Abstract
Background
MicroRNAs (miRNAs) are small noncoding RNA molecules that regulate the posttranscriptional expression of target genes and are important regulators in immune responses. Previous studies demonstrated that the miRNA, miR-182 was significantly increased during allograft rejection. Further, the transcription factor Forkhead box (FOX) protein 1, (FOXO1) was shown to be a target of miR-182. The aim of this study is to further examine the role of miR-182 in alloimmune responses.
Methods
Transplantation of BALB/c cardiac allografts was performed in C57BL/6, miR-182−/−, B6.129S-H2dlAb1-Ea (MHC II− and CD4+ T cell-deficient) and B6.129S2-Tap1tm1Arp (MHC I− and CD8+ T cell-deficient) mice, with or without CTLA-4Ig administration. T cell phenotype, FOXO1 protein levels and graft infiltrating lymphocytes were determined in C57BL/6 or miR-182−/− mice by flow cytometric analysis, western blot and immunohistochemistry, respectively.
Results
We now show that T cells, mainly CD4+ are the main cellular source of miR-182 during allograft rejection. In the absence of miR-182, CTLA-4Ig treatment significantly increased allograft survival (31.5 days C57BL/6 vs. 60 days miR-182−/− , P<0.01). Further, CTLA4-Ig treatment inhibits miR-182 expression, increases FOXO1 levels, and reduces the percentage of CD4+CD44hi T cells after transplantation. Fewer T cells infiltrate the cardiac allografts and memory T cells are significantly decreased in allograft recipients deficient in miR-182 with CTLA4-Ig Treatment P<0.01).
Conclusions
Our findings suggest miR-182 contributes to the T cell responses to alloantigen especially under costimulation blockade. Therapeutics that target specific miRNAs may prove beneficial in transplantation.
Introduction
MicroRNAs (miRNAs) are small noncoding RNA molecules that regulate the posttranscriptional expression of target genes1-4. There is ample evidence that miRNAs are involved in the regulation of the immune response including after transplantation5-7. In previous studies, we demonstrated that miR-182 was significantly increased in mononuclear cells that infiltrate rejecting allografts8. Furthermore, as miR-182 increases after transplant, there is a concomitant posttranscriptional decrease in the mRNA target, FOXO18. FOXO1 acts as a master cellular regulator of a variety of cellular processes and plays a critical role in the homeostasis of cells of the immune system including neutrophils, T and B cells9-12. We, and others, have demonstrated that miR-182 is induced by IL-2 and represses FOXO1 to promote clonal expansion of activated helper T lymphocytes. Expression of miR-182 is dependent on combined T cell receptor (TCR) and IL-2 signaling through STAT513. Further, recent studies have demonstrated that miR-182 in increased in both antibody-mediated rejection and delayed graft function of human renal allografts14. Since miR-182 has been demonstrated to affect T cell responses and to be increased during graft rejection in both heart and kidney in experimental models and human transplants, we sought to further probe the role of this miRNA in alloimmune responses.
Costimulation blockade of T cell – antigen presenting cell (APC) responses has been identified as an effective treatment strategy for a variety of conditions15-18. Biologics that target either CTLA-4 (monoclonal antibody Human IgG1, Ipilimumab) or CD80/CD86 (recombinant fusion protein CTLA-4 human IgG1, Abatacept or Belatacept) are being successfully used for metastatic melanoma, rheumatoid arthritis (RA) and renal transplantation, respectively19-23. We now demonstrate that combined costimulation blockade and absence of miR-182 is superior to costimulation blockade alone, in decreasing alloimmune T cell responses, leading to a significant prolongation of allograft survival. Thus, our findings demonstrate a role for miR-182 in T cell activation during allograft rejection.
Materials and Methods
Animals and transplantation model
Ten-week-old C57BL/6 and BALB/c mice were purchased from Charles River Laboratories (Hollister, CA). B6.129SH2dlAb1-Ea and B6.129S2-Tap1tm1Arp mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Homozygous miR-182 deficient mice (miR-182−/−) on the C57BL/6 background were obtained from Dr. Iwai, National Cerebral and Cardiovascular Center, Japan24 and confirmed in our hands to not express miR-182 (data not shown). All experimental procedures were performed in accordance with a Stanford Institutional Animal Care and Use Committee approved protocol. Heterotopic heart transplantation was performed in groups (n=4-8; see specific experiments) of syngeneic C57BL/6→C57BL/6 and allogeneic BALB/c→C57BL/6; (wild-type, WT) BALB/c→miR-182−/−, , BALB/c→B6.129SH2dlAb1-Ea and, BALB/c→B6.129S2-Tap1tm1Arp mice as reported previously25. Some groups of recipients were treated with CTLA4-Ig (Abatacept, a gift from Bristol Myers Squibb) at dose of 0.5 mg i.p. on day 0, followed by a dose of 0.25 mg on days 2, 4, and 6. Function of the grafts was assessed through abdominal palpation and confirmed by histopathological analyses using H&E and Masson Trichrome staining.
