism requiring the release of H2O2 from endothelial cells and subsequent smooth muscle hyperpolarization.4–6 In the present study, we demonstrate a reduced smooth muscle-mediated dilation resulting from a functional transition from BKCa- and KV-mediated vasodilation to predominantly BKCa-mediated mechanism of dilation in human arterioles during CAD. These results thus reveal another potentially important aspect of microvascular dysfunction where cardiovascular disease not only changes the primary endothelial vasodilators (NO to H2O2) but also affects smooth muscle K+ channels that respond to vasodilator factors. Our unpublished observations also show altered kinetics of dilation in healthy vessels in the presence of 4-AP, suggesting that in addition to reduced overall peak dilation in CAD versus health, the time-to-peak dilation may be altered due to changes in the expression/function of KV channels.
Impaired KV channel function will negatively impact local blood flow regulation in response to not only endothelial factors but also to tissue factors such as β-adrenergic transmitters and other metabolic factors.9, 48, 58 This may induce deficit in regional blood supply and have detrimental effect on the function of tissues such as the heart where a tight coupling of blood perfusion and cell metabolism is essential.58, 59 Regional organ perfusion, especially in the heart where near-maximal extraction of oxygen occurs at rest, requires tight, beat-to-beat regulation of blood flow in order to nearly instantaneously match oxygen supply with tissue’s metabolic demand. In health, expression of different types and sub-types of K+ channels and their axillary subunits with varying activation/inactivation kinetic properties contribute to this precise vasoregulatory control. With the onset/progression of disease, changes in function/expression of these channels would result in a dysregulation of blood flow and, in consequence, lead to a mismatch in oxygen supply and demand. Over time, these brief but repetitious states of tissue hypoxia could induce local inflammation, fibrosis and eventually adverse tissue remodeling.19 The molecular mechanisms of impaired KV function in CAD, as well as exact causal factors responsible for the K+ channel remodeling, remain to be determined. A better understanding of these mechanisms may provide new strategies to improve or even restore normal KV channels and cardiovascular function.
Supplementary Material
Novelty and Significance.
What Is Known?
The primary mediator of shear stress-induced endothelium-dependent vasodilation is NO in patients without coronary artery disease (CAD) and hydrogen peroxide (H2O2) in those with CAD.
The large-conductance Ca2+-activated K+ (BKCa) channel and the voltage-gated K+ (KV) channel have been variably implicated in H2O2-induced dilation in both animals and humans.
Kv1 family channels are known to be redox regulated.
Kv1.5 channels in preclinical models mediate the actions of H2O2 and connect cardiac metabolism to myocardial blood flow
What New Information Does This Article Contribute?
H2O2 induces potent smooth muscle-mediated vasodilation through BKCa and KV (especially KV1.5) channels in adipose arterioles from human subjects without CAD.
H2O2-elicited dilation is reduced in arterioles from patients with CAD, and is accompanied by a loss of KV1.5- but not BKCa-dependent dilation.
KV1.5 cell surface localization and channel currents are reduced in vascular myocytes from subjects with CAD, with no change of mRNA or total cellular protein expression.
Endothelium-derived vasodilator factors such as NO and PGI2 play a key role in regulation of vascular tone and homeostasis under normal conditions. In subjects with CAD, the primary mediator changes to H2O2 in various vasodilator responses especially flow-mediated dilation. The present study shows a reduced smooth muscle-mediated dilation to H2O2 in arterioles from CAD compared with non-CAD subjects, resulting from a transition from a combined BKCa- and KV (KV1.5)-mediated vasodilation to a dilation mediated predominantly by BKCa. Therefore, the onset of cardiovascular disease not only changes the primary endothelial vasodilators (NO to H2O2) but also affects smooth muscle K+ channels that respond to vasodilator factors. The loss of KV1.5-mediated dilation to H2O2 or other vasodilators may represent an important mechanism contributing to microvascular dysfunction in humans with CAD or other cardiovascular diseases.
Acknowledgments
The authors thank the Division of Cardiothoracic Surgery at the Medical College of Wisconsin, the Cardiothoracic Surgery Group of Milwaukee, the Cardiovascular Surgery Associates of Milwaukee, the Midwest Heart Surgery Institute, Cardiothoracic Surgery division at the Zablocki VA Medical Center in Milwaukee, Froedtert Memorial Lutheran Hospital, Aurora St Luke’s Medical Center, Wheaton Franciscan Healthcare’s Elmbrook Memorial Hospital, St Joseph’s Hospital, and the Wisconsin Heart Hospital, as well as the Wisconsin Donor Network, for providing human tissues. The authors thank Victoria Nasci for organizing tissue sample data, Dr. Paul Goldspink for assistance with qPCR, Dr. Jian Zhang for assistance with immunohistochemistry, and Dr. Yanping Liu for critical review.
