SUMMARY
Compositional measures of articular cartilage are accessible in vivo by magnetic resonance imaging (MRI) based relaxometry and cartilage spin-spin transverse relaxation time (T2) has been related to tissue hydration, collagen content and orientation, and mechanical (functional) properties of articular cartilage. The objective of the current study was therefore to evaluate subregional variation, and sex- and age-differences, in laminar (deep and superficial) femorotibial cartilage T2 relaxation time in healthy adults. To this end, we studied the right knees of 92 healthy subjects from the Osteoarthritis Initiative reference cohort (55 women, 37 men; age range 45–78 years; BMI 24.4±3.1) without knee pain, radiographic signs, or risk factors of knee osteoarthritis in either knee. T2 of the deep and superficial femorotibial cartilages was determined in 16 femorotibial subregions, using a multi-echo spin-echo (MESE) MRI sequence. Significant subregional variation in femorotibial cartilage T2 was observed for the superficial and for the deep (both p<0.001) cartilage layer (Friedman test). Yet, layer- and region-specific femorotibial T2 did not differ between men and women, or between healthy adults below and above the median age (54y). In conclusion, this first study to report subregional (layer-specific) compositional variation of femorotibial cartilage T2 in healthy adults identifies significant differences in both superficial and deep cartilage T2 between femorotibial subregions. However, no relevant sex- or age-dependence of cartilage T2 was observed between age 45–78y. The findings suggest that a common, non-sex-specific set of layer-and region-specific T2 reference values can be used to identify compositional pathology in joint disease for this age group.
Keywords: Cartilage Composition, Transverse Relaxation Time (T2), Magnetic Resonance Imaging, Osteoarthritis Initiative, Healthy Reference, Knee
1. Introduction
Compositional and morphological changes are known to occur in human articular cartilage with age-related structural pathology, such as osteoarthritis (OA) (Grushko et al., 1989; Liess et al., 2002; Meachim, 1971; Meachim et al., 1977). Differences in the risk of developing knee OA between women and men (Neogi and Zhang, 2013) may be suggestive of potential differences in cartilage composition between sexes. Characterization of sex-specific and age-related cartilage composition in healthy subjects is a prerequisite for distinguishing between normal aging processes and disease related pathological alterations, such as those occurring in OA.
One of the most robust techniques for the in vivo assessment of the cartilage composition is the magnetic resonance imaging (MRI)-based spin-spin (transverse) (T2) relaxometry (Dardzinski and Schneider, 2013; Mosher et al., 2011; Mosher and Dardzinski, 2004) and cartilage T2 times have been reported to be associated with cartilage composition, in particular hydration, collagen integrity and orientation (Baum et al., 2013; Liess et al., 2002; Mosher and Dardzinski, 2004). Although not specific to a single compositional measure, cartilage T2 was shown to correlate with histological grading (David-Vaudey et al., 2004; T. Kim et al., 2014) and with the mechanical properties (Lammentausta et al., 2006; Mosher and Dardzinski, 2004) of articular cartilage, providing a link between cartilage composition and function. Therefore, cartilage T2 has gained interest as an imaging biomarker for “early” stages of OA (Baum et al., 2013; Joseph et al., 2011; Jungmann et al., 2013; Mosher and Dardzinski, 2004), in which therapeutic intervention is potentially more successful than at more advanced disease stages.
In accordance with marked differences in collagen orientation between the superficial and deep cartilage layer (Glaser and Putz, 2002), cartilage T2 times have been shown to vary substantially between the bone interface and cartilage surface in healthy cartilage (Dardzinski and Schneider, 2013; Smith et al., 2001), and to differ between cartilage plates in the knee (Dardzinski and Schneider, 2013; Joseph et al., 2015).
In vitro studies have reported change in human cartilage composition with age, such as a decline of proteoglycan synthesis and content (DeGroot et al., 1999) and a reduction in interstitial water content (Grushko et al., 1989). Biomechanical experiments have described a reduction in compressive (Armstrong et al., 1979; Armstrong and Mow, 1982) and tensile (Kempson, 1991) stiffness of cartilage with age, whereas other studies suggested cartilage may become stiffer due to age-related alterations in matrix composition (Bank et al., 1998). Early T2 relaxometry studies failed to identify sex-differences in cartilage T2 in young healthy participants (Mosher et al., 2004a), but reported T2 values to become longer with advanced age in the superficial layer of patellar cartilage (Mosher et al., 2004b). In a cross sectional study, skeletal maturation in children was reported to result in a sequential decrease in cartilage T2 relaxation times that was sex-dependent (H. K. Kim et al., 2014). Following adolescent athletes longitudinally, a decrease in cartilage T2 was confirmed in the deep layers of the medial femorotibial compartment cartilages, that did not differ between males and females (Wirth et al., 2014). No such compositional change during maturation was, in contrast, observed in the superficial layers, or in the deep or superficial layers of knee cartilages of mature athletes in the same study (Wirth et al., 2014).
Reference databases of normal values are an important prerequisite for the diagnosis or for grading the disease severity. In osteoporosis, for instance, bone mineral density reference data from young healthy subjects (t-scores) and from age-matched healthy subjects (z-scores) are used to classify an individual as “normal”, “osteopenic” or “osteoporotic” and to express the severity of osteoporosis. A recent paper provided reference data for knee cartilage T2 in participants without (MRI) evidence of cartilage degeneration based on WORMS (Peterfy et al., 2004) cartilage scorings (Joseph et al., 2015) and two studies previously reported cartilage T2 times from larger subsamples of the OAI healthy reference cohort (Pan et al., 2011; Wirth et al., 2016). However, two of these studies (Joseph et al., 2015; Pan et al., 2011) examined “bulk” T2 averages throughout the full depth of the cartilage instead of laminar cartilage T2 times and examined T2 times in the entire femur without taking potential compositional differences between the central, weight-bearing part and the non-weight-bearing parts of the femur into account, and none of these studies assessed cartilage T2 times in subregions (e.g. central vs. peripheral) of knee cartilage plates. In addition, the study by Joseph et al. involved subjects from the incident cohort of the Osteoarthritis Initiative (OAI) with dedicated risk factors of incident OA, which can therefore not be regarded as being strictly healthy (Joseph et al., 2015).
The objective of the current study was therefore, to provide MRI-based T2 relaxation time reference data of layer- and subregion-specific knee cartilage composition in a cohort of healthy adult reference subjects without knee pain, radiographic evidence of OA, and risk factors of OA, and to study the relationship of the layer- and region-specific T2 times with sex and age in cartilage laminae and subregions in this adult healthy reference population.
2. Material and methods
2.1 Study participants
The participants for this study were selected from the healthy reference cohort of the Osteoarthritis Initiative (OAI; http://www.oai.ucsf.edu/, clinicaltrials.gov identifier: NCT00080171)(Eckstein et al., 2012), a large epidemiological study designed to study the incidence and progression of knee OA. All OAI participants provided written informed consent, and the study was carried out in accordance with the IRB-approved OAI data user agreement, approved by the Committee on Human Research of the Institutional Review Board for the University of California, San Francisco (UCSF).
The OAI recruited 4796 participants aged 45–79 years, with (or with risk of) knee OA (Eckstein et al., 2012). All participants were free of rheumatoid or other inflammatory arthritis, bilateral end-stage knee OA, inability to walk without aids, and MRI contraindications at the time of enrollment (Eckstein et al., 2012). For reference purposes, the OAI also included a “non-exposed” reference cohort of 122 healthy participants. These participants were free of clinical signs of knee OA (e.g. knee pain), were not exposed to risk factors for developing knee OA (including obesity, knee injury, knee surgery, a family history of TKA in a biological parent or sibling, Heberden’s nodes, or repetitive knee bending during daily activities) and had no signs of radiographic abnormalities in either knee according to the OAI clinical site readings (Eckstein et al., 2012). Of these 122 reference cohort participants, 23 were later found to have doubtful (Kellgren & Lawrence grade [KLG] 1) or definite (KLG 2) radiographic OA in at least one knee based on central radiographic readings performed by expert readers from Boston University (Eckstein et al., 2012), resulting in 99 participants, who were confirmed to be bilaterally free of radiographic OA. For the current study, we used data and MR images from 92 of the 99 participants that also had at least one follow-up time point. This sample (n=92) comprised 37 men and 55 women, aged 54.7 ± 7.5 years (range: 45 – 78 years) with a BMI of 24.4 ± 3.1 kg/m2.