Cell isolation and purification
Splenocytes were isolated from transplant recipients on day 5, day 7 or day 28 posttransplant. PBMC and graft infiltrating lymphocytes (GILs) were isolated from blood and heart grafts respectively on day 5 posttransplant as described previously8. To isolate GILS, grafts were perfused with PBS before recovery. After removal, the cardiac tissue was minced and placed in RPMI 1640 containing collagenase (2mg/ml), incubated at 37°C for 2 h and strained through a 70μm nylon cell strainer. GILs were purified using Lympholyte (Cedarlane, Ontario, Canada) prior to RNA extraction as described previously8. PBMC were isolated from graft recipients using Lympholyte. CD4+ T cells were enriched from spleens of mice using EasySep mouse CD4+ T cell isolation kit (Stemcell Technologies, BC, Canada). B cells were isolated from spleens of mice using EasySep mouse B cell negative isolation kit (StemCell Technologies, BC, Canada).
CD4+ T cell activation
Purified CD4+ T cells (2 × 105 cells/well) were plated in 96-well plates precoated with 5 μg/mL anti-CD3 Ab (eBioscience, San Diego, CA), then soluble anti-CD28 Ab (eBioscience, San Diego, CA) was added (final concentration 2μg/mL). Cells were cultured for 48 h and the collected for RNA isolation.
Quantitative RT-PCR
miR-182 expression was quantitated in RNA samples isolated from PBMC, GILs, CD4+ T cells, B cells or splenocytes by TaqMan miRNA assays (Applied Biosystems, Carlsbad, CA). SnoRNA202 expression was used for normalization. To determine the expression of mouse IFN-γ, IL-2 and β-actin, cDNA was synthesized from total RNA samples using SuperScript II reverse transcriptase with random hexamers (Invitrogen, Carlsbad, CA). Real-time PCR was performed using iQ SYBR Green Supermix (Bio-rad, Hercules, CA). The primers were as follows (forward and reverse primers): mouse IFN-γ: 5' CAG CAA CAA CAT AAG CGT CAT 3' and 5' ACC TCA AAC TTG GCA ATA CTC 3'; mouse IL-2: 5' GGC ATG TTC TGG ATT TGA CT 3' and 5' TCA TCA TCG AAT TGG CAC TC 3'; mouse β-Actin: 5' CAG CCT TCC TTC TTG GGT AT 3' and 5' GGT CTT TAC GGA TGT CAA CG 3'.
Flow cytometry
Splenocytes were collected and washed with cold FACS buffer (PBS, 1% FBS, 0.1% sodium azide) before staining with immunofluorescent markers. Briefly, 2 ×106 cells were incubated with 0.5 μl LIVE/DEAD® Fixable Aqua Dead Cell Stain (Thermofisher Scientific, Waltham, MA) and incubated for 15-20 min on ice. Cells were then labeled with the following antibodies (as per manufacturer’s instructions): FITC rat anti-mouse CD44 mAb, PE rat anti-mouse CD4 mAb (BD PharMingen, San Diego, CA); and BV570 rat anti-mouse CD4 mAb, BV785 rat anti-mouse CD44 mAb and Alexa700 rat anti-mouse CD62L mAb (Biolegend, San Diego, CA). FITC Rat IgG2b, κ mAb and PE Rat IgG2a, κ mAb (BD PharMingen); and BV570 Rat IgG2a κ mAb, BV785 Rat IgG2b κ mAb and Alexa700 Rat IgG2a κ mAb (Biolegend) were used as isotype controls. Cells were washed twice with cell staining buffer (Biolegend), resuspended in 200 μl of cell staining buffer and analyzed on FACScan flow cytometer or LSR II.2 (BD Biosciences, San Jose, CA). Data was analyzed using FlowJo software (Tree Star, Ashland, OR).
FOXO1 Quantitation
Protein was extracted from splenocytes using Cell Extraction Buffer (Invitrogen, Carlsbad, CA) containing 1.0 mM Phenylmethylsulfonyl fluoride (PMSF) and a protease inhibitor cocktail (Sigma, St. Louis, MO). The following antibodies were used: rabbit anti-mouse FOXO1 mAb (clone C29H4, Cell Signaling Technology, Beverly, MA), anti-β-Actin mAb (clone AC-15, Sigma), donkey anti-rabbit, and donkey anti-mouse IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Blots were visualized with SuperSignal West Pico Substrate (Thermofisher Scientific, Waltham, MA) and quantification was performed using ImageJ software (NIH, Bethesda, MD).
