Abstract
BACKGROUND
Identification of antibodies against high-prevalence Scianna (Sc; ERMAP) antigens, like Sc1 and Sc5, is difficult and may incur delays in blood procurement and costs. The detection of additional clinically significant alloantibodies is hampered in the presence of anti-Scianna. Soluble recombinant Scianna protein is demonstrated to facilitate antibody diagnostics in both cases.
STUDY DESIGN AND METHODS
Soluble recombinant Scianna protein (Sc:1,-2,3,-4,5,6,7) was produced comprising the antigenic extracellular domain fused to a V5-His tag. The protein was isolated from eukaryotic cell culture supernatants of stably transfected HEK293 cells. Seven serum samples with anti-Sc1, anti-Sc2, and anti-Sc5 and 30 serum samples with antibodies to other blood group antigens were evaluated in hemagglutination inhibition assays. Antisera with mixed antibody specificities and autoantibodies were also tested.
RESULTS
Soluble Scianna protein inhibited specifically antibodies to the high-prevalence Scianna antigens Sc1 and Sc5. No antibodies were neutralized that were directed to the low-prevalence Sc2 antigen or to a large representative set of antigens from other blood group systems. Clinically relevant antibodies could be identified despite being masked by anti-Sc1 and anti-Sc5. A mixture of Scianna and JMH proteins allowed detecting a common antibody despite the presence of antibodies to high-prevalence antigens of the Scianna or JMH blood group systems.
CONCLUSION
Antibody detection systems comprising soluble recombinant Scianna protein provide an easy single-step method for detection and identification of antibodies to high-prevalence Scianna antigens. Reagents with Scianna and other recombinant blood group proteins and mixtures of such proteins would be useful routine reagents in immunohematology.
Many antibodies of little or no clinical significance are directed against red blood cell (RBC) antigens of high prevalence. These antibodies infrequently cause minor hemolytic transfusion reaction or hemolytic disease of the fetus and newborn, if any at all. They still may need proper identification before transfusion to differentiate them from clinically significant antibodies against a high-prevalence antigen. They can also mask antibodies against common blood group antigens of major clinical significance. The specific identification is often difficult, labor-intensive, and time-consuming, because it may require a large panel of rare RBC specimens lacking the corresponding high-prevalence antigens.
Antibody detection systems for rapid identification of clinically insignificant antibodies to high-prevalence antigens could ease the serologic work and facilitate the blood supply to patients with such antibodies. A method for selective removal of antibodies to distinct high-prevalence antigens would save much time, effort, and costs. Some of these antibodies may not need to be identified specifically, if their clinical insignificance is assured. Various body fluids, like plasma, urine, or saliva containing soluble antigenic substances, are used to eliminate the reactivity, which enables detection and identification of admixed clinically significant antibodies and provide serum that is suitable for cross-matching.1–3 For example, inhibition tests for anti-Cha and anti-Rga are well established.4
Soluble recombinant blood group proteins have been introduced since 19965 for single-step antibody detection systems and antibody inhibition.5–11 For example, recombinant JMH, Kna, or Lub proteins enabled to identify alloantibodies to high-prevalence antigens.5–7,10
Antibodies against the high-prevalence antigens in the Scianna blood group system,12–16 like Sc1 and Sc5, are among those specificities with limited clinical significance, but may cause infrequently hemolytic disease of the fetus and newborn.17 Resolving patient samples with Scianna antibodies often requires involving specialized reference laboratories. Here we produced eukaryotic soluble recombinant Scianna protein and assessed its suitability as antigen in the clinical diagnosis for difficult to-identify Scianna antibodies.
