Abstract
The current classification system presents challenges to the diagnosis and treatment of patients with diabetes mellitus (DM), in part due to its conflicting and confounding definitions of type 1 DM, type 2 DM, and latent autoimmune diabetes of adults (LADA). The current schema also lacks a foundation that readily incorporates advances in our understanding of the disease and its treatment. For appropriate and coherent therapy, we propose an alternate classification system. The β-cell–centric classification of DM is a new approach that obviates the inherent and unintended confusions of the current system. The β-cell–centric model presupposes that all DM originates from a final common denominator—the abnormal pancreatic β-cell. It recognizes that interactions between genetically predisposed β-cells with a number of factors, including insulin resistance (IR), susceptibility to environmental influences, and immune dysregulation/inflammation, lead to the range of hyperglycemic phenotypes within the spectrum of DM. Individually or in concert, and often self-perpetuating, these factors contribute to β-cell stress, dysfunction, or loss through at least 11 distinct pathways. Available, yet underutilized, treatments provide rational choices for personalized therapies that target the individual mediating pathways of hyperglycemia at work in any given patient, without the risk of drug-related hypoglycemia or weight gain or imposing further burden on the β-cells. This article issues an urgent call for the review of the current DM classification system toward the consensus on a new, more useful system.
A Classification System That Has Petered Out?
The essential function of a classification system is as a navigation tool that helps direct research, evaluate outcomes, establish guidelines for best practices for prevention and care, and educate on all of the above. Diabetes mellitus (DM) subtypes as currently categorized, however, do not fit into our contemporary understanding of the phenotypes of diabetes (1–6). The inherent challenges of the current system, together with the limited knowledge that existed at the time of the crafting of the current system, yielded definitions for type 1 DM, type 2 DM, and latent autoimmune diabetes in adults (LADA) that are not distinct and are ambiguous and imprecise.
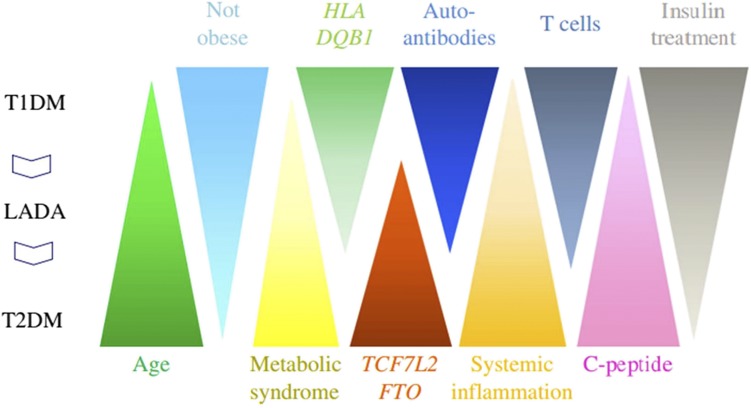
Discovery of the role played by autoimmunity in the pathogenesis of type 1 DM created the assumption that type 1 DM and type 2 DM possess unique etiologies, disease courses, and, consequently, treatment approaches. There exists, however, overlap among even the most “typical” patient cases. Patients presenting with otherwise classic insulin resistance (IR)-associated type 2 DM may display hallmarks of type 1 DM. Similarly, obesity-related IR may be observed in patients presenting with “textbook” type 1 DM (7). The late presentation of type 1 DM provides a particular challenge for the current classification system, in which this subtype of DM is generally termed LADA. Leading diabetes organizations have not arrived at a common definition for LADA (5). There has been little consensus as to whether this phenotype constitutes a form of type 2 DM with early or fast destruction of β-cells, a late manifestation of type 1 DM (8), or a distinct entity with its own genetic footprint (5). Indeed, current parameters are inadequate to clearly distinguish any of the subforms of DM (Fig. 1). Discussions and critiques of the current DM classification system are found in the literature (1–6).
Figure 1.
Qualitative illustration of the spectrum of factors associated with different forms of DM, including the variable age at onset, lack of obesity, metabolic syndrome, genetic associations, different forms of immune changes, C-peptide secretion, and the need for insulin therapy. T1DM, type 1 DM; T2DM, type 2 diabetes. Adapted with permission from Leslie et al. (1).
The use of IR to define type 2 DM similarly needs consideration. The fact that many obese patients with IR do not develop DM indicates that IR is insufficient to cause type 2 DM without predisposing factors that affect β-cell function (9).
Classification Schema Can Raise Barriers to Optimal Patient Care
The current classification schema imposes unintended constraints on individualized medicine. Patients diagnosed with LADA who retain endogenous insulin production may receive “default” insulin therapy as treatment of choice. This decision is guided largely by the categorization of LADA within type 1 DM, despite the capacity for endogenous insulin production. Treatment options that do not pose the risks of hypoglycemia or weight gain might be both useful and preferable for LADA but are typically not considered beyond use in type 2 DM (10). Incretins and sodium–glucose cotransporter 2 (SGLT-2) inhibitors are examples of newer agents that have demonstrated potential and are being rigorously evaluated in the treatment of type 1 DM and LADA (10–17).