2.2 MR imaging and femorotibial cartilage T2 analysis
The OAI acquired sagittal multi-echo spin-echo (MESE) MR images in one of the knees (usually the right one) of all OAI participants (Figure 1) using 3T MRI scanner (Siemens Magnetom Trio, Erlangen, Germany) and quadrature transmit/receive knee coils (USA Instruments, Aurora, OH) (Eckstein et al., 2012; Peterfy et al., 2008). The slice thickness of the MESE acquisitions was 3 mm, the field of view was 120 mm (matrix: 269 [phase] × 384 [frequency] interpolated to 384 × 384 pixels, in-plane resolution 0.3125 × 0.3125 mm), the repetition time was 2700 ms, and the echo times were 10, 20, 30, 40, 50, 60, and 70 ms (Peterfy et al., 2008).
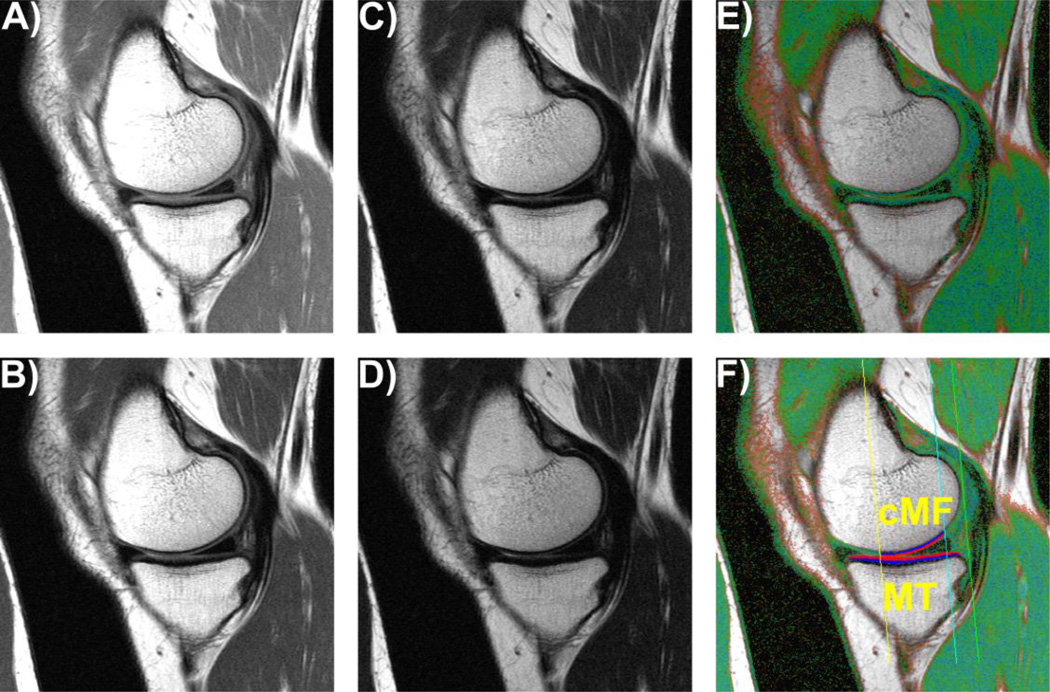
Fig. 1.
Sagittal multi-echo spin-echo (MESE) images showing the cartilages in the medial compartment; A) – D) MESE images acquired at echo times of 10, 30, 50, and 70 ms; E) Color-coded T2map; F) T2 map as in E), showing the femoral region of interest (ROI) and the segmented cartilage of the medial tibia (MT) and the central medial femur (cMF) divided into the superficial (red) and deep (blue) cartilage layers.
The femorotibial cartilages, i.e. the medial and lateral tibia (MT/LT) and the central, weight-bearing femoral part of the medial and lateral femoral condyles (cMF/cLF) were manually segmented by an experienced reader (S.M) using the MESE MRIs (Wirth et al., 2014). The tibial cartilage was segmented entirely, whereas the weight-bearing, central part of the femoral condyles was defined as 75% of the distance between the inter-condylar notch and the most posterior aspect of the condyles (Eckstein et al., 2009) (Fig. 1F).
Cartilage T2 times (in ms) were computed for each (segmented) voxel using a non-linear method by fitting a mono-exponential decay curve to the measured signal intensities (Li and Hornak Joseph P, 1994). The 1st echo (10 ms) was excluded from the fit, in order to reduce the impact of stimulated echoes (Mosher and Dardzinski, 2004). Voxels with R2<0.66 for the curve fitting were not included in the analysis, to avoid contribution from voxels with low image quality (Wirth et al., 2014).
After computing bulk T2 times for each of the 4 segmented cartilage plates, the MT and LT were each computationally divided into one central (cMT/cLT), one external (eMT/eLT), one internal (iMT/iLT), one anterior (aMT/aLT), and one posterior (pMT/pLT) subregion, using a previously published methodology for cartilage thickness measurements (Eckstein et al., 2014, 2012; Wirth and Eckstein, 2008) (Figure 1). Similarly, the cMF and cLF were divided into central (ccMF/ccLF), external (ecMF/ecLF, and internal (icMF/icLF) subregions (Wirth and Eckstein, 2008) (Figure 1). To account for the spatial association between cartilage T2 times and tissue depth (Dardzinski and Schneider, 2013; Mosher and Dardzinski, 2004), each of the four cartilage plates and 16 subregions was computationally divided into a superficial and a deep layer after segmentation has been completed, comprising 50% of the distance between the segmented cartilage surface and the bone interface, respectively (Wirth et al., 2014). For reference purposes, we also report the cartilage thickness for each cartilage plate and subregion, as determined from a morphometry-specific cartilage imaging sequence described in an earlier publication (Eckstein et al., 2010).
The average deep and superficial layer cartilage T2 times in the entire femorotibial joint (FTJ) were computed as the average T2 times of all 4 cartilages (FTJ = Average(MT, LT, cMF, cLF)). The average deep and superficial layer cartilage T2 times in the medial and lateral compartment (MFTC/LFTC) were computed as the average deep and superficial layer cartilage T2 times of the respective cartilages (MFTC = Average(MT, cMF), LFTC = Average(LT, cLF)). The average deep and superficial layer cartilage T2 times in the combined central subregion of the medial and lateral compartment (cMFTC/cLFTC) were computed as the average deep and superficial layer cartilage T2 times of the respective central subregions (cMFTC = Average(cMT, ccMF), cLFTC = Average(cLT, ccLF)).
2.2 Statistical analysis
All statistical analyses were performed using IBM SPSS 22 (IBM Corporation, Armonk, NY). The deep and superficial layer T2 times in combined measures, cartilage plates, and subregions were described using the mean, the standard deviation, and the 95% confidence intervals.
To test, whether cartilage composition differed significantly between femorotibial subregions within each cartilage plate, non-parametric Friedman tests were applied to superficial and deep cartilage, respectively. To explore the specific pattern of the subregional deep and superficial cartilage T2 times, subregional T2 was compared to the average T2 in the same cartilage plate, using a non-parametric Wilcoxon signed rank tests. The significance level was set to p=0.01 for this explorative analysis, to account for the multiple parallel comparisons.
