Abstract
Background
CD59 is a cell surface glycoprotein of approximately 20 kDa limiting the lytic activity of the terminal complement complex C5b-9. Although CD59 is known as a red blood cell (RBC) antigen defined by monoclonal antibodies, it so far has not been identified as a blood group antigen, since the description of a human alloantibody was missing. In this study we show the presence of an anti-CD59 in a patient affected by a homozygous CD59 deficiency.
Study Design and Methods
RBC CD59 and CD55 were determined by flow cytometry or by the column agglutination technique using monoclonal antisera. Commercially available His-tagged recombinant soluble CD59 protein was used to inhibit anti-CD59.
Results
Seven cases of an isolated CD59 deficiency due to three distinct null alleles of the CD59 gene have been published so far. Recently we described the CD59-null allele c.146delA in a young child of heterozygous parents. Her plasma contained an alloantibody directed against the high-prevalence RBC antigen CD59. The antibody specificity was identified using soluble recombinant human CD59 protein, which blocked the reactivity of the patient's antibody and of monoclonal anti-CD59 but not of monoclonal anti-CD55. In addition, RBC alloantibodies such as anti-K, anti-C, anti-c, or anti-Fya remained unaffected. Therefore, inhibition by recombinant CD59 is a useful diagnostic tool to detect alloantibodies in the presence of anti-CD59.
Conclusion
This is the first demonstration of a human anti-CD59 alloantibody, which defines CD59 as an RBC blood group antigen. CD59 represents a candidate for a new blood group system.
Homologous restriction factor (HRF20), membrane inhibitor of reactive lysis (MIRL), membrane attack complex inhibitory factor (MACIF), protectin, and CD59 are a few of the many synonyms for the same protein that was discovered to interfere with complement-related lysis of red blood cells (RBCs) in a homologous test system.1-3 CD59 is a glycoprotein of approximately 20 kDa that binds to the terminal complement complex C5b-9. CD59 is attached by a glycosylphosphatidylinositol (GPI) anchor to the membranes of many different cell types, including hematopoietic cells. At a low concentration CD59 occurs in soluble form in plasma, urine, and other body secretions.4,5
Patients with paroxysmal nocturnal hemoglobinuria (PNH) harbor varying percentages of hematopoietic cells with a deficiency of all GPI-linked proteins, among these CD59. The disorder is confined to hematopoietic cells and acquired by somatic mutations in the phosphatidylinositol glycan A (PIG-A) gene. The deficiency in the complement regulatory proteins CD59 and CD55 causes hemolytic anemia. Further common clinical signs are thromboembolism and bone marrow failure.6,7
Isolated deficiencies of CD59 due to inherited homozygous mutations in the CD59 gene are very rare. Only seven cases have been published so far. One patient from Japan carried the two single-base deletions, c.123delC and c.361delG (p.Val42Serfs*38; p.Ala121Glnfs).8,9 Five CD59-deficient children originating from four unrelated North-African Jewish families all harbored the nucleotide substitution c.266G>A (p.Cys89Tyr),10 which likely is a founder mutation in Jews of North-African ancestry. We recently reported a case of an isolated complete CD59 defect caused by the homozygous deletion c.146delA (p.Asp49Valfs*31).11 The carrier of this allele was a child of Turkish origin. In this study we give evidence that she had formed an anti-CD59 and describe an easily applicable method to identify anti-CD59.
Materials and Methods
Immunohematology
Serologic testing was performed using a gel matrix test to detect agglutination (Scangel cards for the indirect antiglobulin test [IAT], antibody screening cells, ID-Card DC Screening I for the direct antiglobulin test [DAT], low-ionic-strength saline [LISS] buffer, and papain-treated RBCs; Bio-Rad, Munich, Germany) according to the manufacturer's instructions. Dithiothreitol (DTT)-treated RBCs were prepared by incubation with 200 mmol/L DTT in phosphate-buffered saline (PBS), pH 8.0, for 30 minutes at 37°C, followed by washes with PBS, pH 7.3.
