Abstract
Background & Aims
Tremelimumab is a fully human monoclonal antibody that binds to cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) on the surface of activated T lymphocytes. Ablative therapies induce a peripheral immune response which may enhance the effect of anti-CTLA4 treatment in patients with advanced hepatocellular carcinoma (HCC). This study aimed to demonstrate whether tremelimumab could be combined safely and feasibly with ablation.
Methods
Thirty-two patients with HCC were enrolled: male:female: 28:4; median age: 62 (range 36–76). Patients were given tremelimumab at two dose levels (3.5 and 10 mg/kg i.v.) every 4 weeks for 6 doses, followed by 3-monthly infusions until off-treatment criteria were met. On day 36, patients underwent subtotal radiofrequency ablation or chemoablation. Staging was performed by contrast-enhanced CT or MRI scan every 8 weeks.
Results
No dose-limiting toxicities were encountered. The most common toxicity was pruritus. Of the 19 evaluable patients, five (26.3%; 95% CI: 9.1–51.2%) achieved a confirmed partial response. Twelve of 14 patients with quantifiable HCV experienced a marked reduction in viral load. Six-week tumor biopsies showed a clear increase in CD8+ T cells in patients showing a clinical benefit only. Six and 12-month probabilities of tumor progression free survival for this refractory HCC population were 57.1% and 33.1% respectively, with median time to tumor progression of 7.4 months (95% CI 4.7 to 19.4 months). Median overall survival was 12.3 months (95% CI 9.3 to 15.4 months).
Conclusions
Tremelimumab in combination with tumor ablation is a potential new treatment for patients with advanced HCC, and leads to the accumulation of intratumoral CD8+ T cells. Positive clinical activity was seen, with a possible surrogate reduction in HCV viral load.
Lay summary
Studies have shown that the killing of tumors by direct methods (known as ablation) can result in the immune system being activated or switched on. The immune system could potentially also recognize and kill the cancer that is left behind. There are new drugs available known as immune checkpoint inhibitors which could enhance this effect. Here, we test one of these drugs (tremelimumab) together with ablation.
Keywords: Immune checkpoint, Hepatocellular carcinoma, Immune, T-Lymphocytes, Liver cirrhosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequently occurring cancers worldwide, ranked 3rd in global incidence by the International Agency for Research on Cancer [1]. HCC typically occurs in the setting of chronic inflammation, such as that induced by viral hepatitis. In contrast to other types of cancer, where surgery, radiation and chemotherapies dominate the therapeutic landscape, in HCC locoregional treatments are widely applied, either with curative (ablative procedures, surgery) or palliative (arterial chemoembolization) intent [2]. Systemic treatments have a comparatively modest role, sorafenib being the only drug to have demonstrated a survival benefit at the phase III level in the modern era [3, 4]. There are several characteristics relating to HCC, which make it amenable to immunotherapy [5]. Spontaneous immune responses including T cell responses, as well as humoral responses to different tumor-associated antigens have been described [6, 7]. Interestingly, both transcatheter arterial chemoembolization (TACE) and ablation (either cryo- [CA], microwave [MVA] or radiofrequency [RFA]) by themselves have been shown to induce a peripheral immune response [7–12].
Tremelimumab is a fully human monoclonal antibody that binds to cytotoxic T-lymphocyte-associated protein (CTLA)-4 and results in inhibition of B7-CTLA-4-mediated downregulation of T cell activation. Tremelimumab is well tolerated when administered as a single agent to patients with HCC [13]. The primary aim of this study was to demonstrate whether tremelimumab could be administered safely and feasibly with TACE, RFA or CA. Whilst RFA and chemoablation (CA) procedures are generally employed in early stage disease, here they were employed subtotally in the advanced setting, the hypothesis being that peripheral immune stimulation induced by the ablative procedure could be amplified by immune checkpoint blockade.
Patients and methods
Patients
Eligible patients were at least 18 years old and had histopathological confirmation of hepatocellular carcinoma (HCC) by the Laboratory of Pathology of the National Cancer Institute (NCI) prior to entering this study. Other eligibility criteria included: Eastern Cooperative Oncology Group (ECOG) performance status score 0–2; disease not amenable to potentially curative liver transplantation, resection or ablation. Patients with Barcelona Clinic Liver Cancer (BCLC) Stage C must have had disease amenable to subtotal ablation in addition to having progressed on or been intolerant of prior sorafenib; BCLC Stage B patients were treated with TACE as per the standard of care; Child-Pugh A or B (no more than 7 points) classification if cirrhosis present; no history of chronic autoimmunity or inflammatory bowel disease. All patients provided written informed consent and the study was approved by the NCI Institutional Review Board. The Clini-calTrials.gov identifier was: NCT01853618.