To determine whether deep and superficial cartilage T2 times differed between and men and women and between participants in the youngest (45 – 48 years) and oldest (58 – 78 years) quartile, non-parametric Mann-Whitney-U tests were used. The primary analytic focus for these two comparisons was the deep and superficial cartilage T2 time in the entire FTJ. Cartilage T2 times in the MFTC and LFTC were considered a secondary focus. Cartilage T2 times in individual cartilage plates and subregions were considered exploratory. The significance level was adjusted to p=0.0083 (p=0.05/6) to account for the six parallel comparisons (2 layers, 3 analytical measures).
3. Results
Cartilage T2 was consistently longer in the superficial than in the deep layer, and longer in femoral than in tibial cartilage (Table 1). The superficial vs. deep layer T2 ratio ranged from 1.22 ± 0.09 (95% CI: [1.20, 1.23]) in cLF to 1.37 ± 0.07 (95% CI: [1.35, 1.38]) in LT.
Table 1.
Superficial and deep layer cartilage transverse relaxation time (T2) and cartilage thickness in various compartments, plates, and subregions of the femorotibial joint
| Superficial Layer | Deep Layer | Cartilage Thickness | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | [95% CI] | P-Value | Mean ± SD | [95% CI] | P-Value | Mean ± SD | [95% CI] | |
| Composite measures: | ||||||||
| FTJ | 45.4 ± 2.3 | [44.9, 45.9] | 35.8 ± 1.8 | [35.4, 36.2] | 7.4 ± 0.9 | [7.2, 7.5] | ||
| MFTC | 45.6 ± 2.8 | [45.0, 46.1] | 36.3 ± 2.2 | [35.8, 36.7] | 3.5 ± 0.5 | [3.4, 3.6] | ||
| LFTC | 45.3 ± 2.4 | [44.8, 45.8] | 35.4 ± 2.0 | [35.0, 35.8] | 3.8 ± 0.5 | [3.7, 3.9] | ||
| cMFTC | 46.2 ± 3.3 | [45.5, 46.9] | 32.1 ± 2.6 | [31.6, 32.6] | 4.6 ± 0.7 | [4.5, 4.7] | ||
| cLFTC | 44.0 ± 3.1 | [43.3, 44.6] | 31.2 ± 2.1 | [30.7, 31.6] | 5.3 ± 0.8 | [5.2, 5.5] | ||
| Medial tibia (MT): | ||||||||
| MT | 41.5 ± 2.9 | [40.9, 42.1] | 33.0 ± 1.9 | [32.6, 33.4] | 1.7 ± 0.2 | [1.7, 1.7] | ||
| cMT | 42.2 ± 4.1 | [41.4, 43.1] | 0.027 | 29.0 ± 2.1 | [28.5, 29.4] | <0.001 | 2.4 ± 0.4 | [2.3, 2.4] |
| eMT | 44.0 ± 4.2 | [43.1, 44.9] | <0.001 | 34.9 ± 3.1 | [34.3, 35.5] | <0.001 | 1.5 ± 0.2 | [1.4, 1.5] |
| iMT | 37.0 ± 3.8 | [36.2, 37.8] | <0.001 | 32.2 ± 3.9 | [31.4, 33.0] | 0.001 | 1.8 ± 0.2 | [1.8, 1.9] |
| aMT | 41.5 ± 3.8 | [40.8, 42.3] | 0.953 | 33.1 ± 2.4 | [32.6, 33.6] | 0.469 | 1.5 ± 0.2 | [1.5, 1.5] |
| pMT | 41.9 ± 3.6 | [41.2, 42.7] | 0.066 | 36.3 ± 2.5 | [35.7, 36.8] | <0.001 | 1.4 ± 0.2 | [1.4, 1.5] |
| Central medial femur (cMF): | ||||||||
| cMF | 49.6 ± 3.7 | [48.8, 50.4] | 39.5 ± 3.5 | [38.8, 40.2] | 1.8 ± 0.3 | [1.8, 1.9] | ||
| ccMF | 50.2 ± 4.2 | [49.3, 51.0] | 0.007 | 35.2 ± 4.0 | [34.4, 36.1] | <0.001 | 2.2 ± 0.4 | [2.2, 2.3] |
| ecMF | 44.6 ± 4.2 | [43.7, 45.4] | <0.001 | 38.0 ± 4.0 | [37.2, 38.8] | <0.001 | 1.3 ± 0.2 | [1.3, 1.3] |
| icMF | 52.8 ± 4.9 | [51.8, 53.8] | <0.001 | 44.9 ± 5.7 | [43.7, 46.1] | <0.001 | 1.9 ± 0.3 | [1.9, 2.0] |
| Lateral tibia (LT): | ||||||||
| LT | 42.4 ± 2.5 | [41.9, 42.9] | 31.0 ± 1.9 | [30.6, 31.4] | 2.1 ± 0.3 | [2. 0, 2.2] | ||
| cLT | 38.5 ± 3.8 | [37.7, 39.3] | <0.001 | 26.7 ± 2.6 | [26.2, 27.2] | <0.001 | 3.3 ± 0.6 | [3.1, 3.4] |
| eLT | 46.2 ± 3.9 | [45.4, 47.0] | <0.001 | 34.9 ± 3.0 | [34.3, 35.5] | <0.001 | 1.7 ± 0.2 | [1.6, 1.7] |
| iLT | 40.9 ± 4.5 | [40.0, 41.9] | <0.001 | 32.0 ± 3.7 | [31.2, 32.7] | 0.002 | 2.0 ± 0.4 | [2.0, 2.1] |
| aLT | 42.5 ± 3.0 | [41.8, 43.1] | 0.738 | 30.5 ± 2.6 | [29.9, 31.0] | 0.020 | 1.7 ± 0.3 | [1.6, 1.7] |
| pLT | 45.4 ± 3.6 | [44.6, 46.1] | <0.001 | 33.4 ± 3.3 | [32.8, 34.1] | <0.001 | 1.9 ± 0.3 | [1.9, 2.0] |
| Central lateral femur (cLF): | ||||||||
| cLF | 48.1 ± 2.9 | [47.5, 48.7] | 39.7 ± 2.8 | [39.2, 40.3] | 1.7 ± 0.2 | [1.7, 1.8] | ||
| ccLF | 49.4 ± 3.8 | [48.7, 50.2] | <0.001 | 35.7 ± 3.2 | [35.0, 36.3] | <0.001 | 2.1 ± 0.3 | [2.0, 2.1] |
| ecLF | 45.5 ± 3.6 | [44.7, 46.2] | <0.001 | 40.8 ± 3.5 | [40.1, 41.6] | 0.002 | 1.5 ± 0.2 | [1.4, 1.5] |
| icLF | 48.6 ± 4.4 | [47.7, 49.6] | 0.241 | 44.2 ± 4.3 | [43.3, 45.1] | <0.001 | 1.6 ± 0.2 | [1.6, 1.7] |
SD: standard deviation; 95% CI: 95% confidence intervals; P: Wilcoxon signed rank test for differences between subregional T2 and T2 in the respective cartilage. Cartilage thickness for the n=92 participants measurements were adapted from (Eckstein et al., 2010). FTJ: femorotibial joint; MFTC/LFTC: medial/lateral femorotibial compartment; cMFTC/cLFTC: central medial/lateral compartment; c/e/i/a/p M/LT: central/external/internal/anterior/posterior medial/lateral tibia; c/e/i cM/LF: central/external/internal medial/lateral femur.
