Abstract
miR-29b and miR-29a transcript levels were reported to increase in exponentially growing CHO-K1 cells. Here, we examine the regulation of miR-29b-1/a in CHO-K1 cells. We observed that 4-hydroxytamoxifen (4-OHT) increased pri-miR-29b-1 and pri-miR-29a transcription in CHO-K1 cells by activating endogenous estrogen receptor α (ERα). DICER, an established, bona fide target of miR-29b-1/a, was shown to be regulated by 4-OHT in CHO-K1 cells. We showed that miR-29b-1 and miR-29a serve a repressive role in cell proliferation, migration, invasion, and colony formation in CHO-K1 cells. To identify other targets of miR-29b-1 and miR-29a, RNA sequencing was performed by transfecting cells with anti-miR-29a, which inhibits both miR-29a and miR-29b-1, pre-miR-29b-1, and/or pre-miR-29a. In silico network analysis in MetaCore™ identified common and unique putative gene targets of miR-29b-1 and miR-29a. Pathway analysis of identified putative miR-29 targets were related to cell adhesion, cytoskeletal remodeling, and development. Further inquiry revealed regulation of pathways mediating responses to growth factor stimulus and cell cycle regulation.
Keywords: miRNAs, 4-Hydroxytamoxifen, CHO-K1, ERα, DICER1, RNA seq
1. Introduction
Chinese hamster ovary cells (CHO, most often CHO-K1, although CHO-S is another cell line used) are the most frequently applied host system in industrial development for the manufacture and production of recombinant therapeutic proteins (Fischer et al., 2015; Hacker et al., 2009; Harreither et al., 2015; Hernandez Bort et al., 2012). CHO-K1 cells are often used to characterize protein-protein and protein-DNA interactions because they are easy to transfect. MicroRNAs are endogenous 22 nt non-coding RNA molecules that control the fate of gene transcripts by binding to the 3’-untranslated regions (UTRs) of target mRNAs to mediate translational repression and/or mRNA degradation (Iorio and Croce, 2009). Each microRNA can target hundreds of genes to coordinately regulate multiple cellular processes (Selbach et al., 2008). There are few studies offering an in-depth mechanistic study of identified microRNAs, their regulation and targets in CHO-K1 cells.
Selected microRNAs have been identified to regulate phenotypic behavior (Hernandez Bort et al., 2012), protein production (Barron et al., 2011), cell growth (Clarke et al., 2012), or increase apoptosis resistance (Druz et al., 2011) in CHO-K1 cells. For example ectopic expression of miR-7 in CHO-K1 cells decreased cell proliferation (Barron et al., 2011) while overexpression of mmu-miR-466h had a pro-apoptotic role in CHO cells adapted to grow in suspension (Druz et al., 2011). Studies of gene and microRNA function in CHO cells have been facilitated by the availability of techniques including microarray, high throughput next generation sequencing (NGS), and RNA sequencing (RNA), (Gammell et al., 2007; Kantardjieff et al., 2009; Trummer et al., 2008). Although the Chinese hamster (Brinkrolf et al., 2013; Lewis et al., 2013) and CHO-K1 (Xu et al., 2011) genomes have been sequenced, definition of these transcriptomes and the function of individual genes is limited when compared to well-studied genomes, i.e., human and mouse. An additional limitation of CHO-K1 cells is that culture conditions lead to epigenetic changes, in particular DNA methylation (Feichtinger et al., 2016), which would certainly affect transcript expression. This has led to a variety of approaches for evaluating transcriptomics within CHO cell lines (Le et al., 2015), including de novo assembly of transcripts (Vishwanathan et al., 2015). For our analysis, like others (Klanert et al., 2016), we used previously identified sequence homology to mouse Ensembl transcripts.
The miR-29 family is conserved across evolution in human, mouse, rat, and hamster (Barron et al., 2011; Diendorfer et al., 2015; Hackl et al., 2011; Johnson et al., 2011; Kriegel et al., 2012). Using the miRBase (Griffiths-Jones et al., 2006) precursor sequences as a guide, in the Chinese hamster genome, miR-29a and miR-29b are separated by approximately 250 base pairs on contig KE378782 in a region with sequence homology to an intron located within the mouse angiopoietin-like 7 (ANGPTL7) gene. A third member of the miR-29 family, miR-29c, is located on contig KE382798 within an intronic region of the APH1 homolog A, gamma secretase subunit (APH1A) based on sequence homology to the mouse APH1A gene. Each of these precursor miRs (pre-miRs) produces a mature miR on both strands of the hairpin, resulting in the six mature miRs cgr-miR-29a-5p, cgr-miR-29a-3p, cgr-miR-29b-5p, cgr-miR-29b-3p, cgr-miR-29c-5p, and cgr-miR-29c-3p (Diendorfer et al., 2015; Hackl et al., 2011). Pre miR-29b-1 and pre-miR-29a are transcribed in the reverse direction and are 78 nt and 83 nt respectively (Hackl et al., 2012). Pre-miR-29c is transcribed in the forward orientation and is 76 nt long (Hackl et al., 2012).
In CHO-K1 cells, miR-29b and miR-29a were identified as upregulated during exponential growth followed by down-regulation in the stationary and decline phases of CHO-K1 growth in suspension culture (Hernandez Bort et al., 2012). Similarly, miR-29a was increased in the 6 d compared to the 3 d time period in a suspension-adapted CHO-K1 cell assay (Gammell et al., 2007). On the other hand, another study reported that miR-29b-3p, miR-29a-3p, and miR-29c-3p were negatively correlated with growth of CHO-K1 cells (Klanert et al., 2016). Thus, it is uncertain whether miR-29 promotes or represses CHO-K1 cell growth. Further, there are limited functional studies on the regulation of miR-29 expression or its endogenous targets in CHO-K1 cells.
Here we examined the regulation of miR-29b-1/a in CHO-K1 cells in response to 4-hydroxytamoxifen (4-OHT), a selective estrogen receptor modulator (SERM) that has mixed agonist/antagonist activities for estrogen receptor α (ERα). We identified DICER, the RNase that cleaves precursor microRNAs to mature microRNAs (Grishok et al., 2001; Hutvagner et al., 2001; Ketting et al., 2001) as a miR-29b-1/a target in CHO-K1 cells. We report that 4-OHT increases miR-29b-1 and miR-29a expression in CHO-K1 cells. We examined the mechanism of 4-OHT-mediated miR-29b-1/a upregulation and identified endogenous ERα as responsible for 4-OHT-stimutlated miR-29b-1/a in CHO-K1. We used RNA seq to identify possible targets of miR-29b-1/a and/or metabolic processes regulated by these microRNAs in CHO-K1 cells, such as cell adhesion, cytoskeletal remodeling, and development. We show that miR-29b-1 and miR-29a serve a repressive role in cell proliferation, invasion, migration and colony formation of CHO-K1 cells. Finally we report DICER as a miR-29 target and identify enrichment pathways regulated by miR-29.
2. Material and methods
2.1. Chemicals
4-hydroxytamoxifen, (4-OHT) and estradiol (E2) were purchased from Sigma-Aldrich (St. Louis, MO). Fulvestrant (ICI 182,780, ICI) was purchased from Tocris (Ellisville, MO).