The categorization of LADA within type 1 DM also leads to myopia on the part of insurers. Medications that could be logical choices as adjunctive or alternative therapies to insulin for candidate patients with LADA are not designated as approved processes of care under the current classification system and accordingly are not covered by insurers.
We believe that there is little rationale for limiting choice of therapy solely on the current definitions of type 1 DM, type 2 DM, and LADA. We propose that choice of therapy should be based on the particular mediating pathway(s) of hyperglycemia present in each individual patient, as will be discussed. Only large clinical trials can fully validate the best use of various agents across the spectrum of DM. In the interim, however, an evidence-based practice approach can allow for broader utility in routine care. Metformin and pioglitazone may be safe and efficacious adjunctive therapies regardless of the current diagnostic category, as may be incretins (11,15,17–23) and SGLT-2 inhibitors (14,24–26). It is reasonable that broader use of existing agents would extend to the management of maturity-onset diabetes of the young (23,27), as well as stress-related and steroid-induced DM.
β-Cell–Centric Construct: A Potential Model for the Classification of DM
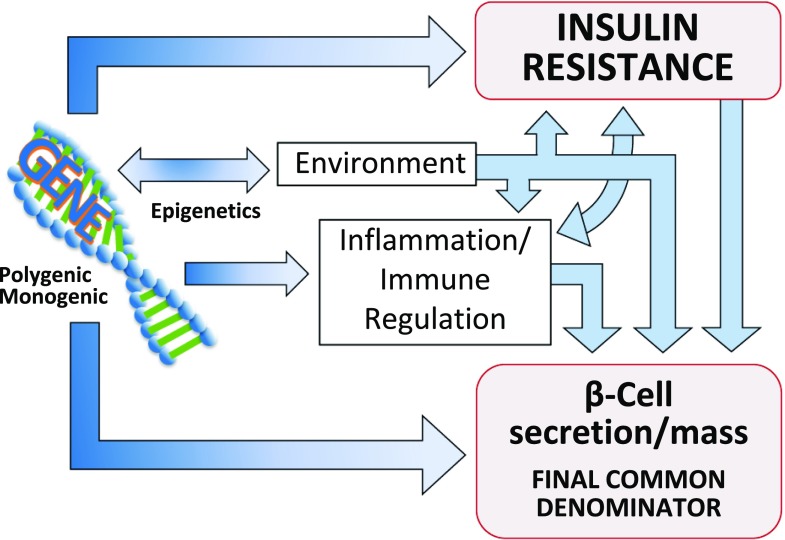
Given the above discussion, the issue is not “what is LADA” or any clinical presentation of DM under the current system. The issue is the mechanisms and rate of destruction of β-cells at work in all DM. We present a model that provides a more logical approach to classifying DM: the β-cell–centric classification of DM. In this schema, the abnormal β-cell is recognized as the primary defect in DM. The β-cell–centric classification system recognizes the interplay of genetics, IR, environmental factors, and inflammation/immune system on the function and mass of β-cells (Fig. 2). Importantly, this model is universal for the characterization of DM. The β-cell–centric concept can be applied to DM arising in genetically predisposed β-cells, as well as in strongly genetic IR syndromes, such as the Rabson-Mendenhall syndrome (28), which may exhaust nongenetically predisposed β-cells. Finally, the β-cell–centric classification of all DM supports best practices in the management of DM by identifying mediating pathways of hyperglycemia that are operative in each patient and directing treatment to those specific dysfunctions.
Figure 2.
Genetic determinants influence IR (whether centrally or peripherally induced), loss of β-cell function and mass, environmental triggers (such as viruses, endocrine disruptors, food advanced glycosylation end products, gut biome), and immune modulation and inflammation. Singly or, more commonly, in various combinations, these factors converge on the genetically susceptible β-cell, impinge on β-cell function and biology, and orchestrate the shift from normoglycemia to hyperglycemia. As this process takes place regardless of subtype of DM, the dysfunctional β-cell is the final common denominator in all DM.
The β-Cell: At the Root and Crossroads of Multiple Mediating Pathways of Hyperglycemia
The β-cell–centric construct suggests a more logical rationale to the eight core defects described by the ominous octet (29). Our model recognizes a total of 11 interlocking pathways that contribute to hyperglycemia (Fig. 3A). These mediating pathways of hyperglycemia are induced by the translation of genetic predispositions to IR, susceptibility to environmental influences, or immune dysregulation and inflammation to genetically predisposed, dysfunctional β-cells. The β-cell construct can incorporate newly discovered pathways to dysglycemia as these evolve, such as emerging research linking osteocalcin levels to A1C and HOMA of β-cell function status (30).