Study design
Patients who satisfied the eligibility criteria were enrolled on a pilot study of tremelimumab at two dose levels (3.5 and 10 mg/kg i.v.) given every 4 weeks for a total of 6 doses, followed by 3-monthly infusions until off-treatment criteria were met (Supplementary Fig. 1). On day 36 (±96 h) patients underwent subtotal RFA or CA. Subtotal ablation means the complete treatment of a single lesion in the setting of multifocal disease, leaving other lesions (both intrahepatic and extrahepatic) intact and untreated. The lesion subjected to ablation was treated with full ablative intent and chosen at the discretion of the interventional radiologist based on technical factors, such as ease of access, proximity to vessels etc. BCLC B patients received TACE as per standard of care. Staging was performed by contrast-enhanced CT or MRI scan every 8 weeks. Objective response was evaluated in lesions not subject to ablation or TACE using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria. Due to the delayed timing of the radiologic procedure the evaluation period for dose-limiting toxicities (DLT) was extended for the first 8 weeks of the study. The primary objective was to determine if it was feasible to administer tremelimumab in combination with locoregional therapy in patients with advanced HCC. Secondary objectives included a variety of immunologic parameters to determine if there was an immune response to treatment, and to assess safety, toxicity and preliminarily efficacy. To demonstrate feasibility, it was desirable if the fraction of the initial 20 patients receiving 10mg/kg who could receive all doses of tremelimumab were consistent with 80% or higher. Safety and toxicity were also evaluated and addressed by tabulating and monitoring the grades of toxicity experienced by patients in the study. Feasibility - the primary endpoint - was established in two small pilot cohorts of up to 10 patients each, based on disease stage and/or type of ablation (advanced HCC receiving RFA or TACE; intermediate HCC receiving TACE). The study was then amended to allow additional recruitment to the TACE and ablation cohorts in order to obtain a preliminary assessment of efficacy. Given that these were all small pilot cohorts, no direct comparison was made regarding relative benefits of ablative method or TACE.
Immune correlative studies
All patients on dose level 2(10 mg/kg) were requested to undergo optional tumor biopsy at the time of the interventional radiologic procedure. Patients underwent blood sampling every cycle with isolation of peripheral blood mononuclear cells (PBMC). T cell subsets were analyzed by multicolor flow cytometry. Activated T cells were analyzed by staining for CD3, CD4, CD8, CD38 and HLA-DR as previously described [14]. Formalin-fixed paraffin-embedded tissue was stained with antibodies for T cell markers CD3 and CD8 (cytotoxic phenotype). Slides were digitally scanned (Aperio ScanScope XT) and analyzed using an automated image analysis algorithm (Aperio Positive Pixel Count v9). The percentage of total positive pixels (corresponding to 3, 3-diaminobenzidine chromogen saturation) in areas of tumor was evaluated between patients stratified by response and pre-and post-treatment biopsies.
Hepatitis monitoring
Patients with HCC in the context of active or chronic hepatitis as well as non-hepatitis etiology were enrolled onto the study. Patients with hepatitis C virus (HCV) were eligible for inclusion whether they had received prior treatment or not. In these patients, HCV viral load was measured every 4 weeks. Patients with chronic hepatitis B were required to be on anti-viral medication. In these patients, hepatitis B virus (HBV) viral load, anti-HBc antibody, anti-HBe antibody, HBeAg and quantitative hepatitis B surface antigen were measured using standard assays every 4 weeks. Serum HBsAg titers were measured by enzyme immunoassay (EIA) using the ARCHITECT platform (Abbott Laboratories, Chicago, IL), as per the manufacturer’s instructions.
Safety
All adverse events and serious adverse events occurring within 30 days of the last dose were reported according to the NCI Common Terminology Criteria for Adverse Events v4.0.
Statistical methods
Efficacy was assessed by response rate, as determined by RECIST 1.1 criteria, and reported along with an exact 95% confidence interval. In addition, the time to tumor progression (TTP) and overall survival (OS) were calculated by the Kaplan-Meier method, and reported along with 95% confidence intervals. TTP was the time from consent until the first documented progression of disease. The actuarial probabilities associated with TTP were referred to as tumor progression free survival probabilities. Regarding the immune correlative studies, the actual levels of changes of immune parameters were determined, and the fraction noted to have a change in the parameter values reported. Wilcoxon signed rank test was used to compare T cell subsets at baseline with samples obtained after 4 and 12 weeks. These were considered secondary and exploratory analyses.