MATERIALS AND METHODS
Scianna expression constructs
We applied a cloning strategy to generate expression constructs encoding for a C-terminally truncated Scianna protein carrying the amino acid sequence coding for the high-prevalence Scianna antigens Sc:1,-2,3,-4,5,6,7. Total RNA was isolated from the human erythroleukemia cell line K562 (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) with a RNA blood mini kit (QIAamp, Qiagen, Hilden, Germany) and reverse transcribed into cDNA (Protoskript, New England Biolabs, Frankfurt/Main, Germany). To generate a eukaryotic expression construct encoding for a soluble Scianna fusion protein, SC cDNA from Nucleotide 1 to 471 encoding the signal peptide and the complete extracellular domain of the Scianna protein was amplified with the primers Sc01s (5′-caccATGGAGATGGCGAGTTCTGC-3′, Nucleotides 1 to 20 in Scianna blood group cDNA, GenBank Accession Number BC099707) and Sc07as (5′-AGCCACTGCTGAGGGGGAG-3′, Nucleotides 471–453) and cloned into the mammalian expression vector pcDNA3.1/V5-His (Invitrogen, Karlsruhe, Germany).18–21 The resulting plasmid encoded for a V5-His-tagged 14-kDa Sc:1,-2,3–4,5,6,7 protein composed of the extracellular domain of the Scianna glycoprotein. All expression constructs were subcloned in Escherichia coli and validated by nucleotide sequence analysis.
Expression of recombinant Scianna protein
Soluble eukaryotic His-tagged fusion protein was expressed in human embryonic kidney HEK293 cells, purified, and analyzed as previously described.7,22 The proteins were bound overnight via their His-tags to nickel-nitrilotriacetic acid–agarose (Sigma-Aldrich Chemie, Steinheim, Germany), loaded in a PD10 column (GE Healthcare, Uppsala, Sweden), washed several times, and eluted with a buffer supplemented with 250 mmol/L imidazole. Purity of the eluted fractions containing soluble protein was assessed by immunoblot and sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis with horseradish peroxidase–labeled (HRP) anti-His antibodies. The protein was further purified and concentrated by tangential flow filtration with membranes (30-kDa molecular mass cutoff; Sartorius, Göttingen, Germany). Quantitative analysis was done with the bicinchoninic acid protein assay kit (Perbio Science, Bonn, Germany) and a sandwich enzyme-linked immunosorbent assay (ELISA) using anti-V5 and HRP–anti-His as capture and detection antibodies and defined amounts of V5-Histagged HLA Class I protein as reference. The final concentration of the soluble recombinant Scianna protein was adjusted to 1 mg/mL before testing.
Hemagglutination inhibition tests
Soluble recombinant Scianna protein (5 µg in 5µL of phosphate buffered saline [PBS]) was incubated for 30 minutes at room temperature with 25 µL antiserum. Then, 50 µL of a 0.8% RBC suspension in Diluent 2 (DiaMed, Cressier sur Morat, Switzerland) or in ScanLiss (Bio-Rad, Marnes-la-Coquette, France) was added as an indicator and incubated for 15 minutes at 37°C and tested for hemagglutination in gel matrix tests (low-ionic-strength saline with indirect antiglobulin test [IAT] at 37°C; ID micro-typing system, DiaMed; or ScanGel, anti-globulin test anti-IgG, -C3d, Bio-Rad). A dilution effect was excluded by testing the antisera with 5 µL of PBS or with 5 µL of Immusol (Immusol Compact, diluted for use, Medion Diagnostics, Düdingen, Switzerland) instead of 5 µL of protein solution.
Agglutination strengths were noted ranging from 0.5+ indicating very weak reactivity to 4+ indicating very strong reactivity. Agglutination strength 0 indicated a negative IAT and reflected complete inhibition or lack of reactivity.
Antiserum
Three anti-Sc1 and three anti-Sc2 samples were provided by members of the Serum, Cells and Rare Fluid (SCARF) exchange program. The anti-Sc5 sample came from the original patient negative for the high-prevalence antigen STAR (provided by R. Thomas).15 Thirty serum and plasma samples containing alloantibodies to common antigens—including two anti-D, one anti-C, two anti-c, one anti-E, one anti-e, two anti-K, one anti-k, one anti-M, one anti-N, two anti-S, two anti-s, two anti-Jka, two anti-Jkb, two anti-Fya, two anti-Fyb, one anti-Kna, one anti-Lub, one anti-Vel, one anti-Jra, one anti-Cha, and one anti-JMH—and 10 serum samples from patients with RBC autoantibodies were used as reference samples.