2.2. Cell culture and treatment
CHO-K1 cells were purchased from ATCC (Manassas, VA). CHO-K1 cells were maintained in phenol red modified IMEM (Life Technologies, Carlsbad CA) supplemented with 5% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA) and 1% penicillin/streptomycin (Pen/Strep; Invitrogen) at 37 C in a humidified atmosphere containing 5% CO2. Cells were ‘serum starved’ in phenol red-free modified IMEM supplemented with 5% charcoal-stripped FBS (DCC-FBS) for 48 h prior to treatments for the times indicated in Figures. In general, treatments included vehicle control (DMSO or ethanol (EtOH)), 100 nM 4-OHT, 10 nM E2, or 100 nM ICI 182,780, alone or after pre-treatment with ICI 182,780 for 6 h. For selected experiments, cells were pretreated with 10 μg/ml actinomycin D (ACTD, a transcriptional inhibitor, Sigma) for 6 h prior to vehicle or 4-OHT treatment.
2.3. Transient transfection
CHO-K1 cells were plated in 6 well plates at 1.0 × 105 cells/well, were serum-starved and transiently transfected for 48 h as indicated in Figure legends. CHO-K1 cells were transfected with pre-miR-29b-1-3p or pre-miR-29a-3p precursor (Pre-miRTMs, Ambion), Anti-miR-29b-1/a inhibitor (Anti-miRTMs, Ambion), and siERα (Silencer®, Ambion) using Lipofectamine RNAiMAX (Invitrogen) and Opti-MEM® Reduced Serum Medium (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. Negative controls were Pre-miR™ negative control #1 (Ambion), Anti-miR ™ negative control #1(Ambion), or Negative Control #1 (Silencer®, Ambion).
2.4. RNA isolation, RT-PCR and quantitative real-time PCR (qPCR)
RNA isolation was performed using the miRCURY RNA isolation kit (Exiqon) according to manufacturer's instructions. RNA concentration and quality was assessed using a NanoDrop spectrophotometer. RNA was converted to cDNA using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems) for microRNA and the High Capacity cDNA Reverse Transcription kit for RNA (PE Applied Biosystems). miR-29b-1/a, pri-miR-29b-1/a (Applied Biosystems) and DICER1 (Kwon et al., 2014) (IDT), expression levels were quantified using TaqMan (Applied Biosystems) or SYBR green (QIAGEN). RNU6B or RNU48 (Applied Biosystems) or GAPDH (SYBR green) served as normalizers. qPCR was performed using ABI Viia 7 (Life Technologies) with each reaction run in triplicate. The comparative threshold cycle (Ct) method (2−ΔΔCT) was used to determine fold change relative to vehicle treated or control transfected cells (Schmittgen and Livak, 2008).
2.5. Luciferase reporter assay
For transfection (1.5 × 105), CHO-K1 cells were plated in each well of a 24-well plate in IMDM without phenol red supplemented with 5% charcoal-stripped calf serum (CSCS) and Pen/Strep. 24 h after plating, the cells were co-transfected with 250 ng reporter (ERE-luciferase) and 5 ng of pRL-CMV (Promega, Madison, WI) as previously described (Thomas et al., 2003) in IMDM without serum. The cells were treated with the vehicle (DMSO), 100 nM 4-OHT, or 1 μM ICI 128,780. Treatments were performed in triplicate within each experiment. After 24 h of treatment, the cells were lysed in reporter lysis buffer (Promega) and the cleared cell extract was used for dual luciferase reporter assay (Promega) (Thomas et al., 2003). Statistical evaluation of the data was performed using GraphPad Prism Software (Graph Pad Software, San Diego, CA).
ERα regulation of miR-29b-1/a promoter was determined by plating CHO-K1 cells in triplicate in 24 well plates. Next day, cells were transfected with pGL3 −1530/þ165 miR-29b-1/miR-29a promoter fragment (Mott et al., 2010) and pGL4-hRluc-TK (Renilla, Promega) using FuGENE HD (Roche) according to manufacturer's protocol. 24 h post transfection, cells were treated for another 24 h prior to performing dual luciferase assay (Promega). Relative expression was determined by dividing averaged values from vehicle control (DMSO) values.
2.6. Functional assays
For all functional assays, CHO-K1 cells were serum-starved and transfected 48 h prior to testing.
2.6.1. Cell proliferation (BrdU) assay
Following serum starvation and transfection in 96 well plates, CHO-K1 cells were treated as indicated for 48 h and the cell proliferation ELISA bromodeoxyuridine (BrdU) assay (Sigma) was performed according to the manufacturer's instructions.
2.6.2. Cell viability (MTS) assay
Following serum starvation and transfection in 96 well plates, CHO-K1 cells were treated and medium changed every 48 h for 4 days. Cell viability was quantified using CellTiter 96 Aqueous One solution Cell proliferation (MTS) assay (Promega).
2.6.3. Colony formation assay
After transfection, CHO-K1 cells were counted, transferred to agarose plates and allowed to grow for 14 days to form colonies. Colonies were stained with crystal violet (0.005% w/v, Sigma) and counted using an inverted Nikon microscope (×10 objective).
2.6.4. Migration and invasion assay
Following transfection, cell were applied to transwell plates (Corning, purchased form VWR); and allowed to migrate across an 8.0 mm pore polycarbonate membrane for 24 h. Migrated cells were stained with 0.2% w/v crystal violet (Sigma), images taken with the Axio Observer.Z1m, (Zeiss,×10 objective) and quantified using imageJ (http://imagej.nih.gov/ij/). Invasion assay was performed similar to migration assay except that the transwell membrane was coated with 0.2 mg/ml/cm2 Matrigel (Sigma).
2.7. Western blotting
CHO-K1 cells were either transfected or treated as indicated in Figure legends. Whole cell extracts (WCE) were prepared in RIPA buffer (Sigma) in the presence of phosphatase and complete protease inhibitors (Roche). After protein quantification (Bio-Rad DC protein assay), 15 μg of protein were electrophoresed on 10% SDS-PAGE gels, transferred to PVDF membranes (Bio-Rad Laboratories, Inc., Hercules, CA), and immunoblotted for ERα (G-20, Santa Cruz Biotechnology, Santa Cruz, CA), DICER1 (Cell signaling Danvers, MA). The membrane was stripped and re-probed for β-actin as a loading control (Sigma). Bands were visualized using Carestream Image Station 4000R PRO running on Carestream Molecular Imaging Software Version 5.0.2.30 and quantified by UN-SCAN-IT Graph Digitizer Software 7.1.