3.1. Subregional distribution of deep and superficial layer cartilage T2 times
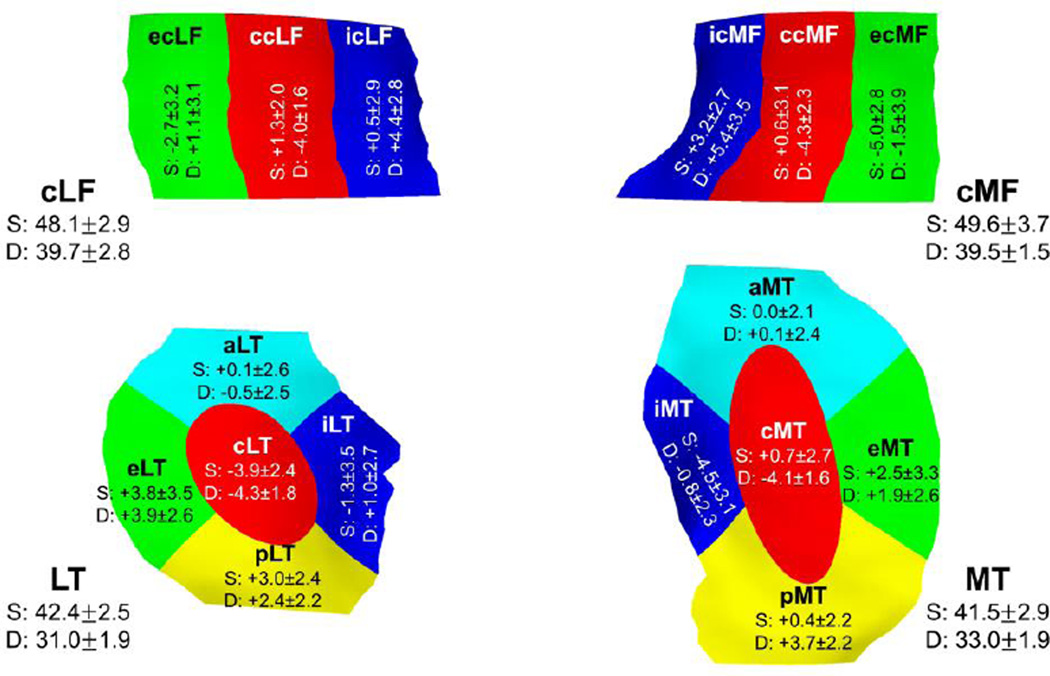
The T2 values differed significantly between cartilage subregions, both for the superficial and for the deep cartilage layer (p<0.001). In the superficial layer, cartilage T2 times were longer in the central femoral subregions when compared to the total cartilages plate (Table 1 & Figure 2). In the LT, T2 times were shorter in the central subregion than for the entire cartilage plate, but were longer in the posterior subregion (Table 1 & Figure 2). Longer T2 times than average were also observed in the external subregions of both the MT and the LT, and in the internal subregion of the cMF (Table 1 & Figure 2). In contrast, shorter T2 times than average were observed in the internal subregions of both the MT and LT, and in the external subregions of both the cMF and cLF (Table 1 & Figure 2).
Fig. 2.
Difference between laminar subregional cartilage T2 times (mean ± SD difference in ms) and the cartilage T2 times in the superficial (S) and deep (D) layers of the respective total cartilages (mean ± SD in ms).The 3D illustration also shows the central (c), external (e), and internal (i) subregions of the central part of the medial (cMF) and lateral (cLF) femur and the central (c), external (e), internal (i), anterior (a), and posterior (p) subregions of the medial (MT) and lateral (LT) tibia.
In the deep layer, the cartilage T2 times were generally shorter than average in the central subregions (Table 1 & Figure 2). Shorter T2 times were also observed in the internal subregion of the MT and the external subregion of the cMF. Longer T2 times than average were observed in the external subregions of MT, LT, and cLF, in the internal subregions of LT, cMF, and cMF, and in the posterior subregions of both MT and LT (Table 1 & Figure 2).
The strongest deviations of superficial layer cartilage T2 times from average were observed in the ecMF (−5.0 ±2.8 [−5.6, −4.4] ms) and eLT (+3.8 ±3.5 [3.1, 4.5] ms), and those of deep layer T2 in ccMF (−4.3±2.2 [−4.7, −3.8] ms), cLT (−4.3±1.8 [−4.7, −3.9] ms) and icMF (+5.4±3.5 [4.7, 6.1] ms, Table 1 & Figure 2).
3.2. Sex differences in cartilage T2 times
Cartilage T2 averaged across the total femorotibial joint did not differ significantly between men and women in either the superficial (45.6±2.1 [44.9, 46.3] ms vs. 45.3 ±2.5 [44.6, 45.9] ms, p=0.26, Table 2) or deep cartilage layers (35.5±1.7 [34.9, 36.1] ms vs. 36.0±1.8 [35.5, 36.5] ms, p=0.32, Table 3). In the MFTC, the superficial T2 times tended to be longer in men (46.2±2.7 [45.3, 47.1] ms) than in women (45.1±2.9 [44.3, 45.9] ms), but the difference did not reach the adjusted significance level (p=0.029, Table 2). Deep layer LFTC T2 times, in contrast, tended to be longer in women (35.7±2.0 [35.2, 36.3] ms) than in men (34.8±1.7 [34.2, 35.4] ms), but again the difference did not meet the adjusted significance level (p=0.018, Table 3).
Table 2.
Sex differences in superficial layer cartilage transverse relaxation time (T2) in various compartments, plates, and subregions of the femorotibial joint
| Men | Women | ||||
|---|---|---|---|---|---|
| Mean ± SD | [95% CI] | Mean ± SD | [95% CI] | P-Value | |
| Composite measures: | |||||
| FTJ | 45.6 ± 2.1 | [44.9, 46.3] | 45.3 ± 2.5 | [44.6, 45.9] | 0.257 |
| MFTC | 46.2 ± 2.7 | [45.3, 47.1] | 45.1 ± 2.9 | [44.3, 45.9] | 0.029 |
| LFTC | 45.0 ± 2.0 | [44.4, 45.7] | 45.4 ± 2.6 | [44.7, 46.1] | 0.491 |
| cMFTC | 46.6 ± 3.3 | [45.5, 47.7] | 45.9 ± 3.3 | [45.0, 46.8] | 0.240 |
| cLFTC | 43.2 ± 2.8 | [42.2, 44.1] | 44.5 ± 3.2 | [43.6, 45.4] | 0.037 |
| Medial tibia (MT): | |||||
| MT | 42.6 ± 2.9 | [41.7, 43.6] | 40.7 ± 2.6 | [40.0, 41.4] | 0.002 |
| cMT | 43.4 ± 4.4 | [41.9, 44.8] | 41.5 ± 3.8 | [40.4, 42.5] | 0.034 |
| eMT | 45.0 ± 3.9 | [43.7, 46.3] | 43.3 ± 4.2 | [42.2, 44.5] | 0.028 |
| iMT | 37.7 ± 3.6 | [36.5, 38.9] | 36.6 ± 4.0 | [35.5, 37.7] | 0.281 |
| aMT | 43.0 ± 3.5 | [41.8, 44.2] | 40.6 ± 3.6 | [39.6, 41.6] | 0.008 |
| pMT | 42.9 ± 3.9 | [41.6, 44.2] | 41.3 ± 3.3 | [40.4, 42.2] | 0.043 |
| Central medial femur (cMF): | |||||
| cMF | 49.8 ± 3.5 | [48.6, 50.9] | 49.5 ± 3.9 | [48.5, 50.6] | 0.438 |
| ccMF | 49.9 ± 4.2 | [48.4, 51.3] | 50.4 ± 4.2 | [49.2, 51.5] | 0.741 |
| ecMF | 44.7 ± 4.5 | [43.2, 46.2] | 44.5 ± 4.0 | [43.5, 45.6] | 0.670 |
| icMF | 53.5 ± 4.6 | [51.9, 55.0] | 52.4 ± 5.1 | [51.0, 53.7] | 0.138 |
| Lateral tibia (LT): | |||||
| LT | 42.5 ± 2.3 | [41.8, 43.3] | 42.3 ± 2.7 | [41.5, 43.0] | 0.636 |
| cLT | 38.1 ± 3.2 | [37.0, 39.2] | 38.7 ± 4.2 | [37.6, 39.8] | 0.442 |
| eLT | 47.4 ± 3.4 | [46.3, 48.6] | 45.4 ± 4.0 | [44.3, 46.4] | 0.014 |
| iLT | 40.4 ± 3.9 | [39.1, 41.7] | 41.3 ± 4.9 | [40.0, 42.6] | 0.438 |
| aLT | 42.8 ± 3.1 | [41.8, 43.9] | 42.2 ± 3.0 | [41.4, 43.0] | 0.438 |
| pLT | 46.0 ± 3.2 | [44.9, 47.0] | 45.0 ± 3.8 | [43.9, 46.0] | 0.162 |
| Central lateral femur (cLF): | |||||
| cLF | 47.5 ± 2.5 | [46.7, 48.3] | 48.6 ± 3.1 | [47.7, 49.4] | 0.110 |
| ccLF | 48.2 ± 3.8 | [47.0, 49.5] | 50.3 ± 3.5 | [49.3, 51.2] | 0.014 |
| ecLF | 45.7 ± 2.7 | [44.8, 46.6] | 45.3 ± 4.1 | [44.2, 46.4] | 0.765 |
| icLF | 47.9 ± 3.9 | [46.6, 49.2] | 49.1 ± 4.6 | [47.9, 50.4] | 0.225 |
For abbreviations used, please see table 1
Table 3.