Spiked antiserum
Antisera containing alloantibodies to common antigens were admixed to the antisera with anti-Sc1 and anti-Sc5 antibodies (both provided by R. Thomas). Because 25 µL of antiserum is used in gel matrix tests, the antibody mixtures were prepared by combining equal volumes of 12.5 µL of serum with antibodies to Scianna antigens and to antigens of other blood group systems, respectively. Some antisera were diluted with Immusol before mixing to achieve approximately equal titers for both antibodies in the mixture. AB plasma without alloantibodies was used as a control.
Protein cocktail
The Scianna protein solution was mixed in equal volumes with the solution of the eukaryotic soluble recombinant JHM protein that we have reported in a previous study.10 The concentration of each protein in the cocktail was 0.5 µg/µL. For hemagglutination inhibition assays, 10 µL of this “high-yield cocktail” was used. Samples with anti-Sc1 or anti-JMH (12.5 µL) were mixed in equal volumes with a patient sample containing anti-Fya or with a donor AB plasma without alloantibodies as control (12.5 µL) for use with 50 µL of a 0.8% RBC and tested for hemagglutination in a gel matrix test (ScanGel, antiglobulin test anti-IgG, -C3d).
RESULTS
A soluble eukaryotic recombinant Scianna protein was produced by expression in human embryonic kidney cells. The protein carried all five defined high-prevalence Scianna antigens but lacked the two low-prevalence antigens Sc2 and Sc4. The corresponding RBC phenotype would be denoted as Sc:1,-2,3,-4,5,6,7. We evaluated the reactivity of this soluble Scianna protein in routine serologic tests.
Specificity
The soluble Scianna protein inhibited specifically antibodies to the high-prevalence antigens Sc1 and Sc5, but did not affect antibodies to the low-prevalence antigen Sc2 (Table 1). The reactivity of 30 samples with alloantibodies to common antigens other than Scianna and 10 samples with autoantibodies was also not inhibited. In particular, antibodies to high-prevalence antigens, like anti-Lub, anti-Cha, anti-Kna, anti-JMH, anti-Vel, and anti-Jra, were not affected (Table 1). We observed no loss of activity of the Scianna protein, when stored as a 1 µg/µL solution in PBS at +4°C for 3 months (data not shown).
TABLE 1.
Inhibition of antibodies to high-prevalence Scianna antigens and lack of inhibition of antibodies to various common blood group antigens by soluble recombinant Scianna protein
| Reactivity in gel matrix test* |
||
|---|---|---|
| Samples† and antibody specificities |
With Scianna protein |
Without Scianna protein |
| Anti-Sc1 | ||
| Sample 1 | 0 | 2+ |
| Sample 2 | 0 | 1.5+ |
| Sample 3 | 0 | 2+ |
| Anti-Sc2 | ||
| Sample 1 | 3.5+ | 3.5+ |
| Sample 2 | 2+ | 2+ |
| Sample 3 | 2.5+ | 2.5+ |
| Anti-Sc5 | 0 | 2+ |
| Anti-D | 3+ | 3+ |
| Anti-C | 3+ | 3+ |
| Anti-c | 3+ | 3+ |
| Anti-E | 3+ | 3+ |
| Anti-e | 3+ | 3+ |
| Anti-K | 3+ | 3+ |
| Anti-k | 2+ | 2+ |
| Anti-M | 3+ | 3+ |
| Anti-N | 2+ | 2+ |
| Anti-S | 3+ | 3+ |
| Anti-s | 2.5+ | 2.5+ |
| Anti-Jka | 1.5+ | 1.5+ |
| Anti-Jkb | 1.5+ | 1.5+ |
| Anti-Fya | 2+ | 2+ |
| Anti-Fyb | 2+ | 2+ |
| Anti-Kna | 1.5+ | 1.5+ |
| Anti-Lub | 2+ | 2+ |
| Anti-Vel | 2.5+ | 2.5+ |
| Anti-Jra | 1.5+ | 1.5+ |
| Anti-Cha | 1+ | 1+ |
| Anti-JMH | 1.5+ | 1.5+ |
Antiglobulin technique.
All samples tested are shown for anti-Scianna specificities. Only representative results are shown for the antibody specificities other than anti-Scianna; for a full list of the antisera tested see Materials and Methods section.