2.8. RNA sequencing
CHO-K1 cells were plated in 100 mm plates, ‘serum-starved’ and transfected with anti-miR-29a, Pre-miR-29b-1 and Pre-miR-29a. Transfections were performed in three separate experiments to increase power and accuracy in RNA seq (Liu et al., 2014). 48 h post transfection, RNA was isolated using the miRCURY RNA isolation kit (Exiqon) according to manufacturer's instructions. RNA concentration and quality was assessed using a NanoDrop spectrophotometer. mRNA libraries were then prepared from 2 mg of RNA using Truseq isolation kit (Woburn, MA) and validated on an Agilent 2100 Bioanalyzer (Santa Clara, CA). Libraries were then quantified using the Illumina Library Quantification Kit, ABI Prism qPCR Mix (Kapa Biosystems) which was ran on an ABI17900HT real-time PCR instrument. The 500 High-output v2 (75cycle) sequencing kit was then used to perform single read sequencing (75–76 cycles) on the Illumina NextSeq500 instrument. The Cricetulus griseus (Chinese hamster) CriGri_1.0 genome was downloaded from the pre-Ensembl ftp site (http://pre.ensembl.org/Cricetulus_griseus/Location/View?r¼JH0000013%A3051578-3509200) (Xu et al., 2011). Sequencing reads were directly aligned to the genome using tophat2 (version 2.0.13) (Trapnell et al., 2012) generating alignment files in bam format. A mapping rate of between 89.2% and 90.2% was observed for all samples (not shown). A gene annotation gtf file for CriGri_1.0 was downloaded from the pre-Ensembl ftp site (Yates et al., 2016). Since this GTF contains annotations from a variety of resources, many of which overlap, the GTF was parsed to contain only homology to mouse Ensembl transcripts. Differentially expressed genes were identified for the three pairwise comparisons: pre-miR-29b-1 vs. anti-miR29a, anti-miR-29a vs. pre-miR-29a, and pre-miR-29b-1 vs. pre-miR-29a using the tuxedo suite of programs (Trapnell et al., 2012, 2013) including cufflinks-cuffdiff2 (version 2.2.1). Our RNA-seq raw data files are available at Gene Expression Omnibus (GEO) database: accession number GSE85710. Entrez gene identifiers for significantly expressed genes had a q-value cutoff of 0.05. Samples were then divided into fastq single end sequencing files representing comparisons of Pre-miR-29b-1 vs. anti-miR-29a and anti-miR-29a vs. pre-miR-29a. CategoryCompare (Flight et al., 2014) was used to report Gene Ontology Biological Processes (GO:BP) (Harris et al., 2004) and KEGG Pathways (Kanehisa and Goto, 2000) that were significantly enriched.
2.9. In silico network analysis
We performed pathway and network analysis of differentially expressed genes in MetaCore™ version 6.27 (GeneGO, Thomson Reuters, New York, N.Y.). MetaCore™ is a web-based software suite for multiple applications in systems biology including RNA seq analysis as used here. For CHO-K1 analysis we used the Mus musculus genome. MetaCore™ analyses are based on MetaBase (http://metadatabase.org/), a 100% manually-curated integrated database of mammalian biology that contains over 6 million experimental findings on protein-protein, protein-DNA, protein-RNA, and protein-compound interactions; metabolic and signaling pathways; and other information (Bolser et al., 2012).
2.10. Statistical analysis
Statistical evaluations of cell-based assays and qPCR used oneway ANOVA followed by Tukey's multiple comparison test using GraphPad Prism 5 (Graph Pad Software, Inc., LaJolla, CA). Differentially expressed genes (expression levels) are in Fragments Per Kilobase of transcript per Megabase (FPKM) and are for differential expression between experimental conditions. A false-discovery rate (FDR) corrected q-value cutoff of 0.05 was used to determine differentially expressed genes.
3. Results
3.1. 4-OHT-activated ERα increased miR-29b-1 and miR-29a expression in CHO-K1 cells
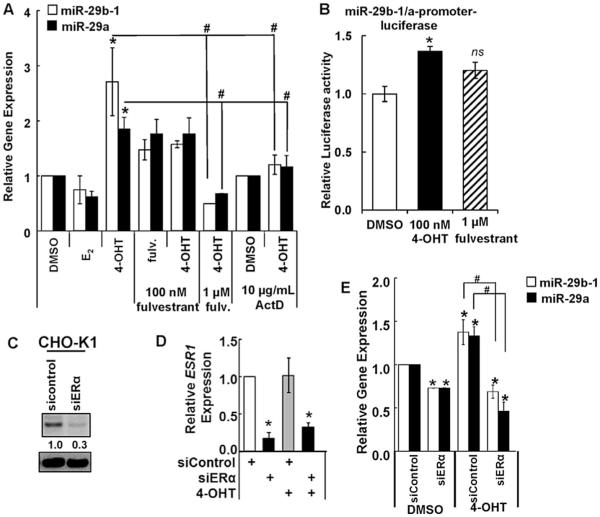
E2 stimulates and 4-OHT inhibits CHO-K1 cell viability (Thomas et al., 2003). miR-29b-1 and miR-29a were downregulated in growing CHO-K1 cells (Klanert et al., 2016). We observed 4-OHT stimulated the expression of miR-29b-1 and miR-29a in CHO-K1 cells (Fig. 1A). Pretreatment of the cells with the transcriptional inhibitor actinomycin D (ActD) inhibited 4-OHT-stimulation of miR-29b-1 and miR-29a expression (Fig. 1A). This suggests that 4-OHT increases transcription of miR-29b-1 and miR-29a in CHO-K1 cells. Fulvestrant, an ER antagonist, non-significantly increased miR-29b-1 and miR-29a expression. The 4-OHT-induced increase in miR-29b-1 and miR-29a was abrogated by pretreatment with1 mM fulvestrant (Fig. 1A); suggesting a role of endogenous ER in mediating the upregulation of miR-29b-1 and miR-29a by 4-OHT in CHO-K1 cells. We did not observe a significant effect of E2 on miR-29b-1/a expression. The 5’ promoter of the human miR-29b-1/a contains half-site EREs (Data not shown). 4-OHT treatment of CHO-K1 cells transiently transfected with a human miR-29b-1/a-luciferase reporter increased luciferase activity (Fig. 1B). The increase with fulvestrant was, like endogenous miR-29b and miR-29a transcript levels, non-significant. Basal expression of miR-29a was higher than miR-29b-1 and we did not detect miR-29c transcript expression (Supplemental Fig. 1). This is consistent with next generation sequencing data (Hackl et al., 2011).
Fig. 1. 4-OHT stimulation of miR-29ab-1/a expression is mediated by ERα in CHO-K1 cells.
A) CHO-K1 cells were serum-starved for 48 h and treated for 6 h with vehicle control (DMSO), 10 nM E2, or 100 nM 4-OHT. Where indicated cells were preincubated with 100 nM or 1 μM fulvestrant or 10 mg/ml actinomycin D for 6 h. B) CHO-K1 cells were transiently transfected with a human miR-29b-1/a promoter-luciferase reporter and a Renilla luciferase reporter. Cells were treated with 100 nM 4-OHT or 1 μM fulvestrant for 24 h prior to dual luciferase assay. Results were normalized to DMSO vehicle control. Values are the avg. ± SEM of 3 transfections. *p = 0.005 versus DMSO. ns = non-significant. (CeD) CHO-K1 cells were serum-starved and transfected with si-control or siERα. Two d post transfection, cells were treated for 6 h with EtOH or 100 nM 4-OHT. WCE was western blotted for ERα (C). For A, C–D: Q-PCR performed as described in Methods. Values are the average of 4–15 (A) and 3 (C–D) separate experiments ± SEM. *p < 0.05 versus vehicle (DMSO) control transfected cells. #p < 0.05 versus control 4-OHT-treated cells.
Q-PCR identified Esr1 (ERα) but not Esr2 (ERβ) transcript expression in CHO-K1 cells (Supplementary Fig. 1B) and we did not detect ERβ protein (data not shown), in contrast to previous results (Thomas et al., 2003), which suggests that different lots of CHO-K1 from ATCC may have variable endogenous ERβ expression. ERα mRNA expression in CHO-K1 cells has previously been reported (Thomas et al., 2003) but not protein expression. To evaluate if ERα is mediating 4-OHT's stimulation of miR-29b-1 and miR-29a transcription, CHO-K1 cells were transfected with pooled ERα siRNAs or a negative control. We show for the first time, ERα protein expression and successful repression ERα at the protein (Fig. 1C) and mRNA (Fig. 1D) levels when CHO-K1 cells were transfected with siERα. siERα reduced basal miR-29b-1 and miR-29a expression in CHO-K1 cells by ~25% (Fig. 1E). 4-OHT increased miR-29b-1 and miR-29a in siControl-transfected cells but this increase was ablated by siERα transfection (Fig. 1E). These data confirm a role for endogenous ERα in mediating 4-OHT stimulation of miR-29b-1 and miR-29a transcription in CHO-K1 cells.
