Abstract
Early stage immune responses can dictate the severity and outcome of inflammatory processes such as tumor growth and viral infection. Cytokines such as the interleukin 17 (IL-17) family and cellular stress defense (e.g., anti-oxidant) pathways have evolved early and regulate disease surveillance in vertebrates and invertebrates as far back as Caenorhabditis elegans. Our group has recently found a new role for nuclear factor erythroid-derived 2-like 2 (Nrf2) in regulating early anti-cancer immune responses by inducing IL-17D and recruiting natural killer (NK) cells. In this Cytokine Stimulus, we discuss recent findings that encourage boosting the Nrf2/IL-17D/NK cell axis for the treatment of cancer and viral infection.
The interleukin (IL)-17 family of cytokines act by inducing genes that recruit and activate immune cells [1]. Additionally, protein sequence analyses and challenge experiments in teleosts and invertebrates have positioned IL-17 cytokines as early evolved immune activators, along with tumor necrosis factor (TNF) [2]. For example, IL-17 homolog expression, similar to TNF, can be found in invertebrates such as the sea urchin that express few other cytokines and lack chemokines and interferons (IFNs) [3]. Given that invertebrates experience both bacterial and viral infection [4, 5], the lack of IFNs and most other cytokine genes suggests that the IL-17s (and TNF) must subserve the primordial surveillance of extracellular and intracellular pathogenic insults. Most notably, sea urchin has over 200 TLRs and over 20 IL-17 genes (more than 3 fold compared to human IL-17s), which suggests synergistic qualities between the two ancient innate immune activators [3].
In fact, several studies have shown IL-17s to participate prominently in vertebrate and invertebrate innate immunity. Teleosts (e.g., trout) and invertebrates (e.g., oyster) respond strongly to bacterial challenge with robust increase in IL-17 homologs [2, 4]. In particular, IL-17D is highly conserved (>50%) in teleost sequence compared to other IL-17s and constitutively expressed throughout the body in some species such as zebrafish and salmon [2]. This observation may indicate a homeostatic role for IL-17D or a distinct function between fish IL-17D and mammalian IL-17D, which is not constitutively expressed in all mammalian tissues [2]. Figure 1 shows a summary of the regulation and function of the IL-17 family members (adapted from [1]. The individual cytokines seem to participate in distinct anti-pathogen responses, are induced by different pathways, and can be secreted by immune and non-immune cells. IL-17A and IL-17F are made by Th17 cells and promote inflammatory responses to combat extracellular bacteria and fungi. IL-17C has autocrine activity on epithelial cells to produce antibacterial peptides as well as pro-inflammatory molecules to mediate downstream activities similar to IL-17A/F [1]. The activities for IL-17B and IL-17E are emerging but seem distinct from the other cytokines.
Figure 1. The family of interleukin 17 cytokines.
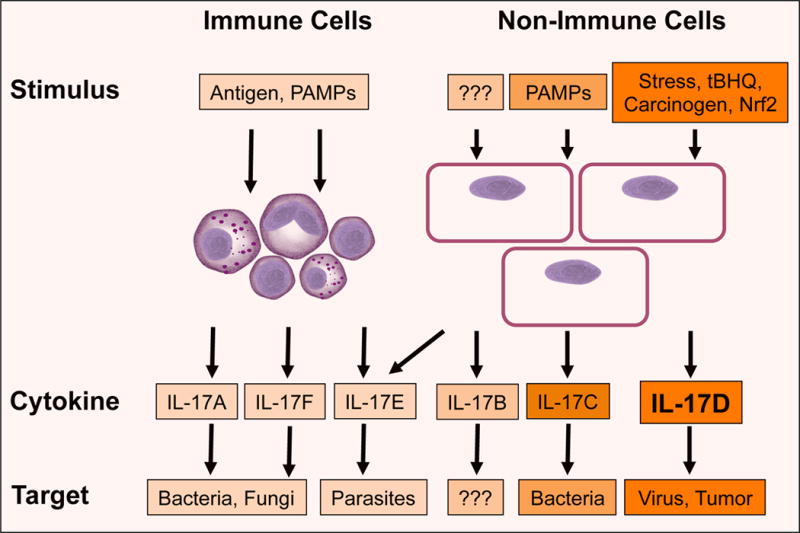
The family of IL-17 cytokines comprises six members (IL-17 A-F) that are induced by different stimuli. The individual cytokines can be expressed by immune or non-immune cells and mediate distinct anti-pathogen responses. PAMPs: pathogen-associated molecular pattern; tBHQ: tert-butylhydroquinone.
We have previously demonstrated that IL-17D offers protection to the host by recruiting NK cells to suppress tumor growth [6]. However, how IL-17D is regulated remained unknown thus far. In our latest study published in Cell Reports [7], we found that IL-17D expression is regulated by the transcription factor nuclear factor erythroid-derived 2-like 2 (Nrf2), a known sensor for oxidative and xenobiotic stress. Given that Nrf2 offers intrinsic protection via antioxidant responses, we sought to investigate whether Nrf2 activity mediates extrinsic immune activation and surveillance mechanisms through IL-17D. Since IL-17s developed early in evolution, suggesting an innate role in anti-pathogen responses before the evolution of adaptive immunity, we also investigated the involvement of the Nrf2/IL-17D pathway during viral infection.