Detection of admixed alloantibodies to common antigens
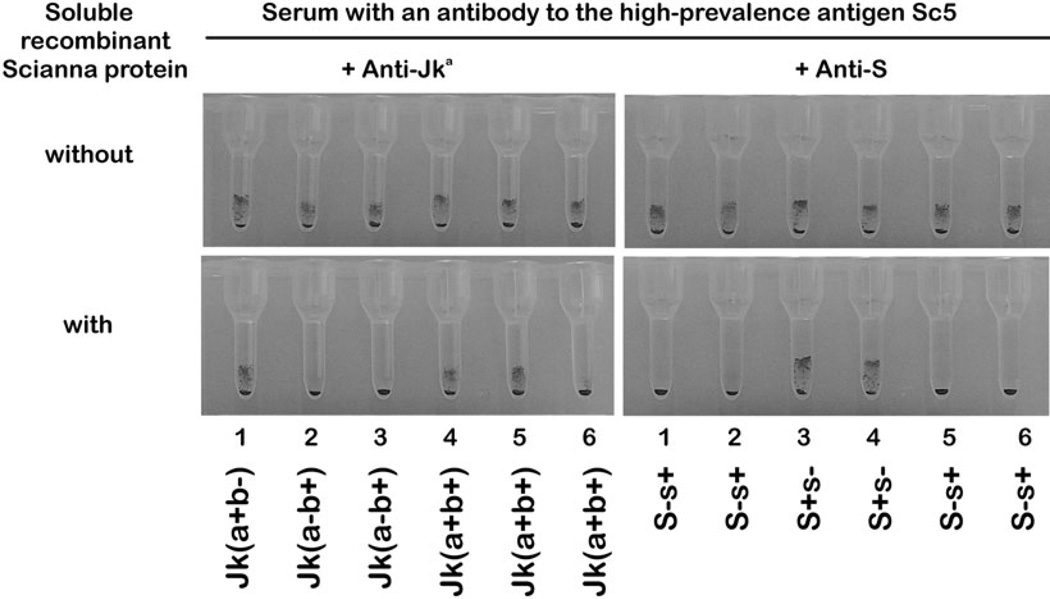
Inhibition of an anti-Sc1 by soluble recombinant Scianna protein allowed the detection of admixed clinically relevant antibodies (Table 2). The detection and identification of the common alloantibody specificities was possible without need for a panel of very rare Sc:-1 RBC samples. Likewise, the identification of admixed anti-Jka and anti-S was possible in the presence of an antibody to the high-prevalence antigen Sc5 (Fig. 1). Comparable results allowed the detection of anti-E, anti-K, anti-Fya, anti-M, anti-Cha, and anti-JHM after inhibition of the anti-Sc5 by soluble Scianna protein (data not shown).
TABLE 2.
Inhibition of anti-Sc1 by soluble recombinant Scianna protein in the presence of antibodies to various common blood group antigens
| Antigens on red blood test cells | Reactivity in gel matrix test with antiglobulin in presence of anti-Sc1 antiserum plus admixed specificities | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhesus | Duffy | Kidd | Chido | With Scianna protein | Without Scianna protein | ||||||||||||||
| D | E | e | Fya | Fyb | Jka | Jkb | Cha | None* | Anti-D | Anti-E | Anti-Fya | Anti-Jka | Anti-Cha | None* | Anti-D | Anti-E | Anti-Fya | Anti-Jka | Anti-Cha |
| + | + | − | + | − | − | + | − | 0 | 2+ | 3+ | 1+ | 0 | 0 | 1+ | 2+ | 3+ | 1+ | 0.5+ | 1+ |
| + | − | + | − | + | − | + | + | 0 | 2+ | 0 | 0 | 0 | 1+ | 1+ | 2+ | 1+ | 1+ | 1.5+ | 1.5+ |
| − | − | + | − | + | + | + | + | 0 | 0 | 0 | 0 | 0.5+ | 1.5+ | 1.5+ | 2+ | 1.5+ | 1+ | 1.5+ | 1.5+ |
AB plasma without anti-RBC alloantibodies.
Fig. 1.
