Abstract
Purpose
Seneca Valley Virus (SVV-001) is a novel naturally occurring replication-competent picornavirus with potent and selective tropism for neuroendocrine cancer cell types, including small cell lung cancer. We conducted a first-in-human, first-in-class phase I clinical trial of this agent in patients with cancers with neuroendocrine features, including small cell lung cancer.
Experimental Design
Clinical evaluation of single intravenous doses in patients with cancers with neuroendocrine features was performed across five log-increments from 107 to 1011 vp/kg. Toxicity, viral titers and clearance, neutralizing antibody development, and tumor response were assessed.
Results
A total of 30 patients were treated with SVV-001, including six with small cell carcinoma at the lowest dose of 107 vp/kg. SVV-001 was well tolerated, with no dose-limiting toxicities observed in any dose cohort. Viral clearance was documented in all subjects and correlated temporally with development of antiviral antibodies. Evidence of in vivo intratumoral viral replication was observed among patients with small cell carcinoma, with peak viral titers estimated to be >103-fold higher than the administered dose. One patient with previously progressive chemorefractory small cell lung cancer remained progression-free for 10 months after SVV-001 administration, and is alive over 3 years after treatment.
Conclusions
Intravenous SVV-001 administration in patients is well tolerated at doses up to 1011 vp/kg, with predictable viral clearance kinetics, intratumoral viral replication, and evidence of antitumor activity in patients with small cell lung cancer. Phase II clinical evaluation in small cell lung cancer is warranted, and has been initiated.
Introduction
Small cell lung cancer is a common, aggressive, and exceptionally lethal malignancy (1). The majority of cases are metastatic at the time of diagnosis, and such cases have a median survival from the time of diagnosis of approximately 9 months (2). There is a critical need for novel and more effective approaches to the treatment of this disease (3).
Seneca Valley Virus (SVV-001) is a replication-competent oncolytic picornavirus, discovered as a contaminant of a laboratory adenovirus preparation (4–7). The replicative potential of SVV-001 has been studied in a large number of human cancer lines. The spectrum of activity is highest in tumor types demonstrating neuroendocrine features, including 13 of 23 SCLC lines tested (4, 8). SVV-001 demonstrates remarkable activity against tumors highly permissive for viral replication. In mice bearing H446 SCLC xenografts, doses ≥108 vp/kg resulted in complete and durable eradication of tumors in 60/60 mice (4).
There have been prior attempts to develop live, replication-competent anticancer viruses, primarily based on retroviral and adenoviral vectors (reviewed in ref. 9). Major problems with oncolytic viral strategies to date have included: difficulty in generating high viral titers; lack of selective tropism for cancer cells; preexisting humoral immunity in a large fraction of the population; potential for reversion of engineered viruses to pathogenic wild type; and potential for inducing additional genomic mutations due to viral DNA integration.
Preclinical analyses of SVV-001 suggest the potential to overcome all of these barriers (4). SVV-001 is systemically bioavailable: it is not inactivated by blood components, does not cause hemagglutination in any blood types, and preexisting antibodies are rare. Unlike other oncolytic viruses, SVV-001 can readily penetrate solid tumors from the vascular system. SVV-001 has a replication cycle of under 12 hours, promoting productive infection within tumors before the development of an immune response. Finally, picornaviruses have no possibility of insertional mutagenesis due to a lack of a DNA intermediate, and do not carry transforming oncogenes.
We sought to evaluate safety, viral kinetics and viral dynamics in a first-in-human first-in-class phase I clinical trial. Because of the observed preferential SVV-001 permissivity observed in tumor cell lines with neuroendocrine differentiation, clinical trial enrollment was limited to patients with advanced solid tumors demonstrating expression of neuroendocrine markers.
Methods
Clinical study design
This was a dose-escalation phase I clinical trial, testing single dose intravenous administration of SVV-001. Five dose cohorts were studied, in log increments ranging from 107 to 1011 vp/kg. SVV-001 was administered on study day 1. No concurrent anticancer treatment was administered. The primary objectives of this study were toxicity assessment and determination of a recommended dose for phase II testing. Secondary objectives included serial evaluation of viral titers in blood, sputum, nasal swabs, urine and stool, and of the development of neutralizing antibody titers at each dose level. Adverse events were graded and assigned a degree of attribution to SVV-001 using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Antitumor response was assessed on serial CT scans according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.