Sex differences in deep layer cartilage transverse relaxation time (T2) in various compartments, plates, and subregions of the femorotibial joint
| Men | Women | ||||
|---|---|---|---|---|---|
| Mean ± SD | [95% CI] | Mean ± SD | [95% CI] | P-Value | |
| Composite measures: | |||||
| FTJ | 35.5 ± 1.7 | [34.9, 36.1] | 36.0 ± 1.8 | [35.5, 36.5] | 0.318 |
| MFTC | 36.2 ± 2.4 | [35.4, 37.0] | 36.3 ± 2.1 | [35.7, 36.9] | 0.990 |
| LFTC | 34.8 ± 1.7 | [34.2, 35.4] | 35.7 ± 2.0 | [35.2, 36.3] | 0.018 |
| cMFTC | 32.1 ± 2.7 | [31.2, 33.0] | 32.1 ± 2.5 | [31.4, 32.7] | 0.777 |
| cLFTC | 30.7 ± 1.8 | [30.1, 31.3] | 31.5 ± 2.3 | [30.9, 32.1] | 0.076 |
| Medial tibia (MT): | |||||
| MT | 33.4 ± 1.9 | [32.8, 34.0] | 32.8 ± 1.8 | [32.3, 33.2] | 0.135 |
| cMT | 29.6 ± 2.3 | [28.8, 30.3] | 28.5 ± 1.8 | [28.0, 29.0] | 0.016 |
| eMT | 35.6 ± 2.7 | [34.7, 36.5] | 34.5 ± 3.2 | [33.6, 35.3] | 0.089 |
| iMT | 31.8 ± 3.1 | [30.8, 32.8] | 32.5 ± 4.4 | [31.3, 33.6] | 0.771 |
| aMT | 33.4 ± 2.5 | [32.6, 34.2] | 32.9 ± 2.4 | [32.2, 33.5] | 0.325 |
| pMT | 36.7 ± 2.5 | [35.9, 37.5] | 36.0 ± 2.5 | [35.3, 36.6] | 0.133 |
| Central medial femur (cMF): | |||||
| cMF | 39.0 ± 3.5 | [37.9, 40.2] | 39.8 ± 3.4 | [38.9, 40.7] | 0.341 |
| ccMF | 34.7 ± 3.8 | [33.4, 35.9] | 35.6 ± 4.2 | [34.5, 36.7] | 0.392 |
| ecMF | 37.4 ± 3.5 | [36.3, 38.6] | 38.4 ± 4.3 | [37.2, 39.5] | 0.257 |
| icMF | 44.9 ± 6.4 | [42.8, 47.0] | 44.8 ± 5.2 | [43.4, 46.3] | 0.833 |
| Lateral tibia (LT): | |||||
| LT | 30.6 ± 1.8 | [30.0, 31.2] | 31.3 ± 2.0 | [30.7, 31.8] | 0.098 |
| cLT | 26.5 ± 2.2 | [25.8, 27.2] | 26.8 ± 2.8 | [26.1, 27.6] | 0.765 |
| eLT | 35.0 ± 3.0 | [34.0, 36.0] | 34.8 ± 3.0 | [34.0, 35.6] | 0.548 |
| iLT | 30.7 ± 2.8 | [29.7, 31.6] | 32.9 ± 3.9 | [31.8, 33.9] | 0.002 |
| aLT | 30.1 ± 2.6 | [29.3, 31.0] | 30.7 ± 2.7 | [30.0, 31.4] | 0.167 |
| pLT | 33.0 ± 3.2 | [31.9, 34.1] | 33.7 ± 3.3 | [32.8, 34.6] | 0.291 |
| Central lateral femur (cLF): | |||||
| cLF | 39.1 ± 2.4 | [38.3, 39.9] | 40.2 ± 2.9 | [39.4, 41.0] | 0.023 |
| ccLF | 34.9 ± 3.0 | [33.9, 35.9] | 36.2 ± 3.3 | [35.3, 37.1] | 0.041 |
| ecLF | 41.8 ± 3.5 | [40.6, 42.9] | 40.2 ± 3.4 | [39.3, 41.1] | 0.031 |
| icLF | 42.5 ± 3.6 | [41.3, 43.7] | 45.3 ± 4.4 | [44.1, 46.5] | 0.002 |
For abbreviations used, please see table 1
In the exploratory analyses, significant differences between men and women were observed in the superficial layer of the MT (Table 2), specifically in aMT (Table 2), and in the deep layer of iLT and icLF (Table 3).
3.3. Age-related differences in cartilage T2 times
Cartilage T2 averaged across the total femorotibial joint did not differ significantly between participants in the youngest versus participants in the oldest quartile in either the superficial (45.8±3.1 [44.4, 47.1] ms vs. 45.7 ±1.8 [44.9, 46.4] ms, p=0.94, Table 4) or the deep cartilage layer (36.3±2.2 [35.3, 37.2] ms vs. 35.9±1.5 [35.2, 36.5] ms, p=0.27, Table 5). Also, no significant age differences were observed in the medial and lateral femorotibial compartment, cartilage plates, and subregions (Tables 4 & 5) or when comparing deep and superficial cartilage T2 times between participants older and younger than the median age of 54 years (data not shown).
Table 4.