Results
Patient characteristics
Thirty-two patients were enrolled onto this pilot study evaluating immune checkpoint inhibition with tremelimumab in combination with either RFA, CA or TACE (Table 1). Four patients experienced rapid disease progression in the first few weeks of study and were evaluated for safety only. The baseline characteristics for the study population patients are shown in Table 1. The median age of the population was 61 (range 36–76). Cirrhosis was present either by clinical or pathologic diagnosis in twenty-two patients with a median Child-Pugh score of 5. The most common etiology for HCC was hepatitis. Nineteen patients had hepatitis C, fourteen of whom had quantifiable viral load. Five patients had hepatitis B, all of whom were on anti-viral medication at the time of enrollment. The majority (75%) of patients progressed on or was intolerant of prior sorafenib. Seven patients (25%) were BCLC B with the remaining N = 21 (75%) being BCLC C. Twenty-five patients (78%) also received other HCC therapies, including surgery, systemic chemotherapy, experimental agents, external beam radiation, as well as multiple interventional radiology procedures (TACE, 90Y radioembolization, radiofrequency ablation) or surgery, with the majority (68%) having received some intervention within the past three months. All patients had evidence of progressive disease at enrollment. All the patients were discussed at an NCI multidisciplinary tumor board to discuss suitability for the protocol-mandated interventional radiology procedure. BCLC B patients were treated with TACE. The first 10 BCLC C patients were treated with RFA followed by 11 patients who received CA.
Table 1.
Patient characteristics.
| Number | 32 (6/14/12)* |
| Age, median (range) | 61 (36–76) |
| Sex | |
| Male | 28 (4/13/11)* |
| Female | 4 (2/1/1)* |
| ECOG | |
| 0 | 8 |
| 1 | 24 |
| Liver cirrhosis | |
| Yes | 22 (3/11/8)* |
| No | 9 (3/2/4)* |
| Cause of liver disease | |
| HBV | 5 (2/1/2)* |
| HCV | 19 (3/11/5)* |
| Baseline Child-Pugh score | |
| 5 | 14 (2/6/6)* |
| 6 | 5 (1/3/1)* |
| 7 | 3 (-/2/1)* |
| Number of lesions | |
| 1 | 5 |
| 2 | 3 |
| 3–5 | 12 |
| >5 | 8 |
| BCLC stage | |
| B | 7 |
| C | 21 |
| Extrahepatic disease | |
| Yes | 14 (2/10/2)* |
| No | 17 (4/4/9)* |
| Prior sorafenib | |
| Yes/no | 21/7 |
| DC due to PD/intolerant | 18/3 |
| Other systemic therapies | 9 |
| Other previous interventions | |
| TACE | 11 |
| Surgery | 5 |
| Ablation | 5 |
| Reason for discontinuation | |
| PD | (5/12/3)* |
| Toxicity | (1/2/1)* |
BCLC, Barcelona Clinic Liver Cancer; DC, discontinued; ECOG, Eastern Cooperative Oncology Group; PD, progressive disease; TACE, transcatheter arterial chemoembolization.
Dose level 1/Dose level 2 ablation/Dose level 2 TACE.
Safety
Treatment-related toxicities for both dose levels are summarized in Table 2. This excludes toxicities which were directly attributable to the interventional radiology procedure (e.g., pain) as per the standard of care experience. There was no clear trend in adverse events (AEs) across the different dose cohorts. No DLT was encountered. The most common clinical toxicity was pruritus which was predominantly grade 1 and frequently accompanied by a rash consistent with immune-related dermatitis. Generally, this responded to treatment with topical pramoxine and 1% clobetasol, although one patient required oral prednisone for this event and remained on steroids for one year despite attempts at weaning and discontinuation of study drug. There was also no significant difference in the event rate for diarrhea between patients in DL1 or 2. There were no episodes of grade 3/4 diarrhea, however one patient with grade 2 persistent diarrhea underwent colonoscopy and, despite grossly normal mucosal appearances was found to have evidence of colitis on biopsy. One patient developed grade 2 autoimmune pneumonitis and was taken off study after three doses of tremelimumab but remained HCC progression free for almost 2 years, with resolution of pneumonitis. One patient developed an episode of angioedema which occurred three weeks following his third dose of tremelimumab. The patient was on an angiotensin converting enzyme (ACE) inhibitor for hypertension. Four patients developed rapid disease progression and came off the protocol in the first 6 weeks of study.