Immunohistochemistry
Infiltrating CD4+ or CD8+ cells in heart grafts were detected by immunofluorescence as described previously 8. Rat anti-mouse CD4 mAb (clone GK1.5) or rat anti-mouse CD8 mAb (clone 53-6.7) (eBioscience, San Diego, CA) were used as primary Abs. Alexa Fluor 488 goat anti-rat IgG (H+L) mAb was used as secondary Ab (Invitrogen, Carlsbad, CA). 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) was used to label nuclei. Representative images (63X magnification) were photographed using a digital camera attached to a Zeiss LSM 510 META inverted laser scanning confocal microscope (Zeiss, Oberkochen, Germany). Infiltrating cells were counted in different fields of vision (7-12 fields) using ImageJ software (NIH, Bethesda, MD).
Statistical Analysis
All of the data are presented as mean±SEM (mean ± standard error of the mean). GraphPad Prism software was used for generating Kaplan-Meier survival curves and data analysis using Student’s t test, and p values of <0.05 were considered statistically significant.
Results
T cells are the cellular source of miR-182 during alloimmune responses
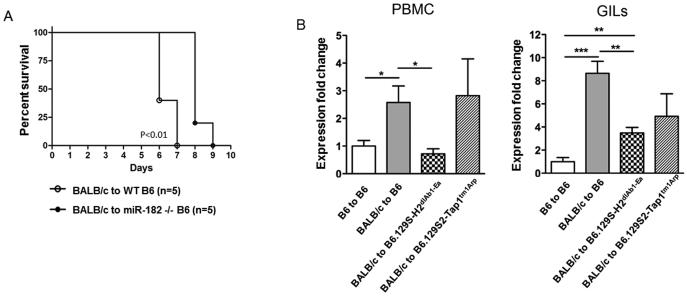
Previously we have demonstrated that cells that infiltrate rejecting cardiac allograft express miR-1828. Further, miR-182 was increased in both the PBMC and splenocytes isolated from recipients of cardiac allografts (BALB/C→C57BL/6) as compared to syngeneic (C57BL/6→C57BL/6) grafts on day 7 posttransplant indicating that miR-182 is increased both within the allograft and the periphery during graft rejection (Figure 1, SDC and SDC, Materials and Methods). These findings support a role for miR-182 in regulating immune cell functions during allograft rejection. To define the role of miR-182 in graft rejection, groups (n=5) of WT C57BL/6 or miR-182−/− mice received cardiac grafts from BALB/c mice and were monitored for graft survival. miR-182−/− recipients had a modest yet statistically significant, 2-day prolongation of graft survival (6.4 days, vs. 8.2 days, P<0.01; Figure 1A). To establish the cellular source of miR-182, BALB/c hearts were transplanted into B6.129S-H2dlAb1-Ea (MHC II− and CD4+ T cell-deficient), B6.129S2-Tap1tm1Arp (MHC I− and CD8+ T cell-deficient) or C57BL/6 recipients (n=4 per group) and miR-182 levels in PBMC and GILs quantitated on day 5 posttransplant. As expected, and consistent with our previous studies8, expression of miR-182 was increased significantly in both the PBMC and GILs of C57BL/6 recipients of allografts as compared to recipients of syngeneic grafts (Figure 1B). In PBMC from B6.129S2-Tap1tm1Arp recipients, the miR-182 levels were similar to that seen in the control allogeneic transplant group of wild-type C57BL/6 recipients indicating that miR-182 was still elevated in the absence of CD8+ T cells. In contrast, in B6.129S-H2dlAb1-Ea recipients that lack CD4+ T cells, miR-182 expression was similar to that detected in the syngeneic transplant group. When GILs were analyzed there was a similar significant decrease in mIR-182 in the absence of CD4+ T cells but the levels of miR-182 were increased over syngeneic suggesting a cell type other than a CD4+ T was also expressing miR-182. We have now added an additional, complementary, approach where we isolated B and T cells from the spleens of syngeneic and rejecting recipients of cardiac allografts and quantitated miR-182 by q-RT-PCR (SDC, Materials and Methods). We clearly demonstrate that all the enhanced miR-182 is from T cells and not from B cells (Figure 2, SDC). Taken together, these results support that miR-182 expression is dependent upon the presence of T cells and suggest that CD4+ T cells are the major cellular source of miR-182 during alloactivation.
Figure 1. The absence of miR-182, expressed by T cells, prolongs graft survival.
(A) Kaplan-Meier survival curves of BALB/c heart allografts in WT C57BL/6 (n=5) or miR-182−/− C57BL/6 (n=5) recipients (p<0.01). (B) BALB/c hearts were transplanted into groups (n=4) of WT C57BL/6, B6.129SH2dlAb1-Ea (MHC II and CD4+ T cell deficient) and B6.129S2-Tap1tm1Arp (MHC I and CD8+ T cell deficient) recipients. C57BL/6 hearts transplanted into C57BL/6 recipients were used as controls. PBMC and GILs were obtained from recipients on day 5 posttransplant and miR-182 expression was detected by quantitative RT-PCR. Expression fold changes of miR-182 were calculated by the 2−ΔΔCt method, normalized to the expression of housekeeping gene snoRNA202 and expression fold changes normalized to the control group. Data shown are the mean ± standard error of all graft recipients, *(p<0.05), ** (p<0.01) or *** (p<0.001).