To seek alloantibodies in the presence of an antibody against a high-prevalence antigen, the plasma of the CD59-deficient patient was absorbed with three RBC suspensions carrying different patterns of antigens allowing the differentiation of all common antibodies whose detection is mandatory according to the German guidelines for hemotherapy. For absorption, aliquots of 0.5 mL of plasma were incubated with aliquots of 4 mL of RBCs in suspension medium (Scanliss, Bio-Rad) for 90 minutes at 37°C.
For agglutination tests, 25 μL of plasma of the CD59-deficient patient was incubated with 50 μL of 0.8% RBCs of unselected donors for 15 minutes at 37°C and centrifuged in gel cards provided for the IAT. In some experiments, alloantibodies were added to plasma samples of the patient. Plasma or serum from surgical IAT containing anti-K, -C, -c, or -Fya were diluted in the plasma of the CD59-deficient patient to a titer of 2. The ID-PNH test kit (Bio-Rad) was used with monoclonal anti-CD59 (Clone MEM43) or anti-CD55 (Clone BRIC216) and gel cards provided by the manufacturer.
Flow cytometry
Blood samples of patients with confirmed PNH were screened by flow cytometry for the fraction of PNH cells as previously described12 using a flow cytometer (FC500, Beckman-Coulter, Krefeld, Germany). Fluorescein isothiocyanate (FITC)-labeled anti-human CD59 Clone p282 (H19) and phycoerythrin (PE)-labeled anti-human CD55 Clone IA10 were from Becton Dickinson PharMingen (Franklin Lakes, NJ). Fifty microliters of a 2% suspension of RBCs in LISS were incubated with 10 μL of FITC-labeled monoclonal anti-CD59 or 0.1 μL of PE-labeled anti-CD55 at room temperature for 30 minutes. Appropriate isotype controls from Becton Dickinson were included (PE-labeled IgGκ Clone MOPC-21 and FITC-labeled IgGκ Clone X40).
When the antibody in the plasma of the CD59-deficient child was investigated, 50 μL of patient plasma was incubated with 50 μL of a 2% suspension of RBCs of a PNH patient in LISS for 1 hour at 37°C followed by staining with FITC-labeled anti-human immunoglobulin (Ig)G secondary antibody (Dianova, Hamburg, Germany). Controls received pooled AB plasma instead of patient plasma.
Inhibition with soluble recombinant CD59
His-tagged, recombinant soluble CD59 protein was reconstituted according to the manufacturer's instructions (Creative Bio Mart, Shirley, NY). For inhibition experiments, 5 μg of recombinant CD59 was added to the antiserum or to the plasma of the CD59-deficient patient followed by incubation for 30 minutes at room temperature before use in agglutination and flow cytometry tests. Buffer controls received PBS instead of recombinant CD59.
Nomenclature
The data will be submitted to the International Society of Blood Transfusion (ISBT) to apply for registration as a blood group system with the proposed name homologous restriction factor.
Results
Evidence for an anti-CD59 alloantibody in the plasma of a patient with isolated CD59 deficiency
Blood samples from a female child of Turkish origin carrying a complete isolated CD59 deficiency were received first at the age of 2 years and occasionally thereafter until the age of 5.11 The first samples already contained an antibody against a high-prevalence antigen with a titer of 4 using the IAT. In the following months the titer dropped and was between 1 and 2. Samples with a titer of 2 or 4 were used for the experiments. At the age of 5, the antibody was only weakly detectable in the IAT, while papain-treated cells still reacted at a titer of 2. The reaction strength was similar with all test panel cells using the antiglobulin technique; the reactivity was enhanced after papain treatment and abrogated after DTT treatment of test cells. RBC incubated with the plasma of the patient were analyzed by the DAT using monospecific anti-IgG, -IgA, -IgM, -C3c, and -C3d. Only IgG was detected on the cells. In all reports available, the DAT was negative, except for one episode where IgG and C3d was present on the patient's cells transiently after an RBC transfusion.
