Abstract
Nitroxyl (HNO) is a simple molecule with significant potential as a pharmacological agent. For example, its use in the possible treatment of heart failure has received recent attention due to its unique therapeutic properties. Recent progress has been made on the elucidation of the mechanisms associated with its biological signaling. Importantly, the biochemical mechanisms described for HNO bioactivity are consistent with its unique and novel chemical properties/reactivity. To date, much of the biology of HNO can be associated with interactions and modification of important regulatory thiol proteins. Herein will be provided a description of HNO chemistry and how this chemistry translates to some of its reported biological effects.
Keywords: Nitroxyl, azanone, thiols, nitrogen oxides
Introduction
Since the discovery of endogenous generation of nitric oxide (NO) as a signaling molecule in the vascular system, interest in related nitrogen oxide species has received significant attention [for example, 1]. It is clear that the biological functions of nitrogen oxides extend beyond the vascular system, with effects in immune response, cell proliferation, neurotransmission and fundamental redox signaling, just to name a few. For the most part, nitrogen oxides that are more oxidized than NO have received the most interest. For example, numerous reports regarding the possible biological effects associated with nitrite (NO2 −), peroxynitrite (ONOO−) and nitrogen dioxide (NO2), among others, abound in the literature. By comparison, species that are reduced compared to NO have received significantly less attention. However, among these reduced species, nitroxyl (HNO, also known as azanone and nitrosyl hydride) has been examined more extensively than others [for example, 2]. Unlike most other biologically relevant small molecule nitrogen species such as nitrate (NO3 −), NO2 −, NO, NO2, ONOO−, hydroxylamine (NH2OH) or ammonia (NH3), HNO cannot be conveniently stored, collected or quantitated due to self reactivity (Reaction 1).
| (1) |
This property of HNO makes studying it, especially in biological systems, difficult and requires the use of HNO-donor species and indirect methods for its detection. In spite of these experimental difficulties, recent studies clearly indicate that HNO possesses unique biological activity that makes it useful as a possible therapeutic agent (vide infra) [3,4]. Indeed, the biological effects of pharmacologically administered HNO have prompted the development of numerous HNO-donor species [5] as well as novel methods for its detection [for example, 6]. Herein will be reviewed the current understanding of the chemistry of HNO along with the targets/chemical biology that may be responsible for some of its reported biological actions and signaling.
HNO Chemistry
Only a brief discussion of the most salient aspects of the biologically relevant chemistry of HNO will be given herein. For more comprehensive and detailed discussions of HNO chemistry several reviews are available [7,8,9 Miranda, 2005; Farmer and Sulc, 2005; Fukuto et al., 2005]. Prior to embarking on a discussion of HNO chemistry, it is worth mentioning briefly something about its nomenclature. The term “nitroxyl” is probably the most common moniker for HNO, in spite of the fact that it poorly describes (and even misrepresents) any aspect of this molecule. Moreover, “nitroxyl” is ambiguous since it has been used to describe paramagnetic “nitroxide” species (R2N-O·). Thus, this term is potentially misleading and confusing and readers need to be aware that a search of the literature for HNO using “nitroxyl” as a keyword may produce references for chemically distinct nitroxide species. More appropriate and less commonly used terms for HNO include hydridooxidonitrogen and azanone [10]. Regardless, the somewhat unfortunate term “nitroxyl” will continue to be used herein since it is embedded in the current chemical and biological lexicon and literature searches will not be complete without recognizing this.
For a species that contains only three elements, the chemistry of HNO is particularly complex. Part of the complexity of HNO chemistry involves one of its most fundamental reactions - the acid-base equilibrium (Reaction 2).
| (2) |
Unlike most all other acid-base equilibria, the electronic ground states of the equilibrium partners are different. That is, the protonated HNO species is a ground state singlet (1HNO) and the deprotonated anion is a ground state triplet (3NO−). Thus, deprotonation of the singlet HNO to the triplet NO− requires a change in the electronic spin state, which, along with nuclear reorganization, makes this process slow [11]. The pKa of HNO has been estimated to be 11.4, indicating that 3NO− is a reasonably strong base [11,12]. However, as with deprotonation of HNO, protonation of 3NO− is also slow (for the same reasons given above). Thus, under biological conditions, HNO and NO− will likely react via other processes before they can deprotonate or protonate to set up an acid-base equilibrium. That is, if HNO or NO− are formed or present in a biological system, numerous other reactions will occur before protonation/deprotonation (vide infra) and chemistry associated with its acid-base equilibrium partner will not likely be observed.
One of the most important chemical properties of HNO is its electrophilicity. Bartberger and coworkers reported that the reaction of HNO with “soft” nucleophiles such as thiols is thermodynamically favorable whereas reaction with “hard” nucleophiles such as water or alcohols is unfavorable [13]. It has also been shown that the reaction of HNO with thiols is relatively fast (k = 2 - 20 × 106 M−1s−1, depending on the nature of the thiol) [14,15]. By comparison, the reactions of thiols with other oxidants are considerably slower (k = 0.87 M−1s−1 for H2O2 [16], k = 1.3 × 103 M−1s−1 for peroxynitrite (ONOO−) at pH 7.4 [17]). Thus, thiols and thiol proteins are considered to be primary targets associated with HNO biological activity (vide infra).