Subject eligibility
Enrollment was limited to adult patients with advanced solid tumors with neuroendocrine features (defined as immunohistochemical expression of CD56, chromogranin A, and/or synaptophysin) for which there were no standard therapies of proven benefit available, and with Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2. Eligible women were either postmenopausal or surgically sterilized and eligible men agreed to use contraception. SVV-001 treatment was initiated at least 4 weeks after any prior anticancer therapy. Exclusion criteria included bone marrow (ANC <1,500/µL; platelets <100,000/µL) or organ dysfunction (bilirubin >1.5 × ULN; AST/ALT >2.5 × ULN; creatinine >1.5 × ULN), continued >grade 1 AE as a result of a prior therapy, presence or history of central nervous system metastasis or concurrent persistent viral infection.
Study subject monitoring
Patients on study were assessed prior to enrollment and for toxicity while on therapy by medical history, physical examination, blood counts, and basic chemistry panel including liver function tests at least weekly. Collection of nasal secretions, sputum, blood, urine, and stool for assessment of viral titers was performed on a daily basis for 5 days starting on the day of SVV-001 administration, thrice weekly in weeks 2 and 3, twice weekly in weeks 4 to 6, and once weekly thereafter until complete clearance of detectable SVV-001 by RT-PCR. CT scans for disease reassessment were performed approximately every 8 weeks. Adverse events were tabulated and categorized both for severity using CTCAE version 3 criteria, and for strength of attribution to the investigational agent. Study investigators and monitors participated in a weekly study safety conference call on which all active study subjects were reviewed.
Quantitation of SVV-001 in patient biospecimens
Patient samples were stored at <−60°C. Samples were thawed and processed (feces—1:10 suspension in PBS and clarified by centrifugation, urine—clarified by centrifugation, sputum—1:1 suspension in PBS and clarified by centrifugation, nasal swabs—vortexed in 1 mL PBS/swab) prior to sterile filtration with a 0.22 µm PVDF membrane syringe filter (Millipore).
RNA was isolated from processed patient samples using the QIAamp Viral RNA Mini Kit (Qiagen) according to manufacturer’s protocol. SVV-001 genomic copy number was determined by quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR) using the Super-Script III Platinum One-Step qRT-PCR kit (Invitrogen). Quadruplicate 15 µL reactions included: upstream primer (5′-CTGTCTCGGTGCACGCTTAC-3′), downstream primer (5′-GATCACATTGTTGAGCACTGTGTTC-3′), probe (5′-6FAM-CGGTTGTGCCGCGAC-MGBNFQ-3; Applied Biosystems) and either RNA standard from purified SVV-001 at 25 to 5 × 109 copies or 5 µL sample RNA. For feces, reactions containing both 5 and 0.5 µL RNA were run due to the frequent presence of PCR inhibitors. The greatest copy number value returned from the 2 conditions was utilized. Reaction conditions were: 50°C for 15 minutes, 95°C for 2 minutes, and 45 cycles of 95°C for 15 seconds and 60°C for 1 minutes.
SVV-001 infectious titer in patient samples was determined using a tissue culture infectious dose-50 (TCID50) titer assay in PER.C6 cells as previously described (10). After incubation for 3 days at 37°C, viral cytopathic effect (CPE) was determined as <50% viability of uninfected cells using the Cell Titer 96 Aqueous One MTS reagent solution (Promega). Because of the growth inhibitory activity of certain sample types on PER.C6 cells, CPE was confirmed by visual inspection.
Quantitation of serum neutralizing antibodies
Serum anti-SVV neutralizing titer was determined by an in vitro infection assay on PER.C6 cells. Serum was serially diluted in 2-fold increments (from 1:4 to 1:106) in growth medium and mixed 1:1 with SVV-001 crude viral lysate (100 TCID50). This mixture was incubated for 1 hour at 37°C and then added to an equal volume of 104 PER.C6 cells. Plates were incubated for 3 days at 37°C. Viral CPE was determined as described earlier. The neutralization titer was defined as the greatest dilution of serum that blocked CPE in >50% of quadruplicate wells.