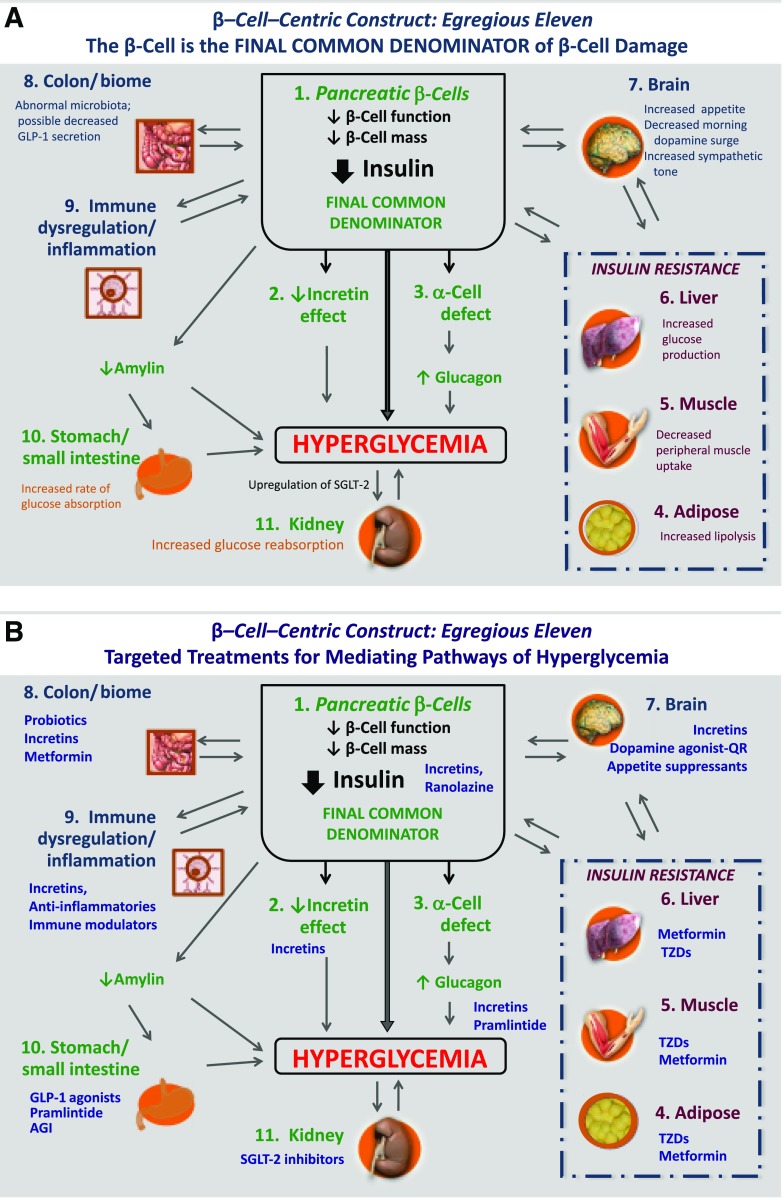
Figure 3.
β-Cell–centric construct: the egregious eleven. Dysfunction of the β-cells is the final common denominator in DM. A: Eleven currently known mediating pathways of hyperglycemia are shown. Many of these contribute to β-cell dysfunction (liver, muscle, adipose tissue [shown in red to depict additional association with IR], brain, colon/biome, and immune dysregulation/inflammation [shown in blue]), and others result from β-cell dysfunction through downstream effects (reduced insulin, decreased incretin effect, α-cell defect, stomach/small intestine via reduced amylin, and kidney [shown in green]). B: Current targeted therapies for each of the current mediating pathways of hyperglycemia. GLP-1, glucagon-like peptide 1; QR, quick release.
The mediating pathways of hyperglycemia that contribute to β-cell dysfunction include liver, muscle, and adipose tissue (organs associated with IR) and brain, colon, and immune dysregulation. This damage results in downstream hyperglycemia arising from increased glucagon secretion, as well as a reduction in insulin production, incretin effect, and amylin levels. Even mild hyperglycemia resulting from β-cell dysfunction can upregulate SGLT-2 protein in the kidney, which further contributes to hyperglycemia (31). Hyperglycemia, regardless of its source, leads to glucotoxicity, which further impairs β-cell function. In a given patient, the specific mediating pathways of hyperglycemia at work are variable, though likely to involve multiple pathways (Fig. 3A).
Three additional mediating pathways of hyperglycemia to those of the ominous octet (29) have been identified. Systemic low-grade inflammation is observed in type 2 DM, type 1 DM, and LADA (32,33) and has been shown to accompany the endoplasmic stress imposed by increased metabolic demand for insulin (34). Early studies show incretins exert anti-inflammatory effects (35,36), which may account in part for their benefit. Inflammation is being clinically evaluated as a therapeutic target. It would be of interest if a recent 1-year trial reporting the ability of a dipeptidyl peptidase 4 inhibitor to delay the progression of disease in LADA patients (17) proved durable and reproducible.
Changes in gut microbiota may contribute to the diabetic state (37–40). Gut microbiota has been shown to be associated with type 1 DM, type 2 DM, and obesity and has been proposed to help explain the observation that only a portion of overweight individuals develop frank DM (38,39). Probiotics and prebiotics may address this mediator of hyperglycemia.