SOURCES OF FUNDING
Support for this research was provided by National Heart, Lung, and Blood Institute (HL096647 to D.X. Zhang; HL113612 to D.D. Gutterman), American Heart Association (0830042N to D.X. Zhang), and Advancing a Healthier Wisconsin Research and Educational program (9520311 to R.A. Sparapani).
Nonstandard Abbreviations and Acronyms
- 4-AP
4-aminopyridine
- BKCa channel
large-conductance Ca2+-activated K+ channel
- CAD
coronary artery disease
- CP-339818
1-benzyl-4-pentylimino-1,4-dihydroquinoline
- COX
cyclooxygenase
- DPO-1
diphenyl phosphine oxide-1
- DTT
DL-dithiothreitol
- EC
endothelial cell
- EDH
endothelium-derived hyperpolarization
- HEK-293 cell
human embryonic kidney 293 cell
- HCA
human coronary artery
- H2O2
hydrogen peroxide
- KCa channel
Ca2+-activated K+ channel
- KV channel
voltage-gated K+ channel
- L-NAME
NG-nitro-L-arginine methyl ester
- NOS
nitric oxide synthase
- PAP-1
phenoxyalkoxypsoralen-1
- Psora-4
5-, (4-phenylbutoxy)psoralen
- PSS
physiological salt solution
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-polymerase chain reaction
- TCEP
tris(2-carboxyethyl)phosphine
- VSMC
vascular smooth muscle cell
Footnotes
DISCLOSURES
None.
References
- 1.Ellis A, Triggle CR. Endothelium-derived reactive oxygen species: Their relationship to endothelium-dependent hyperpolarization and vascular tone. Can J Physiol Pharmacol. 2003;81:1013–1028. doi: 10.1139/y03-106. [DOI] [PubMed] [Google Scholar]
- 2.Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: Issues and considerations. Curr Opin Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations; quo vadis? Acta Physiol (Oxf) 2016 doi: 10.1111/apha.12657. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 4.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 5.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during cad. Am J Physiol Heart Circ Physiol. 2007;292:H93–100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2o2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2o2-induced dilation in human coronary arterioles: Role of protein kinase g dimerization and large-conductance ca2+-activated k+ channel activation. Circ Res. 2012;110:471–480. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimokawa H, Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol. 2005;39:725–732. doi: 10.1016/j.yjmcc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Gutterman DD, Miura H, Liu Y. Redox modulation of vascular tone: Focus of potassium channel mechanisms of dilation. Arterioscler Thromb Vasc Biol. 2005;25:671–678. doi: 10.1161/01.ATV.0000158497.09626.3b. [DOI] [PubMed] [Google Scholar]
- 10.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating bkca channel activity. Am J Physiol. 1998;275:H1283–1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- 11.Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by ca2+-activated k+ channels. Heart Vessels. 1998;13:9–17. doi: 10.1007/BF02750638. [DOI] [PubMed] [Google Scholar]
- 12.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. H2o2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive k+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 13.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive kv currents through s-glutathionylation. Pflugers Arch. 2015;467:285–297. doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berwick ZC, Moberly SP, Kohr MC, Morrical EB, Kurian MM, Dick GM, Tune JD. Contribution of voltage-dependent k+ and ca2+ channels to coronary pressure-flow autoregulation. Basic Res Cardiol. 2012;107:264. doi: 10.1007/s00395-012-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gollasch M, Ried C, Bychkov R, Luft FC, Haller H. K+ currents in human coronary artery vascular smooth muscle cells. Circ Res. 1996;78:676–688. doi: 10.1161/01.res.78.4.676. [DOI] [PubMed] [Google Scholar]
- 16.Rusch NJ. Bk channels in cardiovascular disease: A complex story of channel dysregulation. Am J Physiol Heart Circ Physiol. 2009;297:H1580–1582. doi: 10.1152/ajpheart.00852.2009. [DOI] [PubMed] [Google Scholar]