SVV-001 tissue immunostaining
Tissue immunostaining for the presence of intratumoral viral replication was performed on formalin-fixed paraffin blocked tissues using anti-SVV mouse serum generated by intravenous inoculation of live SVV-001 in CD-1 mice. Antigen retrieval was performed by microwaving sections in sodium citrate buffer prior to primary antibody labeling. Visualization was performed using mouse IgG Vectastain ABC kit (Vector Laboratories).
Results
Patient demographics
A total of 30 patients were treated on study. Two patients in the first dose cohort, both with advanced, chemorefractory and extensively pretreated small cell carcinoma, died of progressive disease within 30 days of SVV-001 administration. Both patients underwent autopsies demonstrating death from progressive metastatic cancer, with no evidence of death related to viral infection. Despite the fact that these 2 early deaths on study were attributable to disease rather than therapy, following an advisory meeting with the Food and Drug Administration, enrollment to subsequent dose escalation cohorts was limited to patients with an expected survival of at least 6 months, for safety and to better insure definitive assessment of therapeutic toxicity. This by definition precluded further enrollment of patients with recurrent SCLC or extrapulmonary small cell carcinoma to study dose escalation cohorts over 107 vp/kg: such patients currently have a median survival of less than 6 months. Thus, only 6 patients with small cell carcinoma were enrolled on study, and all 6 were enrolled in the very lowest dose cohort (107 vp/kg). The majority of the other 24 patients treated had carcinoid tumors (Table 1). Almost all of the patients, both in the small cell and in the other neuroendocrine cohorts, were heavily pretreated (≥3rd line).
Table 1.
Patient demographics
| Patient demographics | Small cell carcinoma* N = 6 |
Mixed neuroendocrine† N = 24 |
|
|---|---|---|---|
| Age | Median (range) | 60 (32–73) | 63 (36–78) |
| Gender | Male:female | 3:3 | 16:8 |
| Ethnicity | White | 6 | 20 |
| Non-white | 0 | 4 | |
| ECOG PS | 0 | 2 (33%) | 10 (42%) |
| 1 | 4 (66%) | 13 (54%) | |
| 2 | 0 | 1 (4%) | |
| No. of prior therapies | Median (range) | 2.5 (2–>4) | 1 (0–>4) |
Five small cell lung cancer, one extrapulmonary small cell.
Six carcinoid, 3 large cell neuroendocrine carcinoma, one islet cell carcinoma, one medullary carcinoma, one olfactory neuroblastoma, 12 unspecified carcinomas with neuroendocrine features.
Safety
SVV-001 was well tolerated with no DLTs observed at any dose. Dose escalation was discontinued at 1011 vp/kg, without defining a maximally tolerated dose, for practical reasons related to viral production and the achievement of dose ranges consistent with significant antitumor efficacy in permissive cancers in preclinical models (4). Observed toxicities are summarized in Table 2. Several patients, notably including those in the lowest dose cohort, experienced flu-like symptoms including low-grade fever, malaise, myalgias, and arthralgias within the first week, lasting 1 to 2 days and peaking on days 3 to 4 after virus administration. These symptoms preceded viral clearance and development of a neutralizing antibody response. After completion of planned dose escalation, a total of 12 patients were treated at the highest dose level to further assess the toxicity profile of SVV-001 administered at 1011 vp/kg. There were no DLTs observed in the expansion cohort (Table 2).
Table 2.
Summary of adverse events*
| Toxicity | Small cell carcinoma | Mixed neuroendocrine | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 107 vp/kg N = 6 |
107–1010 vp/kg N = 12 |
1011 vp/kg N = 12 |
|||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| Pyrexia | 2 (33%) | 1 (17%) | 0 | 4 (33%) | 0 | 0 | 4 (33%) | 1 (8%) | 0 |
| Fatigue | 0 | 0 | 0 | 5 (42%) | 1 (8%) | 0 | 2 (17%) | 1 (8%) | 0 |
| Headache | 0 | 0 | 0 | 2 (17%) | 1 (8%) | 0 | 0 | 1 (8%) | 0 |
| Lymphopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8%) | 1 (8%) |
| Muscle spasms | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8%) | 0 |
| Cough | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8%) | 0 |
All toxicities that reached CTCAE grade ≥2 in at least 1 study subject. For these toxicites, number of grade 1 events is also reported.