We identified Nrf2 as a regulator of IL-17D expression. Chromatin immunoprecipitation (ChIP) followed by qPCR revealed two Nrf2 binding sites in the murine il17d gene, corresponding to predicted anti-oxidant response elements (AREs). Accordingly, the oxidative stress inducer H2O2 and the known Nrf2 activator tert-butylhydroquinone (tBHQ) induced IL-17D in vitro and in vivo, which was prevented by nrf2 knockdown via RNA inference. Nrf2 and IL-17D were also upregulated after vaccinia virus (VV) and mouse cytomegalovirus (MCMV) infection of cultured cells and in vivo VV scarification. In primary tumorigenesis experiments, in which cancer was induced by the chemical 3-methylcholanthrene (MCA), Nrf2, its known target gene heme oxygenase (hmox) 1 and il17d were induced in the harvested tumors. Notably, IL-17D−/− mice were more susceptible to MCA-induced cancer, cleared VV-infected scars slower, and lost more weight after systemic MCMV infection compared to WT animals.
Given that IL-17D deficiency predisposed animals to cancer and viral infection, we tested whether inducing IL-17D with Nrf2 agonists could demonstrate therapeutic efficacy. We made use of a previously developed system to locally activate Nrf2 in vivo [8]. By applying a tBHQ-containing skin cream, we could verify IL-17D induction by Nrf2 in established tumors. Remarkably, tumor growth was significantly inhibited by the treatment, and this depended on both Nrf2 and IL-17D. We could narrow down the effect to be tumor-intrinsic because it was prevented if nrf2 knockdown or il17d deficient cancer cells were transplanted. We had previously shown that forced expression of IL-17D in tumor cells induced rejection by recruiting tumor-fighting NK cells [6, 9]. Similarly, topical application of tBHQ cream recruited NK cells to tumors, and this was prevented if the tumors lacked Nrf2 or IL-17D.
We had hypothesized that the presence of IL-17D orthologs in invertebrate species that lack most other cytokines, including IFNs, meant that IL-17D must have anti-pathogen activity. Our recent studies confirm that IL-17D is required for optimal antiviral immunity. Whether it also participates in immunity against other pathogens, such as intracellular bacteria, is not known. Although we have identified NK cells as a primary target for IL-17D, it is likely that other antiviral pathways are induced by IL-17D. Further studies on the genes induced downstream of IL-17D will elucidate the mechanism of how it protects the host from viral infection. We speculate that similar to IL-17C’s induction of defensins and anti-bacterial cathelicidins, IL-17D should induce antiviral genes such as nucleases or other IFN-induced genes. Moreover, as we have found that tBHQ can induce Nrf2 and IL-17D in the therapy of cancer, this strategy could work for local viral infection.
Outside of infection, Nrf2 and IL-17D also influence cancer progression. Notably, Nrf2 has been puzzling researchers for decades as a “double-edged” sword that can either promote or inhibit cancer [10]. It is proposed that Nrf2 can either act anti-tumorigenic, by mediating protection of normal cells against carcinogens, or pro-tumorigenic, by protecting cancer cells from oxidative stress. Our finding that Nrf2 also induces IL-17D adds a further layer of complexity to its pro- and anti-tumor activities. Importantly, our studies have confirmed that inducing Nrf2 and IL-17D in established cancers can lead to recruitment of NK cells and cancer regression. Therefore, we conclude that the anti-tumor activities of Nrf2, when bolstered through induction of IL-17D, can outweigh its pro-tumor activities in our mouse model. As we elucidate other regulators of IL-17D and its effector pathways, we can tailor specific therapies to activate only the anti-tumor activities of Nrf2 and/or IL-17D.
In summary, by showing that Nrf2 and IL-17D are induced after viral infection and that IL-17D is required for optimal anti-viral responses, we propose that the Nrf2/IL-17D axis evolved as a mediator of immunity, specifically against intracellular pathogens. Inducing IL-17D via the activation of Nrf2 by tBHQ might be broadly applicable for the treatment of viral infection and serve as an immune therapy similar to the observed application in murine cancer. The specific involvement of Nrf2 and IL-17D in other types of infection as well as other types of (human) cancers still needs to be determined and will remain an active area of investigation in the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pappu R, Rutz S, Ouyang W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 2012;33(7):343–9. doi: 10.1016/j.it.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Kono T, Korenaga H, Sakai M. Genomics of fish IL-17 ligand and receptors: a review. Fish Shellfish Immunol. 2011;31(5):635–43. doi: 10.1016/j.fsi.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Hibino T, et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 2006;300(1):349–65. [Google Scholar]
- 4.Roberts S, et al. Rapid accumulation of an interleukin 17 homolog transcript in Crassostrea gigas hemocytes following bacterial exposure. Dev Comp Immunol. 2008;32(9):1099–104. doi: 10.1016/j.dci.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.He Y, et al. Transcriptome analysis reveals strong and complex antiviral response in a mollusc. Fish Shellfish Immunol. 2015;46(1):131–44. doi: 10.1016/j.fsi.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 6.O’Sullivan T, et al. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep. 2014;7(4):989–98. doi: 10.1016/j.celrep.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saddawi-Konefka R, et al. Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell Rep. 2016;16(9):2348–58. doi: 10.1016/j.celrep.2016.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schafer M, et al. Activation of Nrf2 in keratinocytes causes chloracne (MADISH)-like skin disease in mice. EMBO Mol Med. 2014;6(4):442–57. doi: 10.1002/emmm.201303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saddawi-Konefka R, et al. Tumor-expressed IL-17D recruits NK cells to reject tumors. Oncoimmunology. 2014;3(12):e954853. doi: 10.4161/21624011.2014.954853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Rev. 2012;64(4):1055–81. doi: 10.1124/pr.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]