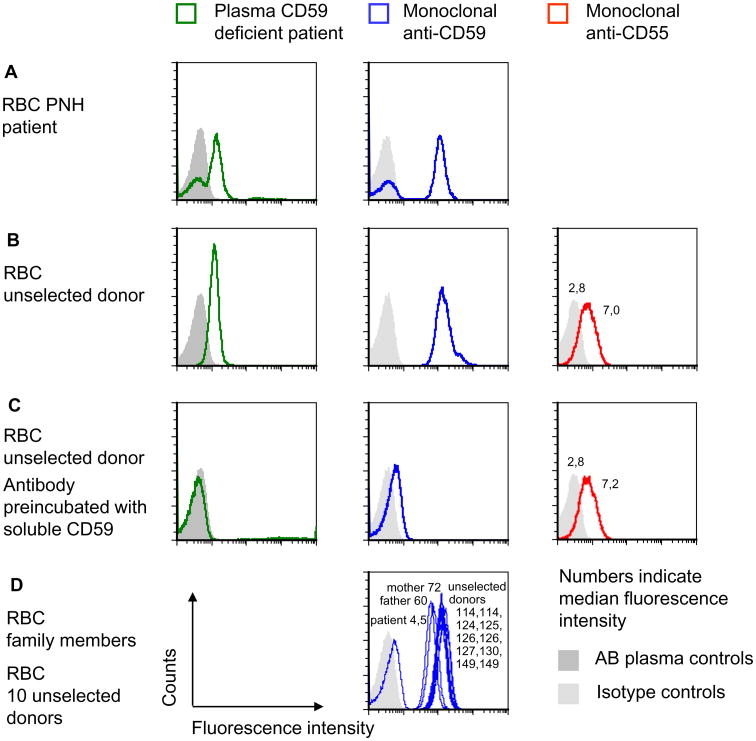
After the CD59 deficiency of the child had been diagnosed,11 it seemed plausible to consider that the antibody in the plasma of the child might be directed against CD59. To identify the specificity of the antibody, we first compared the reaction profile of monoclonal anti-CD59 with that of the patient antibody by flow cytometry. PNH cells are deficient in the GPI anchor causing the absence of all GPI-linked proteins from the RBC surface, including CD59. We selected samples of patients with confirmed PNH known to carry different percentages of PNH cells and tested these with the plasma of the child and for comparison with monoclonal anti-CD59. Similar percentages of CD59-negative PNH cells were observed using the monoclonal anti-CD59 and the patient antibody (Fig. 1A).
Fig. 1.
Identification of anti-CD59 alloantibody by flow cytometry. (A) The fractions of CD59-positive and -negative RBCs of patients with PNH were determined by flow cytometry using plasma of the patient with CD59 deficiency or monoclonal anti-CD59. Controls received either isotype control or pooled AB plasma instead of the patient plasma. The data shown are representative of three independent experiments with similar results. (B) RBCs of an unselected donor were incubated with plasma of the CD59-deficient patient, with FITC-labeled anti-CD59 or PE-labeled anti-CD55, respectively, and analyzed by flow cytometry. The data shown are representative for two independent experiments with similar results. (C) The same assays were performed as in B except that recombinant CD59 was added to the antiserum or to the plasma of the patient. The amount of anti-CD55 used was lower than the amount of anti-CD59 to demonstrate the complete absence of a blocking effect of soluble CD59. The data shown are representative of two independent experiments with similar results. (D) The RBCs of the mother and the father of the CD59-deficient patient were tested by flow cytometry using FITC-labeled monoclonal anti-CD59. Ten unselected donors were analyzed as controls; each sample was accompanied by an isotype control, and the filled light gray area indicates the isotype controls of all experiments depicted in D. The data shown are representative of two experiments with similar results.
While this experiment made it likely that the antibody of the patient was directed against a GPI-linked protein, the specificity was still unknown. Therefore, we used soluble recombinant CD59 protein in an attempt to block the antibody. The patient plasma and, as controls, monoclonal anti-CD59 and monoclonal anti-CD55, were incubated with soluble CD59. Flow cytometric analysis demonstrated inhibition of the patient antibody and of commercial monoclonal anti-CD59 by the recombinant CD59 protein, while the reactivity of anti-CD55 remained unaffected (Figs. 1B and 1C).