Although formally not part of protocol mandated safety assessments, at the request of the Johns Hopkins Biosafety Committee, analysis of blood serum from the study chair and lead research nurse at Johns Hopkins were performed, and showed no detectable neutralizing antibody titers, consistent with absence of viral transmission despite close patient contact.
Viral dynamics and development of neutralizing antibodies
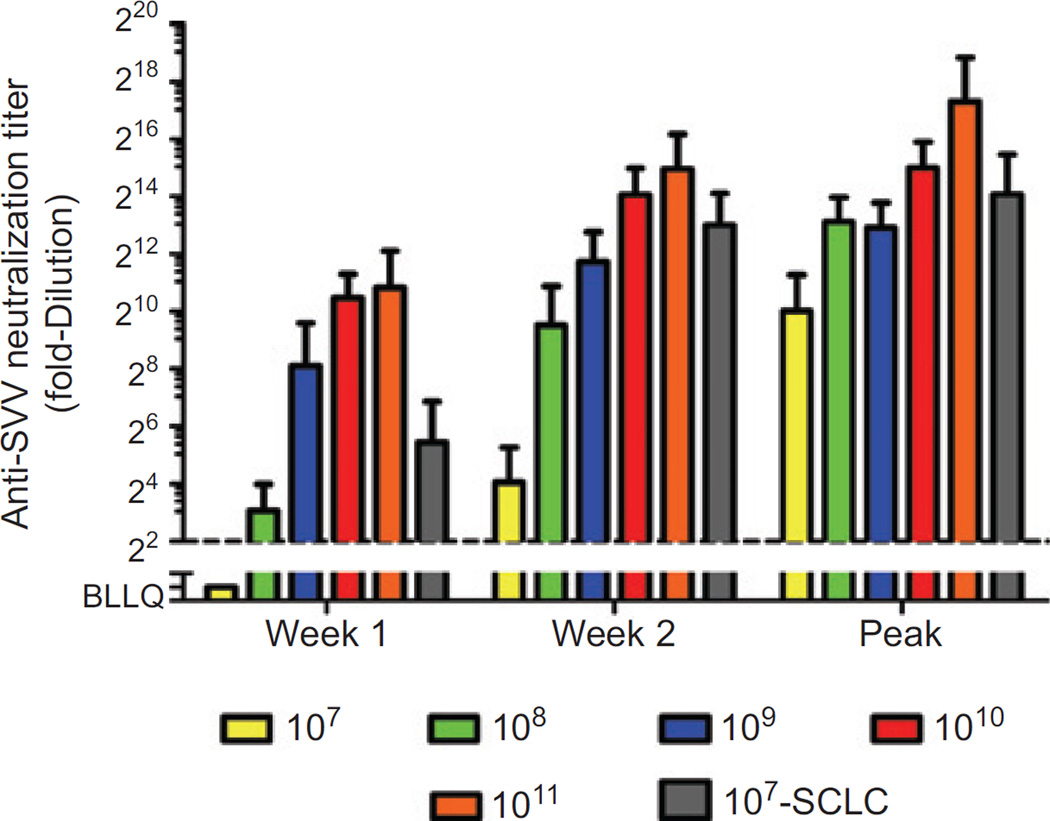
Two study subjects were found to have detectable neutralizing antibodies at baseline prior to SVV-001 administration. All study subjects developed neutralizing antibodies to SVV-001 within the first 2 weeks of viral administration, with the rapidity and titer of the humoral immune response appearing to increase with increasing SVV-001 dose (Fig. 1). Posttreatment neutralizing antibody titers in small cell carcinoma patients were higher than those in non–small cell patients treated at the same dose, consistent with the markedly higher viral titers seen in these patients (described later). Elimination of detectable infectious virus in nasal secretions, sputum, blood, urine, and stool was seen in all dose cohorts, concurrent with or following the neutralizing antibody response.
Figure 1.
Development of anti-SVV-001 neutralizing antibodies. Quantitative assessment of serum SVV-001 neutralizing antibodies was assessed over time in all human subjects treated with SVV-001. Data are reported at week 1 (day 7–9) and week 2 (day 14–16), and as peak neutralizing antibody titer, by dose administered. The cohort of small cell carcinoma patients treated at 107 vp/kg is shown separately. Error bars: standard deviation.
