Abstract
Th17 cells contribute to several inflammatory conditions and increasing evidence supports that Th17 cells are glucocorticoid resistant. However, Th17 cells in psoriasis and related diseases are glucocorticoid sensitive. We compare glucocorticoid sensitive and resistant immunological diseases and suggest that several aspects in Th-17 related diseases alter glucocorticoid sensitivity of Th17 cells. We identify molecular pathways that are implicated in glucocorticoid sensitivity of Th17 cells in the literature, as this information is useful for developing approaches to overcome glucocorticoid-resistant immunopathology.
Keywords: Th17 cells, glucocorticoids, glucocorticoid resistance, asthma, autoimmunity
Introduction
CD4+ helper T (Th) cells play a crucial role in immunity against diverse pathogenic insults. In addition to their protective role, Th cells are also key players in autoimmune disorders, allergies, and asthma. Th17 cells are a subset of Th cells capable of producing IL-17A, IL-17F, IL-22, GM-CSF, and other proinflammatory cytokines (1). IL-17A, the signature cytokine of Th17 cells, is pivotal in immunity against fungi and extracellular bacteria. In addition, Th17 cells have been proposed to contribute to multiple chronic inflammatory diseases. Psoriasis, for instance, has been successfully treated with anti-IL-17A therapy (2, 3).
Glucocorticoids are extensively used for inflammatory conditions. However, glucocorticoid resistance occurs in a subset of patients. Multiple mechanisms have been proposed to underlie glucocorticoid resistance (4) and recently the distinct glucocorticoid sensitivity of Th subsets has been suggested to underlie the distinct glucocorticoid sensitivity of different patient subsets (5). Th1 and Th2 cells are sensitive to glucocorticoid inhibition whereas Th17 cells are resistant to glucocorticoid suppression (6). However, Th17 cells in certain diseases such as psoriasis appear to be sensitive to glucocorticoid inhibition while Th17 cells in some other diseases such as Crohn’s disease are resistant to glucocorticoids (7, 8). Therefore, certain aspects of diseases appear to influence glucocorticoid sensitivity of Th17 cells. We propose that mechanisms altering glucocorticoid sensitivity of Th17 cells originate from molecules necessary for Th17 differentiation, proliferation, recruitment, survival, and signaling. We begin by considering some key features of Th17 cells and Th17 cytokines.
Th17 cytokine redundancy, synergism and antagonism
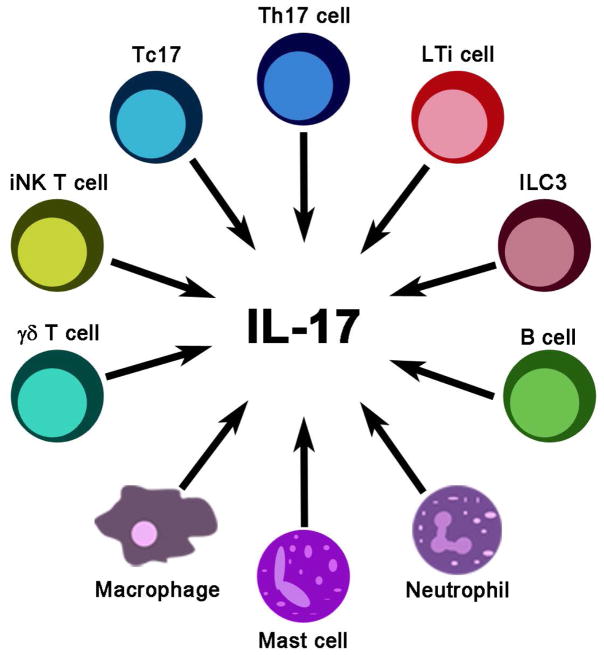
While the focus of this article is to review the role and glucocorticoid sensitivity of Th17 cells in diseases, it is important to appreciate that accumulating evidence indicates the ability of other immune cells to produce IL-17A, IL-17F, and IL-22 (Figure 1). Patients with multiple sclerosis have elevated CD8+ IL-17A-producing (Tc17) cells in the cerebrospinal fluid (9). γδT cells, a T cell subset that acts as a bridge between innate and adaptive responses, can also produce IL-17A and promote multiple sclerosis-like symptoms in animal models (10). Interestingly, IL-17A produced by γδT cells helps to clear inflammation in the airways (11). Invariant natural killer T cells (iNKT) are another subset of T cells that can produce IL-17A and recruit neutrophils to the airways in response to alpha-galactosylceramide or ozone challenges (12, 13). RAG-deficient mice lacking T cells still produce IL-17A, suggesting myeloid cells are also able to secrete IL-17A (14). In an ischemia-reperfusion kidney injury model, IL-17A from neutrophils promotes tissue damage (15). In a mouse model of arthritis, neutrophils from wild type animals, but not from IL-17A knockout littermates, exacerbate the disease (16). Type 3 innate lymphoid cells (ILC3) in the oral mucosa respond to Candida albicans and act as the main and rapid source of IL-17A and IL-17F (17). Depletion of these cells leads to uncontrolled C. albicans infection (17). In addition, ILC3 and associated cytokines are increased in inflammatory bowel disease (18) and asthma (19). Lymphoid tissue inducer (LTi) cells and LTi-like cells are subsets of ILC3 that contribute to lymphoid tissue development (20). LTi-like cells can respond to zymosan, a yeast wall product, and produce IL-17A (21). In an airway inflammation model, macrophages secrete IL-17A that promotes allergen-induced airway inflammation (22). Mast cells stimulated with TNFα, IgG complexes, C5a, or LPS produce IL-17A (23). Mast cells producing IL-17A are elevated in rheumatoid arthritis synovium (23). Mast cells also increase IL-17A production in macrophages via releasing IL-6 and other cytokines (22). In addition, B cells have also been identified as IL-17A producers (24). This redundancy in cellular sources of IL-17A supports that IL-17A is indispensable in immune responses. Multiple sources of IL-17A and their wide anatomical distributions allow for a rapid rise of IL-17A and related cytokines before Th17 cells arrive. Although pivotal in disease development, these non-Th17 IL-17A producing cells are relatively scarcely studied for their glucocorticoid sensitivity.
Figure 1.
A multitude of immune cells are capable of producing IL-17A. IL, interleukin; ILC, innate lymphoid cell; iNK, invariant natural killer; LTi, lymphoid tissue inducer; Th, helper T cell.
Cytokines produced by Th17 cells synergistically strengthen innate immunity. For example, epithelial cells respond to both IL-17A and IL-22. IL-17A increases production of IL-6, CXCL1, and CCL20 (25) and IL-22 promotes epithelial proliferation (26). In diseases, Th17 cytokines other than IL-17A have been identified as culprits. Thus, IL-22 is overexpressed in psoriasis and can induce epidermal thickening, a characteristic of plaque psoriasis (26). GM-CSF is a pro-inflammatory cytokine produced by Th1 and Th2 as well as Th17 cells (27, 28). Pathogenic Th17 cells produce more GM-CSF than non-pathogenic Th17 cells (29). GM-CSF deficient Th17 cells are unable to induce experimental autoimmune encephalitis, highlighting the importance of Th17-derived GM-CSF in driving disease pathology (29, 30).
