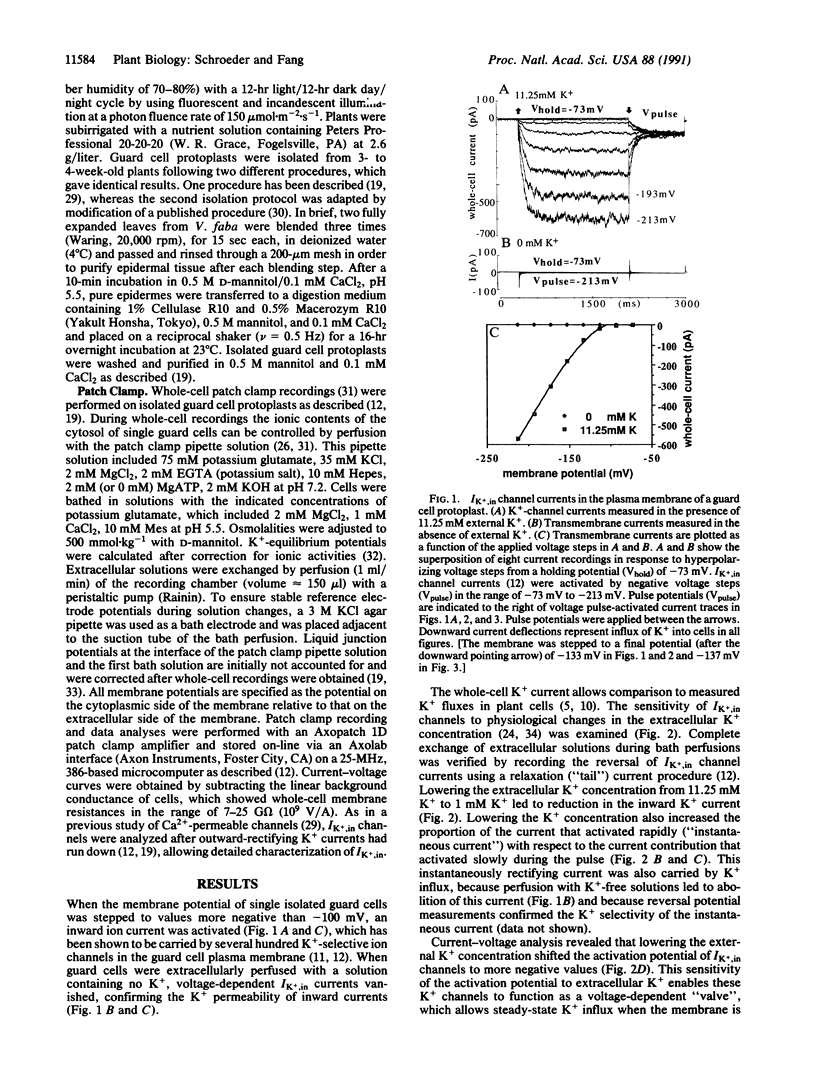
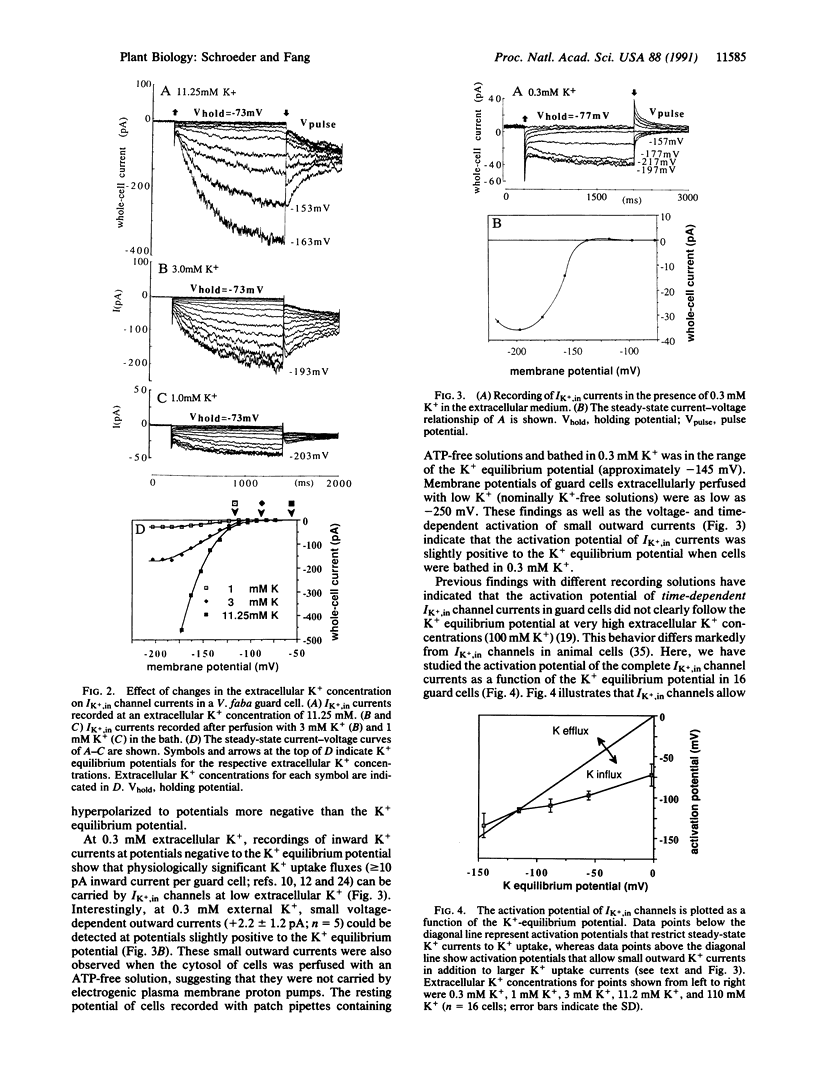
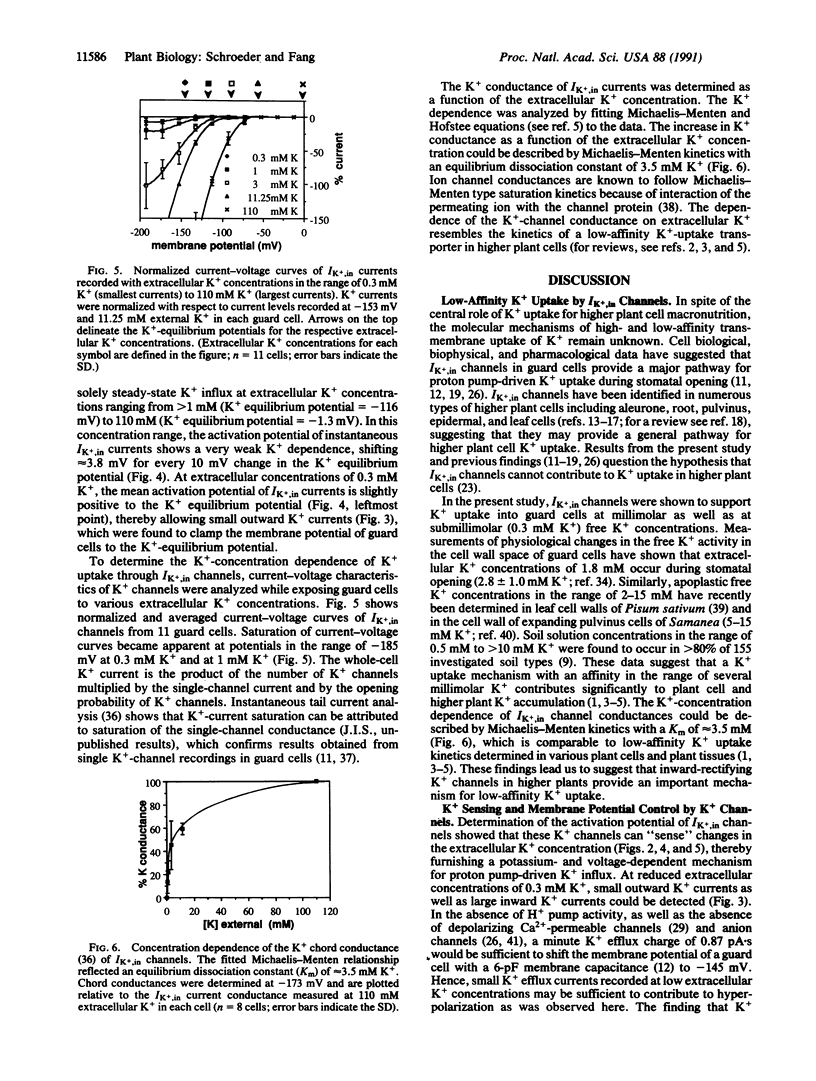
Abstract
The molecular mechanisms by which higher plant cells take up K+ across the plasma membrane (plasmalemma) remain unknown. Physiological transport studies in a large number of higher plant cell types, including guard cells, have suggested that at least two distinct types of K(+)-uptake mechanisms exist, permitting low-affinity and high-affinity K+ accumulation, respectively. Recent patch clamp studies have revealed the presence of inward-conducting (inward-rectifying) K+ channels in the plasma membrane of higher plant cells. Research on guard cells has suggested that these K+ channels provide a major pathway for proton pump-driven K+ uptake during stomatal opening. In the present study the contribution of inward-rectifying K+ channels to higher plant cell K+ uptake was investigated by examining kinetic properties of guard cell K+ channels in Vicia faba in response to changes in the extracellular K+ concentration. Increasing the extracellular K+ concentration in the range from 0.3 mM to 11.25 mM led to enhancement of inward K+ currents and changes in current-voltage characteristics of K+ channels. The increase in K+ conductance as a function of the extracellular K+ concentration revealed a K(+)-equilibrium dissociation constant (Km) of approximately 3.5 mM, which suggests that inward-rectifying K+ channels can function as a molecular mechanism for low-affinity K+ uptake. Lowering the extracellular K+ concentration in the range from 