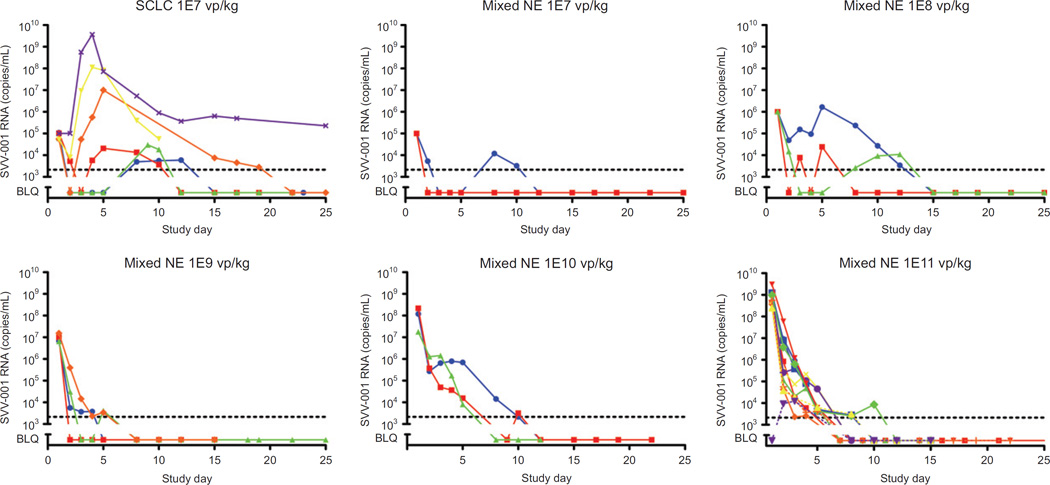
SVV-001 viral titer in most study subjects was highest on the day of SVV-001 administration, with the day 1 peak increasing by cohort, reflecting the log increments in administered dose (Fig. 2). This pattern appears consistent with an absence of, or at best minimal, intratumoral viral replication. However, in the patients with small cell carcinomas treated at the lowest dose level, a quite different pattern emerged, with several patients demonstrating marked increases in viral titer peaking on days 3 to 4, at levels several orders of magnitude higher than the administered dose (Fig. 2, upper left). In these patients, there also appeared to be a delay in the trajectory of viral clearance. This pattern was evident in half of small cell carcinoma patients treated, consistent with observations in small cell carcinoma cell lines suggesting that about half of these lines are permissive for viral replication (4).
Figure 2.
SVV-001 viral titers following IV administration, by cohort. Evaluation of circulating SVV-001 virus in serum was assessed over time by quantitative RT-PCR. Each linear plot represents titers measured in an individual patient. Data are presented by dose administered.
Intratumoral viral replication
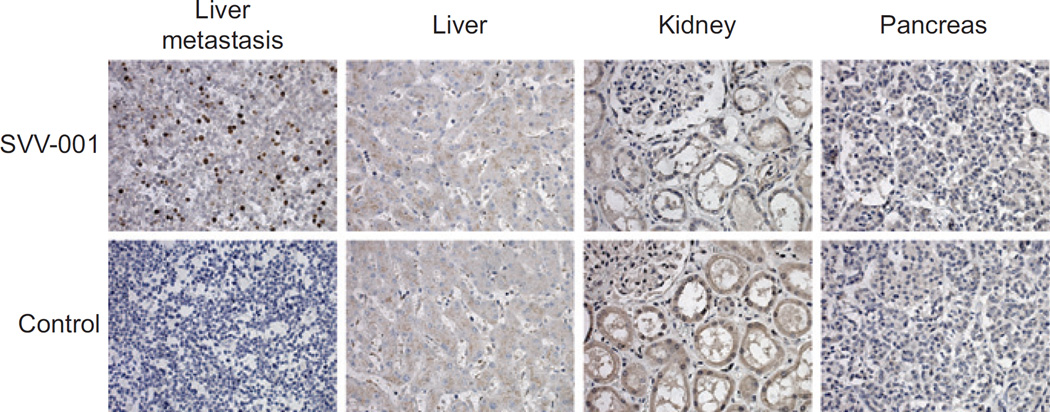
As noted earlier, the observed pattern of peak viral titers in small cell carcinoma patients was suggestive of intratumoral viral replication. Alternatively, it was possible that SVV-001 replication was taking place in another tissue, or without discrete localization. Autopsy material was available from one of the patients with small cell carcinoma who died of progressive disease on day 28 after SVV-001 administration. The peak of SVV-001 titer in this individual was on day 3, and measured over 109 vp/mL of blood. The patient had extensive liver metastases. Immunohistochemical analyses of sections of organs not involved with cancer, including kidney and pancreas, demonstrated no detectable SVV-001 particles. In contrast, immunostaining of the metastatic liver lesions demonstrated intense and specific intracellular staining for viral particles in the tumor cells, but no evidence of virus in adjacent normal liver (Fig. 3). Thus, intravenous administration of SVV-001 was associated with highly specific intratumoral infection and replication of the oncolytic virus, without evident involvement of surrounding normal cells. In addition, despite the low dose administered and the development of systemic neutralizing antibodies, intratumoral SVV-001 replication was persistent in this patient, with ongoing viral particle production up to 4 weeks after dosing.