Table 2.
Toxicity.
| 3.5 mg/kg (N = 6), n |
10 mg/kg (N = 26), n |
All patients (N = 32), n |
||||
|---|---|---|---|---|---|---|
| Toxicity | ≥Grade 2 | Grades 3–4 | ≥Grade 2 | Grades 3–4 | ≥Grade 2 | Grades 3–4 |
| Hyperbilirubinemia | 2 | 1 | 5 | 2 | 7 | 3 |
| Aspartate aminotransferase increased | 6 | 4 | 5 | 3 | 11 | 7 |
| Alanine aminotransferase increased | 1 | - | 5 | 3 | 6 | 3 |
| Pruritus | - | - | 3 | 1 | 3 | 1 |
| Rash | 3 | - | 2 | - | 5 | - |
| Pneumonitis | 1 | - | - | - | 1 | - |
| Colitis | - | - | 2 | - | 2 | - |
| Angioedema | - | - | - | 1 | - | 1 |
| Thyroid dysfunction | - | - | 1 | 1 | 1 | 1 |
| Adrenal insufficiency | - | - | - | 1 | - | 1 |
| Discontinued due to toxicity | 1/6 | 3/25 | 4 (13%) | |||
Efficacy
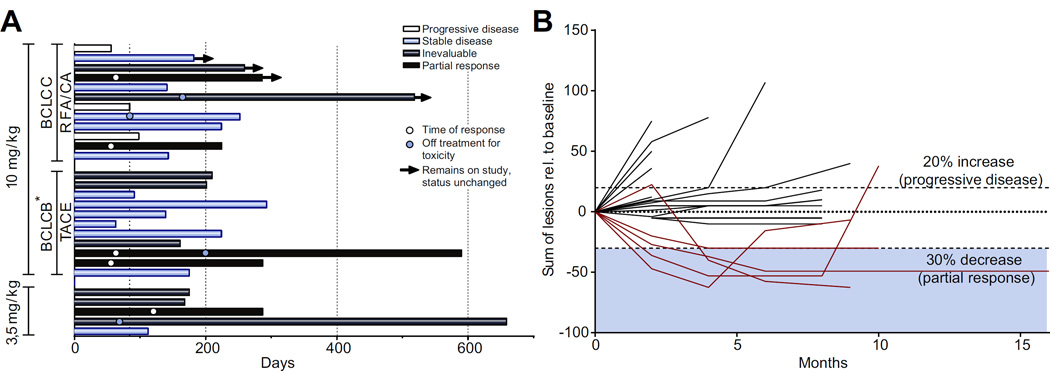
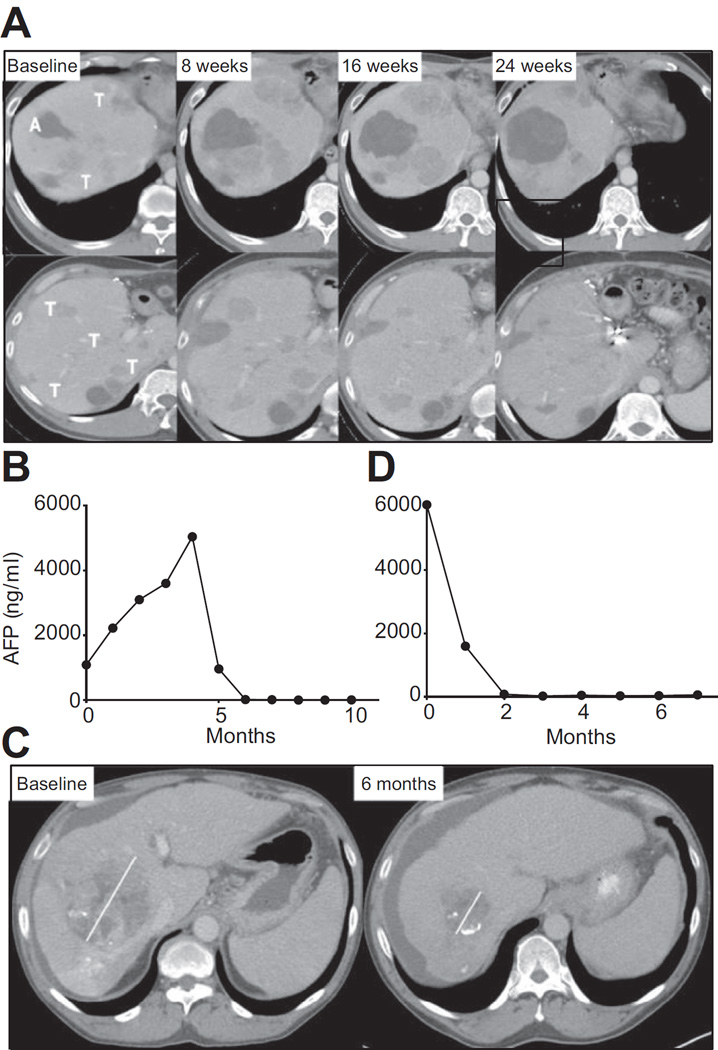
Fig. 1 shows efficacy data for the study population including time on study (Fig. 1A) in addition to quality and duration of objective responses (Fig. 1B). Nineteen patients had lesions that were evaluable for response outside of the areas treated with ablation or TACE. Of these patients five (26%; 95% CI: 9.1–51.2%) achieved confirmed partial responses. Of note, all five patients had hepatic disease only. Fig. 2 shows the CT scans and alpha fetoprotein (AFP) dynamics of two patients who obtained a durable response to treatment, with more detailed summary of lesions in all five responding patients in Supplementary Table 1. The dynamics of response for subject 3 (Fig. 2A, B) were noteworthy in that he had clinical and radiological disease progression at week 8. However, in the subsequent weeks he experienced dramatic improvement. Of the nine patients evaluable for safety but not response, reasons for non-evaluable disease were either because the sole measurable disease was subjected to ablation or TACE or technical factors, such as diffuse enhancement. The median TTP was 7.4 months, both for those undergoing either TACE (N = 11; 95% CI: 2.2–19.4 months) or ablation (N = 12; 95% CI: 2.8 months-undefined) respectively (Table 3 and Supplementary Fig. 2A, B). The median TTP for the total evaluable study population (N = 28) was 7.4 months (95% CI 4.7 to 9.4 months). Six and 12-month tumor progression free survival probabilities for this refractory HCC population were 57.1% (95% CI: 37.1–72.9%) and 33.1% (95% CI: 16.2–51.2%) respectively. Median OS was 12.3 months (95% CI 9.3–15.4 months) with a median potential follow-up (from on study date until analysis) of 18.8 months.
