Abstract
The cell cycle in pluripotent human embryonic stem cells is governed by unique mechanisms that support unrestricted proliferation and competency for endodermal, mesodermal and ectodermal differentiation. The abbreviated G1 period with retention of uncompromised fidelity for genetic and epigenetic mechanisms operative in control of proliferation support competency for expansion of the pluripotent cell population that is fundamental for initial stages of development. Regulatory events during the G1 period of the pluripotent cell cycle are decisive for the transition from pluripotency to lineage commitment. Recent findings indicate that a G2 cell cycle pause is present in both endodermal and mesodermal lineage cells, and is obligatory for differentiation to endoderm.
Keywords: Cell cycle, Embryonic stem cells, Differentiation, Endoderm, G2 pause
Human embryonic stem cells
Human embryonic stem cells (hESCs) are an informative model for the study of early embryonic development. They are isolated from the inner cell mass of a blastocyst-stage embryo, which forms within the five days after fertilization of an oocyte (Reubinoff et al., 2000; Thomson et al., 1998). hESCs have unlimited replicative potential and are pluripotent—capable of differentiating into any somatic cell type (Thomson et al., 1998). Like transformed and tumor cells, hESCs exhibit unrestricted proliferation. There is a striking contrast however, in regulatory control; hESCs exhibit stringent cell cycle control, whereas cancer and cancer-like cells are characterized by compromised genetic and epigenetic regulatory control. hESCs maintain unlimited replicative potential through high levels of telomerase that result in retention of telomere ends on chromosomes through cell division, preventing cells from reaching the Hayflick limit and undergoing senescence (Amit et al., 2000). The expression of telomerase is part of a larger regulatory network that maintains the pluripotency and capacity for self-renewal of hESCs. This regulatory network contains both auto-regulatory and feed-forward loops, and is dominated by three transcription factors, OCT4 (Niwa et al., 2000), NANOG (Chambers et al., 2003), and SOX2 (Avilion et al., 2003; Fong et al., 2008) that are essential for maintenance of pluripotency and self-renewal (Boyer et al., 2005).
Recognition of the vital contributions of hESCs to development and a growing appreciation for the capabilities of hESCs in cell-based therapy and regenerative medicine underscores the need to mechanistically understand fidelity of cellular proliferation and control of the cell cycle in hESCs. This review focuses on regulatory events during the G2 phase of the cell cycle that are obligatory for commitment of pluripotent hESCs to differentiation and the consequences of perturbing G2 physiological control.
Unique Cell Cycle Regulation in hESCs
Maintenance of pluripotency in hESCs is tightly linked to cell cycle control (Kapinas et al., 2013; White and Dalton, 2005). The G1 phase of the cell cycle in pluripotent hESCs is approximately 3 hours, significantly shorter than the 10-hour G1 phase in somatic cells (Becker et al., 2006). Loss of pluripotency and the onset of differentiation are associated with lengthening of the G1 phase (Calder et al., 2013; Filipczyk et al., 2007). Additionally, endo- and mesodermal differentiation are thought to initiate in early G1 and the neuroectodermal lineage in late G1 phase (Pauklin and Vallier, 2013; Sela et al., 2012). Differentiation cues have been shown to elicit minimal response in the G2/M/S phases of the hES cell cycle (Pauklin and Vallier, 2013).
Negative regulators of proliferation in somatic cells, including p21 and p27, have been suggested to have roles in regulating the hESC cell cycle (Calder et al., 2013; Egozi et al., 2007; Zhu et al., 2014). The levels of p21 and p27 remain low in pluripotent hESCs, and increase as cells differentiate. A recent study found that to maintain pluripotency, the expression of p21 and p27 must be repressed, as elevated levels of these cyclin-dependent kinase inhibitors resulted in a higher proportion of cells in the G1 phase (Menchon et al., 2011; Zhu et al., 2014). Additionally, p27 can directly repress SOX2 during differentiation (Li et al., 2012). These results and others establish a correlation between the loss of the abbreviated pluripotent cell cycle and the initiation of hESC differentiation, however, the mechanisms that coordinate changes in the cell cycle and the onset of differentiation are not fully understood.