3.2. Upregulation of miR-29b-1 and miR-29a decreases cell proliferation and colony formation
miR-29b and miR-29a negatively correlate with CHO-K1 cell growth (Klanert et al., 2016) and tamoxifen (TAM) has previously been associated with cell cycle arrest (Lykkesfeldt et al., 1984). Since 4-OHT inhibits CHO-K1 cell growth depending on the culture medium used (Thomas et al., 2003), and because 4-OHT increased miR-29b-1 and miR-29a in CHO-K1 cells (Fig. 1), we hypothesized that the 4-OHT-mediated increase in miR-29b-1 and miR29a contributes to reduced CHO-K1 proliferation. Therefore we sought to determine the functional roles of miR-29b-1/a on CHO-K1 cells alone and in response to 4-OHT.
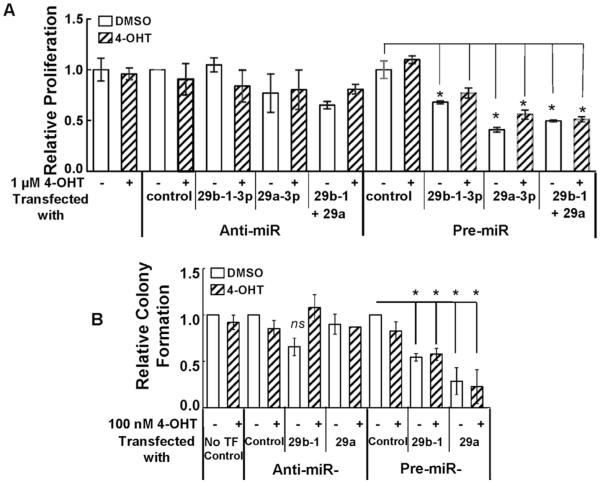
To determine the role of miR-29b-1/a on cellular growth and proliferation, we increased miR-29b-1, miR-29a, or a combination of both by pre-miR-29b-1/a transfection. Alternately, we decreased miR-29b-1, miR-29a, or a combination of both with anti-miR antagonists. 48 h post transfection, the cells were treated with 1 μM 4-OHT for 48 h and BrdU assays were performed to examine cell proliferation. The 48 h treatment is required to allow the miRNA regulation of its targets, which can, in turn, have functional consequences, e.g., cell proliferation. Transfection with anti-miRs or pre-miRs was successful in increasing or repressing miR-29b-1 and miR-29a expression, respectively (Supplementary Fig. 2). 1 μM 4-OHT alone had no effect on CHO-K1 proliferation (Fig. 2A). Transfection with anti-miR-29b-1 or anti-miR-29a, individually or in combination, had no significant effect on cell proliferation relative to anti-miR control. However, relative to pre-miR control transfected cells, pre-miR-29b-1 and pre-miR-29a, individually or in combination, inhibited cell proliferation (Fig. 2A). 4-OHT treatment gave no further reduction in the number of cells. Similar results were observed when cell viability was determined using an MTT assay (Supplementary Fig. 3).
Fig. 2. Ectopic expression of miR-29b-1/a decreases CHO-K1 cell proliferation and colony formation.
Cells were serum-starved and transfected with anti-miR-control, anti-miR-29b-1/a (3p), pre-miR control or pre-miR-29b-1/a. 48 h post transfection, the media was changed and cells treated with 4-OHT, as indicated, for 48 h prior to BrdU assay (A) and colony formation assay counts were performed 14 d after transfection (B). Values are the ±SEM of 3 separate experiments. *p < 0.05 versus vehicle control-transfected cells; #p < 0.05 versus control transfected cells treated with 4-OHT. ns = non-significant.
Since miR-29b-1 and miR-29a repressed CHO-K1 cell proliferation, we examined if these microRNAs would inhibit CHO-K1 colony formation. CHO-K1 cells were transfected with either anti-miRs or pre-miRs for miR-29b-1 or miR-29a and colony formation was examined after 14 d ± 100 nM 4-OHT. Compared to the anti-miR control, neither anti-miR-29b-1 nor anti-mIR-29a had a significant effect on colony formation in the presence or absence of 4-OHT treatment (Fig. 2B). Interestingly, compared to the pre-miR control, both pre-miR-29b-1 and pre-miR-29a decreased colony formation. These results support an anti-proliferative activity of miR-29b-1 and miR-29a in CHO-K1 cells.
3.3. Upregulation of miR-29b-1 and miR-29a decreased cell invasion
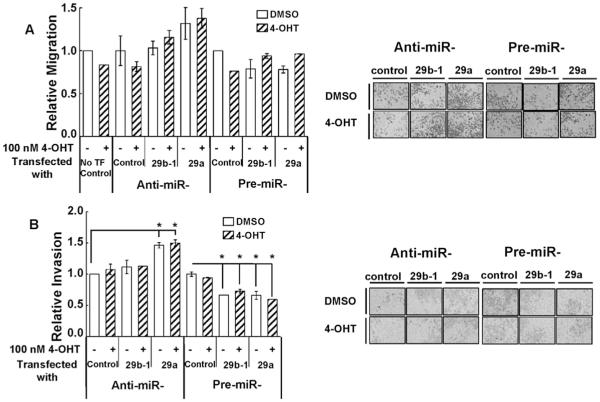
miR-29b promoted the migration/invasion of MCF-7 and MDA-MB-231 breast cancer cells (Wang et al., 2011). To further investigate the functional role of miR-29 in CHO-K1 cells, transwell migration assays were performed with filters coated with or without Matrigel to determine migration and invasion respectively. In addition cells were treated with 100 nM 4-OHT. No effect of 4-OHT was seen on CHO-K1 cell migration or invasion (Fig. 3A and B). Neither anti-miR-29b-1/a nor pre-miR-29b-1/a affected cell migration (Fig. 3A). However, transfection with anti-miR-29a, but not anti-miR-29b-1, promoted CHO-K1 cell invasion (Fig. 3B). Transfection of CHO-K1 with pre-miR-29b-1 and pre-miR-29a decreased cell invasion, but no additive effect of 4-OHT was detected (Fig. 3B).
Fig. 3. Over expression of miR-29b-1/a decreases CHO-K1 cell invasion.
Cells were serum-starved and transfected with anti-miR-control, anti-miR-29b-1/a (3p), pre-miR control, or pre-miR-29b-1/a (3p). A and B) Two d post transfection cells were plated into transwell plates without (A) or with (B) Matrigel coating of the filter. Cells treated with vehicle (DMSO) or 100 nM 4-OHT for 24 h. Images of representative regions of transwells are shown. Values are the mean ± SEM of 4 wells in two separate experiments. *p < 0.05 versus control-transfected cells.