Specific detection of admixed alloantibodies in the presence of anti-Sc5. All RBC samples are agglutinated by mixtures of anti-Sc5 with anti-Jka and with anti-S (top panels). Hence, the admixed antibodies with common specificities are masked. Adding soluble recombinant Scianna protein inhibits the anti-Sc5 component of the serum. The clinically relevant alloantibodies to Jka and to S can be identified easily (bottom panels).
Protein mixture
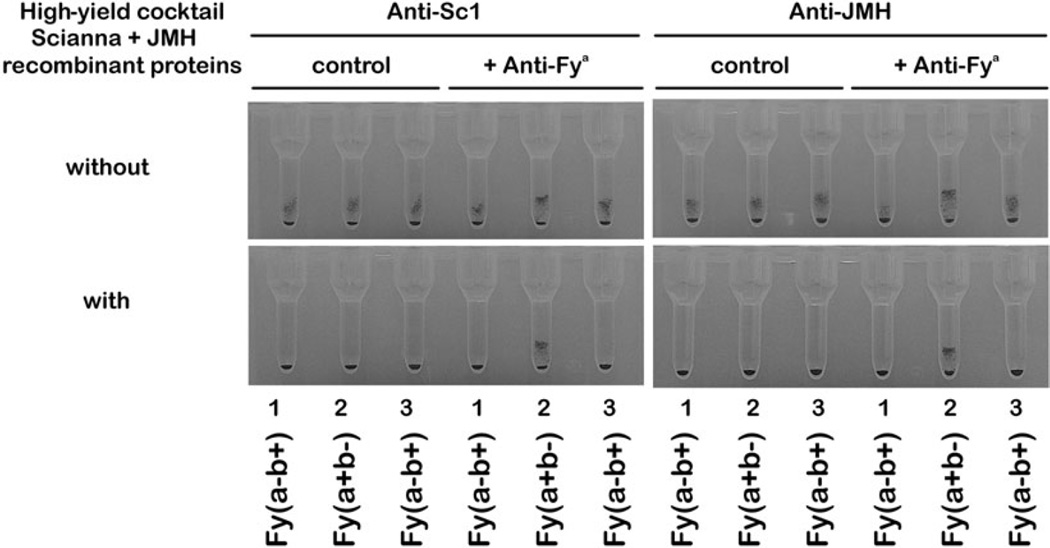
The recently described soluble recombinant JMH protein10 was mixed with the Scianna protein. This protein cocktail inhibited anti-Sc1 and anti-JMH and allowed detecting an admixed anti-Fya (Fig. 2).
Fig. 2.
Mixture of Scianna and JMH proteins. The three RBC samples for antibody screening are agglutinated by anti-Sc1 and anti-JMH (top panels), which can mask an anti-Fya. Adding a mixture of soluble recombinant Scianna and JMH proteins reveals the clinically relevant anti-Fya (bottom panels). It is detected in a single step with the use of one high-yield cocktail without prior knowledge of the involved antibodies to high-prevalence antigens in the Scianna and JMH blood group systems.
DISCUSSION
The presence of anti-Scianna was identified in a single-step procedure, which has the potential to greatly simplify antibody diagnostics in case of antibodies to high-prevalence antigens of unknown specificity. By inhibiting anti-Scianna in sera containing several antibody specificities, the soluble recombinant Scianna protein allowed us to recognize easily clinically relevant alloantibodies that had been masked by anti-Scianna before. We demonstrated the application of a protein cocktail to facilitate the inhibition of several high-prevalence antibodies from different blood groups with a single serologic reagent.
Antibodies against antigens of the Scianna blood group system are difficult to detect specifically, because RBC of routine serologic panels are not typed for Scianna antigens. No commercial Scianna antisera are available to determine Scianna antigens in patients or donors. Alloantibodies other than anti-Scianna may be masked and hence missed in routine serology (Table 2 and Fig. 1). For these reasons the presence of Scianna antibodies still causes problems in the transfusion management of immunized patients, although these antibodies as such are of limited clinical significance. Patients carrying the antibodies can usually be treated easily, once anti-Scianna and lack of admixed clinically relevant alloantibodies are assured. Diagnostic difficulties will often occur, if an alloantibody to the high-prevalence antigen Sc1 is present; antibodies to the other high-prevalence antigens Sc3, Sc5, Sc6, and Sc7 have been reported in isolated cases only,12–16 but may have been missed more often than anticipated, because suitable serologic routine procedures were hitherto lacking.