Fig. 1. Efficacy data for study population.
(A) Swimmer’s plot showing time on study, off-treatment reason, current status and time of response if applicable; the arrow indicates patients still on study; white circle, time of response; blue circle, time of discontinuation due to toxicity. (B) Plot showing magnitude of change in target lesion sum over time as per RECIST; patients evaluable in target lesions not treated by RFA/TACE.
Fig. 2. Example of clinical response.
(A) CT scan over 6-month time period for subject 3 showing an increase in the ablated area (denoted A) in addition to changes in tumor size (denoted T) on two separate cuts of the same scan demonstrating worsening appearances at 8 weeks with subsequent improvement and in some cases resolution at 24 weeks. (B) AFP over time for subject 3. (C) CT scan over 6-month time period for subject 10 showing a marked reduction in the tumor area (denoted T) from 10.5 cm at baseline to 4.3 cm after 2 months. Of note, whilst this patient did undergo subtotal TACE the tumor mass shown in the figure was not embolized. (D) AFP over time for subject 10.
Table 3.
Summary of efficacy.
| Median TIP (95% CI) |
6-month tumor PFS# (95% CI) |
12-month tumor PFS# (95% CI) |
Median OS (95% CI) |
6-month survival (95% CI) |
12-month survival (95% CI) |
|
|---|---|---|---|---|---|---|
| Ablation (n = 12) | 7.4 months (2.8 months- undefined) | 58.3% (27.0–80.1%) | 31.3% (8.5–57.8%) | 10.1 months (4.6–15.7 months) | 75.0% (40.8–91.2%) | 46.9% (17.6–71.8%) |
| TACE (n = 11) | 7.4 months (2.2–19.4 months) | 63.6% (29.7–84.5%) | 29.1% (5.4–59.3%) | 13.6 months (7.5 months- undefined) |
90.9% (50.8–98.7%) | 80.8% (42.4–94.9%) |
| Total population, excluding N = 5 on phase I (n = 23) |
7.4 months 4.6–19.4 months) | 60.9% (38.3–77.4%) | 30.9% (12.8–51.1%) | 12.8 months (7.8–15.7 months) | 82.6% (60.9–93.1%) | 62.3% (38.1–79.3%) |
| Total population (n = 28) | 7.4 months (4.7–19.4 months) | 57.1% (37.1–72.9%) | 33.1% (16.2–51.2%) | 12.3 months (9.3–15.4 months) | 85.7% (66.3–94.4%) | 50.8% (29.1–68.9%) |
CI, confidence interval; OS, overall survival.