3.4. miR-29b-1 mediates 4-OHT decrease in DICER protein expression
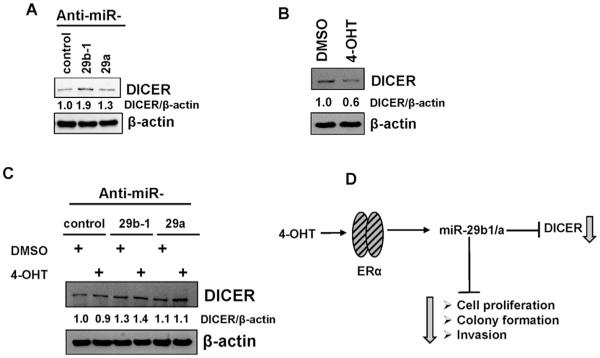
Because 4-OHT increased miR-29b-1/a, and microRNAs bind and repress translation of their mRNA targets, we hypothesized that repression of miR-29 will block 4-OHT-induced miR-29 repression of miR-29 target genes. DICER1 is a target of miR-29b and miR-29a in breast cancer cells (Cochrane et al., 2010) and miR-29b-1 in prostate cancer cells (Bian et al., 2015). Anti-miR-29b-1 and anti-miR-29a increased DICER1 mRNA (Supplementary Fig. 4A) and protein expression in CHO-K1 cells (Fig. 4), consistent with DICER1 as a target of miR-29b and miR-29a in CHO-K1 cells.
Fig. 4. miR-29b-1/a regulates DICER protein expression.
A-C) CHO-K1 cells were serum-starved and transfected with anti-miR-29b-1/a-3p, premiR-29b-1/a-3p and their respective controls for 48 h. B-C) Cells were then treated for 24 h with 100 nM 4-OHT. Membranes were probed for DICER and stripped and reproved for β-actin as a loading control. Representative western blots are shown. Values (DICER/β-actin) are the mean of 2–3 separate experiment (A), a single experiment performed in duplicates (B and C). D) Model of a proposed mechanism of 4-OHT activation of ERα increasing miR-29b-1 and miR-29a, decreasing DICER, and phenotypic effects in CHO-K1 cells.
To determine whether the 4-OHT decrease in DICER protein is mediated through miR-29, CHO-K1 cells were ‘serum-starved’ and transfected with anti-miR control, anti-miR-29b-1 and anti-miR-29a. 48 h post transfection, cells were treated for 6 h with 100 nM 4-OHT. Anti-miR-29b-1 blocked the 4-OHT-mediated decrease in DICER protein expression (Fig. 4C). No significant difference was observed in DICER protein expression between DMSO-treated anti-miR control and 4-OHT-treated anti-miR-29a (Fig. 4C). These data suggest that the increase in miR-29b-1 by 4-OHT decreases DICER protein in CHO-K1 cells.
3.5. Differential regulation of miR-29 target genes in several cellular processes
To identify other putative miR-29 targets in CHO-K1 cells, we performed RNA sequencing of CHO-K1 cells transfected with pre-miR-29b-1, pre-miR-29a, or anti-miR-29a followed by bioinformatics analysis. We observed that anti-miR-29a also repressed miR-29b-1 (Supplementary Fig. 2).
3.6. RNA seq analysis of miR-29b-1- and miR-29a-regulated mRNAs in CHO-K1 cells
To identify potential mRNA targets of miR-29b-1 and miR-29a in CHO-K1 cells, cells were transfected with pre-miR-29b-1, pre-miR-29a, or AS-miR-29a (which inhibits both miR-29b-1 and miR-29a (Supplementary Fig. 2)), followed by RNA seq of total RNA. For target analysis, only transcripts that showed a log2 fold-change greater than 1 (or −1 for repressed mRNAs) were included. Additionally, the statistical threshold for significance was a q value of less than 0.05. RNA seq identified 659 and 725 differentially expressed genes in CHO-K1 cells transfected anti-miR-29a versus pre-miR-29b-1 and with anti-miR-29a versus pre-miR-29a, respectively (Table 1). We analyzed the transcriptome data from RNA seq using MetaCore™ to identify network processes, pathway maps, and shared gene ontologies (GO processes) between pre-miR-29b-1 vs. anti-miR-29a and pre-miR-29a vs. anti-miR-29a data sets. Fig. 5 shows Venn diagrams of these data and identifies the top network pathways identified in MetaCore™. A complete list of the differentially expressed genes, including the changes and statistical differences, is included as Supplementary Information (Supplementary Tables 1 and 2). 357 downregulated and 181 upregulated genes were common to both (anti-miR-29a versus pre-miR-29b-1) and (anti-miR-29a versus pre-miR-29a) transfection comparisons (Fig. 5A and B, Supplementary Tables 3 and 4). These data suggest that miR-29b-1 and miR-29a have more similar than different putative targets in CHO-K1 cells. Enrichment pathway analysis was performed for each data set and the top Pathways are listed in Fig. 5 for commonly or uniquely miR-29b-1 and miR-29a down- or upregulated pathways.
Table 1.
Differentially expressed genes (DEGs) identified in RNA seq. (p < 0.05, q < 0.05, logFC (fold change) ≥ 1.0).
| Comparison | Total DEGs | Downregulated DEGs | Upregulated DEGs |
|---|---|---|---|
| Anti-miR-29a versus Pre-miR-29b-1 | 659 | 390 | 269 |
| Anti-miR-29a versus Pre-miR-29a | 725 | 476 | 249 |
| Pre-miR-29b-1 versus Pre-miR-29a | 10 | 0 | 10 |
Fig. 5. Enrichment analysis of RNA seq data.
Differentially expressed genes were identified in pairwise comparisons: Anti-miR-29a vs. Pre-miR29a and Anti-miR-29a vs. Pre-miR-29b-1 using the tuxedo suite of programs including cufflink-cuff diff2. The Venn diagrams show the number of common and differentially expressed genes significantly down-regulated (A) and upregulated (B). Pathway analysis was performed using GeneGo Pathways Software (MetaCore™). The pathways identified for each comparison are listed in the order provided by MetaCore™ analysis.
The top 10 GO cellular processes commonly downregulated or upregulated are show in Supplementary Figs. 5A and 5B. Top highest scoring commonly downregulated GO processes included cellular component organization (or biogenesis), extracellular matrix and structure organization, and collagen metabolic process (Supplementary Fig. 5A). The top commonly upregulated GO processes included cGMP metabolic, cGMP catabolic, muscle system process, and muscle contraction (Supplementary Fig. 5B). Network analysis identified several miR-29b-1/a targets with potential roles in cell adhesion and cell matrix interactions with specific targets including TGFbeta3 (Supplementary Figs. 6A and 6C). We specifically evaluated the miR-29b-1/a targets that mediate response to growth factor stimulus. Of the several potential growth factor response pathways, MetaCore™ identified the SKP2, STAT3, CDK6, LARP5, and M33 as potential miR-29b-1/a targets that interact within a single network (Supplementary Fig. 6B) and Cyclin E, CDK6, TAB1, TGFβ3, IDH2 which are involved in cell cycle regulation (Supplementary Fig. 6C). We then confirmed miR-29b-1/a binding sites within the STAT3, CDK6, LARP5, M33, TGFβ3, IDH2 30UTR region using microRNA.org; suggesting they are putative direct miR-29b-1/a targets. Additional experiments will be required to experimentally validate their identity as bona fide miR-29b-1/a targets in CHO-K1 cells.
When comparing the 357 commonly downregulated mRNA transcripts by miR-29b-1 and miR-29a in CHO-K1 cells (Fig. 5A) with the set of 1079 miR-29-3p predicted Mus musculus targets from TargetScan ver. 7.1 in MetaCore™, only 113 (31.7%) were in common. Network analysis identified common pathway maps and GO cellular processes (Supplementary Fig. 7). Included in this common gene list is DNMT3A, a bona fide miR-29 target (Fabbri et al., 2007; Morita et al., 2013). Since a repression of DNMT3A would be expected to decrease methylation of its targets and thus possibly increase their transcription, this observation may account for at least some of the upregulated genes detected (Fig. 5B). For example, one upregulated gene (FLT1, EVGFR-1) is known to be regulated by promoter methylation (Kim et al., 2009). Representative unique genes and potential pathways downregulated by miR-29b-1 and miR-29a in CHO-K1 cells that were not included in the Mus miR-29-3p predicted target gene set are shown in Supplementary Fig. 8.