Age differences in superficial layer cartilage transverse relaxation time (T2) in various compartments, plates, and subregions of the femorotibial joint
| 1st Quartile | 4th Quartile | ||||
|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | P-Value | |
| Composite measures: | |||||
| FTJ | 45.8 ± 3.1 | [44.4, 47.1] | 45.7 ± 1.8 | [44.9, 46.4] | 0.939 |
| MFTC | 46.0 ± 3.7 | [44.4, 47.6] | 46.0 ± 2.8 | [44.8, 47.2] | 0.956 |
| LFTC | 45.6 ± 3.0 | [44.3, 46.8] | 45.3 ± 1.7 | [44.6, 46.0] | 0.733 |
| cMFTC | 46.7 ± 4.3 | [44.9, 48.5] | 46.4 ± 3.3 | [45.0, 47.9] | 0.818 |
| cLFTC | 44.3 ± 3.4 | [42.8, 45.7] | 44.0 ± 2.6 | [42.9, 45.1] | 0.956 |
| Medial tibia (MT) | |||||
| MT | 42.2 ± 2.9 | [41.0, 43.5] | 41.9 ± 3.6 | [40.3, 43.5] | 0.531 |
| cMT | 43.3 ± 4.3 | [41.4, 45.1] | 42.5 ± 4.8 | [40.4, 44.6] | 0.546 |
| eMT | 45.8 ± 3.6 | [44.2, 47.4] | 43.7 ± 4.4 | [41.7, 45.6] | 0.036 |
| iMT | 37.3 ± 3.6 | [35.8, 38.9] | 37.7 ± 4.5 | [35.7, 39.6] | 0.869 |
| aMT | 41.9 ± 3.3 | [40.4, 43.3] | 42.1 ± 4.0 | [40.3, 43.8] | 0.701 |
| pMT | 42.3 ± 3.4 | [40.8, 43.8] | 42.6 ± 4.4 | [40.7, 44.5] | 0.974 |
| Central medial femur (cMF): | |||||
| cMF | 49.8 ± 4.9 | [47.6, 51.9] | 50.1 ± 3.3 | [48.7, 51.6] | 0.684 |
| ccMF | 50.1 ± 5.6 | [47.7, 52.5] | 50.3 ± 3.3 | [48.9, 51.7] | 0.784 |
| ecMF | 44.6 ± 5.2 | [42.3, 46.8] | 45.1 ± 4.2 | [43.3, 46.9] | 0.921 |
| icMF | 53.3 ± 6.3 | [50.6, 56.0] | 53.7 ± 4.7 | [51.7, 55.8] | 0.652 |
| Lateral tibia (LT): | |||||
| LT | 42.7 ± 2.8 | [41.5, 43.9] | 42.8 ± 2.8 | [41.6, 44.0] | 0.575 |
| cLT | 39.0 ± 3.6 | [37.5, 40.6] | 38.9 ± 4.3 | [37.1, 40.8] | 0.784 |
| eLT | 46.1 ± 3.5 | [44.6, 47.6] | 45.2 ± 3.6 | [43.7, 46.7] | 0.215 |
| iLT | 41.3 ± 4.6 | [39.3, 43.3] | 43.0 ± 4.3 | [41.2, 44.9] | 0.150 |
| aLT | 42.8 ± 2.9 | [41.6, 44.0] | 43.4 ± 3.2 | [42.0, 44.8] | 0.475 |
| pLT | 45.5 ± 3.9 | [43.8, 47.1] | 44.8 ± 3.5 | [43.3, 46.4] | 0.606 |
| Central lateral femur (cLF): | |||||
| cLF | 48.4 ± 3.8 | [46.8, 50.1] | 47.8 ± 1.5 | [47.2, 48.4] | 0.448 |
| ccLF | 49.5 ± 4.5 | [47.5, 51.5] | 49.2 ± 2.5 | [48.1, 50.2] | 0.668 |
| ecLF | 46.8 ± 3.8 | [45.1, 48.4] | 44.3 ± 2.8 | [43.1, 45.5] | 0.013 |
| icLF | 48.6 ± 4.8 | [46.6, 50.7] | 49.0 ± 3.7 | [47.3, 50.6] | 0.818 |
For abbreviations used, please see table 1
Table 5.
Age differences in deep layer cartilage transverse relaxation time (T2) in various compartments, plates, and subregions of the femorotibial joint
| 1st Quartile | 4th Quartile | ||||
|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | P-Value | |
| Composite measures: | |||||
| FTJ | 36.3 ± 2.2 | [35.3, 37.2] | 35.9 ± 1.5 | [35.2, 36.5] | 0.267 |
| MFTC | 36.7 ± 2.5 | [35.6, 37.8] | 36.2 ± 2.0 | [35.4, 37.1] | 0.362 |
| LFTC | 35.8 ± 2.1 | [34.9, 36.7] | 35.5 ± 2.0 | [34.7, 36.4] | 0.560 |
| cMFTC | 32.7 ± 2.5 | [31.7, 33.8] | 32.0 ± 2.7 | [30.8, 33.1] | 0.258 |
| cLFTC | 31.6 ± 2.1 | [30.7, 32.6] | 31.3 ± 1.8 | [30.5, 32.1] | 0.560 |
| Medial tibia (MT) | |||||
| MT | 33.1 ± 1.9 | [32.3, 33.9] | 33.3 ± 1.9 | [32.5, 34.1] | 0.590 |
| cMT | 29.5 ± 1.9 | [28.7, 30.3] | 29.1 ± 2.7 | [28.0, 30.3] | 0.475 |
| eMT | 35.2 ± 3.1 | [33.8, 36.5] | 34.6 ± 2.8 | [33.4, 35.8] | 0.339 |
| iMT | 32.1 ± 4.0 | [30.4, 33.8] | 32.5 ± 3.1 | [31.1, 33.8] | 0.621 |
| aMT | 33.0 ± 2.7 | [31.9, 34.2] | 33.5 ± 2.3 | [32.5, 34.5] | 0.489 |
| pMT | 36.0 ± 2.4 | [35.0, 37.1] | 36.7 ± 2.6 | [35.6, 37.8] | 0.410 |
| Central medial femur (cMF): | |||||
| cMF | 40.3 ± 3.9 | [38.6, 42.0] | 39.2 ± 3.2 | [37.8, 40.6] | 0.223 |
| ccMF | 35.9 ± 4.0 | [34.2, 37.7] | 34.8 ± 4.2 | [33.0, 36.6] | 0.184 |
| ecMF | 37.7 ± 3.9 | [36.0, 39.4] | 37.7 ± 4.3 | [35.9, 39.6] | 0.991 |
| icMF | 46.5 ± 6.2 | [43.8, 49.1] | 44.7 ± 5.6 | [42.3, 47.1] | 0.199 |
| Lateral tibia (LT): | |||||
| LT | 31.4 ± 2.1 | [30.5, 32.3] | 30.9 ± 2.0 | [30.1, 31.8] | 0.637 |
| cLT | 27.2 ± 2.7 | [26.0, 28.4] | 26.4 ± 2.2 | [25.4, 27.3] | 0.307 |
| eLT | 35.5 ± 3.4 | [34.1, 37.0] | 34.4 ± 2.7 | [33.2, 35.5] | 0.106 |
| iLT | 31.9 ± 3.3 | [30.5, 33.3] | 33.1 ± 4.3 | [31.2, 34.9] | 0.503 |
| aLT | 30.6 ± 2.6 | [29.4, 31.7] | 30.7 ± 2.6 | [29.6, 31.8] | 0.733 |
| pLT | 34.0 ± 3.8 | [32.3, 35.6] | 32.8 ± 3.1 | [31.4, 34.1] | 0.287 |
| Central lateral femur (cLF): | |||||
| cLF | 40.2 ± 2.9 | [39.0, 41.5] | 40.1 ± 2.9 | [38.8, 41.4] | 0.684 |
| ccLF | 36.1 ± 3.3 | [34.7, 37.6] | 36.2 ± 3.4 | [34.7, 37.7] | 0.701 |
| ecLF | 41.7 ± 3.5 | [40.2, 43.2] | 41.0 ± 4.4 | [39.1, 42.9] | 0.621 |
| icLF | 44.4 ± 4.8 | [42.3, 46.4] | 45.0 ± 4.3 | [43.1, 46.9] | 0.886 |
For abbreviations used, please see table 1
4. Discussion
This is the first study to characterize subregional (layer-specific) compositional differences in femorotibial cartilage by MRI spin-spin (transverse) relaxation time (T2) in healthy adults, and to relate T2 systematically throughout different subregions and layers with sex and age. The study identified significant differences in both superficial and deep cartilage T2 between subregions, whereas no relevant sex- or age-dependency was observed for the age range of adult healthy subjects examined here. These findings suggest that men and women, and subjects aged 45–78 years may be pooled in analyses of subregional and layer specific cartilage T2, if these are acquired in truly healthy knees without symptoms, signs or risk factors of OA.
The limitations of the current study are that cartilage T2 was examined in only two layers, each comprising 50% of the full cartilage thickness, whereas histologically, at least 3 zones can be identified based on the collagen orientation and other structural features, that cover various percentages of the cartilage thickness (Glaser and Putz, 2002). However, the in plane resolution of 0.3125 mm (and 3 mm slices thickness) of the MESE sequence precluded the analysis of a higher number of cartilage laminae, in view of cartilage thickness values of < 2 mm in some of the peripheral femorotibial subregions. Yet, a strength of the study was the use of 3T MRI that permitted acquisition of MESE images at a high in-plane resolution (Peterfy et al., 2008). Another limitation is the small sample size, but the subjects included were rigorously selected to be free of symptoms, signs and risk factors of knee OA, and thus can be viewed as “super-normals” when compared to reference groups commonly used in epidemiological studies. Finally, limitations arise from the number of parallel comparisons that had to be performed in exploring potential differences between regions, and for the comparison between men and women, and youngest vs. oldest subjects, but the significance level was adjusted to account for the parallel tests, and a clear hierarchical set of tests was defined upfront for the comparison between men and women, and youngest vs. oldest participants.