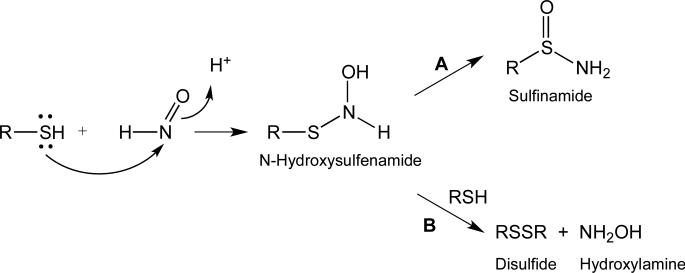
The reaction of HNO with thiols proceeds via initial attack of the sulfur atom of the thiol at the electrophilic nitrogen of HNO, giving a fleeting N-hydroxysulfenamide intermediate (Figure 1) [18,19].
Figure 1.
Reaction of HNO with thiols.
The N-hydroxysulfenamide intermediate has not been isolated as it can spontaneously rearrange to a sulfinamide (Figure 1, pathway A) or, in the presence of excess thiol, will produce a disulfide and hydroxylamine (Figure 1, pathway B). The generation of a sulfinamide product in a biological system represents a nearly irreversible (or at least very slowly reversible) thiol modification. However, disulfide formation represents a biologically reversible modification. Thus, an isolated thiol (i.e. remote from other thiols or at low concentration) will give predominantly an irreversible sulfinamide adduct whereas a thiol in the presence of high concentrations of other thiols or in close proximity to another thiol will react with HNO to give a reversible disulfide adduct.
Recent work regarding the reactivity of HNO with C-terminal cysteine residues (which have a free carboxylate group) has shed light on an alternate reaction pathway to those previously discussed for the reactivity of HNO with thiols [20]. Rather than typical formation of disulfide and sulfinamide products (depending on the conditions), treatment of C-terminal cysteine residues with excess HNO results in formation of thiosulfonate (RS(O)2SR and sulfohydroxamic acid (RS(O)2NHOH) derivatives. Formation of the thiosulfonate and sulfohydroxamic acid products are speculated to proceed through carboxylate-assisted formation of a sulfenic (RSOH) intermediate. However, due to the instability of sulfenic acids (similar to that of N-hydroxysulfenamide intermediates), isolation of the sulfenic acid intermediate could not be achieved.
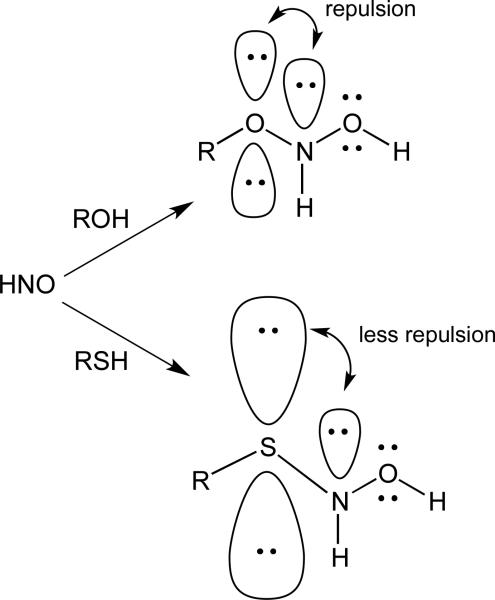
As mentioned above, HNO reacts readily with “soft” nucleophiles such as thiols and phosphines and much less so with “hard” nucleophiles such as alcohols or water (amines are intermediate between these two extremes). This propensity is thought to be due to the repulsion between the lone pair on nitrogen and the lone pairs on oxygen when the hydrate forms [13]. In the case of a thiol nucleophile, the longer bond distances and the greater size of the orbitals on sulfur decrease the repulsion between the nitrogen lone pair and the electrons on the thiol sulfur (Figure 2).
Figure 2.
Electron pair repulsion with HNO/ROH and HNO/RSH adducts.
The recent discovery of high levels of hydropersulfides (RSSH) in biological tissue and fluids [21] alludes to a possible (and likely favorable) reaction of HNO with these oxidized thiol species, especially since it is reported that hydropersulfides possess enhanced nucleophilicity and reducing capability compared to the corresponding thiol [22,23]. Thus, it may be expected that the reaction of HNO with a persulfide is significantly faster than with the corresponding thiol. Indeed, it was found that HNO was capable of reacting with a hydropersulfide [24], although the chemical details of this interaction await clarification. Interestingly, when compared to the corresponding thiol, the HNO-modified persulfide was more easily reduced back to the thiol by an exogenous reductant (dithiothreitol, DTT). That is, HNO-mediated modification of a thiol led to significant irreversible modification (Figure 1, pathway A) whereas HNO-mediated modification of a hydropersulfide led to products that could largely be reduced back to the thiol using thiol-based reductants. Although the detailed chemistry responsible for the DTT-mediated reversal of HNO-modified persulfides is not known, Figure 3 schematically illustrates how reversal of an HNO-modified persulfide may be possible via reductive cleavage of a sulfur-sulfur bond. However, HNO-mediated modification of a thiol generates a sulfinamide, which cannot easily be reductively converted to the corresponding thiol by, for example, DTT.
Figure 3.