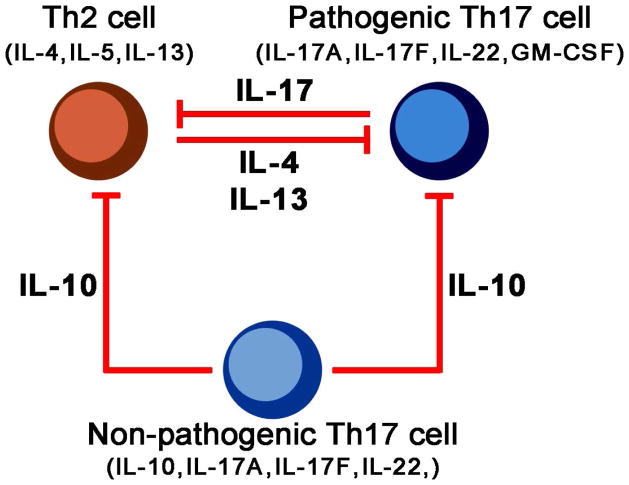
Whilst pathogenic Th17 cells are proinflammatory and produce proinflammatory cytokines indicated above, non-pathogenic Th17 cells produce more IL-10, which limits Th17-driven inflammation (31) (Figure 2). Pathogenicity of Th17 cells can be enhanced by certain stimuli such as NaCl and IL-23 (32–34) while inhibited by other signals such as IL-4 and IL-13 (35–37). Thus, multiple pathways determine the function of a Th17 cell.
Figure 2.
Th-2 and non-pathogenic Th17 cells exert antagonistic effects towards pathogenic Th17 cells. Th2 cell-derived IL-4 or IL-13 can inhibit Th17 cell functions. Conversely, IL-17A can inhibit Th2 cell responses. Th17 cells have pathogenic or non-pathogenic subsets. Non-pathogenic Th17 cell-derived IL-10 can act on Th2 or Th17 cells and inhibit their pro-inflammatory activities. GM-CSF, Granulocyte macrophage colony-stimulating factor; IL, interleukin; Th, helper T cell.
Th17 differentiation
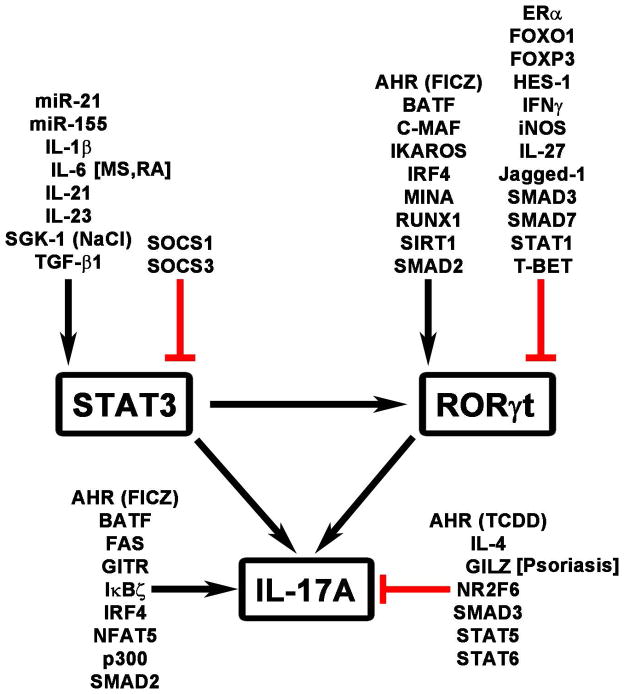
IL-6, TGF-β, IL-21, and IL-1β are key cytokines while RORγt and STAT3 are the pivotal transcription factors for Th17 differentiation (Figure 3). IL-6 directly activates STAT3 whereas TGF-β1 inhibits SOCS3, a negative regulator of STAT3 signaling, and activates SMAD2, which promotes RORγt and IL-17A expression (38–40). TGF-β1 can also have a negative effect on Th17 differentiation by activating SMAD3, an inhibitor of Th17 differentiation (40). ERK signaling, downstream of the IL-6R, promotes phosphorylation of SMAD2 and Th17 differentiation. Together, IL-6 and TGF-β1 induce the expression of RORγt, the master regulator of transcription for Th17 cells (41). IL-6, in a STAT3-dependent manner, induces the expression of IL-21, which acts in an autocrine feed forward loop to further promote STAT3 activation and RORγt expression (42, 43). IL-1β can promote Th17 differentiation by inducing the expression of IRF4, which stimulates the expression of RORγt and IL-17A (44, 45). In addition, IL-1β, via NF-κB activation, also inhibits SOCS3, leading to STAT3 activation (46). While promoting RORγt, STAT3 activation also induces IL-23R and IL-23 is important in the maintenance and stability of Th17 cells (47, 48).
Figure 3.
Th17 regulators. Some of the regulators, e.g., GILZ, GITR, and IL-6, have been identified as candidates that alter glucocorticoid sensitivity of IL-17A in select diseases. Related diseases are shown in brackets. Black arrowhead, positive regulators. Red flat line, inhibitory factors. AHR, aryl hydrocarbon receptor; BATF, basic leucine zipper transcription Factor, ATF-like; C-MAF, v-MAF avian musculoaponeurotic fibrosarcoma oncogene homolog; ER, estrogen receptor; FICZ, 6-formylindolo (3, 2-b) carbazole; FOX, forkhead box protein; GILZ, glucocorticoid-induced leucine zipper; GITR, glucocorticoid-induced TNFR-related protein; HES, HES family BHLH transcription factor; IFN, interferon; IκBζ, NFκB inhibitor ζ; IL, interleukin; iNOS, inducible nitric oxide synthase; IRF, interferon regulatory factor; miR, micro RNA; MS, multiple sclerosis; NaCl, sodium chloride; NFAT, nuclear factor of activated T cells; NR2F6, nuclear receptor subfamily 2, group F, member 6; RA, rheumatoid arthritis; RORγt, RAR-related orphan receptor γ; RUNX, Runt-related transcription factor; SGK, serum and glucocorticoid-regulated kinase; SIRT1, sirtuin 1; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; T-BET, T-box expressed in T cells; TGF, transforming growth factor.
In addition to the key cytokines and transcription factors mentioned above, a myriad of factors have been identified to enhance or suppress Th17 differentiation (Figure 3). Among the Th17-enhancing molecules are transcription factors BATF (49), IKAROS (50, 51), FICZ-mediated activation of aryl hydrocarbon receptor (AHR) (52), RUNX1 (53), C-MAF (54), and NFAT5 (33), signaling molecules and enzymes FAS (55), MINA, a histone lysine demethylase (55), SIRT1 (56), IκBζ (57), p300 (58), and SGK1 (33, 34), and micro RNAs (miRNA) miR-21 (59) and miR-155 (60). Several of these Th17 enhancing molecules work by increasing the activity of the master transcription factors STAT3 and RORγt. In contrast, negative regulators of Th17 differentiation generally inhibit STAT3 and RORγt. They include Jagged-1 (61), HES-1 (61), ER-α (62), iNOS (63), FOXP3 (64), FOXO1 (65), STAT1 (66), STAT5 (67), STAT6 (36), T-BET (53), NR2F6 (68), and GILZ (glucocorticoid-induced leucine zipper) (55, 69). AHR bound to TCDD, in contrast to that bound to FICZ, inhibits Th17 differentiation (52). These multiple regulatory factors orchestrate Th17 cell differentiation in three phases: the early (up to 4 h), the intermediate (4–20 h), and the late (20–72 h) phase (24). The early phase is characterized by an IL-6 and TGF-β1 signature and transcription factors such as STAT3, IRF4, and BATF, and expression of the IL-23R (55). The intermediate phase involves the expression of RORγt and AHR among others (55). By the late phase, key Th17 cytokines are expressed (55). As indicated below, some of these Th17-regulatory molecules have been implicated in altering Th17 glucocorticoid sensitivity in diseases.