Figure 3.
Immunohistochemical staining for viral particles in tumor and noninvolved tissues. Tissue sections from a patient with extensively pretreated advanced small cell carcinoma, who died of progressive disease on day 28 after SVV-001 administration (107 vp/kg) were subject to immunohistochemical staining using mouse SVV-001 antiserum as primary antibody or nonimmune mouse serum as control. Extensive intracellular viral replication is demonstrated in tumor tissue, but no nonmalignant tissue sections (400×).
Evidence of clinical activity
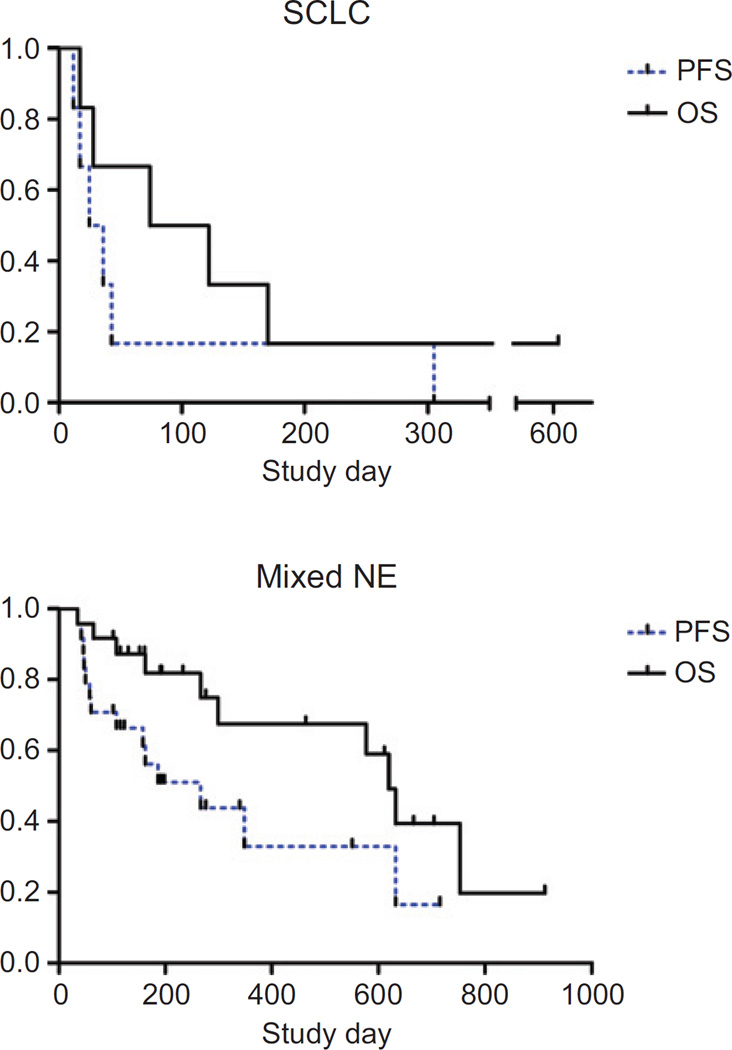
None of the patients treated experienced objective antitumor responses (partial or complete response) by RECIST criteria. However, in the cohort of small cell carcinoma patients treated with 107 vp/kg, one patient with previously rapidly progressive disease after cisplatin/etoposide and irinotecan therapies experienced disease stabilization which persisted for ten months after SVV-001 infusion. This patient remains alive after more than 3 years after SVV-001 treatment. Five patients with other neuroendocrine tumors experienced minor responses, including the patient whose CT scans are shown in the Supplementary Figure. The tumor reduction in this individual with a poorly differentiated neuroendocrine tumor did not meet criteria for RECIST response due to persistence of disease in the abdomen. A carcinoid patient with stable disease, who received serial PET scans prior to and after therapy (not protocol-mandated) was noted to have a >50% decrease in tumor SUV following SVV-001 administration. Progression-free and overall survival curves for the small cell carcinoma and other neuroendocrine tumor groups are shown in Figure 4A and B, respectively.