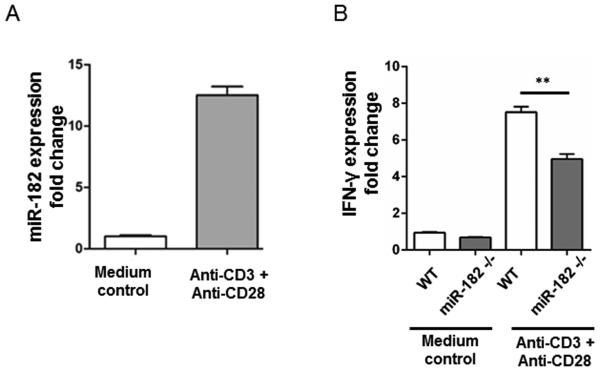
Figure 2. Activation of CD4+ T cells increases miR-182 and IFN-γ expression.
CD4+ T cells were isolated from spleens of WT C57BL/6 mice and activated with anti-mouse CD3 and anti-mouse CD28 antibodies (n=6 wells). (A) miR-182 levels were detected by quantitative RT-PCR after 48 h. Expression fold changes of miR-182 were calculated by 2−ΔΔCt method, normalized to the expression of housekeeping gene snoRNA202 and compared to the medium (without activation) control group. (B) IFN-γ mRNA levels in CD4+ T cells were detected by quantitative RT-PCR at 48 h after stimulation. Expression fold changes of IFN-γ were calculated by 2−ΔΔCt method, normalized to the expression of β-actin and compared to the group without activation. Data shown are the mean ± standard error of the mean of 3 independent experiments, (**, p<0.01).
miR-182 contributes to optimal T cell activation and IFN-γ expression
To understand the role of miR-182 in T cell function, purified CD4+ T cells were isolated from C57BL/6 mice and activated with anti-CD3 and anti-CD28 antibodies. Forty-eight hours after activation, miR-182 and IFN-γ mRNA were significantly increased in CD4+ T cells (Figures 2A and 2B, respectively). However, in the absence of miR-182, IFN-γ was significantly decreased as compared to WT controls, suggesting that miR-182 contributes to optimal, IFN-γ production by CD4+T cells (Figure 2B).
CTLA4-Ig inhibits miR-182 expression and alters the alloimmune response
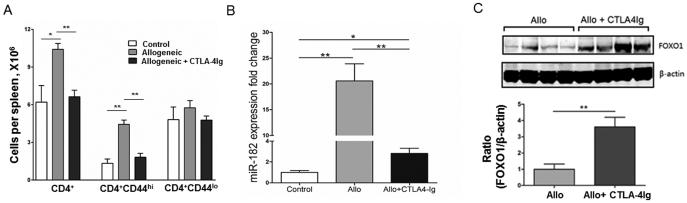
To further understand the relationship between miR-182 expression and T cell activation after transplantation, groups of C57BL/6 recipients received BALB/c heart allografts with or without CTLA4-Ig treatment. CTLA4-Ig blocks the CD28/CTLA4 ligands CD80 and CD86, thus inhibiting costimulation between T cells and APCs. As expected, on day 7 posttransplant, the number of CD4+ T cells was significantly increased in recipients of heart allografts that did not receive CTLA4-Ig, with CD4+CD44hi T cells but not CD4+CD44lo T cells elevated (Figure 3A, grey bars). CTLA4-Ig treatment prevented an increase in the number of CD4+ and CD4+CD44hi T cells, with numbers similar to control group (Figure 3A, black bars). We did not observe any significant changes in the numbers of CD4+CD44lo T cells in either allogeneic transplant groups, without or with CTLA4-Ig treatment, which were not elevated after transplantation as compared to control group.
Figure 3. CTLA4-Ig inhibits miR-182 expression, increases FOXO1 levels, and reduces the CD4+CD44hi T cell population.
Groups (n=4) of BALB/c→C57BL/6 recipients of heart allografts were left untreated or treated with CTLA4-Ig and splenocytes isolated on day 7 post-transplant, (A) The absolute numbers of CD4+, CD4+CD44hi, and CD4+CD44lo T cells in the spleen were quantitated, by flow cytometry on day 7 post-transplant, (B) miR-182 expression was quantitated in splenocytes as detailed previously and compared to control C57BL/6 mice, (C) Lysates were prepared from splenocytes and FOXO1 protein levels determined by western blot (upper panel) with the densitometry of the ratio of FOXO1 to β-actin shown below (lower panel). Data represent mean ± standard error of the mean (*, P<0.05; **, P<0.01).