When taking the average of the deep and superficial T2 times as an approximation of the bulk T2 times in the cartilage plates, the bulk T2 times observed in the tibial cartilages were up to 3ms longer and the bulk T2 times observed in the central medial femur were up to 4 ms shorter than the medial femoral condyle T2 times reported by Pan et al. for a different subset of participants from the OAI reference cohort (Pan et al., 2011). These differences can potentially be attributed to methodological factors including different segmentation methods or the inclusion of the 1st echo for the calculation of the cartilage T2 times in the study by Pan et al. (Pan et al., 2011), whereas in the current study, the 1st echo was excluded to reduce the impact of stimulated echoes (Mosher and Dardzinski, 2004).
Cartilage T2 relaxation times are known to depend on the orientation of the collagen fibers relative to the main magnetic field (magic-angle effect) (Mosher and Dardzinski, 2004), in particular in the deep, radial zone (Xia et al., 2002). In the current study, we have calculated the vector normal to the subchondral bone surface area as an approximation of the collagen orientation in the deep layer, to estimate the impact of the angular dependency between the collagen orientation and the orientation of the main magnetic field on subregional T2 times (data not shown). The orientation of the central, external, and anterior subregions in the main magnetic field was observed to be similar to the orientation of the respective entire cartilage plates, whereas somewhat larger angles were observed for the posterior and the internal subregions. When compared to the laminar T2 times in the respective entire cartilage plates, we observed both shorter and longer T2 times in subregions with similar orientations (e.g. internal subregions) indicating that the orientation of the subregions relative to the main magnetic field is unlikely to explain the variation between subregional T2 times. This can probably be attributed to the fact that the average angles observed for the subregions (4° – 26°) were much smaller than the magic angle (54.7°), where the cartilage T2 times reach their maximum (Xia et al., 2002). Previous studies have also shown that the relative proportion of radial, transitional, and superficial cartilage varies between the central and the peripheral parts of the cartilages (Clark, 1991) and that the composition and biomechanical properties differ between areas of high and low weight-bearing (Akizuki et al., 1986; Gomez et al., 2000; Xia, 2000). The observed spatial pattern of subregional cartilage T2 times is therefore most likely caused by a combination of the above effects, but the specific contribution of these effects needs to be quantified in future studies. It is also worth noting that the combined central medial femorotibial compartment displayed longer cartilage T2 than the combined central lateral femorotibial compartment, with the medial compartment commonly taking more of the load transfer compared with the lateral one (Johnson et al., 1980). We have no reasonable explanation, however, as to why, from a biomechanical point, femoral cartilage subregions displayed substantially longer T2 than tibial subregions, both for the superficial and for the deep cartilages.
The reference values reported in the current study were based on normal-weight OAI healthy reference cohort participants aged 45 to 78 years, which had no signs or risk factors for developing knee OA. Although no significant association was observed between age and cartilage T2 times in the current study, reference values for other, younger cohorts may potentially differ from the reference values reported in the current study. The age of the participants included in the current study, however, covered the age range typically analyzed in knee osteoarthritis studies. Cartilage T2 times for cohorts with different BMI characteristics will most likely also differ from the reference values reported here, given that previous studies reported significant associations between BMI and cartilage T2 times (Joseph et al., 2015; Serebrakian et al., 2015). Obesity has, however, been identified as an important risk factor for developing knee OA (Felson et al., 1997) and cohorts with higher BMI should therefore not be used as basis for providing normal reference values.
Previous reports have been inconsistent with regard to reporting sex- and age-differences in cartilage T2. Our present study shows that, if strictly healthy (i.e. supernormal) subjects are studied, no significant association of cartilage T2 with sex or age are observed. In particular, the variation between men and women, and that of older vs. younger adults was much smaller than that between cartilage layers (superficial vs. deep), between plates (femoral vs. tibial), and between subregions. The inconsistent findings previously reported (H. K. Kim et al., 2014; Mosher et al., 2004a, 2004b) may be due to OA related structural pathology, in particular cartilage lesions, becoming more frequent with age, even in the absence of radiographic signs of knee OA (Guermazi et al., 2012). Hence, the age-dependency of cartilage T2 observed in previous studies may be due to cartilage pathology rather than normal cartilage aging, and may not be observed when risk factors of knee OA are rigorously eliminated. Similar considerations apply to sex-differences previously reported for cartilage T2, given that women have a higher prevalence of knee OA than men (Neogi and Zhang, 2013). The lack of sex-differences as well as that of an age-dependence in cartilage T2 across the age range studied here greatly simplifies the identification of pathological compositional change of cartilage T2 in joint disease (OA), as it suggests that the same reference values can be used for men and women and for an age range of 45–78 years that applies to most studies of knee OA.
In conclusion, this is the first study to report subregional, layer-specific compositional variation in femorotibial cartilage T2 (spin-spin/transverse) relaxation time in healthy adults, and the first study to report significant differences between femorotibial subregions in both the superficial and deep cartilage layers. No relevant sex- or age-dependency of cartilage T2 was, however, detected between age 45 and 78, suggesting that a common set of layer-and region-specific T2 reference values can be used for this age range to identify compositional pathology in joint disease.
Acknowledgments
Acknowledgements and funding
We would like to thank the OAI participants, OAI investigators, OAI clinical and technical staff, the OAI coordinating center and the OAI funders for providing this unique public data base. The OAI, a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262), was funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners of the OAI include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. The sponsors were not involved in the design and conducting of this particular study, in the analysis and interpretation of the data, and in the preparation, review, or approval of the manuscript. We would further like to thank the Paracelsus Medical University research fund (PMU FFF E-13/17/090-WIR) for supporting the image analysis of the MESE images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Wolfgang Wirth is co-owner and has a part time employment at Chondrometrics GmbH (Ainring, Germany), a company providing medical image analysis service to academic researchers and to industry. He has provided consulting services to MerckSerono.
- Susanne Maschek is co-owner and has a part time employment at Chondrometrics GmbH.
- Felix Eckstein is CEO and co-owner of Chondrometrics GmbH. He provides consulting services to MerckSerono, Synarc and Servier, and has held educational lectures for Medtronic. He has received funding support (for studies not related to the current one) from Pfizer, Eli Lilly, Stryker, Novartis, MerckSerono, Glaxo Smith Kline, Wyeth, Contocor, Abbvie, Kolon, Synarc, Ampio, and Orthotrophix.