Th17 cells in diseases
Mutations of genes necessary for Th17 function and blockade of Th17 pathways in clinical trials definitively indicate the role of Th17 cells in several autoimmune diseases. An arginine381 to glutamine (R381Q) in the cytoplasmic domain of IL-23R is highly associated with low incidence of Crohn’s disease (70). R381Q impairs IL-17A production and has also been inversely correlated to psoriasis (71) and rheumatoid arthritis (72). In addition, Th17 cells have been found at inflamed tissues in rheumatoid arthritis (73, 74), multiple sclerosis (75, 76), psoriasis (77), inflammatory bowel diseases (78), and severe asthma (79–82) among others. These diseases with Th17 involvement have varied glucocorticoid sensitivity, from psoriasis being glucocorticoid sensitive to subsets of Crohn’s disease on the other end of the spectrum. As discussed below, Th17-driven diseases have varied glucocorticoid sensitivity likely because each disease has varying amounts of glucocorticoid-sensitive components that intersect with Th17 cell functions. Because the term glucocorticoid sensitivity is context-dependent, examining glucocorticoid sensitivity begins with defining the entity to be questioned as well as the context. We focus on the glucocorticoid sensitivity of Th17 cell counts and their ability to produce signature cytokines such as IL-17A in immunopathology. One caveat of this focus is that changes in Th17 cell numbers in blood could be interpreted in several ways. Increased Th17 cell numbers in circulation might reflect increased inflammation. However, reduced numbers could indicate greater recruitment to disease tissues, rather than reduced inflammation. Therefore, we also reference studies that examine whether glucocorticoids alleviate symptoms of Th17-related diseases.
Th17 cells are intrinsically glucocorticoid resistant
Th17 cells isolated from various tissues express memory T cell markers and are highly proliferative (83). Memory Th17 cells are relatively resistant to activation- or chemotherapy drug (i.e., cisplatin and paclitaxel)-induced apoptosis due to elevated Notch, hypoxia-inducible factor 1-α, and BCL-2 (83, 84). Th17 cells isolated from peripheral blood of healthy subjects or from donors with mild allergies are resistant to glucocorticoid killing, the mechanism of which has also been linked to high levels of BCL-2 in Th17 cells (6). ROR-γt, the master transcription factor of Th17 cells, counteracts glucocorticoid-induced apoptosis (85) and STAT3, another key transcription factor in Th17 cells, can antagonize glucocorticoid receptor (GR) functions (86). Glucocorticoids promote Th17 differentiation in vitro (87). When glucocorticoid sensitivity is compared among Th1, Th2, and Th17 cells, Th2 and Th17 cells are resistant to glucocorticoid-induced apoptosis (6). A prerequisite for tissue Th17 activity is their recruitment to site of inflammations. CCL20, the Th17 chemokine, is increased by glucocorticoids in the sputum of asthmatics (88).
IL-17A, but not IL-22 or GM-CSF, from the same Th17 cells is resistant to glucocorticoid suppression, suggesting that gene promoter-specific mechanisms mediate glucocorticoid sensitivity in Th17 cells (6). Glucocorticoids can even elevate IL-17A in certain diseases (81, 89). Once secreted, IL-17A promotes recruitment of neutrophils via CXCL8 (90). Neutrophils and Th17 cell co-localize in gut tissues isolated from patients with Crohn’s disease, synovial fluid from rheumatoid arthritis patients (90), and sputum from asthmatics (91). Glucocorticoids elevate blood neutrophil counts (92) and inhibit neutrophil apoptosis (93). It has been suggested that neutrophils, in turn, promote Th17 cells (94). Neutrophils stimulated with a combination of IFN-γ and LPS produce Th17 and Th1 chemokines CCL20, CCL2 and CXCL10 (90). Therefore, there is a feedforward loop between Th17 cell activity and neutrophils, which is enhanced by glucocorticoids. In epithelial cells and other IL-17A targets cells, IL-17A receptors signal through p38 MAPK, extracellular signal-related kinase, and phosphoinositide-3-kinase pathways that antagonize glucocorticoid signaling (95). Thus, IL-17A reduces the sensitivity of TNF-α-induced IL-8 production to budesonide in airway epithelium (95). In addition, IL-17A reduces HDAC activity and overexpression of HDAC2 can reverse IL-17A-induced glucocorticoid insensitivity (95). Even though these findings support that Th17 cells are intrinsically resistant to glucocorticoid suppression, recent evidence indicates that Th17 cells in certain diseases are sensitive to glucocorticoids (Table 1).
Table 1.
Th17 cells are sensitive to glucocorticoid suppression in certain diseases.
| Glucocorticoid effects | References | |
|---|---|---|
| Psoriasis | ||
| Th17 cell number | Decrease | (7) |
| IL-17A level | Decrease | (97) |
| Symptoms | Reduce plaques and relieve associated symptoms | (96, 97) |
| Proposed mechanisms | Inhibit overall inflammation | |
| Inhibit Th17 via inducing GILZ | (55, 69) | |
| Multiple Sclerosis | ||
| Th17 cell number | Decrease | (106) |
| IL-17A level | Decrease | (106) |
| Symptoms | Improve functional recovery during relapse | (104, 106) |
| Proposed mechanisms | Inhibit overall inflammation | |
| Inhibit Th17 via suppressing IL-6 | (107) | |
| Do not inhibit Th17 when IL-6, dopamine, LPS are high | (107–109) | |
| Rheumatoid arthritis | ||
| Th17 cell number | Decrease | (119) |
| IL-17A level | Decrease | (119) |
| Symptoms | Suppress morning stiffness and flares | (111, 112) |
| Do not prevent loss of cartilage and bone | (113–115) | |
| Proposed mechanisms | Inhibit Th1 inflammation | (121) |
| Do not inhibit neutrophils, which cause damage to joints | (93) | |
Wide-ranging glucocorticoid sensitivity of diseases and Th17 cells
Psoriasis
Topical glucocorticoids are the most frequently prescribed medication for controlling outbreaks of psoriasis (96). Glucocorticoids, independently or together with vitamin D3, through their anti-inflammatory actions reduce plaques and relieve associated symptoms (96, 97). Recent clinical trials targeting Th17 pathways have been remarkably successful. Anti-IL-17A mAbs secukinumab (2) and ixekizumab (3) are highly effective for psoriasis. Other Th17 targeting therapeutics, brodalumumab (anti-IL-17RA) (98) and guselkumab, tildrakizumab, BI-655066, AMG139, and LY3074828 (anti-IL-23p19 mAbs) (99–102) are also excellent for psoriasis. Since Th17 cells play a major role in psoriasis and glucocorticoids are effective in controlling the symptoms of psoriasis, it is not surprising that glucocorticoids decrease the frequency of Th17 cells in the circulation in psoriatic patients (7). In an ex vivo psoriatic skin cell culture system, betamethasone suppresses IL-17A and IL-22 (97). Therefore, the majority of symptoms of psoriasis are glucocorticoid sensitive and both Th17 cell number and function are sensitive to glucocorticoid inhibition in psoriasis (Table 1).