Regulation of the G2/M Transition of the Cell Cycle
The focus on cell cycle regulation in hESCs has been primarily on the role of the G1 phase and the G1/S transition. However, evidence is emerging for significant contributions to control of hESC proliferation during the G2 phase of the cell cycle. The G2 phase of the cell cycle begins at the completion of DNA replication and includes a period of rapid protein production and cell growth that concludes upon initiation of prophase in mitosis. The events that modulate the transition from G2 to mitosis form a checkpoint that prevents premature mitosis in cells with defects such as incomplete DNA replication or DNA damage repair (O’Connell and Cimprich, 2005; Zhou and Elledge, 2000). Progress through the mammalian cell cycle is mediated by the sequential activation of cyclin-dependent kinases (Pines, 1995). Activation of CDKs is regulated by both cell-cycle stage–specific cyclin binding and phosphorylation events.
A key regulatory event in the activation of CDKs and the transition from G2 to mitosis is the formation of a complex comprised of CDK1 and one of the five cyclin B isoforms (Nigg, 1995). This complex was originally identified as the maturation-promoting factor or M phase-promoting factor (MPF) in meiotic frog eggs, because it was capable of inducing mitosis in immature G2 phase oocytes (Masui and Markert, 1971). In humans it is generally thought that CDK1 levels remain constant throughout the cell cycle, while the levels of cyclin B1 mRNA and protein change with cell-cycle progression (Smits and Medema, 2001). Transcription from the cyclin B1 promoter increases after S phase and decreases during the beginning of G1, and cyclin B1 protein is degraded during the metaphase-anaphase transition during mitosis (Piaggio et al., 1995; Pines and Hunter, 1989). Figure 1 shows an overview of regulation of the G2 to M-phase transition.
Figure 1.
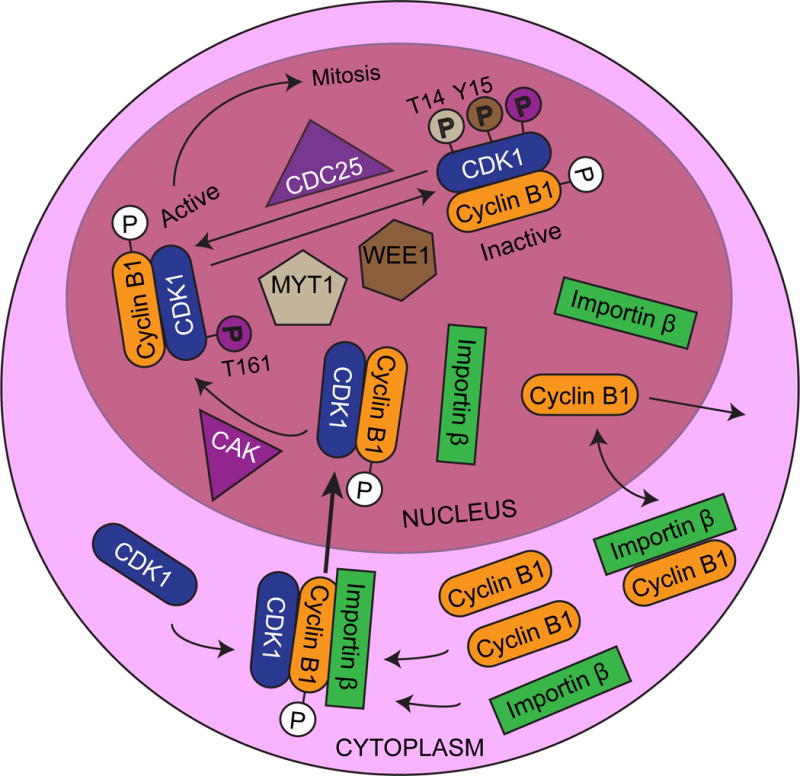
Overview of the Regulation of the G2/M Transition
During interphase, cyclin B interacts with importin β, is imported into the nucleus, and then is swiftly shuttled out of the nucleus due to its N-terminal nuclear export signal (Hagting et al., 1998; Takizawa et al., 1999; Toyoshima et al., 1998). Once the levels of cyclin B increase, MPF complex levels in the cytoplasm increase accordingly. The cyclin B portion of the complex is then phosphorylated near the nuclear export signal on residues S133 and S147, creating a nuclear localization signal. This increases the rate of nuclear import beyond what would be caused by simply blocking the nuclear export signal (Hagting et al., 1998; Toyoshima-Morimoto et al., 2001).