Reductions in amylin production in the diabetic state are a consequence of β-cell dysfunction. Decreased amylin levels lead to accelerated gastric emptying and increased glucose absorption in the small intestine, with corresponding increases in postprandial glucose levels. This pathway of hyperglycemia could theoretically be addressed, at least in part, by the ability of incretins to slow gastric emptying.
A key premise is that the mediating pathways of hyperglycemia are common across prediabetes, type 1 DM, type 2 DM, and other currently defined forms of DM. Accordingly, we believe that the current antidiabetes armamentarium has broader applicability across the spectrum of DM than is currently utilized.
The ideal treatment paradigm would be one that uses the least number of agents possible to target the greatest number of mediating pathways of hyperglycemia operative in the given patient. It is prudent to use agents that will help patients reach target A1C levels without introducing drug-related hypoglycemia or weight gain. Despite the capacity of insulin therapy to manage glucotoxicity, there is a concern for β-cell damage due to IR that has been exacerbated by exogenous insulin-induced hyperinsulinemia and weight gain (41). Sulfonylureas have been shown to induce apoptosis of β-cells in culture (42,43). In contrast, early data on some newer agents are suggestive of β-cell–sparing abilities. An improvement of early and late β-cell response to glucose load has been reported with dipeptidyl peptidase 4 inhibitor treatment (18,21). Incretins have been shown, in preclinical evaluations, to halt apoptosis, stimulate proliferation of β-cells, increase insulin availability, improve α-cell response to insulin (44–46), and, in animal studies, preserve β-cells (47).
Genetic Influences on the β-Cell
The β-cell–centric model recognizes that the final common denominator of DM is the genetically predisposed, dysfunctional β-cell, which ultimately leads to compromised β-cell function, loss in β-cell mass, or depleted insulin content in the face of IR. These may include monogenic or polygenic defects that predispose to hyperinsulinemia, IR, more recently understood mechanisms such as inflammation by the immune system (48–51), susceptibility to environmental factors (37,51,52), or other physiological factors that increase demand on or otherwise damage β-cells such as elevated circulating lipids (37,53–55) (Fig. 2). As not all carriers of genes associated with DM develop DM, susceptibility likely relies on combinations of genetic abnormalities, environment, and lifestyle factors to exacerbate underlying genetic predispositions. Though research is nascent, implicated environmental factors have included endocrine disruptors (56), food additives (52), abnormal gut biome (38,39,57), and ingested advanced glycation end products (58). There is also evidence that certain environmental factors may epigenetically alter the genotype in reproductive cells, producing inheritable DM factors in future generations (59,60) (Fig. 2).
Clinically evident DM ensues at or after the juncture when the combined gene–environment trigger reaches a tipping point for sufficient β-cell compromise to be expressed as phenotypic hyperglycemia. This fundamental concept applies to all forms of DM, substantiating that the final common denominator in DM is at the level of the β-cell.
In our model, as typical in obesity, IR is a monogenic or, more commonly, a polygenic disorder (59). Additional contributing factors to IR may include inflammation (48–51), changes in the gut microbiota (37–40), and brain-modulated changes in metabolism (51,61,62). Resulting hyperinsulinemia feeds back to the hypothalamus to further exacerbate peripheral IR (61,62). Downstream effects of IR cause detriment to β-cell function by mechanisms that may include inflammatory cytokines, adipocytokines, lipotoxicity, and decreased adiponectin, potentially representing a physiological scenario similar to that induced by hyperinsulinemia (63,64).
β-Cell–Centric Schema and Individualized Care
We propose that the β-cell–centric model is a conceptual framework that could help optimize processes of care for DM. A1C, fasting blood glucose, and postprandial glucose testing remain the basis of DM diagnosis and monitoring. Precision medicine in the treatment of DM could be realized by additional diagnostic testing that could include C-peptide (1), islet cell antibodies or other markers of inflammation (1,65), measures of IR, improved assays for β-cell mass, and markers of environmental damage and by the development of markers for the various mediating pathways of hyperglycemia.
We uphold that there is, and will increasingly be, a place for genotyping in DM standard of care. Pharmacogenomics could help direct patient-level care (66–69) and holds the potential to spur on research through the development of DM gene banks for analyzing genetic distinctions between type 1 DM, LADA, type 2 DM, and maturity-onset diabetes of the young. The cost for genotyping has become increasingly affordable.
Lifestyle modification is the starting point for intervention in prediabetes and DM as is normalization of dyslipidemia, given the links of prolonged lipid exposure with β-cell dysfunction (9,53–55). Our approach advocates intervention early in the process of β-cell dysfunction. It is intuitively obvious that the constellation of mediating pathways of hyperglycemia in frank DM is likely the same as those in prediabetes. Pharmacotherapy for prediabetes should be considered if lifestyle approaches do not produce normoglycemia. Preferential use of agents with proven or strong evidence for β-cell preservation is logical (70).
The optimal strategy is to use the least number of agents to target the greatest number of mediating pathways of hyperglycemia operative in the given patient. It would use regimens that stabilize hyperglycemia across multiple causes, act synergistically to reduce cardiovascular and other risk factors, and preserve β-cells. Figure 3B illustrates the mediating pathways of hyperglycemia addressed by various available agents and provides a logic for the selection of complementary modes of action in combination therapy.