Activated keratinocytes and multiple immune cells including Th1 and dendritic cells are involved in pathogenesis psoriasis (103). Reducing overall inflammation via inhibition of these other immune cells by glucocorticoids provides a means to reduce signals that promote Th17 cells. In addition, recent reports suggest that GILZ is a direct mediator of glucocorticoid sensitivity of Th17 cells in psoriasis. Low levels of GILZ have been found in lesional skin of psoriasis patients and GILZ is inversely correlated with levels of IL-23, IL-17A, IL-22, and STAT3, suggesting GILZ could be inhibitory to Th17 activity (69). GILZ null mice subjected to the imiquimod model of psoriasis have worse inflammation than wild type animals (69). Correspondingly, dendritic cells lacking GILZ produce more IL-1, IL-23 and IL-6 in response to imiquimod stimulation than those with intact GILZ (69). GILZ has also been suggested to limit Th17 cell differentiation by acting as a negative regulator of the Th17 transcriptional program via directly binding to promoter regions of key Th17 genes BATF, STAT3, IRF4 and ROR-γt (55). Glucocorticoids highly elevate GILZ in Th17 cells (6), suggesting a role of GILZ in the ability of glucocorticoids to decrease Th17 cell number and activity in psoriasis (Table 1).
Multiple sclerosis
Glucocorticoids are the mainstay in managing relapse of multiple sclerosis (104) and the role of Th17 cells in pathogenesis of multiple sclerosis is accumulating. IL-17A and IL-6 are among the most highly expressed genes in brain lesions (75) and IL-17A is elevated in serum and cerebrospinal fluid of multiple sclerosis patients (76, 105). Glucocorticoids not only improve functional recovery in patients with multiple sclerosis during relapse, but also decrease IL-17A production and Th17 cell counts in circulation (106). Since IL-6 is upstream of Th17 cell activity and glucocorticoids effectively inhibit IL-6 in multiple sclerosis, this cytokine may mediate the glucocorticoid sensitivity of Th17 cells in multiple sclerosis. When high levels of IL-6 persists, Th17 cells become glucocorticoid resistant in multiple sclerosis (107). Additional factors including dopamine (108) and endotoxin levels (109) have also been suggested to promote glucocorticoid resistance of Th17 cells in multiple sclerosis (Table 1). T cells from patients with progressive multiple sclerosis are resistant to glucocorticoid-induced apoptosis, whereas T cells from relapse-remitting multiple sclerosis patients are sensitive (110), the mechanism of which has not been examined.
Rheumatoid arthritis
Glucocorticoids are indispensable in managing inflammation in rheumatoid arthritis (111, 112). Morning stiffness and flares both are suppressed by glucocorticoids. However, clinical studies indicate that despite the antiinflammatory benefits of glucocorticoids in rheumatoid arthritis, they are not effective in managing several aspects of arthritis including loss of cartilage and bone (Table 1). Early treatment of undifferentiated arthritis with glucocorticoids provides only limited benefits with regard to remission rates (113). The SAVE trial (114) indicates that a single 120 mg dose of methylprednisolone in patients with arthritis of less than 16 weeks helps only 17% of patients to achieve remission, comparable to placebo. The STIVEA trial (115) indicates that three injections of 80 mg methylprednisolone over 3 weeks also have a low (20%) remission rate. In contrast, anti-IL-17A secukinumab (116), AIN457 (117), and ixekizumab (118) are effective in patients who are inadequate responders to anti-TNFα therapy. Glucocorticoids reduce blood Th17 cells in active rheumatoid arthritis (119). Therefore, the inflammation aspect of rheumatoid arthritis and Th17 cell counts are sensitive to glucocorticoids whereas other key aspects of the disease are glucocorticoid resistant (Table 1).
Th17-driven diseases such as rheumatoid arthritis have significant underpinnings from additional T helper cell types. Thus, Th1 cells play a major role in rheumatoid arthritis, psoriasis, multiple sclerosis, and Crohn’s disease and may collaborate with Th17 cells in exacerbating inflammation (120). Glucocorticoids induce apoptosis of Th1 cells (6) and decrease IFN-γ production by T cells from patients with rheumatoid arthritis (121). Inhibition of Th1 activity by glucocorticoids may help to reduce overall inflammation. On the other hand, the glucocorticoid resistant aspects of rheumatoid arthritis may be driven by neutrophils. Neutrophils are the culprit of cartilage and bone damage (122) and as mentioned above, neutrophils are expanded by glucocorticoids (93).
Crohn’s disease
While glucocorticoids are effective to control exacerbations and induce remission of Crohn’s disease in the majority of patients, approximately 20% of patients with Crohn’s disease are resistant to glucocorticoids (123) (Table 2). Elevated Th17 cells (78, 124, 125) and increased IL-17A levels have been found in lesions of Crohn’s disease (126, 127). Th17 cells isolated from the gut of active Crohn’s diseases are resistant to glucocorticoid suppression (8). It is not known whether Th17 cells underlie the subgroup of glucocorticoid-resistant Crohn’s disease.
Table 2.
Th17 cells are resistant to glucocorticoid suppression in certain diseases.
| Glucocorticoid effects | References | |
|---|---|---|
| Crohn’s disease | ||
| Th17 cell number | Do not decrease | (8) |
| IL-17A level | Do not decrease | (8) |
| Symptoms | Control exacerbations and induce remission in ~80% patients | (123) |
| Proposed mechanisms | Inhibit overall inflammation | |
| Do not inhibit pathogenic Th17 cells in gut | (8) | |
| Th17 high asthma | ||
| Th17 cell number | Do not decrease | (80–82, 91, 138) |
| IL-17A level | Do not decrease | (80–82, 91, 138) |
| Decrease | (133, 139) | |
| Symptoms | Do not improve lung function | (81, 138) |
| Proposed mechanisms | Glucocorticoid resistant Th17 cells/neutrophils | (80–82, 91, 138) |
Th17-high asthma
It has been recently recognized that the etiology of asthma varies and Th2 cells and eosinophils underlie only approximately 50% of asthma (128). Th2 activity, eosinophils, and related asthma are effectively controlled by glucocorticoids (129). In contrast, Th17 cells have been suggested to contribute to subsets of glucocorticoid-resistant asthma (130) (Table 2). His161Arg mutation in IL-17F has been inversely correlated with asthma (131) and a significant association of asthma with polymorphisms in IL-1R1 and RORA has been reported (132). Increased Th17 cells are found in asthmatic tissues (77). Airway or blood IL-17A and IL-17F levels are positively correlated to asthma severity (133–137). Elevated IL-17A is correlated with increased neutrophils (91, 135) and methacholine induced airway hyperresponsiveness in asthmatics (80). Although several studies suggest that Th17 cell number and IL-17A levels in asthmatics are resistant to glucocorticoid inhibition (80–82, 91, 138), airway IL-17A detected by immunocytochemistry has been found to be decreased by 2 weeks of oral glucocorticoids in a cohort of moderate-to-severe asthmatics (133). The source of the glucocorticoid-sensitive IL-17A in those patients was not identified. Glucocorticoid inhibition of IL-6 could underlie glucocorticoid inhibition of IL-17A in some asthma patients as methylprednisolone downregulates IL-6, IL-17A, and IFN-γ in stimulated PBMCs from asthmatic children (139). A recent trial using brodalumab (anti-IL-17RA) suggests possible benefits in a select group of patients with highly reversible asthma (140). It will be interesting to determine whether blocking Th17 activity is effective in the Th17-high asthmatics. A recent preclinical study indicates that neutralizing IL-17A and IL-13 together, but not each cytokine alone, abolishes airway hyperreactivity in house dust mite sensitized and challenged mice (37).