CD59 expression on RBC of both parents was investigated by flow cytometry. RBCs of 10 unselected donors served as controls. While the 10 donors showed only minor differences in CD59 expression, CD59 was diminished on the RBCs of both parents (Fig. 1D). The child lacked CD59 on RBCs (Fig. 1D).
Serologic method for the determination of anti-CD59
To confirm the specificity of the antibody in the plasma of the CD59-deficient child and to design an assay for antibody detection which is easier to perform we chose the gel agglutination technique.
A commercial PNH test was used supplying monoclonal anti-CD59 and anti-CD55 different from the clones used in flow cytometry experiments. First, we tested whether the reactivity of the monoclonal anti-CD59 MEM 43 was blocked by soluble CD59. Indeed, the agglutinating capacity of anti-CD59 was completely lost after incubation with soluble CD59 while anti-CD55 remained unaffected (Fig. 2). Also the reactivity of the plasma of the CD59-deficient child with RBCs of an unselected donor could be completely inhibited by soluble CD59 (Fig. 2, Column 7 from the left).
Fig. 2.
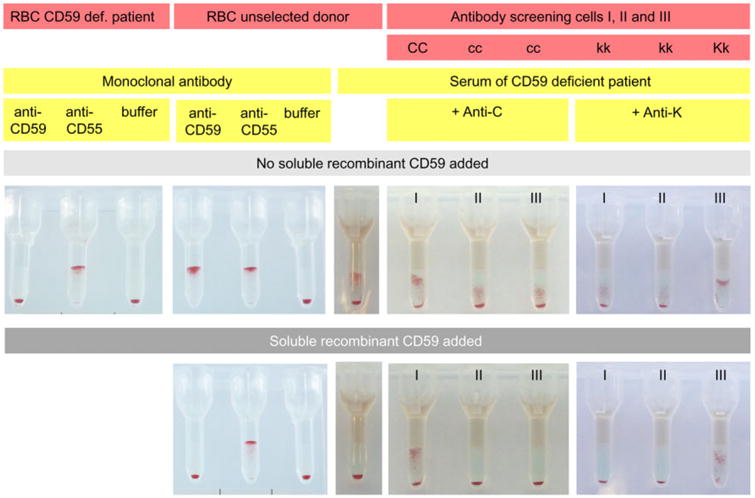
Application of the column agglutination technique for the identification of anti-CD59. RBCs of the CD59-deficient patient of an unselected donor or antibody screening cells were incubated with antibodies at 37°C as indicated. Either MoAbs or plasma of the CD59-deficient patient were used or plasma of the CD59-deficient patient to which human alloantibodies had been added. After centrifugation of the gel cards, agglutination indicated reactivity.
In further experiments the irregular RBC antibodies anti-K, anti-C, anti-c, or anti-Fya were added to the plasma of the CD59-deficient patient before cross-match with three different RBCs of an antibody screening test. The added antibodies were difficult to identify in the presence of the high-prevalence anti-CD59 (examples shown in Fig. 2). Therefore, the plasma of the child with the added antibodies was incubated with soluble CD59 before cross-match with the antibody screening cells. While the antibody directed against the high-prevalence antigen CD59 was blocked by recombinant CD59, the added alloantibodies were still detectable. Furthermore, the CD59 defect of the child was easily detectable with the agglutination technique (Fig. 2).
Discussion
Only seven individuals with an inherited CD59 deficiency have been described so far.8-11 Among these, three different CD59-null alleles were found in correlation to ethnic origin. All CD59-deficient individuals were severely ill. Common to all homozygous carriers of CD59-null alleles is the occurrence of episodes of PNH-like hemolysis while other manifestations of disease may differ. All the five North African Jewish children and the single Turkish child showed an early onset of the disease already within 3 to 7 months while the single Japanese patient was affected by the first hemolytic episode not before the age of 13. The North African Jewish children and the patient of Turkish origin presented with severe peripheral neurologic disease. The Japanese patient suffered from two cerebral infarctions but apparently no peripheral neurologic symptoms. The reason for differences in clinical presentation is presently unknown and more cases may be necessary to draw definite conclusions.