Our approach for using combination therapy is consistent with the recommendations within the 2015 American Diabetes Association (71) and 2015 American Association of Clinical Endocrinologists (72) guidelines. We advocate the introduction of combination therapy early in the pharmacological management of the disease. Critically, we avoid stratifying first-, second-, and third-line treatment sequencing. This stratification establishes undue competition between classes, which should more rightly be viewed as complementary options rather than salvage therapy after inevitable treatment failure (19,73).
The ideal treatment regimens should not be potentially detrimental to the long-term integrity of the β-cells. Specifically, sulfonylureas and glinides should be ardently avoided. Any benefits associated with sulfonylureas and glinides (including low cost) are not enduring and are far outweighed by their attendant risks (and associated treatment costs) of hypoglycemia and weight gain, high rate of treatment failure and subsequent enhanced requirements for antihyperglycemic management, potential for β-cell exhaustion (42), increased risk of cardiovascular events (74), and potential for increased risk of mortality (75,76). Fortunately, there are a large number of classes now available that do not pose these risks. Empagliflozin has been recently shown to reduce cardiovascular outcomes and mortality in type 2 DM, while reducing weight and posing a low risk for hypoglycemia (24).
Newer agents present alternatives to insulin therapy, including in patients with “advanced” type 2 DM with residual insulin production. Insulin therapy induces hypoglycemia, weight gain, and a range of adverse consequences of hyperinsulinemia with both short- and long-term outcomes (77–85). Newer antidiabetes classes may be used to delay insulin therapy in candidate patients with endogenous insulin production (19). In patients requiring basal insulin, clinical research on novel combinations of classes, such as pramlintide (86) and incretins (19,22), may reduce or eliminate the need for bolus insulin. Bolus insulin accounts for most of the hypoglycemia seen with basal–bolus insulin therapy (87). When insulin therapy is needed, we suggest it be incorporated as add-on therapy rather than as substitution for noninsulin antidiabetes agents. Outcomes research is needed to fully evaluate various combination therapeutic approaches, as well as the potential of newer agents to address drivers of β-cell dysfunction and loss.
The principles of the β-cell–centric model provide a rationale for adjunctive therapy with noninsulin regimens in patients with type 1 DM (7,12–16). Thiazolidinedione (TZD) therapy in patients with type 1 DM presenting with IR, for example, is appropriate and can be beneficial (17). Clinical trials in type 1 DM show that incretins (20) or SGLT-2 inhibitors (25,88) as adjunctive therapy to exogenous insulin appear to reduce plasma glucose variability.
Further Experimental and Translational Research
This article highlights the need to replot the classification of DM, recognizing the β-cell as the final common denominator of glucose dysregulation and the mediating pathways of hyperglycemia surrounding the β-cell as the basis for treatment decisions. A β-cell–focused schema can integrate knowledge to date and incorporate new discoveries. It can provide sage advice for preferential use of pharmacological interventions that address the mechanisms of hyperglycemia operative in an individual patient, avoid hypoglycemia and weight gain, and appear to be β-cell sparing. Preferred therapies will be those that affect multiple mediators of hyperglycemia. Novel anti-inflammation agents currently in phase 2 and 3 clinical development should be evaluated for safety and efficacy, and we should further explore suggestions that this approach could effectively treat, reverse, or even prevent DM with an inflammatory component (89).
The β-cell–centric classification schema was envisioned as a stimulus to guide basic research, as well as clinical and translational research. It is hoped to help direct research on the genes involved in DM, the functions that these genes serve, the mechanisms that lead to β-cell damage, the downstream effects of reduced β-cell function, and any novel mechanisms of β-cell pathophysiology. Also needed is research toward improved diagnostic markers for the development of DM.
The β-cell–centric model can be readily retrofitted into the terminology of the existing classification system. However, we submit that an entirely new nomenclature may likely best fulfill the imperative of bringing the classification in line with the known etiology and disease course.
A Call to Action
For all the above-stated reasons, we urge that the time is right to convene a committee of diabetes community leaders and researchers to reevaluate the current outmoded DM classification system. Members of the American Diabetes Association, American Association of Clinical Endocrinologists, European Association for the Study of Diabetes, International Diabetes Federation, and World Health Organization should come together to address this immense, but vital, task toward delivering state-of-the-art, optimal patient care and directing future research.
Article Information
Acknowledgments. The authors acknowledge Dr. Mary E. Herman, Montclair State University, Montclair, NJ, for her editorial assistance in the crafting of the manuscript.
Funding. S.S.S. and S.F.A.G. are partially supported by National Institutes of Health (NIH) grant R01 DK085212. S.F.A.G. holds the Daniel B. Burke Endowed Chair for Diabetes Research. B.E.C. is partially supported by NIH grants DK99618, DK56690, DK74778, and DK35914. No funding was received by any of authors for the work in the manuscript.