The complete inhibition of anti-Sc1 and anti-Sc5 and the lack of inhibition of anti-Sc2 by the Scianna protein (Table 1) corroborated that the corresponding epitopes are expressed on the extracellular domain of the Scianna (ERMAP) protein and documented that the proper folding of the extracellular protein segment and its antigen expression is independent of the presence of a membrane or of other membrane proteins. The fact that antibody inhibition by the Scianna protein was highly specific suggested that the protein was expressed in its native structural form. Eukaryotic protein expression systems have the advantage of producing proteins with posttranslational modifications that may be required for proper protein folding and, thus, for conformation-dependent discontinuous epitopes. The Scianna protein is similar to the Lutheran protein in having a relatively simple structure and a low degree of glycosylation. Thus, the extracellular domain of the Scianna protein would be a promising candidate for the more economical prokaryotic expression systems, which we have applied to the Lub protein before.7
Blood group antigens with a single extracellular domain, like Scianna, may be good candidates for expression as soluble recombinant molecules. They comprise the 10 single-pass proteins Lu, Xg, LW, In, Ok, Kell, Knops, MNS, and Ge including Scianna and also the four glycosylphosphatidylinositol-linked proteins JMH, Dombrock, Yt, and Cromer.23 Hence, panels of these recombinant blood group proteins could be produced and become useful serologic routine reagents, because many clinically significant as well as insignificant high-prevalence blood group antigens are located on single-pass and glycosylphosphatidylinositol-linked proteins. Mixtures of two or more recombinant proteins, which we would like to dub high-yield cocktails (Fig. 2), may be customized to inhibit several clinically insignificant antibodies against high-prevalence antigens at once and essentially allow eliminating such specificities from routine serology. Patients with a clinically significant alloantibody that is admixed and directed to a regular or to a high-prevalence antigen may be readily recognized and subjected to detailed antibody identification, because they need compatible, sometimes very rare RBC units, which still represents a clinical problem.24,25 The identification or exclusion of antibodies to antigens of a certain blood group system can also be accomplished by panels of suitably composed high-yield cocktails comprising several different sets of blood group proteins.
We envisage soluble recombinant blood group proteins as a widely applicable tool in blood group serology. Besides soluble protein reagents, the proteins can be immobilized in solid-phase assays such as ELISA, color-coded microspheres, and protein microarray chip–based techniques.10,11 Recombinant protein–based assays are already routine for anti-HLA and could be adapted for blood group proteins.26 Because hemagglutination remains the standard in blood group serology, inhibition assays with soluble recombinant proteins may facilitate an immediate implementation, particularly for routine laboratories.
Antibody detection systems comprising soluble recombinant blood group proteins allow for the identification of corresponding antibodies and also the detection of admixed antibodies. This single-step method can be very rapid and cost-efficient compared to currently available serologic methods. Reagents with soluble Scianna protein or with mixtures of soluble blood group proteins have the potential to become a routine reagent for the serologic laboratory. Such reagents would enable the serologists to identify antibodies directed against a host of distinct high-prevalence blood group antigens.
Acknowledgments
We thank Rebecca Thomas (Memorial Blood Centers, Minneapolis, MN) for anti-Sc1, anti-Sc2, and plentiful anti-Sc5 antisera;15 Cornelie B. Lonicer (Blutspendedienst des Bayerischen Roten Kreuzes, München, Germany) for two anti-Sc2 antisera; Ronda Perguson (Gulf Coast Regional Blood Center, Houston, TX) and Michel Bizot (Etablissement de Transfusion Sanguine de Languedoc-Roussillon, Montpellier, France) for one anti-Sc1 antiserum each; and Joann M. Moulds (Lifeshare Blood Centers, Shreveport, LA) for rare serum and RBC samples and for facilitating their exchange by the SCARF program. We acknowledge the technical assistance of Gabriele Braun and Nadine Trost in Ulm.