HNO-mediated modification of a thiol versus a persulfide and possible mechanism of reductive conversion of the adduct to the corresponding thiol.
To be sure, the generation of a persulfide sulfinamide (Figure 3, the persulfide/HNO intermediate shown) has not been demonstrated and its intermediacy is based purely on extrapolation of the known thiol chemistry. With regards to persulfide/HNO reactivity, it is intriguing to speculate that HNO reacts preferentially with persulfides compared to thiols since persulfides are superior nucleophiles [23] and HNO is known to be highly reactive towards sulfur species. If this is true, this may explain the high apparent specificity of HNO biological activity. That is, HNO demonstrates extremely potent and specific biological activities, consistent with the idea that it reacts preferentially with only specific biological targets (as opposed to having general reactivity with numerous thiol proteins). Since numerous protein persulfides have been detected [21], it is possible that the specificity of HNO signaling may be a function of the presence of a persulfide. Of course, this idea is highly speculative at this time and further experimentation will be required to test this hypothesis.
The reaction of HNO with thiols (and possibly with hydropersulfides) likely represents a primary mechanism by which HNO elicits its biological activity. That is, modification of thiol proteins (or protein hydropersulfides) by HNO can lead to changes in function and/or activity. It should also be considered that selenoproteins can also be primary targets for HNO reactivity. Selenium is directly below sulfur on the periodic table and, like hydropersulfides, selenols are considered to be more nucleophilic and more reducing compared to the corresponding thiol [25,26,27]. Currently, only a few studies have examined the interaction between HNO and a selenoprotein. One study examined the ability for HNO to inhibit the selenoprotein glutathione peroxidase (GPx) [28] and reports that HNO was capable of inhibiting the activity of purified GPx under both aerobic and anaerobic conditions, with slightly more inhibition observed under aerobic conditions. Moreover, approximately 50% of the inhibition of GPx by HNO could not be reversed by the thiol reductant DTT, alluding to a significant population of an irreversibly modified adduct. It needs to be stressed, however, that this work is primarily descriptive and although it seems likely that HNO-mediated modification of the active site selenocysteine occurs, this chemistry has not been established and the irreversible portion of this inhibition may be due to interactions at protein cysteine residues as opposed to interactions with selenocysteine.
More recently, the reactivity of HNO with small organoselenium compounds has been studied [29]. In this work it was determined that organic selenol species are resistant towards irreversible HNO-induced modification. Whereas the treatment of thiols with HNO can result in disulfide (high thiol concentration) or sulfinamide (low thiol concentration) formation, treatment of selenols with HNO results in diselenide formation only under all conditions (both high and low selenol concentration). This implies that while HNO is able to inactivate thiol proteins, selenoproteins have the ability to revert easily to an active state following treatment with HNO. Additionally, organic selenol species displayed an enhanced ability to trap HNO, in comparison to analogous thiols. Again, as mentioned in regard to hydropersulfides, these findings may indicate a preferential specificity of HNO for selenoproteins over thiol proteins. Indeed, the presence of selenoproteins may play a significant role in the biological signaling pathway of HNO although further analysis is needed.
The N-H bond dissociation energy (BDE) for H-NO is relatively weak (approximately 47 kcal/mole) [30]. Thus, HNO is a reasonable H-atom donor capable of quenching oxidizing radical intermediates (Reaction 3). Reaction of HNO with one-electron oxidants generates NO, which is also capable of reacting with and quenching oxidizing radical species (Reaction 4).
| (3) |
| (4) |
Thus, HNO is an efficient anti-oxidant capable of limiting free-radical chain reactions (vide infra) [31]. The facility of Reaction 3 is evidenced by reports that HNO can donate a hydrogen atom to even relatively stable radicals and weak oxidants such as 4-hydroxy-2,2,6,6-tetramethyl-1-piperinyloxyl and related nitroxides (R2NO·) generating NO and the corresponding hydroxylamine [32,33]. To be sure, the physiological/pharmacological relevance or importance of this chemical property remains to be demonstrated.
The ability for HNO to act as a reductant (via H-atom donation) is consistent with the idea that NO is a relatively poor oxidant (i.e. a poor hydrogen-atom abstractor). The lack of NO oxidizing activity is supported by the low reduction potential for NO. The NO,H+/HNO couple is estimated to be −0.55 V (pH7) and the NO/NO− couple is estimated to be −0.81 V (both versus NHE) [11]. Also, as mentioned previously, the H-NO BDE is only 47 kcal/mole, indicating that abstraction of a hydrogen atom by NO will only be favorable if the hydrogen atom being abstracted has a BDE lower than 47 kcal/mol. Given that the BDEs most C-H and O-H bonds are nearly twice this value, direct hydrogen abstraction by an NO radical (yielding HNO) is expected to be unlikely or at least extremely slow. Conversely, HNO can potentially serve as a capable hydrogen atom donor to carbon- or heteroatom-based radicals.
In spite of the apparent difficulty in NO reduction via abstraction of a hydrogen atom (radical) or electron transfer, several recent reports indicate that NO is capable of being reduced by alcohols (especially phenols or “activated” –OH groups) to give HNO [34, 35]. Since the O-H BDEs for HO-H and phenyl-O-H are 119 and 88 kcal/mole, respectively, it remains to be determined how this chemistry occurs. NO-mediated oxidations (giving HNO) can also be evaluated using redox potentials. As mentioned above, the NO,H+/HNO reduction potential is estimated to be −0.55 V (vs NHE at pH 7). The reduction potentials for the HO·,H+/H2O and Phenyl-O·,H+/Phenyl-OH couples are 2.3 and 0.9 V (vs NHE, pH 7.0), respectively. Thus, these values also indicate a highly unfavorable reaction between NO and alcohols (at least under standard conditions).