Duality of Interest. S.S.S. is a speaker and advisor to Novo Nordisk, Merck, Takeda, Johnson & Johnson, AstraZeneca/Bristol-Myers Squibb, Eli Lilly and Co., and Boehringer Ingelheim/Eli Lilly and Co. and is a speaker for Eisai and GlaxoSmithKline. J.R.G. has received consultant fees from Abbott Diabetes Care, Intarcia Pharmaceuticals, AstraZeneca, and Novo Nordisk; has served on the advisory boards of Janssen Pharmaceuticals and AstraZeneca; and has served on the speakers’ bureaus of AstraZeneca, Janssen Pharmaceuticals, and Boehringer Ingelheim/Eli Lilly and Co. R.B.A. sits on the advisory board and speakers’ bureaus of Eli Lilly and Co., Boehringer Ingelheim, Janssen Pharmaceuticals, and Takeda. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.S.S. conceived the β-cell–centric concept and crafted the first draft of the manuscript. S.E., B.E.C., S.F.A.G., J.R.G., and R.B.A. critically reviewed, provided incisive input, edited, and approved the final version of the manuscript. B.E.C., in particular, lent important critical analysis of the concept. R.B.A. additionally contributed graphic design.
References
- 1.Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia. 24 October 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Grant SFA, Hakonarson H, Schwartz S. Can the genetics of type 1 and type 2 diabetes shed light on the genetics of latent autoimmune diabetes in adults? Endocr Rev 2010;31:183–193 [DOI] [PubMed] [Google Scholar]
- 3.Rolandsson O, Palmer JP. Latent autoimmune diabetes in adults (LADA) is dead: long live autoimmune diabetes! Diabetologia 2010;53:1250–1253 [DOI] [PubMed] [Google Scholar]
- 4.Redondo MJ. LADA: time for a new definition. Diabetes 2013;62:339–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile KJ, Guy VC, Schwartz S, Grant SFA. Overlap of genetic susceptibility to type 1 diabetes, type 2 diabetes, and latent autoimmune diabetes in adults. Curr Diab Rep 2014;14:550. [DOI] [PubMed] [Google Scholar]
- 6.Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am 2015;99:1–16 [DOI] [PubMed] [Google Scholar]
- 7.Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2015;22:277–282 [DOI] [PubMed] [Google Scholar]
- 8.Guy VC, Chesi A, Hawa M, et al. The role of GWAS-implicated type 1 and type 2 diabetes loci in the pathogenesis of latent autoimmune diabetes in adults (LADA) (Abstract) Diabetes 2015;64(Suppl. 1):A80 [Google Scholar]
- 9.Corkey BE. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and type 2 diabetes? Diabetes Care 2012;35:2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guglielmi C, Palermo A, Pozzilli P. Latent autoimmune diabetes in the adults (LADA) in Asia: from pathogenesis and epidemiology to therapy. Diabetes Metab Res Rev 2012;28(Suppl. 2):40–46 [DOI] [PubMed] [Google Scholar]
- 11.Ghazi T, Rink L, Sherr JL, Herold KC. Acute metabolic effects of exenatide in patients with type 1 diabetes with and without residual insulin to oral and intravenous glucose challenges. Diabetes Care 2014;37:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebovitz HE. Adjunct therapy for type 1 diabetes mellitus. Nat Rev Endocrinol 2010;6:326–334 [DOI] [PubMed] [Google Scholar]
- 13.Munir KM, Davis SN. The treatment of type 1 diabetes mellitus with agents approved for type 2 diabetes mellitus. Expert Opin Pharmacother 2015;16:2331–2341 [DOI] [PubMed] [Google Scholar]
- 14.Perkins BA, Cherney DZ, Partridge H, et al. . Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014;37:1480–1483 [DOI] [PubMed] [Google Scholar]
- 15.Renukuntla VS, Ramchandani N, Trast J, Cantwell M, Heptulla RA. Role of glucagon-like peptide-1 analogue versus amylin as an adjuvant therapy in type 1 diabetes in a closed loop setting with ePID algorithm. J Diabetes Sci Technol 2014;8:1011–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tafuri KS, Godil MA, Lane AH, Wilson TA. Effect of pioglitazone on the course of new-onset type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol 2013;5:236–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Yang L, Xiang Y, et al. . Dipeptidyl peptidase 4 inhibitor sitagliptin maintains β-cell function in patients with recent-onset latent autoimmune diabetes in adults: one year prospective study. J Clin Endocrinol Metab 2014;99:E876–E880 [DOI] [PubMed] [Google Scholar]
- 18.Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab 2008;10:376–386 [DOI] [PubMed] [Google Scholar]
- 19.Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015;17:268–275 [DOI] [PMC free article] [PubMed]
- 20.Arnolds S, Dellweg S, Clair J, et al. . Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care 2010;33:1509–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nonaka K, Kakikawa T, Sato A, et al. . Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008;79:291–298 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz S. Evidence-based practice use of incretin-based therapy in the natural history of diabetes. Postgrad Med 2014;126:66–84 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz SS, DeFronzo RA, Umpierrez GE. Practical implementation of incretin-based therapy in hospitalized patients with type 2 diabetes. Postgrad Med 2015;127:251–257 [DOI] [PubMed] [Google Scholar]