Mechanisms underlying Th17 cell glucocorticoid sensitivity in diseases
From available data on glucocorticoid sensitivity of Th17 cells in several diseases detailed above, some key factors can be identified that sensitize Th17 cells to glucocorticoids. Suppression of cytokines such as IL-6 may sensitize Th17 cells to glucocorticoid suppression. Glucocorticoids are highly effective in suppressing IL-6 and anti-IL-6R mAbs potentiate the ability of glucocorticoids to suppress Th17 cytokines (141). Tocilizumab, a humanized anti-IL-6 receptor monoclonal antibody, has outstanding efficacy against rheumatoid arthritis (142). Since dendritic cells are a major producer of IL-6 and other Th17 promoting cytokines (143), inhibition of dendritic cells will allow glucocorticoids to suppress Th17 differentiation. Dendritic cells have maturational stage-specific glucocorticoid sensitivity (144). Immature dendritic cells are resistant and mature dendritic cells are sensitive to glucocorticoid killing due to a switch of GR isoforms (144). Furthermore, dendritic cells are biased by glucocorticoids to expand Tregs (145). Thus, glucocorticoid sensitivity of Th17-promoting dendritic cells could underlie the glucocorticoid sensitivity of Th17 cells. In addition, recent reports indicate that GITR (glucocorticoid-induced TNFR family-related protein) and its ligand play a critical role in the pathogenesis of rheumatoid arthritis by enhancing the Th17 cell response via p38 MAPK and STAT3 (146). High levels of p38 phosphorylation have been detected in rheumatoid arthritis patients, which correlate with the serum level of anti-cyclic citrullinated peptide antibody (146). GITR is significantly increased by glucocorticoids (147) and could underlie the glucocorticoid-resistant aspect of Th17 cells in diseases.
Th17 cells and the other Th subsets have an extensive network of cross talk. IL-17A, IFN-γ, and IL-4 are thought to be mutually antagonistic (35, 37). IL-4 inhibits STAT3 binding at the IL-17A promoter (36) and neutralizing IL-13 in a mouse asthma model elevates IL-17A and vice versa (37). However, it has been demonstrated that in severe/chronic diseases Th17 and other Th cells can synergize with each other. Th17 cells from collagen immunized animals or those differentiated in vitro for 3 weeks are resistant to suppression by IL-4 due to impaired IL-4R signaling in mature Th17 cells (36). Double-positive IL-4/IL-17A T cells have been found in asthmatics (148, 149). In addition, an endotype of glucocorticoid-resistant asthma with elevated levels of both IL-17A and IFN-γ has been reported (138). In an adoptive transfer model of airway inflammation, transfer of Th2 and Th17 cells induces eosinophil- and neutrophil-dominant inflammation, respectively, while co-transfer of Th2 and Th17 cells enhances the Th2 response, eosinophil infiltration, and airway hyperresponsiveness [95]. Similarly, IFN-γ and IL-17A producing Th1/17 cells are increased in multiple sclerosis patients, cross the blood brain barrier, and accumulate in the central nervous system (150). Reducing overall inflammation via blocking Th1/Th2 cell activity could provide another means for glucocorticoids to inhibit Th17 recruitment and function.
It is possible that there are distinct subsets of Th17 cells with distinct glucocorticoid sensitivity. It has been reported that glucocorticoid-resistant and pathogenic Th17 cells are restricted to a subset expressing MDR1 (multi-drug resistant type 1, ABCB1) (8). Glucocorticoids increase the level of MDR1 in brain endothelial cells (151) and in lymphocytes from rheumatoid arthritis patients (152). Polymorphisms that increase the level and activity of MDR1 have been suggested to contribute to glucocorticoid resistance in inflammatory bowl diseases (153). However, blocking MDR1 in Th17 cells does not increase glucocorticoid sensitivity (8). Nonetheless, glucocorticoids specifically expand MDR1+ Th17 subsets within mixed T cell cultures (8), suggesting that the underlying Th subsets in diseases can be altered by glucocorticoids. Long-term glucocorticoids that eliminate glucocorticoid-sensitive T cells will select and favor Th17 subsets that are glucocorticoid resistant, which in turn exacerbate disease.
Perspective
It will be interesting to determine whether in the same individual, long-term glucocorticoid treatment shifts the etiology of a disease from glucocorticoid-sensitive to a resistant Th subset. Another intriguing question is whether rheumatoid arthritis and Crohn’s disease, like asthma, can be categorized into endotypes with distinct underlying T helper cell subsets and glucocorticoid sensitivity. Further research is also needed to identify the role of Th17 regulatory factors, for example those presented in Figure 3, in glucocorticoid sensitivity in order to overcome glucocorticoid resistance of Th17 cells in diseases. Vitamin D3 has been suggested to be effective in inhibiting Th17 cytokines in patients with glucocorticoid refractory asthma (81). In addition, vitamin D3 has been frequently given together with glucocorticoids to effectively manage psoriasis (97). Continued investigation in novel approaches to overcome glucocorticoid resistance of Th17 cells will help to improve the quality of life for patients with glucocorticoid resistant immunopathology.
Acknowledgments
Fundings: This study was funded by the National Institute of Allergy and Infectious Diseases, (R21AI113935), National Heart, Lung, and Blood Institute (R37HL068546), and the Ernest S. Bazley Foundation.
LIST OF ABBREVIATIONS
- AHR
aryl hydrocarbon receptor
- BATF
basic leucine zipper transcription Factor, ATF-like
- BCL-2
B-cell lymphoma 2
- CCL
CC chemokine ligands
- CCR
CC chemokine receptor
- CXCL
CXC chemokine ligand
- CXCR
CXC chemokine receptors
- ER
estrogen receptor
- ERK
extracellular-signal-regulated kinase
- FICZ
6-formylindolo (3, 2-b) carbazole
- FOXO1
forkhead box protein O1
- FOXP3
forkhead box P3
- G-CSF
granulocyte-colony stimulating factor
- GILZ
glucocorticoid-induced leucine zipper
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GR
glucocorticoid receptor
- HDAC
histone deacetylase
- HES1
HES family BHLH transcription factor 1
- IFNγ
interferon γ
- IκBζ
NFκB inhibitor ζ
- IL
interleukin
- ILC
innate lymphoid cell
- INKT
invariant NKT
- INOS
inducible nitric oxide synthase
- IRF4
interferon regulatory factor 4
- LPS
lipopolysaccharide
- LTI
lymphoid tissue inducer
- MAPK
mitogen-activated protein kinase
- MDR1
multi-drug resistance 1
- MEK1
mitogen-activated protein kinase kinase 1
- miRNA
micro RNA
- MS
multiple sclerosis
- NFAT
nuclear factor of activated T cells
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PI3K
phosphoinositide 3-kinase
- RA
rheumatoid arthritis
- RAG
recombination activating gene
- RORγt
RAR-related orphan receptor γ
- RUNX
Runt-related transcription factor
- SGK1
serum and glucocorticoid-regulated kinase 1
- SIRT1
sirtuin 1
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription
- TGFβ
transforming growth factor β
- Th
helper T cell
- TNF
tumor necrosis factor
- Treg
regulatory T cells
Footnotes
Conflict of interest
All authors declare that they have no relevant conflict of interests.