4. Discussion
Little is known about the effects of hormonal regulation of microRNAs in CHO-K1 cells. SERMs, including 4-OHT, regulate microRNA expression in other cell lines but no one has evaluated their effects in CHO-K1 cells (Jan et al., 2003). Here, we report that 4-OHT increases miR-29b-1 and miR-29a expression in CHO-K1 cells. For the first time, we show CHO-K1 cells express ERα protein and that the 4-OHT-induced increase in miR-29b-1 and miR-29a is mediated by ERα. Ectopic expression of miR-29b-1 and miR-29a decreased CHO-K1 cell proliferation, colony formation and cell invasion. These data agree with a report showing that miR-29b and miR-29a negatively correlated with CHO-K1 cell growth (Klanert et al., 2016) and corroborates miR-29's repressive functional role in cells (Garzon et al., 2009; Kwon et al., 2014; Mott et al., 2007; Xiong et al., 2010). Earlier we reported that 4-OHT increased miR-29b-1/a expression in LY2 endocrine-resistant breast cancer cells based on microarray analysis of miRNA expression (Manavalan et al., 2011) and recently confirmed this observation by qPCR (Muluhngwi et al., 2017). In addition, we observed that 4-OHT increased pri-miR-29b-1/a transcription in an ERα-dependent manner in LCC9 and LY2 cells (Muluhngwi et al., 2017). Thus, the 4-OHT stimulation of miR-29b-1/a in the CHO-K1 cells resembles that seen in the endocrine-resistant LCC9 and LY2 breast cancer cells. The 4-OHT-mediated increase in miR-29b-1/a results in a decrease in their target DICER at the mRNA and protein level. We also note that MetaCore™ analysis of our RNA seq data identified regulation of translation as a GO process inhibited by overexpression of both miR-29b-1 and miR-29a.
RNA seq has provided identification of new putative miR-29b-1/a targets in CHO-K1 cells. These data reveal possible cellular and metabolic processes regulated by miR-29. Importantly, in agreement with our observation that overexpression of miR-29b-1/a inhibited cell viability, some enriched categories associated with apoptosis were identified in MetaCore™ analysis of our RNA seq data. Likewise, we observed that overexpression of miR-29b-1/a inhibited cell invasion through Matrigel and GO categories related to invasion, cell adhesion, and extracellular remodeling were also identified in the MetaCore™ analysis. An improved understanding of the signaling pathways and cellular processes and/or mechanisms including microRNA regulation by hormones and hormonal regulators will assist in the designing and production of therapeutic proteins-which are currently in high demand.
TAM concentrations greater than 5 μM were reported to decrease viability of CHO-K1 cells (Jan et al., 2003). Here we showed that 100 nM and 1 μM 4-OHT treatment did not have any significant effect on the viability, migration, invasion, or colony forming ability of CHO-K1 cells-even when miR-29b-1 or miR-29a were overexpressed or their activity repressed by anti-miR transfection. These results suggest that CHO-K1 cells have higher tolerance to TAM than human derived cells and this is tolerance is independent of miR-29b-1/a regulation.
TAM was reported to have non-ER-mediated effects in MCF-7 cells at 0.5–10 μM that resulted in organelle acidification, although the mechanism(s) were not defined (Altan et al., 1999). This is significantly higher than the concentration of 100 nM 4-OHT used in our study. We examined the impact of 4-OHT an extracellular acidification rate (ECAR). Supplemental Fig. 9 shows that 4-OHT significantly increased basal ECAR in CHO-K1 cells, but the relationship of ECAR to the transcriptional activity of 4-OHT in CHO-K1 cells remains to be resolved.
microRNAs generally mediate their effects by regulating translational activity of target mRNA transcripts by binding to their seed elements in the 3′ UTR. Here we confirmed DICER as a miR-29b-1/a target in CHO-K1 cells and our results suggest a pathway for 4-OHT-ERα stimulation of miR-29b-1/a targeting DICER. DICER cleaves pre-microRNAs to mature microRNAs. Downregulation of DICER by miR-29b-1/a would be thus expected to reduce mature microRNAs, but increase pre-microRNAs, in CHO-K1 cells. Transient DICER repression was reported to decrease in CHO-K1 cell growth (Hackl et al., 2014). However, moderately overexpressed DICER increased CHO-K1 maximum growth rate although there was a decrease in cell viability while strong overexpression impaired maximum growth rate and decreased viability (Hackl et al., 2014). Ectopic DICER upregulation also increased a subset of microRNAs positively correlated with growth rate (Hackl et al., 2014). The dual roles of DICER overexpression on CHO-K1 cells growth may account for why repression of miR-29 had no significant effect on CHO-K1 functional outcomes measured in this study. Further studies are warranted to ascertain the veracity of these speculations.
Our study also identifies common and unique mRNA targets altered by miR-29b-1 and miR-29a expression in CHO-K1 cells. The 357 commonly downregulated and 181 commonly upregulated mRNA transcripts (70% and 53% respectively of all up- or down-regulated transcripts (Fig. 5A and B)) suggest that targeted regulatory networks of miR-29b-1 and miR-29a are similar, although each regulates unique complex networks that are distinct from each other. Analysis of the common transcripts revealed that miR-29b-1 and miR-29a regulate cellular response to growth factor stimulus in CHO-K1. This supports our finding that miR-29 is regulated by the SERM 4-OHT. The observation that E2 had no significant effect on miR-29 expression suggests that the activation of ERα by 4-OHT regulates miR-29b-1/a transcription by a mechanism distinct from E2 in CHO-K1 cells. Possible explanations involve altered ERα conformation and consequent interaction with coregulators and other transcription factors (reviewed in (Klinge, 2000)). A network analysis of some transcripts identified as miR-29 target genes were involved in apoptosis, protein folding and maturation, and cell adhesion, among others. Interestingly, the 7th pathway identified in the commonly downregulated 357 mRNAs was “Membrane-bound ESR1: interaction with G-proteins signaling”. It is possible that 4-OHT activation of membrane bound-ERα (and not just the nuclear receptor) increases miR-29b-1/a in CHO-K1, but further experiments will be necessary to dissect this pathway.
5. Conclusions
Overall, results from our study demonstrate that 4-OHT increases miR-29 by ERα-dependent transcriptional activation. We observed that miR-29b-1/a represses DICER, an endonuclease required for microRNA processing. miR-29b-1/a regulates the CHO-K1 transcriptome and pathways predicted to lead to altered functional properties including cell proliferation, colony formation, and cell invasion. RNA seq identified multiple miR-29b-1/a targets in CHO-K1 cells, including genes involved in cell adhesion, cytoskeletal remodeling, and development. Additionally, we found alterations in pathways that have a possibility of mediating responses to growth factors which may be important for investigators using CHO-K1 for protein production.
Supplementary Material
Acknowledgements
This work was supported in part by National Institutes of Health R01 CA138410 to C.M.K. and grants from the University of Louisville (UofL) School of Medicine and the UofL Center for Genetics and Molecular Medicine (CGeMM) Next Generation Pilot Grant to C.M.K. Bioinformatics support for this work by E.C.R. was provided by National Institutes of Health grants P20GM103436 (Nigel Cooper, PI). J.N. was supported by National Institutes of Health T35 DK072923 (to C.M.K). We thank Dr. Marsha Cole for the use of her microscope.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.mce.2017.01.044.