While the idea that NO can serve as an electron acceptor toward nucleophiles under typical synthetic conditions remains surprising, and perhaps even provocative, evidence does exist for the participation of NO in reactions involving oxygen and nitrogen-based nucleophiles (although the biological relevance of this chemistry remains in doubt, vide infra). The unpaired electron of neutral NO resides in a doubly-degenerate, antibonding π* orbital; thus reactions involving NO and the electron pair of a nucleophile would necessarily result in the occupancy of an antibonding orbital by one electron of the adduct. In principle, such an initial reaction step would be expected to be disfavored without a thermodynamic basin or other driving force. However, evidence exists for nucleophile-NO adducts, such as the formation of N-nitroso compounds resulting from reaction of NO with carbazolide anions as reported by Zhu et al. [36 1999] (chemistry performed under non-physiological conditions). In this instance, anion/NO addition is proposed followed by reduction of free NO by the resultant radical anion. While this might be expected to be energetically prohibitive based on the best current estimates of NO reduction onset potential (vide supra) under conditions involving an excess of NO, participation of the radical anion of the NO dimer and/or other multimeric states may facilitate this process [for example, 37]. Also, Janzen et al. [38] have reported the reaction of phenolic antioxidants with an excess of NO (also under non-physiological conditions), yielding products characteristic of reversible O-nitroso formation, and EPR signals consistent with phenoxyl radicals were observed upon removal of NO from the solution. As mentioned above, the idea of NO reduction by alcohols has been recently revisited [34,35], and the proposal of a proton-coupled nucleophilic attack of phenolic or ascorbate oxygen upon the nitrogen of NO with concurrent or concomitant proton transfer has been put forth. It is proposed that ensuing O-N cleavage yields the resultant phenoxyl radical and HNO. While the initial steps of this process are, as expected, energetically uphill as demonstrated by density functional theory calculations, subsequent HNO dimerization to N2O is proposed as a thermodynamic driving force. Regardless, the biological relevance of this chemistry remains to be established.
Besides reaction with sulfur species, HNO can also react with metals/metalloproteins. This topic has been extensively reviewed previously [8] and only a brief discussion of this topic will be given. Classic examples of HNO-metalloprotein interactions are its reactions with iron heme proteins. For example, HNO is capable of reacting with both ferric (Fe3+) and ferrous (Fe2+) iron heme systems. Some of the earliest reports of the interaction of HNO with ferric heme indicate the ability of HNO to react with metmyoglobin (MbFe3+) and/or methemoglobin (HbFe3+) giving ultimately the ferrous nitrosyl complex (MbFe2+-NO) [18,39] (Reaction 5).
| (5) |
This is a relatively fast reaction with a reported rate constant of 8 × 105 M−1s−1 [14]. Later it was shown by the Farmer lab that HNO can coordinate a ferrous heme protein, e.g. ferrous myoglobin (MbFe2+) to generate a surprisingly stable and essentially irreversible adduct whereby HNO coordinates via the nitrogen atom and with a rate constant of >1.4 × 104 M−1s−1 (Reaction 6) [40,41,42].
| (6) |
The formation of a ferrous-nitrosyl species with the enzyme soluble guanylate cyclase (sGC) leads to a tremendous increase in enzyme activity resulting in significant increases in intracellular cyclic guanosine monophosphate (cGMP) and, in vascular tissue, vasorelaxation [43]. Indeed, this is the primary mechanism by which NO regulates vascular tone. The normal state of the iron heme group in sGC is the ferrous form (sGC-Fe2+), which can directly coordinate NO, and binding of NO to the Fe2+ heme results in activation via sGC-Fe2+NO formation. Since HNO is capable of reacting with ferric hemes to form the corresponding ferrous-nitrosyl complex (Reaction 5), it was hypothesized that HNO may be capable of activating the ferric enzyme to the same extent that NO activates the ferrous enzyme. However, this is not the case as ferric sGC was found to be substitutionally inert (six-coordinate with little or no ligand dissociation to open up a coordination site) and will not directly interact with HNO [44].
There have been several reports that indicate a reaction between HNO and O2 can produce a potent oxidant [45,46]. Interestingly, the uncharacterized oxidant produced appears to be distinct from the established oxidant peroxynitrite (ONOO−), a species that could be made from the iso-electronic reaction of NO and O2 − [17]. The biological relevance of this chemistry is, as yet, undetermined.
Currently, it is thought that the major biological targets responsible for HNO bioactivity (vide infra) are thiols/thiol proteins, although selenoproteins and protein hydropersulfides need to be seriously considered as possible, if not primary, sites as well. Clearly, metalloproteins are also possible sites of interaction, although to date there is only scant speculation that HNO bioactivity involves metal centers. It needs to be stressed, however, that in spite of the numerous biological effects associated with HNO, only a few have an established biological target or a well-defined and specific mechanism of action. Some of the better-defined examples are presented below.