In addition to the regulation of MPF complex formation and localization, there are multiple phosphorylation events on CDK1 that control the transition from G2 to mitosis. An activating phosphorylation of CDK1 occurs on T161 in the T-loop by cyclin activating kinase (CAK), which is a heterotrimeric complex composed of CDK7, cyclin H, and menage a trois 1 (MAT1) (Tassan et al., 1994). This activating phosphorylation is opposed by a series of inhibitory phosphorylations. The Wee1-like protein kinase (WEE1) and myelin transcription factor 1 (MYT1) kinases are responsible for placing these inhibitory phosphorylations on CDK1 in the MPF complex. WEE1 primarily phosphorylates Y15 of CDK1, while the MYT1 kinase can phosphorylate either T14 or Y15 (Booher et al., 1997; Liu et al., 1997; Parker and Piwnica-Worms, 1992). Phosphorylation of Y15 on CDK1 interferes with phosphate transfer to a bound substrate, while the phosphorylation of T14 interferes with ATP binding (Atherton-Fessler et al., 1993; Endicott et al., 1994). Cyclin B binding mediates these inhibitory phosphorylations on CDK1 (Meijer et al., 1991).
Once CDK1 is bound to Cyclin B and phosphorylated by CDK-activating kinase (CAK) and WEE1/MYT1, it is primed and ready to be activated through the dephosphorylation of T14 and Y15, which triggers the initiation of mitosis. Dephosphorylation of CDK1 T14 and Y15 is performed by the dual specificity phosphatase CDC25, which can dephosphorylate phosphotyrosine as well as phosphothreonine residues (Gautier et al., 1991; Kumagai and Dunphy, 1991; Strausfeld et al., 1991). Conversely, the MPF complex can then phosphorylate WEE1 and CDC25, thereby inhibiting WEE1 and further activating CDC25, which can then further dephosphorylate CDK1 on T14 and Y15 (Hoffmann et al., 1993; Mueller et al., 1995). This amplifies the signal and contributes to a burst of MPF activity, which initiates mitosis through the phosphorylation of downstream targets. Importantly, these targets include nuclear lamins that cause nuclear-envelope breakdown, microtubule-associated proteins that promote assembly of the mitotic spindle assembly, and condensin subunits that are responsible for chromosome condensation (Heald and McKeon, 1990; Kimura et al., 1998; Miake-Lye et al., 1983; Tombes et al., 1991).
Interestingly, in mice, CDK1 is the only CDK that is essential for survival—its absence results in embryonic lethality by day E1.5 (Santamaria et al., 2007). Of the cyclins, only disruption of cyclin A2 and cyclin B1, which have roles in the S/G2/M phases, results in embryonic lethality early in development (E5.5 and before E10.5/not determined, respectively) (Brandeis et al., 1998; Gong and Ferrell, 2010; Gong et al., 2007; Murphy et al., 1997). The role of WEE1 in mammalian development is especially difficult to evaluate because WEE1 is required prior to blastocyst formation. Without WEE1, embryonic lethality occurs before day E3.5; an accumulation of DNA damage results in apoptosis and embryo death] (Tominaga et al., 2006). Together, these studies indicate the importance of the regulation of G2/M transition.
Lineage-Specific Early Differentiation of Human Embryonic Stem Cells Requires a G2 Cell Cycle Pause
Recent results have provided evidence for the stringent requirement of a unique G2 regulatory mechanism to support competency for differentiation in hESCs. (VanOudenhove et al., 2016) have observed a cell cycle pause in G2 during differentiation to several lineages that is regulated by WEE1. Functionality was established by demonstrating that WEE1 inhibition disrupts the G2 cell cycle pause and compromises lineage-determinate gene expression. These findings provide a novel mechanistic dimension to functional relationships between control of proliferation and induction of differentiation.
This G2 pause is mediated by CDK1 phosphorylation at Y15 due to an increase in WEE1 kinase levels. Up-regulation of WEE1 and the cell cycle pause are observed during differentiation to both endodermal and mesodermal lineages, but only endoderm specification is compromised as a consequence of WEE1 inhibition. By 72 hours of differentiation, the level of WEE1 begins to decrease, as does the extent of inhibiting phosphorylation of CDK1 Y15 and, in turn, the number of cells that exhibit paused cell cycle progression (VanOudenhove et al., 2016).
Consequently, the balance between the opposing influences of the inactivating phosphorylation of WEE1 and the activating dephosphorylation allows cells to begin to progress through the cell cycle. These findings provide compelling evidence for a WEE1-mediated cell cycle pause in G2, that when disrupted, selectively compromises lineage commitment to endoderm.
Conclusion and Perspectives
The abbreviated, but remarkably regulated, cell cycle of rapidly proliferating hESCs supports competency for unrestricted proliferation of pluripotent progenitor cells with full capacity for development in response to physiological cues. This is in striking contrast to transformed and tumor cells, in which unrestricted proliferation is associated with compromised genetic and epigenetic regulation. Regulatory mechanisms that are operative in this tightly controlled pluripotent cell cycle provide informative insight into the biology and pathology of the unique requirements for proliferation that support pluripotency and the initiation of lineage commitment.