- 17.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 18.Joseph BK, Thakali KM, Moore CL, Rhee SW. Ion channel remodeling in vascular smooth muscle during hypertension: Implications for novel therapeutic approaches. Pharmacol Res. 2013;70:126–138. doi: 10.1016/j.phrs.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation: Regulation of flow and beyond. Circ Res. 2016;118:157–172. doi: 10.1161/CIRCRESAHA.115.305364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. Trpv4-mediated endothelial ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang DX, Gauthier KM, Chawengsub Y, Campbell WB. Ach-induced relaxations of rabbit small mesenteric arteries: Role of arachidonic acid metabolites and k+ Am J Physiol Heart Circ Physiol. 2007;293:H152–159. doi: 10.1152/ajpheart.00268.2006. [DOI] [PubMed] [Google Scholar]
- 22.Zhang DX, Fryer RM, Hsu AK, Zou AP, Gross GJ, Campbell WB, Li PL. Production and metabolism of ceramide in normal and ischemic-reperfused myocardium of rats. Basic Res Cardiol. 2001;96:267–274. doi: 10.1007/s003950170057. [DOI] [PubMed] [Google Scholar]
- 23.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 24.Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005;42:167–195. doi: 10.1385/CBB:42:2:167. [DOI] [PubMed] [Google Scholar]
- 25.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated k+ channels in rat small cerebral arteries: Molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi G, Trimmer JS. Differential asparagine-linked glycosylation of voltage-gated k+ channels in mammalian brain and in transfected cells. J Membr Biol. 1999;168:265–273. doi: 10.1007/s002329900515. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J, Watanabe I, Gomez B, Thornhill WB. Trafficking of kv1.4 potassium channels: Interdependence of a pore region determinant and a cytoplasmic c-terminal vxxsl determinant in regulating cell-surface trafficking. Biochem J. 2003;375:761–768. doi: 10.1042/BJ20030885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bkaily G, Al-Khoury J, Jacques D. Nuclear membranes gpcrs: Implication in cardiovascular health and diseases. Curr Vasc Pharmacol. 2014;12:215–222. doi: 10.2174/1570161112666140226120837. [DOI] [PubMed] [Google Scholar]
- 29.Jang SH, Byun JK, Jeon WI, Choi SY, Park J, Lee BH, Yang JE, Park JB, O’Grady SM, Kim DY, Ryu PD, Joo SW, Lee SY. Nuclear localization and functional characteristics of voltage-gated potassium channel kv1.3. J Biol Chem. 2015;290:12547–12557. doi: 10.1074/jbc.M114.561324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishijima Y, Cao S, Chabowski D, Ge C, Gutterman D, Zheng X, Zhang D. Expression of shaker-type voltage-gated k+ channels in human conduit and resistance arteries. FASEB J. 2016;30:1281–1285. [Google Scholar]
- 31.Berwick ZC, Dick GM, Tune JD. Heart of the matter: Coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2012;52:848–856. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: An update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scalia R. The microcirculation in adipose tissue inflammation. Rev Endocr Metab Disord. 2013;14:69–76. doi: 10.1007/s11154-013-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: Role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285:H2255–2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- 35.Tang H, Viola HM, Filipovska A, Hool LC. Ca(v)1.2 calcium channel is glutathionylated during oxidative stress in guinea pig and ischemic human heart. Free Radic Biol Med. 2011;51:1501–1511. doi: 10.1016/j.freeradbiomed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, Trower TC, Zhang S, Jiang C. Molecular basis and structural insight of vascular k(atp) channel gating by s-glutathionylation. J Biol Chem. 2011;286:9298–9307. doi: 10.1074/jbc.M110.195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vennekamp J, Wulff H, Beeton C, Calabresi PA, Grissmer S, Hansel W, Chandy KG. Kv1.3-blocking 5-phenylalkoxypsoralens: A new class of immunomodulators. Mol Pharmacol. 2004;65:1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 38.Lagrutta A, Wang J, Fermini B, Salata JJ. Novel, potent inhibitors of human kv1.5 k+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther. 2006;317:1054–1063. doi: 10.1124/jpet.106.101162. [DOI] [PubMed] [Google Scholar]
- 39.Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol. 2006;291:C348–356. doi: 10.1152/ajpcell.00086.2006. [DOI] [PubMed] [Google Scholar]