HNO Signaling/Biological Activity
The brief discussion of HNO chemical biology indicates that sulfur- and/or selenium-containing sites, and possibly metals, are likely targets for HNO-mediated biological effects. Not surprisingly, the majority of proposed activities associated with HNO involve interaction at these targets. Below are discussed specific examples of HNO-mediated effects on signaling systems and/or biochemical processes. To be sure, the examples given below do not represent a comprehensive listing of all of the biological actions attributed to HNO. For a more in depth treatment of this topic other reviews are available [3,4]. It is hoped that the following examples will suffice in illustrating the range of possible effects associated with HNO and the nature of the likely targets.
Inhibition of aldehyde dehydrogenase
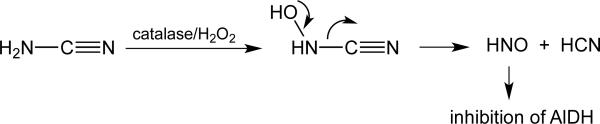
Some of the earliest reports of HNO biological activity are from the Nagasawa lab, which discovered that the anti-alcoholic drug cyanamide (NH2CN) was capable of inhibiting the enzyme aldehyde dehydrogenase (AlDH) via oxidative metabolism to HNO [for example, 47 Nagasawa et al., 1990]. That is, the ubiquitous peroxidase catalase is capable of oxidizing NH2CN to the corresponding N-hydroxycyanamide, which can spontaneously decomposes to give HCN and HNO (Figure 4).
Figure 4.
Mechanism of HNO generation from NH2CN.
HNO-Mediated inhibition of AlDH thus led to a buildup of the ethanol metabolite acetaldehyde and subsequent adverse physiological effects, providing a specific mechanism for the use of cyanamide in alcohol aversion therapy. In support of this idea, other HNO-donors species were also capable of inhibiting AlDH [48]. This work on the mechanism of action of cyanamide represents the first published example of the ability of HNO to elicit a biological effect via inhibition of a thiol protein, in this case AlDH. It was also found that HNO-mediated inhibition of AlDH exhibited both reversible (with the addition of DTT or glutathione (GSH)) and irreversible behavior [49]. These observations led to the idea that HNO could react with thiol proteins to give either reversible or irreversible adducts and prompted subsequent studies describing the chemistry shown in Figure 1 [19]. Thus, the work describing the mechanism of cyanamide-mediated inhibition of AlDH (via initial HNO generation) represents one of the few clear and established examples of HNO interacting at a thiol protein to elicit a specific biological effect.
Effects on sGC
The discovery of NO as a vasorelaxant (vide supra) prompted the examination of the cardiovascular effects of other related or derived nitrogen oxides. An early study using the HNO-donor Angeli's salt indicated that HNO could activate sGC, leading to increases in intracellular cGMP and, ultimately, vasorelaxation [50]. More detailed subsequent examinations of the interaction of HNO with sGC by two groups gave conflicting results. Miller and coworkers [44] found that HNO could activate purified sGC (albeit to a lesser degree than NO) and proposed a possible interaction of HNO with the ferrous heme form of the enzyme. However, Zeller and coworkers [51] reported that HNO-mediated activation of sGC requires prior oxidation of HNO to NO (by, for example, SOD). Significantly, both studies report that HNO is incapable of activating the ferric form of sGC. Regardless, whether HNO activates sGC directly or indirectly via conversion of NO, cGMP levels are elevated in the presence of HNO and thus some of the biological actions of HNO could be a result of sGC activation and increased levels of cGMP. A more recent study using cardiomyocytes reports that HNO (from Angeli's salt) is capable of activating sGC, leading to increases in steady-state levels of cGMP and a protection from cardiac hypertrophy [52].
Effect on failing hearts
One of the more exciting and impressive actions of HNO, at least from a therapeutic perspective, is its effect on failing hearts. Indeed, HNO-mediated treatment for heart failure has received significant and recent attention [for example, 53,54,55]. In the scenario of heart failure, HNO is capable of enhancing Ca2+ cycling (i.e. simultaneous increases in Ca2+ release and reuptake by the sarcoplasmic reticulum (SR)), increasing the Ca2+ sensitivity of myofilaments, eliciting a combined arterial and venous dilation and all without an increase in heart rate [53]. Taken altogether, these effects represent a near ideal therapeutic profile for the treatment of heart failure.