Further, on day 7 posttransplant, miR-182 was significantly increased in splenocytes from mice that received allografts (Figure 3B, grey bars). Treatment with CTLA4-Ig significantly decreased miR-182 expression (Figure 3B, black bars). Since CTLA4-Ig treatment alters miR-182 expression and FOXO1 is a target of miR-1828, we determined if FOXO1 protein levels were modulated after transplant in recipients treated with CTLA4-Ig (Figure 3C). Splenocytes from graft recipients treated with CTLA4-Ig had significantly more FOXO1 that untreated allograft recipients consistent with a concomitant decrease in miR-182 and increase of the FOXO1 protein. Taken together, these studies indicate that CTLA4-Ig results in a decrease in CD4+CD44hi T cells, decreased levels of miR-182 and a significant increase in the transcription factor, FOXO1.
Absence of miR-182 augments efficacy of CTLA4-Ig treatment posttransplant
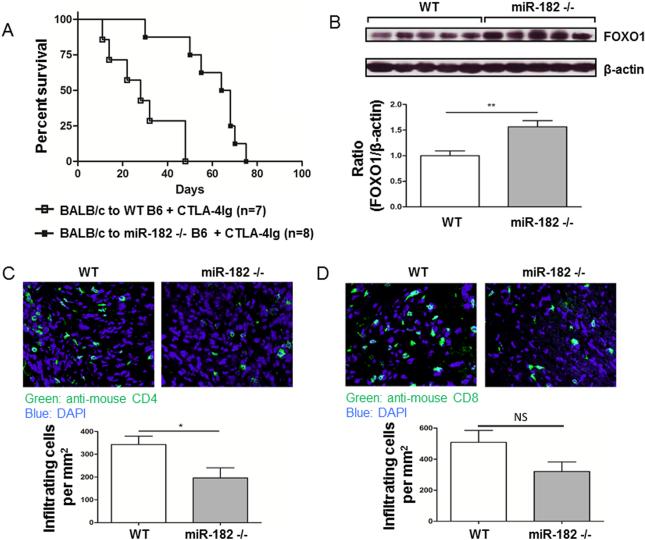
Since the absence of miR-182 prolonged graft survival, we then asked whether co-stimulation blockade would further prolong graft survival in the absence of miR-182. Groups of WT C57BL/6 (n=7) or miR-182−/− (n=8) recipients of BALB/c hearts were treated with CTLA4-Ig and graft survival monitored. In WT, CTLA4-Ig-treated C57BL/6 recipients, graft survival was significantly increased to a mean of over 30 days as compared to 7 days in untreated WT C57BL/6 recipients (see Figure 1A for comparison). Graft survival was further prolonged (mean of 60.0 days miR-182−/− versus 30.5 days WT, P<0.01) in the absence of miR-182 (Figure 4A). Splenocyte lysates analyzed by western blot, demonstrated that FOXO1 protein levels were significantly increased in mice deficient in miR-182−/−, that had significantly prolonged graft survival, as compared to WT C57BL/6 recipients of (Figure 4B) consistent with the finding that graft survival is enhanced in the presence of increased levels of FOXO1 protein.
Figure 4. Absence of miR-182 prolongs graft survival by increasing FOXO1, which decreases T cell infiltration into allografts.
BALB/c hearts were transplanted into groups of WT C57BL/6 or miR-182−/− recipients and treated with CTLA4-Ig (0.5 mg i.p. on day 0; 0.25 mg on days 2, 4, and 6). (A) Kaplan-Meier survival curves of treated allograft groups (p<0.001). (B) Lysates prepared from splenocytes on day 28 posttransplant from groups (n=5) of WT or miR-182−/− allograft recipients, treated with CTLA4-Ig, were probed for FOXO1 expression by western blot (upper panel). The ratio of FOXO1 to β-actin was determined by densitometry (lower panel). Data shown are the mean ± standard error of the mean (**, P<0.01). (C, D) Heart grafts from groups (n=3) of transplanted mice were sectioned and stained with anti-mouse CD4 (B; clone GK1.5) or anti-mouse CD8 (C; clone 53-6.7) on day 28 posttransplant. Representative images of infiltrating T cells (upper panel) and the average number of infiltrating cell per mm2 (lower panel) are shown. Data shown are the mean ± standard error of the mean (*, p<0.05; NS, Not Significant).
Sections of the cardiac allografts were examined at 28 days posttransplant, a time-point when graft survival was 100% in the miR-182 −/− CTLA4-Ig treated group. Both CD4+ T and CD8+ T cells were decreased (CD4+T cells, 42.9±13.1%, P<0.05; CD8+ T cells, 36.9±12%, P=0.08) in miR-182−/− allograft recipients as compared to WT C57BL/6 allograft recipients (Figure 4C and 4D). The observation that both CD4+ and CD8+ T cells were reduced in the heart grafts of miR-182−/− recipients, suggest that miR-182 regulation of FOXO1 (increased FOXO1 as a result of the lack of miR-182) reduces the infiltration of lymphocytes into allografts leading to reduced inflammatory response and prolonged graft survival.