The CD59 protein has a length of 128 amino acids.1 The Japanese and the Turkish patient harbored frameshift mutations leading to terminating codons at Amino Acids 38 and 31, respectively. Therefore, the carriers of these two alleles probably generate no functional protein. The North African Jewish allele is characterized by an amino acid substitution from Cys to Tyr in Position 89. The altered protein was present in cells but absent on membranes.10 Since the change involves the substitution of a cysteine that participates in the formation of a disulfide bond, the authors speculated that a disturbed tertiary structure may interfere with the localization of the protein to the membrane.
CD59 represents an antigen of high prevalence. This report describes for the first time the occurrence of a human anti-CD59 alloantibody found in the Turkish patient. From the available medical history, the immunization event remained unclear. Before we received the first sample at the age of 2, intravenous immunoglobulin had been given. An antibody in the plasma of the child had already been recognized at the occasion of pretransfusion analyses at the age of 1½ and several RBC units had been transfused. No major adverse clinical signs of a transfusion reaction were evident from anamnestic records. All available reports on serologic analyses indicated a negative DAT. Only at one instance, approximately at the age of 3, a transiently positive DAT was reported shortly after transfusion of RBCs, probably due to a serologic transfusion reaction. At the age of 5, approximately 2 years after the last transfusion, the antibody titer of the child had fallen to almost undetectable levels.
In case the presence of anti-CD59 poses a transfusion problem, the application of eculizumab11 and the use of the blood of heterozygous carriers of a CD59 deficiency may aid transfusion therapy. The consanguineous parents of the CD59-deficient child carried the same single-nucleotide deletion in heterozygous form11 and showed a lowered expression of CD59 on their RBCs (Fig. 1D).
The antibody in the plasma of the CD59-deficient patient could be easily identified by an inhibition test using soluble recombinant CD59 (Figs. 1 and 2). Analyses by flow cytometry or by the gel agglutination technique are suitable, but the serologic test is easier to perform and can be established in most immunohematologic laboratories. Characteristic of the antibody were enhanced reaction with enzyme-treated RBCs, no reaction with DTT-treated RBCs, and a homogeneous reaction profile with all panel cells. Only IgG was detected on the cells after cross-match; hence, the antibody was at least in part IgG.
The antibody specificity could not be solved either by cross-matches of the patient's plasma with a variety of rare test cells negative for high-prevalence antigens or by determination of the patient's antigens or by specialized techniques of an experienced reference laboratory. Previous reports have established the use of soluble recombinant JMH, Kna, Lub, and Scianna proteins for the identification of antibodies with rare specificities.13 Also soluble CD59 protein proved to be a useful diagnostic tool not only for the identification of anti-CD59 (Figs. 1C and 2) but also for the detection of additional alloantibodies (Fig. 2). While the antibody against the high-frequency antigen CD59 was blocked by recombinant CD59, other alloantibodies admixed to the plasma of the patient were still detectable. The CD59 deficiency of the child could also be easily identified by the agglutination test (Fig. 2).
CD59 is well known as an antigen of the RBC membrane defined by monoclonal antibodies (MoAbs) and is characterized at the molecular level.1-3,14 However, CD59 had not been identified as a blood group antigen, since the description of a human alloantibody was missing. Our present report now for the first time gives evidence for the presence of a human anti-CD59 alloantibody and thereby identifies CD59 as a blood group antigen. The following criteria established by the ISBT for constituting a new blood group system are fulfilled: the blood group antigen is defined by a human alloantibody (this article) and is inherited;11 the encoding gene is sequenced1 and is different from other blood group genes and its chromosomal location is known.15 The new system currently comprises one antigen and three null alleles. [Correction added after online publication 03-Jan-2014: Paragraph updated for clarification.]