References
- Altan N, Chen Y, Schindler M, Simon SM. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4432–4437. doi: 10.1073/pnas.96.8.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron N, Kumar N, Sanchez N, Doolan P, Clarke C, Meleady P, O'Sullivan F, Clynes M. Engineering CHO cell growth and recombinant protein productivity by overexpression of miR-7. J. Biotechnol. 2011;151:204–211. doi: 10.1016/j.jbiotec.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Bian X, Shen Y, Zhang G, Gu C, Cai Y, Wang C, Zhu Y, Zhu Y, Zhang H, Dai B, Ye D. Expression of dicer and its related miRNAs in the progression of prostate cancer. PLoS One. 2015;10:e0120159. doi: 10.1371/journal.pone.0120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DM, Chibon PY, Palopoli N, Gong S, Jacob D, Del Angel VD, Swan D, Bassi S, Gonzalez V, Suravajhala P, Hwang S, Romano P, Edwards R, Bishop B, Eargle J, Shtatland T, Provart NJ, Clements D, Renfro DP, Bhak D, Bhak J. MetaBaseethe wiki-database of biological databases. Nucleic Acids Res. 2012;40:D1250–D1254. doi: 10.1093/nar/gkr1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkrolf K, Rupp O, Laux H, Kollin F, Ernst W, Linke B, Kofler R, Romand S, Hesse F, Budach WE, Galosy S, Muller D, Noll T, Wienberg J, Jostock T, Leonard M, Grillari J, Tauch A, Goesmann A, Helk B, Mott JE, Puhler A, Borth N. Chinese hamster genome sequenced from sorted chromosomes. Nat. Biotechnol. 2013;31:694–695. doi: 10.1038/nbt.2645. [DOI] [PubMed] [Google Scholar]
- Clarke C, Henry M, Doolan P, Kelly S, Aherne S, Sanchez N, Kelly P, Kinsella P, Breen L, Madden SF, Zhang L, Leonard M, Clynes M, Meleady P, Barron N. Integrated miRNA, mRNA and protein expression analysis reveals the role of post-transcriptional regulation in controlling CHO cell growth rate. BMC genomics. 2012;13:656. doi: 10.1186/1471-2164-13-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane DR, Cittelly DM, Howe EN, Spoelstra NS, McKinsey EL, LaPara K, Elias A, Yee D, Richer JK. MicroRNAs link estrogen receptor alpha status and Dicer levels in breast cancer. Horm. Cancer. 2010;1:306–319. doi: 10.1007/s12672-010-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diendorfer AB, Hackl M, Klanert G, Jadhav V, Reithofer M, Stiefel F, Hesse F, Grillari J, Borth N. Annotation of additional evolutionary conserved microRNAs in CHO cells from updated genomic data. Biotechnol. Bioeng. 2015;112:1488–1493. doi: 10.1002/bit.25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druz A, Chu C, Majors B, Santuary R, Betenbaugh M, Shiloach J. A novel microRNA mmu-miR-466h affects apoptosis regulation in mammalian cells. Biotechnol. Bioeng. 2011;108:1651–1661. doi: 10.1002/bit.23092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feichtinger J, Hernandez I, Fischer C, Hanscho M, Auer N, Hackl M, Jadhav V, Baumann M, Krempl PM, Schmidl C, Farlik M, Schuster M, Merkel A, Sommer A, Heath S, Rico D, Bock C, Thallinger GG, Borth N. Comprehensive genome and epigenome characterization of CHO cells in response to evolutionary pressures and over time. Biotechnol. Bioeng. 2016;113:2241–2253. doi: 10.1002/bit.25990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Handrick R, Otte K. The art of CHO cell engineering: a comprehensive retrospect and future perspectives. Biotechnol. Adv. 2015;33:1878–1896. doi: 10.1016/j.biotechadv.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Flight RM, Harrison BJ, Mohammad F, Bunge MB, Moon LD, Petruska JC, Rouchka EC. categoryCompare, an analytical tool based on feature annotations. Front. Genet. 2014;5:98. doi: 10.3389/fgene.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammell P, Barron N, Kumar N, Clynes M. Initial identification of low temperature and culture stage induction of miRNA expression in suspension CHO-K1 cells. J. Biotechnol. 2007;130:213–218. doi: 10.1016/j.jbiotec.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Garzon R, Heaphy CE, Havelange V, Fabbri M, Volinia S, Tsao T, Zanesi N, Kornblau SM, Marcucci G, Calin GA, Andreeff M, Croce CM. MicroRNA 29b functions in acute myeloid leukemia. Blood. 2009;114:5331–5341. doi: 10.1182/blood-2009-03-211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, Mello CC. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Hacker DL, De Jesus M, Wurm FM. 25 years of recombinant proteins from reactor-grown cells - where do we go from here? Biotechnol. Adv. 2009;27:1023–1027. doi: 10.1016/j.biotechadv.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Hackl M, Jadhav V, Jakobi T, Rupp O, Brinkrolf K, Goesmann A, Pühler A, Noll T, Borth N, Grillari J. Computational identification of microRNA gene loci and precursor microRNA sequences in CHO cell lines. J. Biotechnol. 2012;158:151–155. doi: 10.1016/j.jbiotec.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl M, Jadhav V, Klanert G, Karbiener M, Scheideler M, Grillari J, Borth N. Analysis of microRNA transcription and post-transcriptional processing by Dicer in the context of CHO cell proliferation. J. Biotechnol. 2014;190:76–84. doi: 10.1016/j.jbiotec.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl M, Jakobi T, Blom J, Doppmeier D, Brinkrolf K, Szczepanowski R, Bernhart SH, Siederdissen CH, Bort JH, Wieser M, Kunert R, Jeffs S, Hofacker IL, Goesmann A, Puhler A, Borth N, Grillari J. Next-generation sequencing of the Chinese hamster ovary microRNA transcriptome: identification, annotation and profiling of microRNAs as targets for cellular engineering. J. Biotechnol. 2011;153:62–75. doi: 10.1016/j.jbiotec.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harreither E, Hackl M, Pichler J, Shridhar S, Auer N, Labaj PP, Scheideler M, Karbiener M, Grillari J, Kreil DP, Borth N. Microarray profiling of preselected CHO host cell subclones identifies gene expression patterns associated with increased production capacity. Biotechnol. J. 2015;10:1625–1638. doi: 10.1002/biot.201400857. [DOI] [PubMed] [Google Scholar]
- Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, Eilbeck K, Lewis S, Marshall B, Mungall C, Richter J, Rubin GM, Blake JA, Bult C, Dolan M, Drabkin H, Eppig JT, Hill DP, Ni L, Ringwald M, Balakrishnan R, Cherry JM, Christie KR, Costanzo MC, Dwight SS, Engel S, Fisk DG, Hirschman JE, Hong EL, Nash RS, Sethuraman A, Theesfeld CL, Botstein D, Dolinski K, Feierbach B, Berardini T, Mundodi S, Rhee SY, Apweiler R, Barrell D, Camon E, Dimmer E, Lee V, Chisholm R, Gaudet P, Kibbe W, Kishore R, Schwarz EM, Sternberg P, Gwinn M, Hannick L, Wortman J, Berriman M, Wood V, de la Cruz N, Tonellato P, Jaiswal P, Seigfried T, White R. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32:D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Bort JA, Hackl M, Hoflmayer H, Jadhav V, Harreither E, Kumar N, Ernst W, Grillari J, Borth N. Dynamic mRNA and miRNA profiling of CHO-K1 suspension cell cultures. Biotechnol. J. 2012;7:500–515. doi: 10.1002/biot.201100143. [DOI] [PubMed] [Google Scholar]