Tumor progression-free survival (PFS) refers to probabilities associated with time to tumor progression (TTP).
Anti-viral responses
In a previous study evaluating tremelimumab in a hepatitis C-related HCC population, an interesting observation was a reduction in viral load associated with an anti-HCV immune response [13]. In our study, sixteen patients had hepatitis C, fourteen of whom had quantifiable viral load. We also found that 1) the median HCV viral load decreased from 1275 × 103 IU/ml to 351 × 103 IU/ml after 3 months; (Supplementary Fig. 3A); 2) the viral load decreased in 12 of 14 patients; 3) three patients who had a reduction in HCV viral load had a subsequent increase in viral load which coincided with disease progression at which point they were taken off-treatment (Supplementary Fig. 4); 4) the two patients who did not experience any reduction in viral load had clearly progressive disease. Hepatitis B is generally an exclusion factor for immunotherapy clinical trials in HCC. Given its global prevalence, we opted to allow patients with hepatitis B to enroll. Five hepatitis B patients were enrolled, four of whom were on entecavir with suppression of detectable viral replication. In these patients no viral reactivation was seen and quantitative hepatitis B surface antigen was found to reduce over time in all four of these measurable patients (Supplementary Fig. 3B). A fifth patient did not go on anti-viral medication and had a reduction in quantitative HBV DNA from baseline 14,200 IU/ml to a nadir, after 3 months on study to 48 IU/ml
Immune correlatives
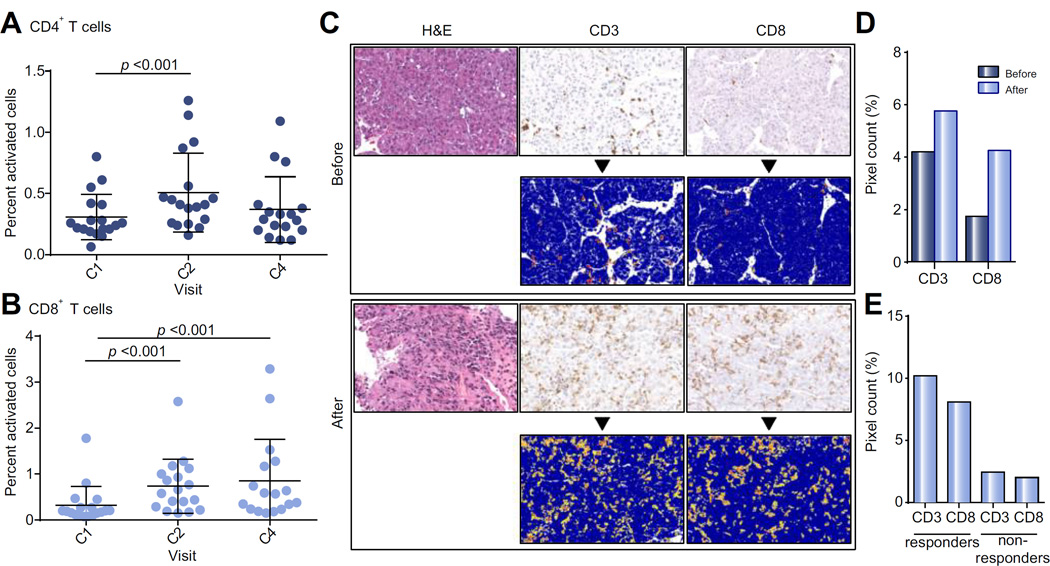
In PBMC of patients, an increase in the number of activated CD4+ and CD8+ T cells was seen (Fig. 3A and B). The frequency of activated CD8+ T cells was increased by 2-fold over baseline and was sustained for at least 12 weeks. The effectiveness of tremelimumab likely depends on the ability of activated immune cells to transit to the tumor interface. HCC does not generally contain tumor-infiltrating lymphocytes (TIL). Thus, tumor biopsies were obtained at the time of ablation and compared to archived samples obtained prior to enrollment. Of the study patients with evaluable material (N = 12), all showed immune cell infiltration within the tumor (Fig. 3C and D). Quantitative analysis was performed. The mean positive pixel count of TILs increased from pre-treatment samples (N = 6) to post-treatment (N = 12) (Fig. 3C). Pre- and post-treatment mean positive pixel count (mPPC) were 4.21% vs. 5.77% (CD3) and 1.75% vs. 4.26% (CD8), respectively (Fig. 3D). The differences were not statistically significant, which is likely to be attributable to the small number of cases. Paired samples (N = 6) also showed increased post-treatment TILs with a CD3 mPPC of 6.17% (vs. 3.3%) and CD8 mPPC of 3.58% (vs. 2.1%). Responders (N = 5) had a post-treatment mPPC for CD3 and CD8 of 10.21% and 8.10%, respectively (vs. 2.44% CD3 and 2.00% CD8 in non-responders, N = 4) (Fig. 3E).