Footnotes
AS designed the study, developed the protein expression system, performed and interpreted serologic testing, and wrote the paper; DG performed the protein expression and serologic testing; RB participated in designing research and analyzed the data; and WAF designed parts of the study, contributed serologic reagents, performed and interpreted serologic testing, and wrote the paper.
CONFLICT OF INTEREST
The authors declare no competing interests relevant to this article.
REFERENCES
- 1.Grubb R. Correlation between Lewis blood group and secretor character in man. Nature. 1948;162:933. doi: 10.1038/162933a0. [DOI] [PubMed] [Google Scholar]
- 2.Morton JA, Pickles MM, Terry AM. The Sda blood group antigen in tissues and body fluids. Vox Sang. 1970;19:472–482. doi: 10.1111/j.1423-0410.1970.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 3.Dzierzkowa-Borodej W, Seyfried H, Nichols M, Reid M, Marsh WL. The recognition of water-soluble I blood group substance. Vox Sang. 1970;18:222–234. doi: 10.1111/j.1423-0410.1970.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 4.Middleton J, Crookston MC. Chido-substance in plasma. Vox Sang. 1972;23:256–261. doi: 10.1111/j.1423-0410.1972.tb03459.x. [DOI] [PubMed] [Google Scholar]
- 5.Moulds JM, Rowe KE. Neutralization of Knops system antibodies using soluble complement receptor 1. Transfusion. 1996;36:517–520. doi: 10.1046/j.1537-2995.1996.36696269510.x. [DOI] [PubMed] [Google Scholar]
- 6.Ridgwell K, Dixey J, Parsons SF, Green CA, Scott ML. Screening human sera for anti-Lu antibodies using soluble recombinant Lu antigens. Transfus Med. 2003;(Suppl):32. [Google Scholar]
- 7.Seltsam A, Grüger D, Blasczyk R. Prokaryotic versus eukaryotic recombinant Lutheran blood group protein for antibody identification. Transfusion. 2007;47:1630–1636. doi: 10.1111/j.1537-2995.2007.01334.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheffield WP, Bhakta V, Denomme GA. Use of recombinant forms of Duffy blood group antigen to detect anti-Fy(a) and anti-Fy(b) Transfusion. 2003;43(Suppl):34A. [Google Scholar]
- 9.Seltsam A, Agaylan A, Grueger D, Bombard S, Blasczyk R, Salama A. Rapid detection of anti-Lu(b) with recombinant Lu(b) protein and the particle gel immunoassay. Transfusion. 2008;48:731–734. doi: 10.1111/j.1537-2995.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 10.Seltsam A, Agaylan A, Grueger D, Meyer O, Blasczyk R, Salama A. Rapid detection of JMH antibodies with recombinant Sema7A (CD108) protein and the particle gel immunoassay. Transfusion. 2008;48:1151–1155. doi: 10.1111/j.1537-2995.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 11.Ridgwell K, Dixey J, Scott ML. Production of soluble recombinant proteins with Kell, Duffy and Lutheran blood group antigen activity, and their use in screening human sera for Kell, Duffy and Lutheran antibodies. Transfus Med. 2007;17:384–394. doi: 10.1111/j.1365-3148.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 12.McCreary J, Vogler AL, Sabo B, Eckstein EG, Smith TR. Another minus-minus phenotype: Bu(a-)Sm—two examples in one family. Transfusion. 1973;13(Suppl):350. [Google Scholar]
- 13.Nason SG, Vengelen-Tyler V, Cohen N, Best M, Quirk J. A high incidence antibody (anti-Sc3) in the serum of a Sc:-1,-2 patient. Transfusion. 1980;20:531–535. doi: 10.1046/j.1537-2995.1980.20581034505.x. [DOI] [PubMed] [Google Scholar]
- 14.Peloquin P, Moulds M, Keenan J, Kennedy M. Anti-Sc3 as an apparent autoantibody in two patients. Transfusion. 1989;29(Suppl):49S. [Google Scholar]