- Hutvagner G, McLachlan J, Pasquinelli A, Balint E, Tuschl T, Zamore P. A cellular function for the RNA-interference enzyme dicer in the maturation of the let-7 small temporal RNA. Sci. (New York, N. Y. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J. Clin. Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan CR, An-Jen C, Chang HT, Roan CJ, Lu YC, Jiann BP, Ho CM, Huang JK. The anti-breast cancer drug tamoxifen alters Ca2þ movement in Chinese hamster ovary (CHO-K1) cells. Archives Toxicol. 2003;77:160–166. doi: 10.1007/s00204-002-0420-0. [DOI] [PubMed] [Google Scholar]
- Johnson KC, Jacob NM, Nissom PM, Hackl M, Lee LH, Yap M, Hu WS. Conserved microRNAs in Chinese hamster ovary cell lines. Biotechnol. Bioeng. 2011;108:475–480. doi: 10.1002/bit.22940. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantardjieff A, Nissom PM, Chuah SH, Yusufi F, Jacob NM, Mulukutla BC, Yap M, Hu WS. Developing genomic platforms for Chinese hamster ovary cells. Biotechnol. Adv. 2009;27:1028–1035. doi: 10.1016/j.biotechadv.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes & Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Hwang JH, Zhou W, Shin J, Noh SM, Song IS, Kim JY, Lee SH, Kim J. The expression of VEGF receptor genes is concurrently influenced by epigenetic gene silencing of the genes and VEGF activation. Epigenetics. 2009;4:313–321. [PubMed] [Google Scholar]
- Klanert G, Jadhav V, Shanmukam V, Diendorfer A, Karbiener M, Scheideler M, Bort JH, Grillari J, Hackl M, Borth N. A signature of 12 microRNAs is robustly associated with growth rate in a variety of CHO cell lines. J. Biotechnol. 2016;235:150–161. doi: 10.1016/j.jbiotec.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000;65:227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. genomics. 2012;44:237–244. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SY, Lee JH, Kim B, Park JW, Kwon TK, Kang SH, Kim S. Complexity in regulation of microRNA machinery components in invasive breast carcinoma. Pathol. Oncol. Res. POR. 2014;20:697–705. doi: 10.1007/s12253-014-9750-5. [DOI] [PubMed] [Google Scholar]
- Le H, Chen C, Goudar CT. An evaluation of public genomic references for mapping RNA-Seq data from Chinese hamster ovary cells. Biotechnol. Bioeng. 2015;112:2412–2416. doi: 10.1002/bit.25649. [DOI] [PubMed] [Google Scholar]
- Lewis NE, Liu X, Li Y, Nagarajan H, Yerganian G, O'Brien E, Bordbar A, Roth AM, Rosenbloom J, Bian C, Xie M, Chen W, Li N, Baycin-Hizal D, Latif H, Forster J, Betenbaugh MJ, Famili I, Xu X, Wang J, Palsson BO. Genomic landscapes of Chinese hamster ovary cell lines as revealed by the Cricetulus griseus draft genome. Nat. Biotechnol. 2013;31:759–765. doi: 10.1038/nbt.2624. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou J, White KP. RNA-seq differential expression studies: more sequence or more replication? Bioinforma. Oxf. Engl. 2014;30:301–304. doi: 10.1093/bioinformatics/btt688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkesfeldt AE, Larsen JK, Christensen IJ, Briand P. Effects of the anti-oestrogen tamoxifen on the cell cycle kinetics of the human breast cancer cell line, MCF-7. Br. J. cancer. 1984;49:717–722. doi: 10.1038/bjc.1984.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan TT, Teng Y, Appana SN, Datta S, Kalbfleisch TS, Li Y, Klinge CM. Differential expression of microRNA expression in tamoxifen-sensitive MCF-7 versus tamoxifen-resistant LY2 human breast cancer cells. Cancer Lett. 2011;313:26–43. doi: 10.1016/j.canlet.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Horii T, Kimura M, Ochiya T, Tajima S, Hatada I. miR-29 represses the activities of DNA methyltransferases and DNA demethylases. Int. J. Mol. Sci. 2013;14:14647–14658. doi: 10.3390/ijms140714647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J, Kobayashi S, Bronk S, Gores G. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JL, Kurita S, Cazanave S, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, Hedgehog, and NF-kappaB. J. Cell. Biochem. 2010;110:1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muluhngwi P, Krishna A, Vittitow SL, Napier JT, Richardson KM, Ellis M, Mott JL, Klinge CM. Tamoxifen differentially regulates miR-29b-1 and miR-29a expression depending on endocrine-sensitivity in breast cancer cells. Cancer Lett. 2017;388:230–238. doi: 10.1016/j.canlet.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Thomas PB, Risinger KE, Klinge CM. Identification of estrogen receptor beta expression in Chinese hamster ovary (CHO) cells and comparison of estrogen-responsive gene transcription in cells adapted to serum-free media. J. Steroid Biochem. Mol. Biol. 2003;86:41–55. doi: 10.1016/s0960-0760(03)00250-4. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trummer E, Ernst W, Hesse F, Schriebl K, Lattenmayer C, Kunert R, Vorauer-Uhl K, Katinger H, Muller D. Transcriptional profiling of phenotypically different Epo-Fc expressing CHO clones by cross-species microarray analysis. Biotechnol. J. 2008;3:924–937. doi: 10.1002/biot.200800038. [DOI] [PubMed] [Google Scholar]
- Vishwanathan N, Yongky A, Johnson KC, Fu HY, Jacob NM, Le H, Yusufi FN, Lee DY, Hu WS. Global insights into the Chinese hamster and CHO cell transcriptomes. Biotechnol. Bioeng. 2015;112:965–976. doi: 10.1002/bit.25513. [DOI] [PubMed] [Google Scholar]
- Wang C, Bian Z, Wei D, Zhang JG. miR-29b regulates migration of human breast cancer cells. Mol. Cell Biochem. 2011;352:197–207. doi: 10.1007/s11010-011-0755-z. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010;51:836–845. doi: 10.1002/hep.23380. [DOI] [PubMed] [Google Scholar]
- Xu X, Nagarajan H, Lewis NE, Pan S, Cai Z, Liu X, Chen W, Xie M, Wang W, Hammond S, Andersen MR, Neff N, Passarelli B, Koh W, Fan HC, Wang J, Gui Y, Lee KH, Betenbaugh MJ, Quake SR, Famili I, Palsson BO, Wang J. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 2011;29:735–741. doi: 10.1038/nbt.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates A, Akanni W, Amode MR, Barrell D, Billis K, Carvalho-Silva D, Cummins C, Clapham P, Fitzgerald S, Gil L, Girón CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Keenan S, Lavidas I, Martin FJ, Maurel T, McLaren W, Murphy DN, Nag R, Nuhn M, Parker A, Patricio M, Pignatelli M, Rahtz M, Riat HS, Sheppard D, Taylor K, Thormann A, Vullo A, Wilder SP, Zadissa A, Birney E, Harrow J, Muffato M, Perry E, Ruffler M, Spudich G, Trevanion SJ, Cunningham F, Aken BL, Zerbino DR, Flicek P. Ensembl 2016. Nucleic Acids Res. 2016;44:D710–D716. doi: 10.1093/nar/gkv1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.