Author contributions
JB, YC, SS, and NZL wrote the manuscript.
References
- 1.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8(5):337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 2.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- 4.Gross KL, Lu NZ, Cidlowski JA. Molecular mechanisms regulating glucocorticoid sensitivity and resistance. Mol Cell Endocrinol. 2009;300(1–2):7–16. doi: 10.1016/j.mce.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banuelos J, Lu NZ. A gradient of glucocorticoid sensitivity among helper T cell cytokines. Cytokine Growth Factor Rev. 2016 doi: 10.1016/j.cytogfr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banuelos J, Shin S, Cao Y, Bochner BS, Morales-Nebreda L, Budinger GR, et al. BCL-2 protects human and mouse Th17 cells from glucocorticoid-induced apoptosis. Allergy. 2016;71(5):640–650. doi: 10.1111/all.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hino R, Kabashima R, Kawakami C, Sugita K, Nakamura M, Tokura Y. Circulating Th17 cell fluctuation in psoriatic patients treated with topical calcipotriol and betamethasone butyrate propionate. J Eur Acad Dermatol Venereol. 2011;25(2):242–244. doi: 10.1111/j.1468-3083.2010.03714.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211(1):89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123(1):247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch JR, Lloyd CM. Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing {gamma}{delta}T cells. Am J Respir Crit Care Med. 2010;182(4):464–476. doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1. 1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205(2):385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eken A, Singh AK, Treuting PM, Oukka M. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 2014;7(1):143–154. doi: 10.1038/mi.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120(1):331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katayama M, Ohmura K, Yukawa N, Terao C, Hashimoto M, Yoshifuji H, et al. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One. 2013;8(5):e62231. doi: 10.1371/journal.pone.0062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190(2):521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 18.Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208(6):1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu S, Kim HY, Chang YJ, DeKruyff RH, Umetsu DT. Innate lymphoid cells and asthma. J Allergy Clin Immunol. 2014;133(4):943–950. doi: 10.1016/j.jaci.2014.02.015. quiz 951. [DOI] [PubMed] [Google Scholar]
- 20.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13(2):75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 21.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181(9):6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 23.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184(7):3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez-Tello A, Halwani R, Li R, Nadigel J, Bar-Or A, Mazer BD, et al. IL-17A and IL-17F expression in B lymphocytes. Int Arch Allergy Immunol. 2012;157(4):406–416. doi: 10.1159/000329527. [DOI] [PubMed] [Google Scholar]
- 25.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 28.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165(11):6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 29.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(1):39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 31.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13(10):991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 36.Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. J Immunol. 2011;187(9):4440–4450. doi: 10.4049/jimmunol.1002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7(301):301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 38.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009;183(1):97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon JH, Sudo K, Kuroda M, Kato M, Lee IK, Han JS, et al. Phosphorylation status determines the opposing functions of Smad2/Smad3 as STAT3 cofactors in TH17 differentiation. Nat Commun. 2015;6:7600. doi: 10.1038/ncomms8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 42.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 43.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282(48):34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mudter J, Yu J, Zufferey C, Brustle A, Wirtz S, Weigmann B, et al. IRF4 regulates IL-17A promoter activity and controls RORgammat-dependent Th17 colitis in vivo. Inflamm Bowel Dis. 2011;17(6):1343–1358. doi: 10.1002/ibd.21476. [DOI] [PubMed] [Google Scholar]
- 46.Basu R, Whitley SK, Bhaumik S, Zindl CL, Schoeb TR, Benveniste EN, et al. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance. Nat Immunol. 2015;16(3):286–295. doi: 10.1038/ni.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 48.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181(9):5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong LY, Hatfield JK, Brown MA. Ikaros sets the potential for Th17 lineage gene expression through effects on chromatin state in early T cell development. J Biol Chem. 2013;288(49):35170–35179. doi: 10.1074/jbc.M113.481440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heller JJ, Schjerven H, Li S, Lee A, Qiu J, Chen ZM, et al. Restriction of IL-22-producing T cell responses and differential regulation of regulatory T cell compartments by zinc finger transcription factor Ikaros. J Immunol. 2014;193(8):3934–3946. doi: 10.4049/jimmunol.1401234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 53.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12(1):96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka S, Suto A, Iwamoto T, Kashiwakuma D, Kagami S, Suzuki K, et al. Sox5 and c-Maf cooperatively induce Th17 cell differentiation via RORgammat induction as downstream targets of Stat3. J Exp Med. 2014;211(9):1857–1874. doi: 10.1084/jem.20130791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496(7446):461–468. doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim HW, Kang SG, Ryu JK, Schilling B, Fei M, Lee IS, et al. SIRT1 deacetylates RORgammat and enhances Th17 cell generation. J Exp Med. 2015 doi: 10.1084/jem.2013237805062015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, et al. IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010;464(7293):1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 58.Hammitzsch A, Tallant C, Fedorov O, O’Mahony A, Brennan PE, Hay DA, et al. CBP30, a selective CBP/p300 bromodomain inhibitor, suppresses human Th17 responses. Proc Natl Acad Sci U S A. 2015;112(34):10768–10773. doi: 10.1073/pnas.1501956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murugaiyan G, da Cunha AP, Ajay AK, Joller N, Garo LP, Kumaradevan S, et al. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest. 2015;125(3):1069–1080. doi: 10.1172/JCI74347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X, et al. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One. 2012;7(10):e46082. doi: 10.1371/journal.pone.0046082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Xing F, Ye S, Xiao J, Di J, Zeng S, et al. Jagged-1 signaling suppresses the IL-6 and TGF-beta treatment-induced Th17 cell differentiation via the reduction of RORgammat/IL-17A/IL-17F/IL-23a/IL-12rb1. Sci Rep. 2015;5:8234. doi: 10.1038/srep08234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen RY, Fan YM, Zhang Q, Liu S, Li Q, Ke GL, et al. Estradiol inhibits Th17 cell differentiation through inhibition of RORgammaT transcription by recruiting the ERalpha/REA complex to estrogen response elements of the RORgammaT promoter. J Immunol. 2015;194(8):4019–4028. doi: 10.4049/jimmunol.1400806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jianjun Y, Zhang R, Lu G, Shen Y, Peng L, Zhu C, et al. T cell-derived inducible nitric oxide synthase switches off Th17 cell differentiation. J Exp Med. 2013;210(7):1447–1462. doi: 10.1084/jem.20122494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laine A, Martin B, Luka M, Mir L, Auffray C, Lucas B, et al. Foxo1 Is a T Cell-Intrinsic Inhibitor of the RORgammat-Th17 Program. J Immunol. 2015;195(4):1791–1803. doi: 10.4049/jimmunol.1500849. [DOI] [PubMed] [Google Scholar]