Figure 4.
Progression-free and overall survival following SVV-001 administration. Kaplan–Meier survival plots are presented, by disease type. All dose cohorts for mixed neuroendocrine are combined. All study subjects are included. PFS, progression-free survival; OS, overall survival.
Discussion
In this first-in-human phase I study of a replication competent oncolytic picornavirus, we demonstrate that intravenous administration of SVV-001 was well tolerated at doses up to 1011 vp/kg, with toxicities primarily limited to a spectrum of transient flu-like symptoms. Even extensively pretreated metastatic cancer patients were able to mount an effective humoral immune response, clearing detectable infectious SVV-001 from bodily fluids within a month of virus administration. Together these observations suggest that clinical administration of intravenous SVV-001 is safe.
Intriguingly, the subset of patients with small cell carcinoma showed definitive evidence of SVV-001 replication, with blood levels of circulating free virus peaking several days after administration at levels in great excess to the number of viral particles administered. This was also associated with markedly higher neutralizing antibody titer than that observed in other patients treated at the same dose. The high level of viral replication specific to the small cell carcinoma group corroborates preclinical observation of the sensitivity of this tumor type to SVV-001 (4). Persistent SVV-001 production was evident in, and specifically limited to, malignant tumor in a patient with metastatic small cell carcinoma. These data serve as important proof-of-principal for this novel anticancer agent, demonstrating tumor cell selectivity of viral infection and a capacity for continued intratumoral viral replication despite systemic immunity.
None of the patients with SCLC on this study were treated with doses over 107 vp/kg. Preclinical experiments in human tumor xenograft models of permissive tumors such as SCLC suggest that doses below 108 vp/kg, even in the context of an immunosuppressed murine host, produce less effective and less durable antitumor responses than doses in the range of 1010 to 1011 vp/kg (4). We have shown in the dose-escalation cohorts of this study that doses 10,000-fold higher than those given to small cell carcinoma patients on this study (i.e. 1011 vp/kg) are safe and well tolerated. Exploration of substantially higher doses in SCLC patients would be of significant interest. To evaluate this, a phase II study of SVV-001 has been initiated in the North Central Cancer Treatment Group, enrolling patients with extensive stage SCLC. This randomized phase II study is evaluating postchemotherapy administration of 1011 vp/kg SVV-001 versus placebo in a total of 90 subjects (45 per arm).
The biological basis of the highly selective permissivity of this oncolytic virus for a subset of human cancer cells is unknown, and would be of major benefit in guiding future clinical development of this agent. Laboratory work to define the essential components of the SVV-001 receptor, of the viral entry pathway, and of viral replication, is ongoing. Results from these investigations may help direct this therapy specifically to the subset of patients most likely to benefit from SVV-001 administration. A key feature of the recently launched phase II study above will be sample collection for correlative analysis of these putative determinants of SVV-001 permissivity.
The mixed response observed in the patient shown in the Supplementary Figure (marked decrease in some tumor masses but not others) suggests that intrapatient tumor heterogeneity should also be explored in future clinical studies of this agent. Explanations for this heterogeneity may include variable expression of components of the viral receptor, or variable viral access to tumor cells in distinct microenvironments. Given the anecdotal observation of decreased tumor metabolic activity based on serial PET scanning in a patient with carcinoid despite stable disease by size criteria, functional imaging should also be considered in future studies of this cytolytic virus.