Bibliography
- Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulu. J. Orthop. Res. 1986;4:379–392. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- Armstrong CG, Bahrani AS, Gardner DL. In vitro measurement of articular cartilage deformations in the intact human hip joint under load. J. Bone Joint Surg. Am. 1979;61:744–755. [PubMed] [Google Scholar]
- Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J. Bone Jt. Surg. Am. 1982;64:88–94. [PubMed] [Google Scholar]
- Bank RA, Bayliss MT, Lafeber FPJG, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem. J. 1998;330:345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr. Cart. 2013;21:1474–1484. doi: 10.1016/j.joca.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM. Variation of Collagen Fiber Alignment in a Joint Surface : A Scanning Electron Microscope Study of the Tibia1 Plateau in Dog, Rabbit, and Man. J Orthop Res. 1991;9:246–257. doi: 10.1002/jor.1100090213. [DOI] [PubMed] [Google Scholar]
- Dardzinski BJ, Schneider E. Radiofrequency (RF) coil impacts the value and reproducibility of cartilage spin-spin (T2) relaxation time measurements. Osteoarthritis Cartilage. 2013;21:710–720. doi: 10.1016/j.joca.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- DeGroot J, Verzijl N, Bank RA, Lafeber FP, Bijlsma JW, TeKoppele JM. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, Kwoh CK, Guermazi A, Hunter D. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: Data from the Osteoarthritis Initiative. Arthritis Rheum. 2009;61:1218–1225. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F, Guermazi A, Gold G, Duryea J, Hellio Le Graverand M-PPM-P, Wirth W, Miller CGG. Imaging of cartilage and bone: promises and pitfalls in clinical trials of osteoarthritis. Osteoarthr. Cartil. 2014;22:1516–1532. doi: 10.1016/j.joca.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging-the Osteoarthritis Initiative. Nat. Rev. Rheumatol. 2012;8:622–630. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F, Yang M, Guermazi A, Roemer FW, Hudelmaier M, Picha K, Baribaud F, Wirth W, Felson DT. Reference values and Z-scores for subregional femorotibial cartilage thickness--results from a large population-based sample (Framingham) and comparison with the non-exposed Osteoarthritis Initiative reference cohort. Osteoarthritis Cartilage. 2010;18:1275–1283. doi: 10.1016/j.joca.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, Levy D. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- Glaser C, Putz R. Functional anatomy of articular cartilage under compressive loading Quantitative aspects of global, local and zonal reactions of the collagenous network with respect to the surface integrity. Osteoarthritis. Cartilage. 2002;10:83–99. doi: 10.1053/joca.2001.0484. [DOI] [PubMed] [Google Scholar]
- Gomez S, Toffanin R, Bernstorff S, Romanello M, Amenitsch H, Rappolt M, Rizzo R, Vittur F. Collagen fibrils are differently organized in weight-bearing and not-weight-bearing regions of pig articular cartilage. J. Exp. Zool. 2000;287:346–352. [PubMed] [Google Scholar]
- Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect. Tissue Res. 1989;19:149–176. doi: 10.3109/03008208909043895. [DOI] [PubMed] [Google Scholar]
- Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, Aliabadi P, McLennan CE, Felson DT. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Leitl S, Waugh W. The distribution of load across the knee. A comparison of static and dynamic measurements. J. Bone Jt. Surg. Br. 1980;62:346–349. doi: 10.1302/0301-620X.62B3.7410467. [DOI] [PubMed] [Google Scholar]
- Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, Lynch JA, McCulloch CE, Majumdar S, Link TM. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls - data from the osteoarthritis initiative. Arthritis Res. Ther. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph GB, McCulloch CE, Nevitt MC, Heilmeier U, Nardo L, Lynch JA, Liu F, Baum T, Link TM. A reference database of cartilage 3T MRI T2 values in knees without diagnostic evidence of cartilage degeneration: Data from the osteoarthritis initiative. Osteoarthr. Cartil. 2015;23:897–905. doi: 10.1016/j.joca.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann PM, Kraus MS, Nardo L, Liebl H, Alizai H, Joseph GB, Liu F, Lynch J, McCulloch CE, Nevitt MC, Link TM. T2 relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: Longitudinal data from the osteoarthritis initiative. J. Magn Reson. 2013;38:1415–1424. doi: 10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the t… - PubMed - NCBI. Biochim Biophys Acta. 1991;1075:223–230. doi: 10.1016/0304-4165(91)90270-q. [DOI] [PubMed] [Google Scholar]
- Kim HK, Shiraj S, Anton CG, Horn PS, Dardzinski BJ. Age and sex dependency of cartilage T2 relaxation time mapping in MRI of children and adolescents. AJR Am. J Roentgenol. 2014;202:626–632. doi: 10.2214/AJR.13.11327. [DOI] [PubMed] [Google Scholar]
- Kim T, Min BH, Yoon SH, Kim H, Park S, Lee HY, Kwack KS. An in vitro comparative study of T2 and T2* mappings of human articular cartilage at 3-Tesla MRI using histology as the standard of reference. Skelet. Radiol. 2014;43:947–954. doi: 10.1007/s00256-014-1872-z. [DOI] [PubMed] [Google Scholar]
- Lammentausta E, Kiviranta P, Nissi MJ, Laasanen MS, Kiviranta I, Nieminen MT, Jurvelin JS. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: Relationships with tissue mechanical properties. J. Orthop. Res. 2006;24:366–374. doi: 10.1002/jor.20041. [DOI] [PubMed] [Google Scholar]
- Li X, Hornak Joseph P. T2 Calculations in MRI: Linear versus Nonlinear Methods. J. Imaging Sci. Technol. 1994;38:154–157. [Google Scholar]
- Liess C, Luesse S, Karger N, Heller M, Glueer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis. Cartilage. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- Meachim G. Effect of age on the thickness of adult articular cartilage at he shoulder joint. Ann. Rheum. Dis. 1971;30:43–46. doi: 10.1136/ard.30.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meachim G, Bentley G, Baker R. Effect of age on thickness of adult patellar articular cartilage. Ann. Rheum. Dis. 1977;36:563–568. doi: 10.1136/ard.36.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher TJ, Collins CM, Smith HE, Moser LE, Sivarajah RT, Dardzinski BJ, Smith MB. Effect of gender on in vivo cartilage magnetic resonance imaging T2 mapping. J. Magn Reson. 2004a;19:323–328. doi: 10.1002/jmri.20013. [DOI] [PubMed] [Google Scholar]
- Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin. Musculoskelet. Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- Mosher TJ, Liu Y, Yang QX, Yao J, Smith R, Dardzinski BJ, Smith MB. Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum. 2004b;50:2820–2828. doi: 10.1002/art.20473. [DOI] [PubMed] [Google Scholar]
- Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, Kwoh CK, Eckstein F, Witschey WR, Borthakur A. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258:832–842. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum. Dis. Clin. North Am. 2013;39:1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Pialat J-BB, Joseph T, Kuo D, Joseph GB, Nevitt MC, Link TM. Knee Cartilage T2 Characteristics and Evolution in Relation to Morphologic Abnormalities Detected at 3-T MR Imaging : A Longitudinal Study of the Normal Control Cohort from the Osteoarthritis Initiative. Radiology. 2011;261:507–515. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis. Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebrakian AT, Poulos T, Liebl H, Joseph GB, Lai A, Nevitt MC, Lynch JA, McCulloch CE, Link TM. Weight loss over 48 months is associated with reduced progression of cartilage T2 relaxation time values: data from the osteoarthritis initiative. J Magn Reson. 2015;41:1272–1280. doi: 10.1002/jmri.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, Schmithorst VJ, Smith MB. Spatial variation in cartilage T2 of the knee. J Magn Reson. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans. Med. Imaging. 2008;27:737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- Wirth W, Eckstein F, Boeth H, Diederichs G, Hudelmaier M, Duda GNN. Longitudinal analysis of MR spin–spin relaxation times (T2) in medial femorotibial cartilage of adolescent vs mature athletes: dependence of deep and superficial zone properties on sex and age. Osteoarthr. Cartil. 2014;22:1554–1558. doi: 10.1016/j.joca.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Wirth W, Maschek S, W Roemer F, Eckstein F. Layer-specific femorotibial cartilage T2 relaxation time in knees with and without early knee osteoarthritis: Data from the Osteoarthritis Initiative (OAI) Sci. Rep. 2016;6:34202. doi: 10.1038/srep34202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. Heterogeneity of cartilage laminae in MR imaging. J Magn Reson. 2000;11:686–693. doi: 10.1002/1522-2586(200006)11:6<686::aid-jmri16>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Xia Y, Moody JB, Alhadlaq H. Orientational dependence of T2 relaxation in articular cartilage: A microscopic MRI (microMRI) study. Magn Reson. 2002;48:460–469. doi: 10.1002/mrm.10216. [DOI] [PubMed] [Google Scholar]