- 66.Villarino AV, Gallo E, Abbas AK. STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and -independent mechanisms. J Immunol. 2010;185(11):6461–6471. doi: 10.4049/jimmunol.1001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermann-Kleiter N, Meisel M, Fresser F, Thuille N, Muller M, Roth L, et al. Nuclear orphan receptor NR2F6 directly antagonizes NFAT and RORgammat binding to the Il17a promoter. J Autoimmun. 2012;39(4):428–440. doi: 10.1016/j.jaut.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones SA, Perera DN, Fan H, Russ BE, Harris J, Morand EF. GILZ regulates Th17 responses and restrains IL-17-mediated skin inflammation. J Autoimmun. 2015;61:73–80. doi: 10.1016/j.jaut.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122(2):201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 72.Farago B, Magyari L, Safrany E, Csongei V, Jaromi L, Horvatovich K, et al. Functional variants of interleukin-23 receptor gene confer risk for rheumatoid arthritis but not for systemic sclerosis. Ann Rheum Dis. 2008;67(2):248–250. doi: 10.1136/ard.2007.072819. [DOI] [PubMed] [Google Scholar]
- 73.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54(4):1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 75.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 76.Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128(Pt 5):988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 77.Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180(11):7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 78.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108(3):430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 80.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97(6):726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 81.Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1alpha,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132(2):297–304. e293. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 82.Schewitz-Bowers LP, Lait PJ, Copland DA, Chen P, Wu W, Dhanda AD, et al. Glucocorticoid-resistant Th17 cells are selectively attenuated by cyclosporine A. Proc Natl Acad Sci U S A. 2015;112(13):4080–4085. doi: 10.1073/pnas.1418316112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3(104):104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35(6):972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Littman DR, Sun Z, Unutmaz D, Sunshine MJ, Petrie HT, Zou YR. Role of the nuclear hormone receptor ROR gamma in transcriptional regulation, thymocyte survival, and lymphoid organogenesis. Cold Spring Harb Symp Quant Biol. 1999;64:373–381. doi: 10.1101/sqb.1999.64.373. [DOI] [PubMed] [Google Scholar]
- 86.Langlais D, Couture C, Balsalobre A, Drouin J. The Stat3/GR interaction code: predictive value of direct/indirect DNA recruitment for transcription outcome. Mol Cell. 2012;47(1):38–49. doi: 10.1016/j.molcel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013;6(2):335–346. doi: 10.1038/mi.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zijlstra GJ, Fattahi F, Rozeveld D, Jonker MR, Kliphuis NM, van den Berge M, et al. Glucocorticoids induce the production of the chemoattractant CCL20 in airway epithelium. Eur Respir J. 2014;44(2):361–370. doi: 10.1183/09031936.00209513. [DOI] [PubMed] [Google Scholar]
- 89.Gupta A, Dimeloe S, Richards DF, Chambers ES, Black C, Urry Z, et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax. 2014;69(6):508–515. doi: 10.1136/thoraxjnl-2013-203421. [DOI] [PubMed] [Google Scholar]
- 90.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115(2):335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 91.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dale DC, Fauci AS, Guerry DI, Wolff SM. Comparison of agents producing a neutrophilic leukocytosis in man. Hydrocortisone, prednisone, endotoxin, and etiocholanolone. J Clin Invest. 1975;56(4):808–813. doi: 10.1172/JCI108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liles WC, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86(8):3181–3188. [PubMed] [Google Scholar]
- 94.Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol. 2010;10(11):1325–1334. doi: 10.1016/j.intimp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 95.Zijlstra GJ, Ten Hacken NH, Hoffmann RF, van Oosterhout AJ, Heijink IH. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur Respir J. 2012;39(2):439–445. doi: 10.1183/09031936.00017911. [DOI] [PubMed] [Google Scholar]
- 96.Augustin M, Mrowietz U, Bonnekoh B, Rosenbach T, Thaci D, Reusch M, et al. Topical long-term therapy of psoriasis with vitamin D(3) analogues, corticosteroids and their two compound formulations: position paper on evidence and use in daily practice. J Dtsch Dermatol Ges. 2014;12(8):667–682. doi: 10.1111/ddg.12396. [DOI] [PubMed] [Google Scholar]
- 97.Lovato P, Norsgaard H, Tokura Y, Ropke MA. Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis. J Dermatol Sci. 2016;81(3):153–164. doi: 10.1016/j.jdermsci.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 98.Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 99.Sofen H, Smith S, Matheson RT, Leonardi CL, Calderon C, Brodmerkel C, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133(4):1032–1040. doi: 10.1016/j.jaci.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 100.Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A Phase 2 Trial of Guselkumab versus Adalimumab for Plaque Psoriasis. N Engl J Med. 2015;373(2):136–144. doi: 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- 101.Kopp T, Riedl E, Bangert C, Bowman EP, Greisenegger E, Horowitz A, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222–226. doi: 10.1038/nature14175. [DOI] [PubMed] [Google Scholar]
- 102.Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136(1):116–124. e117. doi: 10.1016/j.jaci.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 103.Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krieger S, Sorrells SF, Nickerson M, Pace TW. Mechanistic insights into corticosteroids in multiple sclerosis: war horse or chameleon? Clin Neurol Neurosurg. 2014;119:6–16. doi: 10.1016/j.clineuro.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 105.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu M, Hu X, Wang Y, Peng F, Yang Y, Chen X, et al. Effect of high-dose methylprednisolone treatment on Th17 cells in patients with multiple sclerosis in relapse. Acta Neurol Scand. 2009;120(4):235–241. doi: 10.1111/j.1600-0404.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 107.Ferreira TB, Hygino J, Barros PO, Teixeira B, Kasahara TM, Linhares UC, et al. Endogenous interleukin-6 amplifies interleukin-17 production and corticoid-resistance in peripheral T cells from patients with multiple sclerosis. Immunology. 2014;143(4):560–568. doi: 10.1111/imm.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferreira TB, Barros PO, Teixeira B, Cassano T, Centuriao N, Kasahara TM, et al. Dopamine favors expansion of glucocorticoid-resistant IL-17-producing T cells in multiple sclerosis. Brain Behav Immun. 2014;41:182–190. doi: 10.1016/j.bbi.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 109.Teixeira B, Bittencourt VC, Ferreira TB, Kasahara TM, Barros PO, Alvarenga R, et al. Low sensitivity to glucocorticoid inhibition of in vitro Th17-related cytokine production in multiple sclerosis patients is related to elevated plasma lipopolysaccharide levels. Clin Immunol. 2013;148(2):209–218. doi: 10.1016/j.clim.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 110.Correale J, Gilmore W, Li S, Walsh J, Bassani MM, Lund B, et al. Resistance to glucocorticoid-induced apoptosis in PLP peptide-specific T cell clones from patients with progressive MS. J Neuroimmunol. 2000;109(2):197–210. doi: 10.1016/s0165-5728(00)00326-x. [DOI] [PubMed] [Google Scholar]
- 111.Neeck G. Fifty years of experience with cortisone therapy in the study and treatment of rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:28–38. doi: 10.1111/j.1749-6632.2002.tb04199.x. [DOI] [PubMed] [Google Scholar]