- 24.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 25.Henry RR, Rosenstock J, Edelman S, et al. . Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care 2015;38:412–419 [DOI] [PubMed] [Google Scholar]
- 26.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group . Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab 2014;16:124–136 [DOI] [PubMed] [Google Scholar]
- 27.Anık A, Çatlı G, Abacı A, Böber E. Maturity-onset diabetes of the young (MODY): an update. J Pediatr Endocrinol Metab 2015;28:251–263 [DOI] [PubMed] [Google Scholar]
- 28.Longo N, Wang Y, Pasquali M. Progressive decline in insulin levels in Rabson-Mendenhall syndrome. J Clin Endocrinol Metab 1999;84:2623–2629 [DOI] [PubMed] [Google Scholar]
- 29.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunutsor SK, Apekey TA, Laukkanen JA. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur J Epidemiol 2015;30:599–614 [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14:5–14 [DOI] [PubMed] [Google Scholar]
- 32.Pietropaolo M, Barinas-Mitchell E, Kuller LH. The heterogeneity of diabetes: unraveling a dispute: is systemic inflammation related to islet autoimmunity? Diabetes 2007;56:1189–1197 [DOI] [PubMed] [Google Scholar]
- 33.Subauste A, Gianani R, Chang AM et al. Islet autoimmunity identifies a unique pattern of impaired pancreatic beta-cell function, markedly reduced pancreatic beta cell mass and insulin resistance in clinically diagnosed type 2 diabetes. PLoS One 2014;9:e106537 [DOI] [PMC free article] [PubMed]
- 34.Oslowski CM, Hara T, O’Sullivan-Murphy B, et al. . Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab 2012;16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhuri A, Ghanim H, Vora M, et al. . Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab 2012;97:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makdissi A, Ghanim H, Vora M, et al. . Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab 2012;97:3333–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allin KH, Nielsen T, Pedersen O. Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol 2015;172:R167–R177 [DOI] [PubMed] [Google Scholar]
- 39.Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord 2015;16:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho BM, Saad MJ Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm 2013;2013:986734 [DOI] [PMC free article] [PubMed]
- 41.Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of type 2 diabetes mellitus and emerging treatment strategies. Diabet Med 2009;26:1185–1192 [DOI] [PubMed] [Google Scholar]
- 42.Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab 2005;90:501–506 [DOI] [PubMed] [Google Scholar]
- 43.Iwakura T, Fujimoto S, Kagimoto S, et al. . Sustained enhancement of Ca(2+) influx by glibenclamide induces apoptosis in RINm5F cells. Biochem Biophys Res Commun 2000;271:422–428 [DOI] [PubMed] [Google Scholar]
- 44.Reimer MK, Holst JJ, Ahrén B. Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur J Endocrinol 2002;146:717–727 [DOI] [PubMed] [Google Scholar]
- 45.Mu J, Woods J, Zhou YP, et al. . Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 2006;55:1695–1704 [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Couto FM, Minn AH, Shalev A. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun 2006;346:1067–1074 [DOI] [PubMed] [Google Scholar]
- 47.Shimoda M, Kanda Y, Hamamoto S, et al. . The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia 2011;54:1098–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 2014;13:465–476 [DOI] [PubMed] [Google Scholar]
- 49.Roep BO, Tree TI. Immune modulation in humans: implications for type 1 diabetes mellitus. Nat Rev Endocrinol 2014;10:229–242 [DOI] [PubMed] [Google Scholar]
- 50.Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest 1995;96:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Straub RH. Insulin resistance, selfish brain, and selfish immune system: an evolutionarily positively selected program used in chronic inflammatory diseases. Arthritis Res Ther 2014;16(Suppl. 2):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmons AL, Schlezinger JJ, Corkey BE. What are we putting in our food that is making us fat? Food additives, contaminants, and other putative contributors to obesity. Curr Obes Rep 2014;3:273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT. Chronic exposure to excess nutrients left-shifts the concentration dependence of glucose-stimulated insulin secretion in pancreatic beta cells. J Biol Chem 2015;290:16191–16201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halban PA, Polonsky KS, Bowden DW, et al. . β-Cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014;37:1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stähli BE, Gebhard C, Tardif JC. Lipid effects and cardiovascular disease risk associated with glucose-lowering medications. Curr Cardiol Rep 2015;17:608. [DOI] [PubMed] [Google Scholar]
- 56.Chevalier N, Fénichel P. Endocrine disruptors: new players in the pathophysiology of type 2 diabetes? Diabetes Metab 2015;41:107–115 [DOI] [PubMed] [Google Scholar]
- 57.Escobedo G, López-Ortiz E, Torres-Castro I. Gut microbiota as a key player in triggering obesity, systemic inflammation and insulin resistance. Rev Invest Clin 2014;66:450–459 [PubMed] [Google Scholar]