Memory T cells are decreased in recipients deficient in miR-182 after cardiac transplantation and CTLA4-Ig treatment
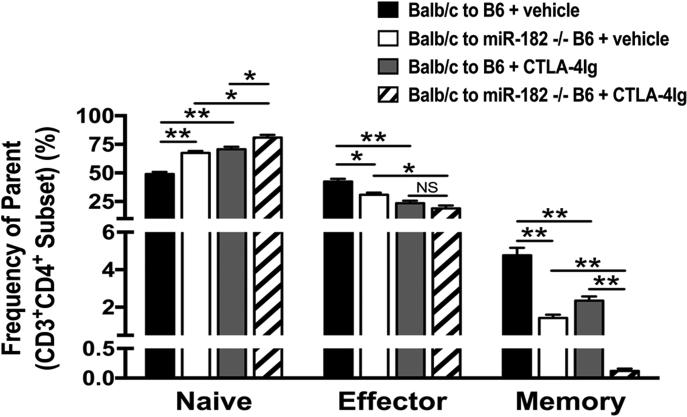
To further define the mechanism for prolonged graft survival by the combination of CTLA4-Ig treatment and the absence of miR-182, we examined the T cell phenotypes from splenocytes in groups (n=3) of C57BL/6 and miR-182−/−, recipients of BALB/c cardiac allografts with and without CTLA4-Ig treatment. As expected, we observed a reduction in the effector (CD4+CD44hiCD62LloT cells, 23.6±2.11 vs 43.4±2.4; P<0.01) and memory (CD4+CD44hiCD62LintT cells, 2.35±0.23 vs 4.77±0.4; P<0.01) T cell types in CTLA-4Ig-treated C57BL/6 WT allograft recipients (grey bars) as compared to untreated (vehicle) control allograft recipients (black bars). While we observed an increase in the naïve CD4+ T cells (CD4+CD44loCD62LhiT cells, 67.5±1.76 vs 80.93±2.44; P<0.05), there was a significant decrease in percentages of both effector (CD4+CD44hiCD62LloT cells, 18.93±2.53 vs 30.93±1.76; P<0.05) and memory (CD4+CD44hiCD62LintT cells, 0.12±0.04 vs 1.42±0.18; P<0.01) T cells in miR-182−/− recipients treated with CTLA4-Ig (diagonal bar) as compared to vehicle control (white bar) (Figure 5). Indeed, when compared to transplant recipient’s treatment with CTLA-Ig alone (grey bars), the absence of miR-182 did not alter the percentage of effector cells but had a dramatic effect on the memory cells (diagonal bars). Our results show that the absence of miR-182 causes a significant reduction in the memory cells in CTLA-4Ig treated allograft recipients.
Figure 5. Absence of miR-182 decreases memory T cells in CTLA4-Ig treated allograft recipients.
BALB/c→C57BL/6 or BALB/c→miR-182−/− recipients of heart allografts were left untreated (black, white bars) or treated with CTLA4-Ig (grey, diagonal bars) and splenocytes isolated on day 7 posttransplant were quantitated by flow cytometry. Percentage of naïve, effector and memory CD4+ T cells (Frequency of parent population, %). Data shown are the mean ± standard error of the mean (*, p<0.05; **, p<0.01; NS, Not Significant), n=3.
DISCUSSION
We demonstrated previously that miR-182 was dramatically increased during rejection and specifically detected in the circulation of allograft recipients8. The IL-2–miR-182 axis has been suggested to be important for the clonal expansion of activated naive helper T cells13. miR-182 was found to be induced in the late phase of clonal expansion and inhibition of this miRNA limited T helper cell expansion and function. Treatment with an antagomir to miR-182 resulted in diminished severity of disease in a mouse model of ovalbumin-induced arthritis further supporting the role of miR-182 in helper T cell–mediated immune responses. We now show prolongation of cardiac allograft survival in the absence of miR-182 during costimulation blockade.
Since activation of T cells results in increased miR-182 and a concomitant decrease in the transcription factor FOXO1, we asked whether blockade of costimulatory pathways alters miR-182 levels. Indeed, we clearly show that in the presence of CTLA4-Ig, miR-182 in decreased in T cells while increasing FOXO1 protein production. CTLA4, a member of the immunoglobulin superfamily, is expressed on the surface of T cells and transmits an inhibitory signal. Specifically, CTLA4 competes with CD28 for binding to CD80 and CD86 and while CD28 transmits a stimulatory signal, CTLA4 suppresses T cell function26. Indeed, 2 pharmacologically modified forms of CTLA4-Ig, Belatacept and Abatacept are FDA approved for treatment of kidney transplantation and rheumatoid arthritis, respectively27,28.