The effect of HNO on enhanced Ca2+ cycling can be attributed to effects on thiol proteins. The release of Ca2+ from the SR occurs via the ryanodine receptor channel (RyR2) and RyR2 stimulation leads to an increase in Ca2+ efflux and inotropy (increased contractility). Interestingly, RyRs are known to be regulated by thiol modifying agents [for example, 56,57] and the ability of HNO to increase Ca2+ efflux is due to affects on RyR2 [58]. Uptake of Ca2+ from the cytosol back into the SR is carried out by the Ca2+ reuptake transporter SR Ca2+ pump (SERCA2a). Importantly, SERCA2a is regulated by another protein phospholamban (PLN) and it has been shown that the cysteine thiols on PLN are vital to its interactions with HNO [59,60]. Using isolated cardiomyocytes and whole hearts from wild-type and PLN knockout mice, the HNO-induced positive inotropy/lusitropy was shown to be lost in PLN null hearts/myocytes, and that HNO actions are PKA-independent, as confirmed by the lack of Ser16 phosphorylation on PLN after HNO treatment. From these studies, it was concluded that HNO functionally uncouples PLN from SERCA2a, in a manner reminiscent of, but independent from cAMP/PKA signaling. A more recent study examining the modification of PLN by HNO employed the use of 15N-edited NMR spectroscopy and has identified two of three possible cysteine residues (Cys41 and Cys46) on PLN as being primary sites for HNO reactivity [61]. In this study, treatment of PLN with HNO lead to HNO-induced thiol modification followed by uncoupling of PLN from SERCA2a, relieving the inhibition of SERCA2a. Finally, Ca2+ sensitization of the myofilaments has been shown to be due to HNO-mediated promotion of disulfide bond formation between critical cysteine residues on the myofilaments themselves [62]. Thus, the effects of HNO on the heart (especially the failing heart) illustrates the importance of cysteine thiol targets in HNO biology. It remains to be determined whether persulfide targets in the myocardium may also play a role in the overall effects of HNO on the heart.
HNO inhibition of cysteine proteases, transcription factors and other thiol proteins
Based on the above discussions of HNO-thiol reactivity, it is not unreasonable to suspect that HNO will interact readily with cysteine proteases and other cysteine-based proteins, altering their function. Indeed, several studies have demonstrated that HNO is capable of inhibiting the cysteine proteases papain, cathepsin B as well as 26s proteosomal activity [63,64]. Significantly, a portion of the HNO-mediated inhibition of these enzymes was found to be irreversible (with thiol reductants), consistent with the chemistry shown in Figure 1.
HNO is also capable of inhibiting the activity of cysteine-based dehydrogenases, such as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [65]. Importantly, inhibition of GAPDH by HNO in yeast occurs at a level that does not alter the GSH/GSSG ratio or lead to GSH depletion [66]. This means that the affect of HNO on GAPDH activity is not merely due to a change in the overall thiol redox status on the cell but that HNO is capable of specific interactions with thiol proteins. Moreover, inhibition of GAPDH by HNO was primarily irreversible and could be protected against by the presence of high concentrations of enzyme substrate. Analysis of the HNO-GAPDH adduct by mass spectroscopy found both disulfide and sulfinamide formation on the protein and the authors proposed that the irreversible nature of HNO-mediated GAPDH inhibition could be due to a disruption of the active tetrameric structure [67]. This interpretation brings up the important point that irreversible inhibition of protein function by HNO is not necessarily an indication of sulfinamide formation as other biophysical alterations may also be responsible.
HNO was also found to inhibit the activity of a thiol-containing, metal-responsive yeast transcription factor, Ace1 [68]. Moreover, Ace1 inhibition by HNO was found to be largely irreversible which could be due to sulfinamide formation. An advantage to examining HNO biochemistry in yeast is that it is possible to distinguish between O2-dependent and O2-independent processes (since yeast can grow and thrive aerobically or anaerobically). Thus, it was also determined that the ability for HNO to inhibit Ace1 activity, presumably by disrupting the metal-binding capacity of the Ace1 thiols, was entirely independent of O2. This was an important distinction since previous literature alluded to the possibility that HNO could react with O2 to generate a potent and as yet uncharacterized oxidant (vide infra) that could have oxidized critical metal-binding thiols [45].
HNO and H2O2
Cellular signaling by H2O2 has been proposed in a number of systems, although this remains somewhat controversial due to the unfavorable kinetics associated with the reaction of H2O2 with presumed targets (i.e. cysteine phosphatases) coupled with its rapid destruction by metabolizing enzymes [for example, 16,69]. The metabolic destruction of H2O2 occurs primarily via the actions of the ferric heme protein catalase, the selenoprotein glutathione peroxidase and the thiol-containing peroxiredoxins. Since HNO is known to react with and presumably disrupt ferric heme proteins, selenoproteins and, of course, activated thiol proteins, it was hypothesized that HNO could elicit at least some of its biological activity by inhibiting H2O2 metabolism. Also, since both H2O2 and HNO presumably signal via electrophilic chemistry with thiols, there is a possibility that their signaling pathways are similar. Indeed, Jackson and coworkers [28] examined the relationship between H2O2 and HNO and found that HNO was capable of increasing cellular levels of H2O2. Moreover, a comparison of the effects of H2O2 and HNO on T-cell activation (a process reported to be regulated by H2O2) found that they had identical signaling profiles. These results are consistent with the idea that HNO increased signaling levels of H2O2 or, more likely, that HNO interacted with otherwise H2O2-reactive targets to elicit similar effects. In the case of T-cell signaling, one target was found to be the cysteine phosphatase CD45, which could be inhibited by both HNO and H2O2. Regardless, the chemistry and putative biological targets for HNO and H2O2 are significantly overlapping and it is not surprising that their signaling effects are similar.