- 15.Hue-Roye K, Chaudhuri A, Velliquette RW, Fetics S, Thomas R, Balk M, Wagner FF, Flegel WA, Reid ME. STAR: a novel high-prevalence antigen in the Scianna blood group system. Transfusion. 2005;45:245–247. doi: 10.1111/j.1537-2995.2004.04226.x. [DOI] [PubMed] [Google Scholar]
- 16.Flegel WA, Chen Q, Reid ME, Martin J, Orsini LA, Poole J, Moulds MK, Wagner FF. SCER and SCAN: two novel high-prevalence antigens in the Scianna blood group system. Transfusion. 2005;45:1940–1944. doi: 10.1111/j.1537-2995.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- 17.Hurstell PJ, Banks J. A case of haemolytic disease of the newborn due to anti-Sc2. Transfus Med. 2005;15(Suppl 1):48. [Google Scholar]
- 18.Ye TZ, Gordon CT, Lai YH, Fujiwara Y, Peters LL, Perkins AC, Chui DH. Ermap, a gene coding for a novel erythroid specific adhesion/receptor membrane protein. Gene. 2000;242:337–345. doi: 10.1016/s0378-1119(99)00516-8. [DOI] [PubMed] [Google Scholar]
- 19.Su YY, Gordon CT, Ye TZ, Perkins AC, Chui DH. Human ERMAP: an erythroid adhesion/receptor transmembrane protein. Blood Cells Mol Dis. 2001;27:938–949. doi: 10.1006/bcmd.2001.0465. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Foltz L, Sha Y, Madlansacay MR, Cain C, Lindemann G, Vargas J, Nagy D, Harriman B, Mahoney W, Schueler PA. Cloning and characterization of human erythroid membrane-associated protein, human ERMAP. Genomics. 2001;76:2–4. doi: 10.1006/geno.2001.6600. [DOI] [PubMed] [Google Scholar]
- 21.Wagner FF, Poole J, Flegel WA. Scianna antigens including Rd are expressed by ERMAP. Blood. 2003;101:752–757. doi: 10.1182/blood-2002-07-2064. [DOI] [PubMed] [Google Scholar]
- 22.Seltsam A, Strigens S, Levene C, Yahalom V, Moulds M, Moulds JJ, Hustinx H, Weisbach V, Figueroa D, Bade-Doeding C, DeLuca DS, Blasczyk R. The molecular diversity of Sema7A, the semaphorin that carries the JMH blood group antigens. Transfusion. 2007;47:133–146. doi: 10.1111/j.1537-2995.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- 23.Rojewski MT, Schrezenmeier H, Flegel WA. Tissue distribution of blood group membrane proteins beyond red cells: evidence from cDNA libraries. Transfus Apher Sci. 2006;35:71–82. doi: 10.1016/j.transci.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Seltsam A, Wagner FF, Salama A, Flegel WA. Antibodies to high-frequency antigens may decrease the quality of transfusion support: an observational study. Transfusion. 2003;43:1563–1566. doi: 10.1046/j.1537-2995.2003.00565.x. [DOI] [PubMed] [Google Scholar]
- 25.Reesink HW, Engelfriet CP, Schennach H, Gassner C, Wendel S, Fontao-Wendel R, de Brito MA, Sistonen P, Matilainen J, Peyrard T, Pham BN, Rouger P, Le Pennec PY, Flegel WA, von Zabern I, Lin CK, Tsoi WC, Hoffer I, Barotine-Toth K, Joshi SR, Vasantha K, Yahalom V, Asher O, Levene C, Villa MA, Revelli N, Greppi N, Marconi M, Tani Y, Folman CC, de Haas M, Koopman MM, Beckers E, Gounder DS, Flanagan P, Wall L, Aranburu Urtasun E, Hustinx H, Niederhauser C, Flickinger C, Nance SJ, Meny GM. Donors with a rare pheno (geno) type. Vox Sang. 2008;95:236–253. doi: 10.1111/j.1423-0410.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith JD, Hamour IM, Banner NR, Rose ML. C4d fixing, luminex binding antibodies—a new tool for prediction of graft failure after heart transplantation. Am J Transplant. 2007;7:2809–2815. doi: 10.1111/j.1600-6143.2007.01991.x. [DOI] [PubMed] [Google Scholar]