- 58.Biswas SK, Mohtarin S, Mudi SR, et al. . Relationship of soluble RAGE with insulin resistance and beta cell function during development of type 2 diabetes mellitus. J Diabetes Res 2015;2015:150325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raciti GA, Longo M, Parrillo L, et al. . Understanding type 2 diabetes: from genetics to epigenetics. Acta Diabetol 2015;52:821–827 [DOI] [PubMed] [Google Scholar]
- 60.Zhao M, Wang Z, Yung S, Lu Q. Epigenetic dynamics in immunity and autoimmunity. Int J Biochem Cell Biol 2015;67:65–74 [DOI] [PubMed]
- 61.Schlaich M, Straznicky N, Lambert E, Lambert G. Metabolic syndrome: a sympathetic disease? Lancet Diabetes Endocrinol 2015;3:148–157 [DOI] [PubMed] [Google Scholar]
- 62.Coomans CP, van den Berg SA, Lucassen EA, et al. . The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 2013;62:1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandrup-Poulsen T. IAPP boosts islet macrophage IL-1 in type 2 diabetes. Nat Immunol 2010;11:881–883 [DOI] [PubMed] [Google Scholar]
- 64.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003;52:1–8 [DOI] [PubMed] [Google Scholar]
- 65.Gottlieb PA. What defines disease in an age of genetics and biomarkers? Curr Opin Endocrinol Diabetes Obes 2015;22:296–299 [DOI] [PubMed] [Google Scholar]
- 66.Groop L, Storm P, Rosengren A. Can genetics improve precision of therapy in diabetes? Trends Endocrinol Metab 2014;25:440–443 [DOI] [PubMed] [Google Scholar]
- 67.Zhou K, Donnelly L, Yang J, et al.; Wellcome Trust Case Control Consortium 2 . Heritability of variation in glycaemic response to metformin: a genome-wide complex trait analysis. Lancet Diabetes Endocrinol 2014;2:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014;383:1084–1094 [DOI] [PubMed] [Google Scholar]
- 69.Wherrett DK, Chiang JL, Delamater AM, et al.; Type 1 Diabetes TrialNet Study Group . Defining pathways for development of disease-modifying therapies in children with type 1 diabetes: a consensus report. Diabetes Care 2015;38:1975–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armato J, DeFronzo RA, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and β-cell dysfunction. Endocr Pract 2012;18:342–350 [DOI] [PubMed] [Google Scholar]
- 71.Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015;58:429–442 [DOI] [PubMed] [Google Scholar]
- 72.Garber AJ, Abrahamson MJ, Barzilay JI, et al. . AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract 2015;21:438–447 [DOI] [PubMed] [Google Scholar]
- 73.DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013;36(Suppl. 2):S127–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Federal Drug Administration. Code of Federal Regulations Title 21. Sec. 310.517: Labeling for oral hypoglycemic drugs of the sulfonylurea class, 49 FR 14331, 11 April 1984. Available from http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=310.517. Accessed 20 September 2015
- 75.Evans JM, Ogston SA, Emslie-Smith A, Morris AD. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 2006;49:930–936 [DOI] [PubMed] [Google Scholar]
- 76.Margolis DJ, Hoffstad O, Strom BL. Association between serious ischemic cardiac outcomes and medications used to treat diabetes. Pharmacoepidemiol Drug Saf 2008;17:753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Currie CJ, Johnson JA. The safety profile of exogenous insulin in people with type 2 diabetes: justification for concern. Diabetes Obes Metab 2012;14:1–4 [DOI] [PubMed] [Google Scholar]
- 78.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Draznin B. Mechanism of the mitogenic influence of hyperinsulinemia. Diabetol Metab Syndr 2011;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karlstad O, Starup-Linde J, Vestergaard P, et al. . Use of insulin and insulin analogs and risk of cancer - systematic review and meta-analysis of observational studies. Curr Drug Saf 2013;8:333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yarchoan M, Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes 2014;63:2253–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev 2015;95:727–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelly CT, Mansoor J, Dohm GL, Chapman WH 3rd, Pender JR 4th, Pories WJ. Hyperinsulinemic syndrome: the metabolic syndrome is broader than you think. Surgery 2014;156:405–411 [DOI] [PubMed] [Google Scholar]
- 84.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 2014;63:2232–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muntoni S, Muntoni S. Insulin resistance: pathophysiology and rationale for treatment. Ann Nutr Metab 2011;58:25–36 [DOI] [PubMed] [Google Scholar]
- 86.Riddle M, Frias J, Zhang B, et al. . Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care 2007;30:2794–2799 [DOI] [PubMed] [Google Scholar]
- 87.Garber AJ, King AB, Del Prato S, et al.; NN1250-3582 (BEGIN BB T2D) Trial Investigators . Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012;379:1498–1507 [DOI] [PubMed] [Google Scholar]
- 88.Matthews D, Fulcher G, Perkovic V, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium glucose co-transporter 2, added on to insulin therapy with or without oral agents in type 2 diabetes. Poster presented at the 48th Annual Meeting of the European Association for the Study of Diabetes, 1–5 October 2012, Berlin, Germany [Google Scholar]
- 89.Larsen CM, Faulenbach M, Vaag A, et al. . Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]