Acknowledgments
The authors acknowledge the technical assistance of Sabine Kaiser and Sabine Zahn. Klaus Schwarz, Ulrich Pannicke, and Markus Rojewski provided information on results of molecular and flow cytometry analyses before publication.
Abbreviations
- GPI
glycosylphosphatidylinositol
- PNH
paroxysmal nocturnal hemoglobinuria
Footnotes
The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
Conflict of Interest: The authors report no conflicts of interest or funding sources.
Web Resources: ISBT (International Society of Blood Transfusion) Working Party on Red Cell Immunogenetics and Blood Group Terminology. http://www.isbtweb.org/working-parties/red-cell-immunogenetics-and-blood-group-terminology/blood-group-terminology/Accessed June 11, 2013.
References
- 1.Davies A, Simmons DL, Hale G, et al. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989;170:637–54. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lachmann PJ. The control of homologous lysis. Immunol Today. 1991;12:312–15. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- 3.Morgan BP. Cellular responses to the membrane attack complex. In: Whaley K, Loos M, Weiler JM, editors. Complement in health and disease. Lancaster (UK): Kluwer; pp. 1993pp. 325–51. [Google Scholar]
- 4.Kimberley FC, Baalasubramanian S, Morgan P. Alternative roles for CD59. Mol Immunol. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Meri S, Lehto T, Sutton CW, et al. Structural composition and functional characterization of soluble CD59: heterogeneity of the oligosaccharide and glycophosphoinositol (GPI) anchor revealed by laser-desorption mass spectrometric analysis. Biochem J. 1996;316:923–35. doi: 10.1042/bj3160923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330:249–55. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 7.Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–8. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 8.Yamashina M, Ueda E, Kinoshita T, et al. Inherited complete deficiency of 20-kilodalton homologous restriction factor (CD59) as a cause of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1990;323:1184–89. doi: 10.1056/NEJM199010253231707. [DOI] [PubMed] [Google Scholar]
- 9.Motoyama M, Okada N, Yamashina M, et al. Paroxysmal nocturnal hemoglobinuria due to hereditary nucleotide deletion in the HRF20 (CD59) gene. Eur J Immunol. 1992;22:2669–73. doi: 10.1002/eji.1830221029. [DOI] [PubMed] [Google Scholar]
- 10.Nevo Y, Ben-Zeev B, Tabib A, et al. CD59 deficiency is associated with chronic hemolysis and childhood relapsing immune mediated polyneuropathy. Blood. 2013;121:129–35. doi: 10.1182/blood-2012-07-441857. [DOI] [PubMed] [Google Scholar]
- 11.Höchsmann B, Dohna-Schwake C, Kyrieleis H, et al. Targeted therapy with Eculizumab for inherited CD59 deficiency. N Engl J Med. 2014;370:90–2. doi: 10.1056/NEJMc1308104. [DOI] [PubMed] [Google Scholar]
- 12.Höchsmann B, Rojewski M, Schrezenmeier H. Paroxysmal nocturnal hemoglobinuria (PNH): higher sensitivity and validity in diagnosis and serial monitoring by flow cytometric analysis of reticulocytes. Ann Hematol. 2011;90:887–99. doi: 10.1007/s00277-011-1177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seltsam A, Grueger D, Blasczyk R, et al. Easy identification of antibodies to high-prevalence Scianna antigens and detection of admixed alloantibodies using soluble recombinant Scianna protein. Transfusion. 2009;49:2090–6. doi: 10.1111/j.1537-2995.2009.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Fedarovich A, Tomlinson S, et al. Crystal structure of CD59: implications for molecular recognition of the complement proteins C8 and C9 in the membrane-attack complex. Acta Crystallogr D Biol Crystallogr. 2007;D63:714–21. doi: 10.1107/S0907444907015557. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg UH, Bazil V, Stefanová I, et al. Gene for human CD59 (likely Ly-6 homologoue) is located on the short arm of chromosome 11. Immunogenetics. 1989;30:188–93. doi: 10.1007/BF02421205. [DOI] [PubMed] [Google Scholar]