11 mM to 1 mM induced negative shifts in the activation potential of K+ channels, such that these channels function as a K+ sensor, permitting only K+ uptake. At low extracellular K+ concentrations of 0.3 mM K+, inward-rectifying K+ channels induce hyperpolarization. Results from the present study suggest that inward-rectifying K+ channels constitute an essential molecular mechanism for plant nutrition and growth control by providing a K(+)-sensing and voltage-dependent pathway for low-affinity K+ uptake into higher plant cells and additionally by contributing to plasma membrane potential regulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheeseman J. M., Lafayette P. R., Gronewald J. W., Hanson J. B. Effect of ATPase inhibitors on cell potential and k influx in corn roots. Plant Physiol. 1980 Jun;65(6):1139–1145. doi: 10.1104/pp.65.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Rains D. W. CARRIER-MEDIATED CATION TRANSPORT IN BARLEY ROOTS: KINETIC EVIDENCE FOR A SPECTRUM OF ACTIVE SITES. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1320–1324. doi: 10.1073/pnas.53.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Rains D. W., Elzam O. E. RESOLUTION OF DUAL MECHANISMS OF POTASSIUM ABSORPTION BY BARLEY ROOTS. Proc Natl Acad Sci U S A. 1963 May;49(5):684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradmann D., Klieber H. G., Hansen U. P. Reaction kinetic parameters for ion transport from steady-state current-voltage curves. Biophys J. 1987 Apr;51(4):569–585. doi: 10.1016/S0006-3495(87)83382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ketchum K. A., Shrier A., Poole R. J. Characterization of potassium-dependent currents in protoplasts of corn suspension cells. Plant Physiol. 