All of the patients treated on this phase I study developed neutralizing antibodies to SVV-001. This could ultimately prove to be a double-edged sword in the development of this agent; a reassuring finding with regard to safety, but one which could limit the capacity for repetitive therapeutic administration. One approach to repetitive dosing would be administration together with immunosuppressive cytotoxic agent: cyclophosphamide, a drug used clinically in the treatment of SCLC, is an effective suppressor of humoral immunity (11, 12). Alternatively, SVV-001 could be administered together with a specific inhibitor of B lymphocytes, such as the anti-CD20 antibody rituximab. These concepts warrant additional testing in relevant immunocompetent preclinical models.
SVV-001 is the first oncolytic picornavirus to be tested in humans. This agent has several theoretical advantages over other replication-competent oncolytic viruses. We have found that SVV-001 administration has minimal toxicity, can home to tumor following intravenous administration, demonstrates specific and persistent intratumoral replication, and is associated with preliminary evidence of anticancer activity in heavily pretreated patients. Continued studies to understand the basis of SVV-001 tumor specificity, and to optimize its clinical utility, offer promise to patients with advanced SCLC and other permissive tumor types.
Supplementary Material
Translational Relevance.
This paper describes the results of a first-in-human first-in-class phase I study of a novel oncolytic picornavirus with selective tropism for cancers with neuroendocrine differentiation. This viral therapy overcomes many of the barriers that have limited prior attempts to develop oncolytic viruses, notably including adenoviral and retroviral vectors. Key pharmacodynamic correlates include demonstration that the picornavirus can replicate in vivo following intravenous administration, that viral replication is specifically limited to tumor, and that active replication persists up to 28 days after viral administration. Antitumor activity was observed in patients with small cell lung cancer. This work has prompted subsequent phase II studies of this agent, both in small cell lung cancer patients, and in pediatric neuroendocrine cancer patients.
Acknowledgments
Disclosure of Potential Conflicts of Interest
KDB, PSR, and PLH were or are employees of Neotropix, Inc.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Murray N, Turrisi AT., III A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 3.Rudin CM, Hann CL, Peacock CD, Watkins DN. Novel systemic therapies for small cell lung cancer. J Natl Compr Canc Netw. 2008;6:315–322. doi: 10.6004/jnccn.2008.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy PS, Burroughs KD, Hales LM, Ganesh S, Jones BH, Idamakanti N, et al. Seneca Valley Virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst. 2007;99:1623–1633. doi: 10.1093/jnci/djm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hales LM, Knowles NJ, Reddy PS, Xu L, Hay C, Hallenbeck PL. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. J Gen Virol. 2008;89:1265–1275. doi: 10.1099/vir.0.83570-0. [DOI] [PubMed] [Google Scholar]
- 6.Venkataraman S, Reddy SP, Loo J, Idamakanti N, Hallenbeck PL, Reddy VS. Structure of Seneca Valley Virus-001: an oncolytic picornavirus representing a new genus. Structure. 2008;16:1555–1561. doi: 10.1016/j.str.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkataraman S, Reddy SP, Loo J, Idamakanti N, Hallenbeck PL, Reddy VS. Crystallization and preliminary X-ray diffraction studies of Seneca Valley virus-001, a new member of the Picornaviridae family. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:293–296. doi: 10.1107/S1744309108006921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadhwa L, Hurwitz MY, Chevez-Barrios P, Hurwitz RL. Treatment of invasive retinoblastoma in a murine model using an oncolytic picornavirus. Cancer Res. 2007;67:10653–10656. doi: 10.1158/0008-5472.CAN-07-2352. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol Ther. 2009;18:243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Reilly D, Miller L, Luckow V. Titering Virus Stocks, Baculovirus Expression Vectors: A Laboratory Manual. Oxford: Oxford University Press; 1994. [Google Scholar]
- 11.Brodsky RA, Jones RJ. Intensive immunosuppression with high dose cyclophosphamide but without stem cell rescue for severe autoimmunity: advantages and disadvantages. Autoimmunity. 2008;41:596–600. doi: 10.1080/08916930802197206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perini P, Calabrese M, Rinaldi L, Gallo P. Cyclophosphamide-based combination therapies for autoimmunity. Neurol Sci. 2008;29(Suppl 2):S233–S234. doi: 10.1007/s10072-008-0947-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.