Treatment with CTLA4-Ig dramatically and significantly prolonged cardiac allograft survival in mice deficient in miR-182; 50% of the allografts survived more than 60.0 days as compared to 30.5 days in mice expressing miR-182, suggesting a role for miR-182 in graft rejection. Analysis of human renal allograft biopsies demonstrated that increased expression of miR-182 is associated with both antibody-mediated rejection and acute kidney injury14,29. Our studies, and others, have demonstrated FOXO1 as a target of miR-182 and indeed FOXO1 was significantly increased in miR-182−/− recipients of cardiac allografts treated with CTLA4-Ig supporting a role for FOXO1 in graft survival8,13,30. FOXO1 suppresses proliferation of resting CD4+ T cells by up-regulating the expression of a Cdk inhibitor, p27Kip131. FOXO1 has been reported to control effector to memory conversion and functional maintenance of CD8+ T cells and memory CD4+ T cells32. This is consistent with the significant decrease in the memory (CD4+CD44hiCD62Lint) CD4+ T cell compartment we observed in the CTLA4-Ig treated miR-182−/− graft recipients.
Previous studies have also shown that the levels of FOXO1 impact lymphocyte infiltration33. Mice with a deletion of FOXO1 in T cells were shown to have increased infiltration of T cells and enhanced Tfh cell differentiation11. Consistent with this finding, we show that there is a significant reduction in CD4+ T cells (and a marked trend towards decreased numbers of CD8+ T cells) in CTLA-4Ig treated miR-182−/− mice as compared to miR-182 expressing mice supporting that an increase of FOXO1 is associated with a decrease of lymphocyte infiltration.
Computational analysis identified miR-182 in Th2-Foxp3+ cells as a potential regulatory miRNA hub34. Further, it was shown that Tregs isolated from Th2 environments following chronic Schistosoma mansoni infection or Th2-driven inflammation downregulated IL-2 associated genes under the control of miR-18235. No differences in Treg numbers (CD4+CD25+FoxP3+T cells) were observed in the absence or presence of miR-182 in our (Th1-mediated) model of cardiac allograft rejection (BALB/c to miR-182−/− + CTLA-4Ig, 4.54±0.54 vs; BALB/c to C57BL/6 + CTLA-4Ig, 4.59±0.28; P< 0.9302). Although a recent study suggested that the immune response to experimental Listeria monocytogenes infection is intact in miR-182−/− mice 36 our results, using miR-182−/− on the same C57BL/6 background, do suggest some alterations on the early immune response after cardiac allograft transplantation. Further, the recent studies of Li et al demonstrate that miR-182 deficiency (using knock-out mice) does impair early T cell-dependent immune responses37. Taken together, these studies suggest that miR-182 does have a role in T cell function, early after stimulation, but suggest after chronic stimulation/exposure the malleability of the miR networks may compensate for the loss of specific microRNAs.
In summary, we have reported for the first time the synergistic effect of absence of a specific miRNA and costimulation blockade on cardiac allograft survival. Further, our results support a mechanism whereby modulation of FOXO1 may be at least partially responsible for the immune alterations that prolong graft survival. Blockade of selective miRNAs may prove to be an efficacious strategy to inhibit the alloimmune response after transplant.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Stanford Shared FACS Facility for providing the LSR II.2 (BD Biosciences, San Jose, CA) for flow cytometry. We would also like to acknowledge Lakshmi M. Kotamarthi and Marc Lucia Perez for assistance with animal experiments.
Funded by an American Heart Association Grant-in-Aid to S.K.
V.K. and L.W. were supported by fellowships from the Transplant and Tissue Engineering Center of Excellence at Lucile Packard Children’s Hospital. A.L. was supported by an American Heart Association Early Career Award and a Pilot Early Career Award from the Stanford Child Health Research Institute.