1989 Apr;89(4):1184–1192. doi: 10.1104/pp.89.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Shaff J. E., Lucas W. J. High affinity k uptake in maize roots: a lack of coupling with h efflux. Plant Physiol. 1989 Nov;91(3):1202–1211. doi: 10.1104/pp.91.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Xin-Zhi J., Lucas W. J. Potassium Transport in Corn Roots : IV. Characterization of the Linear Component. Plant Physiol. 1985 Nov;79(3):771–776. doi: 10.1104/pp.79.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse T., Tallman G., Zeiger E. Isolation of Guard Cell Protoplasts from Mechanically Prepared Epidermis of Vicia faba Leaves. Plant Physiol. 1989 Aug;90(4):1382–1386. doi: 10.1104/pp.90.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. M., Widders I. E. Quantification of Apoplastic Potassium Content by Elution Analysis of Leaf Lamina Tissue from Pea (Pisum sativum L. cv Argenteum). Plant Physiol. 1990 Nov;94(3):1040–1047. doi: 10.1104/pp.94.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro A., Blatt M. R., Slayman C. L. A potassium-proton symport in Neurospora crassa. J Gen Physiol. 1986 May;87(5):649–674. doi: 10.1085/jgp.87.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I. K+ transport properties of K+ channels in the plasma membrane of Vicia faba guard cells. J Gen Physiol. 1988 Nov;92(5):667–683. doi: 10.1085/jgp.92.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]