- 40.Zhong XZ, Abd-Elrahman KS, Liao CH, El-Yazbi AF, Walsh EJ, Walsh MP, Cole WC. Stromatoxin-sensitive, heteromultimeric kv2.1/kv9.3 channels contribute to myogenic control of cerebral arterial diameter. J Physiol. 2010;588:4519–4537. doi: 10.1113/jphysiol.2010.196618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodwill AG, Fu L, Noblet JN, Casalini ED, Sassoon D, Berwick ZC, Kassab GS, Tune JD, Dick GM. Kv7 channels contribute to paracrine, but not metabolic or ischemic, regulation of coronary vascular reactivity in swine. Am J Physiol Heart Circ Physiol. 2016;310:H693–704. doi: 10.1152/ajpheart.00688.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiecha J, Schlager B, Voisard R, Hannekum A, Mattfeldt T, Hombach V. Ca(2+)-activated k+ channels in human smooth muscle cells of coronary atherosclerotic plaques and coronary media segments. Basic Res Cardiol. 1997;92:233–239. doi: 10.1007/BF00788518. [DOI] [PubMed] [Google Scholar]
- 43.Lu T, He T, Katusic ZS, Lee HC. Molecular mechanisms mediating inhibition of human large conductance ca2+-activated k+ channels by high glucose. Circ Res. 2006;99:607–616. doi: 10.1161/01.RES.0000243147.41792.93. [DOI] [PubMed] [Google Scholar]
- 44.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary bk(ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated k+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobin AA, Joseph BK, Al-Kindi HN, Albarwani S, Madden JA, Nemetz LT, Rusch NJ, Rhee SW. Loss of cerebrovascular shaker-type k(+) channels: A shared vasodilator defect of genetic and renal hypertensive rats. Am J Physiol Heart Circ Physiol. 2009;297:H293–303. doi: 10.1152/ajpheart.00991.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent k(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52:912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs camp-mediated dilation by reducing kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 49.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward k+ current densities and kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 50.Gao Y, Balut CM, Bailey MA, Patino-Lopez G, Shaw S, Devor DC. Recycling of the ca2+-activated k+ channel, kca2.3, is dependent upon rme-1, rab35/epi64c, and an n-terminal domain. J Biol Chem. 2010;285:17938–17953. doi: 10.1074/jbc.M109.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leo MD, Bannister JP, Narayanan D, Nair A, Grubbs JE, Gabrick KS, Boop FA, Jaggar JH. Dynamic regulation of beta1 subunit trafficking controls vascular contractility. Proc Natl Acad Sci U S A. 2014;111:2361–2366. doi: 10.1073/pnas.1317527111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-gtpase-dependent endocytic recycling of kv1.5 in atrial myocytes. J Biol Chem. 2007;282:29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- 53.Svoboda LK, Reddie KG, Zhang L, Vesely ED, Williams ES, Schumacher SM, O’Connell RP, Shaw R, Day SM, Anumonwo JM, Carroll KS, Martens JR. Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel kv1.5. Circ Res. 2012;111:842–853. doi: 10.1161/CIRCRESAHA.111.263525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimizu S, Paul RJ. The endothelium-dependent, substance p relaxation of porcine coronary arteries resistant to nitric oxide synthesis inhibition is partially mediated by 4-aminopyridine-sensitive voltage-dependent k+ channels. Endothelium. 1997;5:287–295. doi: 10.3109/10623329709052593. [DOI] [PubMed] [Google Scholar]
- 55.Quignard JF, Feletou M, Edwards G, Duhault J, Weston AH, Vanhoutte PM. Role of endothelial cell hyperpolarization in edhf-mediated responses in the guinea-pig carotid artery. Br J Pharmacol. 2000;129:1103–1112. doi: 10.1038/sj.bjp.0703175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dittrich M, Daut J. Voltage-dependent k(+) current in capillary endothelial cells isolated from guinea pig heart. Am J Physiol. 1999;277:H119–127. doi: 10.1152/ajpheart.1999.277.1.H119. [DOI] [PubMed] [Google Scholar]
- 57.Fancher IS, Butcher JT, Brooks SD, Rottgen TS, Skaff PR, Frisbee JC, Dick GM. Diphenyl phosphine oxide-1-sensitive k(+) channels contribute to the vascular tone and reactivity of resistance arteries from brain and skeletal muscle. Microcirculation. 2015;22:315–325. doi: 10.1111/micc.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canty JM, Jr, Iyer VS. Hydrogen peroxide and metabolic coronary flow regulation. J Am Coll Cardiol. 2007;50:1279–1281. doi: 10.1016/j.jacc.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 59.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM. Requisite role of kv1.5 channels in coronary metabolic dilation. Circ Res. 2015;117:612–621. doi: 10.1161/CIRCRESAHA.115.306642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.