Abbreviations
- miRNA
microRNA, miR
- UTR
untranslated region
- AR
acute rejection
- FOXO1
Forkhead Box O1
- APC
Antigen Presenting Cells
- GILs
Graft infiltrating lymphocytes
Footnotes
L.W. and S.K. are responsible for the initial research design. L.W. and V.K. performed experiments. X.Q. performed the transplants. X.X. performed immunohistochemistry, and provided some mice. A.L. assisted with Flow Cytometry. N.I. provided miR-182−/− mice. O.M.M. provided critical review of the experimental design and manuscript. L.W. S.K. and V.K. wrote the manuscript. All authors reviewed and approved the final manuscript.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duong Van Huyen JP, Tible M, Gay A, et al. MicroRNAs as non-invasive biomarkers of heart transplant rejection. EurHeart J. 2014;35(45):3194–3202. doi: 10.1093/eurheartj/ehu346. [DOI] [PubMed] [Google Scholar]
- 6.Kaul V, Krams S. MicroRNAs as master regulators of immune responses in transplant recipients. Curr Opin Organ Transplant. 2015;20(1):29–36. doi: 10.1097/MOT.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 7.Lu LF, Gasteiger G, Yu IS, et al. A Single miRNA-mRNA Interaction Affects the Immune Response in a Context- and Cell-Type-Specific Manner. Immunity. 2015;43(1):52–64. doi: 10.1016/j.immuni.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei L, Wang M, Qu X, et al. Differential expression of microRNAs during allograft rejection. Am J Transplant. 2012;12(5):1113–1123. doi: 10.1111/j.1600-6143.2011.03958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong G, Wang Y, Xiao W, et al. FOXO1 regulates dendritic cell activity through ICAM-1 and CCR7. J Immunol. 2015;194(8):3745–3755. doi: 10.4049/jimmunol.1401754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu P, Santner-Nanan B, Hu M, et al. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. J Immunol. 2015;195(8):3665–3674. doi: 10.4049/jimmunol.1402898. [DOI] [PubMed] [Google Scholar]
- 11.Kerdiles YM, Stone EL, Beisner DR, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33(6):890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng SL. Foxo in the immune system. Oncogene. 2008;27(16):2337–2344. doi: 10.1038/onc.2008.26. [DOI] [PubMed] [Google Scholar]
- 13.Stittrich AB, Haftmann C, Sgouroudis E, et al. The microRNA miR-182 is induced by IL-2 and promotes clonal expansion of activated helper T lymphocytes. Nature Immunol. 2010;11(11):1057–1062. doi: 10.1038/ni.1945. [DOI] [PubMed] [Google Scholar]
- 14.Wilflingseder J, Regele H, Perco P, et al. miRNA profiling discriminates types of rejection and injury in human renal allografts. Transplantation. 2013;95(6):835–841. doi: 10.1097/TP.0b013e318280b385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedi B, Li JY, Grassi F, Tawfeek H, Weitzmann MN, Pacifici R. Inhibition of antigen presentation and T cell costimulation blocks PTH-induced bone loss. Ann N Y Acad Sci. 2010;1192:215–221. doi: 10.1111/j.1749-6632.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10(1):14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circulation research. 2008;103(11):1220–1231. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podojil JR, Turley DM, Miller SD. Therapeutic blockade of T-cell antigen receptor signal transduction and costimulation in autoimmune disease. Adv Exp Med Biol. 2008;640:234–251. doi: 10.1007/978-0-387-09789-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12(2):130–146. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu CC, Fornoni A, Weins A, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369(25):2416–2423. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincenti F, Blancho G, Durrbach A, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21(9):1587–1596. doi: 10.1681/ASN.2009111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin ZB, Hirokawa G, Gui L, et al. Targeted deletion of miR-182, an abundant retinal microRNA. Molecular vision. 2009;15:523–533. [PMC free article] [PubMed] [Google Scholar]
- 25.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Fresnay S, Welty E, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11(8):1599–1609. doi: 10.1111/j.1600-6143.2011.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pieper J, Herrath J, Raghavan S, Muhammad K, Vollenhoven R, Malmstrom V. CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC immunology. 2013;14:34. doi: 10.1186/1471-2172-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snanoudj R, Frangie C, Deroure B, et al. The blockade of T-cell co-stimulation as a therapeutic stratagem for immunosuppression: Focus on belatacept. Biologics. 2007;1(3):203–213. [PMC free article] [PubMed] [Google Scholar]
- 29.Wilflingseder J, Sunzenauer J, Toronyi E, et al. Molecular pathogenesis of post-transplant acute kidney injury: assessment of whole-genome mRNA and miRNA profiles. PloS one. 2014;9(8):e104164. doi: 10.1371/journal.pone.0104164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. The Journal of biological chemistry. 2009;284(35):23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl M, Dijkers PF, Kops GJ, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168(10):5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 32.Tejera MM, Kim EH, Sullivan JA, Plisch EH, Suresh M. FoxO1 controls effector-to-memory transition and maintenance of functional CD8 T cell memory. J Immunol. 2013;191(1):187–199. doi: 10.4049/jimmunol.1300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529(7587):532–536. doi: 10.1038/nature16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vickers KC, Shoucri BM, Levin MG, et al. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology. 2013;57(2):533–542. doi: 10.1002/hep.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelada S, Sethupathy P, Okoye IS, et al. miR-182 and miR-10a are key regulators of Treg specialisation and stability during Schistosome and Leishmania-associated inflammation. PLoS pathogens. 2013;9(6):e1003451. doi: 10.1371/journal.ppat.1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pucella JN, Yen WF, Kim MV, et al. miR-182 is largely dispensable for adaptive immunity: lack of correlation between expression and function. J Immunol. 2015;194(6):2635–2642. doi: 10.4049/jimmunol.1402261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li YF, Ou X, Xu S, Jin ZB, Iwai N, Lam KP. Loss of miR-182 affects B-cell extrafollicular antibody response. Immunology. 2016 doi: 10.1111/imm.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.