A fundamental property of the pluripotent human embryonic cell cycle is the striking abbreviation of the G1 cell-cycle phase, with retention of physiological control, despite the absence of the full complement of G1 checkpoint activities. In contrast, the S and G2 periods of the pluripotent cell cycle are temporally, biochemically and presumably, functionally retained.
Several lines of evidence indicate the contribution of G1 regulatory mechanisms to the transition from pluripotency, to initiation of lineage commitment and development of phenotype. This initiation of differentiation is accompanied by, and potentially functionally linked with, an expanded G1 period and establishment of the full complement of G1 checkpoint mechanisms. The discovery of a G2 pause in the hES cell cycle that is obligatory for establishing phenotype, expands understanding of modifications in cell cycle control that are operative in pluripotent cells that require functionally important modifications for differentiation. Insight into the impact of G1 and G2 cell cycle control on the proliferation/differentiation transition is informative within the context of developmental regulation as well as for tissue remodeling and regeneration. Exploring the contributions of epigenetic control and the extent to which mechanisms operative during initial lineage commitment can be leveraged to expand understanding of the biology and pathology of lineage-committed stem cells will be informative at the fundamental regulatory and clinically relevant levels.
Acknowledgments
Contract grant sponsor: National Cancer Institute
Contract grant number: R01 CA139322; P01 CA082834
Footnotes
The authors declare no conflicts of interest.
References
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Atherton-Fessler S, Parker LL, Geahlen RL, Piwnica-Worms H. Mechanisms of p34cdc2 regulation. Mol Cell Biol. 1993;13:1675–1685. doi: 10.1128/mcb.13.3.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–893. doi: 10.1002/jcp.20776. [DOI] [PubMed] [Google Scholar]
- Booher RN, Holman PS, Fattaey A. Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J Biol Chem. 1997;272:22300–22306. doi: 10.1074/jbc.272.35.22300. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis M, Rosewell I, Carrington M, Crompton T, Jacobs MA, Kirk J, Gannon J, Hunt T. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc Natl Acad Sci U S A. 1998;95:4344–4349. doi: 10.1073/pnas.95.8.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder A, Roth-Albin I, Bhatia S, Pilquil C, Lee JH, Bhatia M, Levadoux-Martin M, McNicol J, Russell J, Collins T, Draper JS. Lengthened G1 phase indicates differentiation status in human embryonic stem cells. Stem Cells Dev. 2013;22:279–295. doi: 10.1089/scd.2012.0168. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Egozi D, Shapira M, Paor G, Ben-Izhak O, Skorecki K, Hershko DD. Regulation of the cell cycle inhibitor p27 and its ubiquitin ligase Skp2 in differentiation of human embryonic stem cells. FASEB J. 2007;21:2807–2817. doi: 10.1096/fj.06-7758com. [DOI] [PubMed] [Google Scholar]
- Endicott JA, Nurse P, Johnson LN. Mutational analysis supports a structural model for the cell cycle protein kinase p34. Protein Eng. 1994;7:243–253. doi: 10.1093/protein/7.2.243. [DOI] [PubMed] [Google Scholar]
- Filipczyk AA, Laslett AL, Mummery C, Pera MF. Differentiation is coupled to changes in the cell cycle regulatory apparatus of human embryonic stem cells. Stem Cell Res. 2007;1:45–60. doi: 10.1016/j.scr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26:1931–1938. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gong D, Ferrell JE., Jr The roles of cyclin A2, B1, and B2 in early and late mitotic events. Mol Biol Cell. 2010;21:3149–3161. doi: 10.1091/mbc.E10-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Pomerening JR, Myers JW, Gustavsson C, Jones JT, Hahn AT, Meyer T, Ferrell JE., Jr Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr Biol. 2007;17:85–91. doi: 10.1016/j.cub.2006.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A, Karlsson C, Clute P, Jackman M, Pines J. MPF localization is controlled by nuclear export. EMBO J. 1998;17:4127–4138. doi: 10.1093/emboj/17.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinas K, Grandy R, Ghule P, Medina R, Becker K, Pardee A, Zaidi SK, Lian J, Stein J, van Wijnen A, Stein G. The abbreviated pluripotent cell cycle. J Cell Physiol. 2013;228:9–20. doi: 10.1002/jcp.24104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Hirano M, Kobayashi R, Hirano T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science. 1998;282:487–490. doi: 10.1126/science.282.5388.487. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Matheu A, Lynch CJ, Canamero M, Rizzoti K, Carneiro C, Martinez G, Vidal A, Lovell-Badge R, Serrano M. p27(Kip1) directly represses Sox2 during embryonic stem cell differentiation. Cell Stem Cell. 2012;11:845–852. doi: 10.1016/j.stem.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Stanton JJ, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol Cell Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Meijer L, Azzi L, Wang JY. Cyclin B targets p34cdc2 for tyrosine phosphorylation. EMBO J. 1991;10:1545–1554. doi: 10.1002/j.1460-2075.1991.tb07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menchon C, Edel MJ, Izpisua Belmonte JC. The cell cycle inhibitor p27Kip(1) controls self-renewal and pluripotency of human embryonic stem cells by regulating the cell cycle, Brachyury and Twist. Cell Cycle. 2011;10:1435–1447. doi: 10.4161/cc.10.9.15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake-Lye R, Newport J, Kirschner M. Maturation-promoting factor induces nuclear envelope breakdown in cycloheximide-arrested embryos of Xenopus laevis. J Cell Biol. 1983;97:81–91. doi: 10.1083/jcb.97.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Stinnakre MG, Senamaud-Beaufort C, Winston NJ, Sweeney C, Kubelka M, Carrington M, Brechot C, Sobczak-Thepot J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat Genet. 1997;15:83–86. doi: 10.1038/ng0197-83. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- O’Connell MJ, Cimprich KA. G2 damage checkpoints: what is the turn-on? J Cell Sci. 2005;118:1–6. doi: 10.1242/jcs.01626. [DOI] [PubMed] [Google Scholar]
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- Pauklin S, Vallier L. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013;155:135–147. doi: 10.1016/j.cell.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaggio G, Farina A, Perrotti D, Manni I, Fuschi P, Sacchi A, Gaetano C. Structure and growth-dependent regulation of the human cyclin B1 promoter. Exp Cell Res. 1995;216:396–402. doi: 10.1006/excr.1995.1050. [DOI] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995;308(Pt 3):697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, Caceres JF, Dubus P, Malumbres M, Barbacid M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- Sela Y, Molotski N, Golan S, Itskovitz-Eldor J, Soen Y. Human embryonic stem cells exhibit increased propensity to differentiate during the G1 phase prior to phosphorylation of retinoblastoma protein. Stem Cells. 2012;30:1097–1108. doi: 10.1002/stem.1078. [DOI] [PubMed] [Google Scholar]
- Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta. 2001;1519:1–12. doi: 10.1016/s0167-4781(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Strausfeld U, Labbe JC, Fesquet D, Cavadore JC, Picard A, Sadhu K, Russell P, Doree M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991;351:242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Takizawa CG, Weis K, Morgan DO. Ran-independent nuclear import of cyclin B1-Cdc2 by importin beta. Proc Natl Acad Sci U S A. 1999;96:7938–7943. doi: 10.1073/pnas.96.14.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan JP, Schultz SJ, Bartek J, Nigg EA. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase) J Cell Biol. 1994;127:467–478. doi: 10.1083/jcb.127.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tombes RM, Peloquin JG, Borisy GG. Specific association of an M-phase kinase with isolated mitotic spindles and identification of two of its substrates as MAP4 and MAP1B. Cell Regul. 1991;2:861–874. doi: 10.1091/mbc.2.11.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga Y, Li C, Wang RH, Deng CX. Murine Wee1 plays a critical role in cell cycle regulation and pre-implantation stages of embryonic development. Int J Biol Sci. 2006;2:161–170. doi: 10.7150/ijbs.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature. 2001;410:215–220. doi: 10.1038/35065617. [DOI] [PubMed] [Google Scholar]
- Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanOudenhove JJ, Grandy RA, Ghule PN, Del Rio R, Lian JB, Stein JL, Zaidi SK, Stein GS. Lineage-Specific Early Differentiation of Human Embryonic Stem Cells Requires a G2 Cell Cycle Pause. Stem Cells. 2016;34:1765–1775. doi: 10.1002/stem.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Dalton S. Cell cycle control of embryonic stem cells. Stem Cell Rev. 2005;1:131–138. doi: 10.1385/SCR:1:2:131. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Zhu H, Hu S, Baker J. JMJD5 regulates cell cycle and pluripotency in human embryonic stem cells. Stem Cells. 2014;32:2098–2110. doi: 10.1002/stem.1724. [DOI] [PubMed] [Google Scholar]