Fig. 3. Correlative studies.
(A) Percent of live activated CD4+ T cells in peripheral blood of patients during first 4 cycles of tremelimumab. Wilcoxon signed rank test was used to compare T cell subsets at baseline with samples obtained after 4 and 12 weeks. (B) Percent of live activated CD8+ T cells in peripheral blood of patients during first 4 cycles of tremelimumab. Wilcoxon signed rank test was used to compare T cell subsets at baseline with samples obtained after 4 and 12 weeks. (C) Representative tumor biopsy at baseline and post 2 doses of tremelimumab showing marked intratumoral CD3+/CD8+ T cell infiltration (bottom rows: positive pixel count overlay). (D) Quantitative assessment of immunohistochemical staining showing average CD3 and CD8 immune cell tumor infiltration before (N = 6) and after 2 doses of tremelimumab (N = 12). (E) Quantitative assessment of immunohistochemical staining showing average CD3 and CD8 immune cell tumor infiltration after 2 doses of tremelimumab for patients divided into responders (defined as stable/partial response of at least 4 months, N = 5) vs. non-responders (defined as PD/partial response of under 4 months, N = 4).
Discussion
Although HCC is amongst the world’s most frequently occurring cancers, progress has been slow in developing effective treatment options for advanced disease [15]. Several factors make HCC difficult to treat, particularly the underlying cirrhosis which is generally present. Immunomodulatory approaches have the enormous advantage of not requiring hepatic metabolism. Con-siderable evidence suggests that HCC is a tumor frequently recognized by the immune system [16]. Spontaneous immune responses to different tumor-associated antigens have been described [6, 7]. In the advanced disease setting, these responses are not likely to be clinically relevant. For localized or locoregional disease ablative therapies and TACE are very commonly applied treatments in HCC. Ablation - by means of alcohol, radiofrequency, microwave or cryoablation - is considered a curative alternative to surgical resection [17, 18]. TACE is a non-curative procedure for patients with liver-localized HCC for whom surgical resection or ablation is not possible [19]. Both modalities have been shown to induce a peripheral immune response which may be clinically relevant [9–12]. For example, Mizukoshi et al. found that an increase in the magnitude of HCC-specific immune response by interferon-γ ELISPOT after RFA in 69 HCC patients was the only relevant prognostic factor on multivariate analysis [20]. Once an immune response is instigated it can be potentially amplified by immune modulating agents [21–23].
We found that combining tremelimumab with these interventional radiologic procedures in this heavily pretreated post-sorafenib patient population was not only feasible, but resulted in objective tumor responses outside of the ablated or embolized zone. Encouraging clinical activity was seen with objective durable responses observed. The duration of responses was noteworthy, with each positive response lasting 7, 8, 9, 9 and 19 months. Two other patients who were non-evaluable by virtue of having multifocal disease previously treated with TACE exhibited long-term disease control without any further intervention for 16 and 21 months. This is also the first study combining immune checkpoint inhibition with TACE. The potential applicability was exhibited in one case where following TACE to the right hepatic lobe – and an objective tumor response in the left lobe – a patient remained free of disease progression for two years until a further TACE was required. Overall TTP was favorably comparable with other studies in similar post-sorafenib populations.