Antioxidant actions of HNO – Comparison with H2O2
As discussed previously, the chemistry of HNO predicts that it should be able to quench radical chain reactions via H-atom donation, which generates NO, followed by further quenching of radical chain-carrying intermediates by NO (Reactions 3 and 4). Thus, HNO has the potential to be a potent antioxidant. Indeed, HNO was found to protect yeast supplemented with polyunsaturated fatty acids from oxidative stress and inhibit lipid peroxidation in a manner similar to alpha-tocopherol [31]. As discussed immediately above, HNO and H2O2 can behave similarly as signaling species. However, the ability for HNO to act as an antioxidant makes it distinct form H2O2. It is well-established that H2O2 can serve as a precursor for the generation of a potent and indiscriminant oxidant, hydroxyl radical (HO·), via Fenton chemistry (Reaction 7).
| (7) |
Thus, H2O2 in the presence of a reduced metal, such as Fe2+ or Cu1+, will generate the potent one-electron oxidant HO·, which can initiate damaging radical chain processes in, for example, membranes containing polyunsaturated fatty acids. Because of the Fenton reaction, H2O2 signaling via thiol modification may also lead to radical-mediated cellular damage (i.e. lipid peroxidation, protein carbonyl formation, etc.). On the other hand, this will not be a problem with HNO signaling since HNO is capable of modifying biological thiols (akin to H2O2, vide supra) but unlike H2O2 will inhibit indiscriminant and potentially deleterious radical processes.
Other effects
The biological effects associated with pharmacological application of HNO donors go beyond the few examples above. HNO has also been shown to inhibit neuropathic pain and inflammatory hyperalgesia [70,71], regulate STAT3 signaling [72], activate glucose uptake [73] and serve to protect myocardial tissue in a manner akin to pre-conditioning [74], just to name a few.
HNO versus NO signaling
Considering the fact that HNO can be oxidized to NO (i.e. HNO is a reasonable reductant, for example Reactions 3 and 5), it is tempting to propose that the signaling associated with HNO could be merely due to conversion to NO. Although this is clearly possible [for example, 51], it is noteworthy that many of the HNO-mediated effects described above have been shown to be due to HNO itself and not dependent on HNO conversion to NO. A comparison of HNO and NO chemistry/signaling indicates some very important differences that make the biological targets for HNO and NO very distinct [75]. For example, concentrating on thiol redox signaling, it is important to realize that NO does not directly react with thiols. NO-mediated modification of a thiol to give an S-nitrosthiol requires a one-electron oxidant to convert NO to a nitrosonium (NO+)-like species. Conversion of a thiol to a reactive thiyl (RS·) species by NO also requires NO to be converted to a more potent oxidant. Thus, all reactions of thiols with NO require NO to be first converted to a more reactive species. On the other hand, HNO directly reacts with thiols and does not require any “pre-activation”. It also should be emphasized that the products of HNO- versus NO-mediated thiol modification are different. HNO-mediated thiol modification leads to either a disulfide or sulfinamide (via N-hydroxysulfenamide intermediacy, Figure 1), whereas NO-mediated thiol modification often results initially in the generation of an S-nitrosothiol [75]. Thus, it is becoming increasingly clear that HNO signaling is distinct from NO signaling, something the chemistries of HNO and NO predict.
Endogenous HNO generation
An overriding question in the HNO field is whether it is endogenously generated for the purposes of signaling. To be sure, there are numerous chemical processes whereby HNO could be made in a biological system. For example, HNO can be made via the reaction of thiols with S-nitrosothiols, by nitric oxide synthases that are deplete in tetrahydrobiopterin and by oxidation of N-hydroxyarginine (the intermediate in NO biosynthesis) or hydroxylamine [for example, 3,4]. Whether any of these processes are regulated and used to intentionally produce HNO as a cell signaling species is not established. Moreover, other pathways for endogenous HNO generation may be discovered in the future. It is worth noting, however, that the specific physiological affects associated with the pharmacological administration of HNO for the treatment of heart failure represents a remarkable scenario that begs the question: Is HNO made endogenously as a means of regulating heart function? That is, the reports that HNO is capable of eliciting simultaneous inotropy and lusitropy on the heart via an enhancement of Ca2+cycling, sensitizes myofilaments to Ca2+, causes a balanced arterial and venous dilation while not eliciting tachycardia or tolerance [76] and all this without any apparent toxicity would represent a near perfect physiological response to a failing heart and a remarkable and unprecedented pharmacological profile for a single drug. A more reasonable explanation for the pharmacological profile of HNO is that it is simply enhancing/supplementing a physiological HNO signaling system that is already in place. In many regards, this is reminiscent of the situation with NO prior to the discovery of its regulated biosynthesis via the nitric oxide synthases. NO and NO-releasing species/drugs were found to be extremely potent vasorelaxants via activation of the heme-protein sGC [77] and the subsequent discovery of NO biosynthesis led directly to the idea that NO is an endogenously generated vasorelaxant. In the case of HNO, there does not appear to be a single signaling target (like sGC and NO) but rather numerous thiol targets, but other than that the similarities can be considered striking. Regardless, the question of regulated and intentional HNO biosynthesis remains an open one, but it is safe to say that discovery of endogenous, regulated HNO generation for the purposes of controlling vascular and heart function would not be at all surprising.
Summary
It seems clear that HNO signaling can occur via interactions with thiol proteins (or as discussed above, selenoproteins or protein persulfides). Many of the examples given above and the known chemistry of HNO are consistent with this idea. From a purely pharmacological perspective, HNO possesses intriguing and important properties that can lead to the development of therapies for, at the very least, cardiovascular ailments and it seems likely that other therapeutic uses for HNO will be forthcoming.