- 112.Kirwan J, Power L. Glucocorticoids: action and new therapeutic insights in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19(3):233–237. doi: 10.1097/BOR.0b013e3280d6471a. [DOI] [PubMed] [Google Scholar]
- 113.Quinn MA, Green MJ, Marzo-Ortega H, Proudman S, Karim Z, Wakefield RJ, et al. Prognostic factors in a large cohort of patients with early undifferentiated inflammatory arthritis after application of a structured management protocol. Arthritis Rheum. 2003;48(11):3039–3045. doi: 10.1002/art.11269. [DOI] [PubMed] [Google Scholar]
- 114.Machold KP, Landewe R, Smolen JS, Stamm TA, van der Heijde DM, Verpoort KN, et al. The Stop Arthritis Very Early (SAVE) trial, an international multicentre, randomised, double-blind, placebo-controlled trial on glucocorticoids in very early arthritis. Ann Rheum Dis. 2010;69(3):495–502. doi: 10.1136/ard.2009.122473. [DOI] [PubMed] [Google Scholar]
- 115.Verstappen SM, McCoy MJ, Roberts C, Dale NE, Hassell AB, Symmons DP, et al. Beneficial effects of a 3-week course of intramuscular glucocorticoid injections in patients with very early inflammatory polyarthritis: results of the STIVEA trial. Ann Rheum Dis. 2010;69(3):503–509. doi: 10.1136/ard.2009.119149. [DOI] [PubMed] [Google Scholar]
- 116.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Aelion JA, et al. One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled study. J Rheumatol. 2014;41(3):414–421. doi: 10.3899/jrheum.130637. [DOI] [PubMed] [Google Scholar]
- 117.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 118.Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS, et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2014;66(7):1693–1704. doi: 10.1002/art.38617. [DOI] [PubMed] [Google Scholar]
- 119.Guggino G, Giardina A, Ferrante A, Giardina G, Schinocca C, Sireci G, et al. The in vitro addition of methotrexate and/or methylprednisolone determines peripheral reduction in Th17 and expansion of conventional Treg and of IL-10 producing Th17 lymphocytes in patients with early rheumatoid arthritis. Rheumatol Int. 2015;35(1):171–175. doi: 10.1007/s00296-014-3030-2. [DOI] [PubMed] [Google Scholar]
- 120.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. [Google Scholar]
- 121.Verhoef CM, van Roon JA, Vianen ME, Lafeber FP, Bijlsma JW. The immune suppressive effect of dexamethasone in rheumatoid arthritis is accompanied by upregulation of interleukin 10 and by differential changes in interferon gamma and interleukin 4 production. Ann Rheum Dis. 1999;58(1):49–54. doi: 10.1136/ard.58.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(10):593–601. doi: 10.1038/nrrheum.2014.80. [DOI] [PubMed] [Google Scholar]
- 123.Gabryel M, Skrzypczak-Zielinska M, Kucharski MA, Slomski R, Dobrowolska A. The impact of genetic factors on response to glucocorticoids therapy in IBD. Scand J Gastroenterol. 2016;51(6):654–665. doi: 10.3109/00365521.2015.1132336. [DOI] [PubMed] [Google Scholar]
- 124.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206(3):525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nielsen OH, Kirman I, Rudiger N, Hendel J, Vainer B. Upregulation of interleukin-12 and -17 in active inflammatory bowel disease. Scand J Gastroenterol. 2003;38(2):180–185. doi: 10.1080/00365520310000672. [DOI] [PubMed] [Google Scholar]
- 127.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology. 2009;137(5):1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 128.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185(6):612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190(10):1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 131.Kawaguchi M, Takahashi D, Hizawa N, Suzuki S, Matsukura S, Kokubu F, et al. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J Allergy Clin Immunol. 2006;117(4):795–801. doi: 10.1016/j.jaci.2005.12.1346. [DOI] [PubMed] [Google Scholar]
- 132.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111(6):1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 134.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 135.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104(8):1131–1137. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 136.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132(5):1194–1204. e1192. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy. 2013;43(9):1018–1026. doi: 10.1111/cea.12119. [DOI] [PubMed] [Google Scholar]
- 138.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, et al. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-gamma(high) immunophenotypes: Potential benefits of calcitriol. J Allergy Clin Immunol. 2015;136(3):628–637. e624. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sarinho ES, Azoubel-Antunes A, Rego MJ, Brayner-Cavalcanti M, Lins ELTU, da Pitta IR, et al. Evaluation of Th17-related cytokines and IFNgamma production from blood mononuclear cells of moderate and severe asthmatic children reveals methylprednisolone does not decrease IL-22 levels. J Asthma. 2015;52(3):227–231. doi: 10.3109/02770903.2014.959129. [DOI] [PubMed] [Google Scholar]
- 140.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188(11):1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 141.Barros PO, Cassano T, Hygino J, Ferreira TB, Centuriao N, Kasahara TM, et al. Prediction of disease severity in neuromyelitis optica by the levels of interleukin (IL)-6 produced during remission phase. Clin Exp Immunol. 2016;183(3):480–489. doi: 10.1111/cei.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang S, Tanaka T, Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol. 2015;27(1):21–29. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]
- 143.Hsia BJ, Whitehead GS, Thomas SY, Nakano K, Gowdy KM, Aloor JJ, et al. Trif-dependent induction of Th17 immunity by lung dendritic cells. Mucosal Immunol. 2015;8(1):186–197. doi: 10.1038/mi.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cao Y, Bender IK, Konstantinidis AK, Shin SC, Jewell CM, Cidlowski JA, et al. Glucocorticoid receptor translational isoforms underlie maturational stage-specific glucocorticoid sensitivities of dendritic cells in mice and humans. Blood. 2013;121(9):1553–1562. doi: 10.1182/blood-2012-05-432336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamdi H, Godot V, Maillot MC, Prejean MV, Cohen N, Krzysiek R, et al. Induction of antigen-specific regulatory T lymphocytes by human dendritic cells expressing the glucocorticoid-induced leucine zipper. Blood. 2007;110(1):211–219. doi: 10.1182/blood-2007-08-107896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang X, Tian J, Ma J, Wang J, Qi C, Rui K, et al. GITRL modulates the activities of p38 MAPK and STAT3 to promote Th17 cell differentiation in autoimmune arthritis. Oncotarget. 2015 doi: 10.18632/oncotarget.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lang F, Voelkl J. Therapeutic potential of serum and glucocorticoid inducible kinase inhibition. Expert Opin Investig Drugs. 2013;22(6):701–714. doi: 10.1517/13543784.2013.778971. [DOI] [PubMed] [Google Scholar]
- 148.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125(1):222–230. e221–224. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 149.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207(11):2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, et al. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66(3):390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 151.Iqbal M, Baello S, Javam M, Audette MC, Gibb W, Matthews SG. Regulation of Multidrug Resistance P-Glycoprotein in the Developing Blood-Brain Barrier: Interplay between Glucocorticoids and Cytokines. J Neuroendocrinol. 2016;28(3) doi: 10.1111/jne.12360. [DOI] [PubMed] [Google Scholar]
- 152.Tsujimura S, Saito K, Nawata M, Nakayamada S, Tanaka Y. Overcoming drug resistance induced by P-glycoprotein on lymphocytes in patients with refractory rheumatoid arthritis. Ann Rheum Dis. 2008;67(3):380–388. doi: 10.1136/ard.2007.070821. [DOI] [PubMed] [Google Scholar]
- 153.De Iudicibus S, Franca R, Martelossi S, Ventura A, Decorti G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol. 2011;17(9):1095–1108. doi: 10.3748/wjg.v17.i9.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]