A major question is the relative contribution of the ablative procedure to the efficacy seen. All patients received tremelimumab, which by itself might account for the antitumor effects. Indeed, of 12 patients in whom we obtained a tumor biopsy at the time of the ablation or TACE (and after 2 doses of tremelimumab) all showed evidence of immune cell infiltration. Whilst this seemed to be a prerequisite for responding to the treatment it is unclear to what extent the ablative procedure or TACE amplified this, if at all. Ongoing analysis of T cell repertoire may partially answer this question, but a randomized study will ultimately be needed. Previously, Sangro et al. administered tremelimumab as monotherapy in a phase II study of HCV-related HCC, achieving a partial response rate of 17.6%, a median TTP of 6.48 months and median OS of 8.2 months. Significant differences between the two studies were the use of a less dose dense schedule (15 mg/kg i.v./90 d) in the study by Sangro et al. as well as a better prognostic population overall (43% BCLC stage A or B compared to 95% BCLC stage C in our study). Another variable of unknown significance was the delayed timing chosen for the interventional procedure. The main reason for this was safety considerations as we were using a new monthly schedule of tremelimumab which we wanted to assess for a short time as a single agent in this relatively co-morbid patient population to ensure tolerability prior to the addition of an experimental IR intervention.
Sangro et al. also observed a reduction in HCV viral load on tremelimumab. Similarly, we found that 12 of 14 patients with evaluable HCV viral load achieved a reduction. Interestingly, the two patients who experienced no reduction derived no antitumor benefit from treatment and three of these initially responding patients subsequently had an elevation in viral load which coincided with the time of disease progression. This suggests that anti-viral immune responses may act as a surrogate for disease control. Hepatitis B is generally an exclusion factor for immunotherapy clinical trials in HCC. Given its global prevalence we opted to allow patients with hepatitis B to enroll. Five hepatitis B patients were enrolled, all of whom were virally suppressed. In these patients no viral reactivation was seen. Quantitative hepatitis B antigen, which is thought to reflect number of infected cells as opposed to active replication, was measured and was found to reduce over time in all patients. Although the numbers are small this is a significant and reassuring finding.
In conclusion, this study combining immune checkpoint inhibition with subtotal TACE or ablation in patients with advanced HCC demonstrated intriguing clinical activity. The relative contribution of the interventional radiology procedure needs further study, specifically whether TACE or ablation, both of which cause significant disruption and tumor necrosis, are truly necessary to stimulate the immune system or if immunomodulation by immune checkpoint inhibition is sufficient for clinical benefit.
Supplementary Material
Acknowledgments
Financial support
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Clinical trial number: ClinicalTrials.gov: NCT01853618.
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Authors’ contributions
All authors contributed to MS writing, data collection and analysis.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/jjhep.2016.10.029.
References
- 1. at http://globocan.iarc.fr.
- 2.European Association for Study of LiverEuropean Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641. doi: 10.1016/j.ejca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunologic perspective. Clin Cancer Res. 2013;19:6678–6685. doi: 10.1158/1078-0432.CCR-13-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korangy F, Ormandy LA, Bleck JS, et al. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–4341. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 7.Mizukoshi E, Nakamoto Y, Arai K, et al. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–1216. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 8.Zerbini A, Pilli M, Laccabue D, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138:1931–1942. doi: 10.1053/j.gastro.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 9.Ayaru L, Pereira SP, Alisa A, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178:1914–1922. doi: 10.4049/jimmunol.178.3.1914. [DOI] [PubMed] [Google Scholar]
- 10.Hiroishi K, Eguchi J, Baba T, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451–458. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 11.Nobuoka D, Motomura Y, Shirakawa H, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2012;40:63–70. doi: 10.3892/ijo.2011.1202. [DOI] [PubMed] [Google Scholar]
- 12.Hansler J, Wissniowski TT, Schuppan D, et al. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol. 2006;12:3716–3721. doi: 10.3748/wjg.v12.i23.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–2079. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 16.Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62:1420–1429. doi: 10.1016/j.jhep.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoem-bolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 20.Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448–1457. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 21.Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430–439. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Brok MH, Sutmuller RP, Nierkens S, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–7292. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 23.Johnson EE, Yamane BH, Buhtoiarov IN, et al. Radiofrequency ablation combined with KS-IL2 immunocytokine (EMD 273066) results in an enhanced antitumor effect against murine colon adenocarcinoma. Clin Cancer Res. 2009;15:4875–4884. doi: 10.1158/1078-0432.CCR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.