The chemical biology of HNO appears to revolve around its electrophilicty, in particular its reaction with nucleophilic thiols/thiolproteins.
The biological activity of HNO can be explained by its ability to interact with specific thiol proteins.
Specific interactions of HNO with thiol proteins in the cardiovascular system give it ideal properties for the treatment of heart failure.
There is a prediction that HNO will also interact extensively with selenoproteins and biological hydropersulfides.
Acknowledgment
The authors wish to acknowledge support from the NSF (CHE-1213438 and CHE-1566065) for JPT and (CHE-1148641) for JMF and the NIH (HL106598) for JMF.
Abbreviations
- RSSH
hydropersulfides
- DTT
dithiothreitol
- GPx
glutathione peroxidase
- BDE
bond dissociation energy
- NHE
normal hydrogen electrode
- MbFe3+
metmyoglobin
- HbFe3+
methemoglobin
- MbFe2+
ferrous myoglobin
- sGC
soluble guanylate cyclase
- cGMP
cyclic guanosine monophosphate
- AlDH
aldehyde dehydrogenase
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- RyR2
ryanodine receptor channel
- SERCA2a
SR Ca2+ pump
- PLN
phospholamban
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basudhar D, Ridnour LA, Cheng R, Kesarwala AH, Heinecke J, Wink DA. Biological signaling by small inorganic molecules. Coord. Chem. Rev. 2016;306:708–723. doi: 10.1016/j.ccr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuto JM, Carrington SJ. HNO Signaling Mechanisms. Antiox. Redox Signal. 2011;14(9):1649–1657. doi: 10.1089/ars.2010.3855. [DOI] [PubMed] [Google Scholar]
- 3.Irvine JC, Ritchie RH, Favaloro JL, Andrews KL, Widdop RE, Kemp-Harper BK. Nitroxyl (HNO): The Ciderella of the nitric oxide story. Trends Pharmacol. Sci. 2008;29:601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Shoman M, Aly OM. Nitroxyl (HNO): A reduced form of nitric oxide with distinct chemical, pharmacological, and therapeutic properties. Oxid. Med. Cellular Longevity. 2016 doi: 10.1155/2016/4867124. 2016, ID 4867124, 15 pages. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Miranda KM, Nagasawa HT, Toscano JP. Donors of HNO. Curr. Top. Med. Chem. 2005;5:649–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- 6.Rivera-Fuentes P, Lippard SJ. Metal-based optical probes for live cell imaging of nitroxyl (HNO) Acc. Chem. Res. 2015;48:2927–2934. doi: 10.1021/acs.accounts.5b00388. [DOI] [PubMed] [Google Scholar]
- 7.Miranda KM. The chemistry of nitroxyl (HNO) and implications in biology. Coord. Chem. Rev. 2005;249:433–455. [Google Scholar]
- 8.Farmer PJ, Sulc F. Coordination chemistry of the HNO ligand with hemes and synthetic coordination complexes. J. Inorg. Biochem. 2005;99:166–184. doi: 10.1016/j.jinorgbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN. The Physiological Chemistry and Biological Activity of Nitroxyl (HNO): The Neglected, Misunderstood and Enigmatic Nitrogen Oxide. Chem. Res. Toxicol. 2005;18:790–801. doi: 10.1021/tx0496800. [DOI] [PubMed] [Google Scholar]
- 10.Connelly NG, Damhus T, Hartshorn RM, Hutton AT. Nomenclature of Inorganic Chemistry. IUPAC Recommendations 2005. Royal Society of Chemistry; Cambridge: 2005. [Google Scholar]
- 11.Shafirovich V, Lymar SV. Nitroxyl and its anion in aqueous solutiions: Spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc. Natl., Acad. Sci., USA. 2002;99:7240–7345. doi: 10.1073/pnas.112202099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartberger MD, Liu W, Ford E, Miranda KM, Switzer C, Fukuto JM, Farmer PJ, Wink DA, Houk KN. The Reduction Potential of Nitric Oxide (NO) and Its Importance to NO Biochemistry. Proc. Natl. Acad. Sci., USA. 2002;99(17):10958–10963. doi: 10.1073/pnas.162095599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartberger MD, Fukuto JM, Houk KN. On the Acidity and Reactivity of HNO in Aqueous Solution and Biological Systems. Proc. Natl. Acad. Sci, USA. 2001;98(5):2194–2198. doi: 10.1073/pnas.041481598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda K,M, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci., USA. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson MI, Han TH, Serbulea L, Dutton A, Ford E, Miranda KM, Houk KN, Wink DA, Fukuto JM. Kinetic Feasibility of Nitroxyl (HNO) Reduction by Physiological Reductants and Biological Implications. Free Radic. Biol. Med. 2009;47:1130–1139. doi: 10.1016/j.freeradbiomed.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone JR. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch. Biochem. Biophys. 2004;422:119–124. doi: 10.1016/j.abb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 18.Doyle MP, Mahapatro SN, Broene RD, Guy JK. Oxidation and reduction of hemoproteins by trioxodinitrate(II): the role of nitrosyl hydride and nitrite. J. Am. Chem. Soc. 1988;110:593–599. [Google Scholar]
- 19.Wong PS-Y, Hyun J, Fukuto JM, Shiroda FN, DeMaster EG, Nagasawa HT. The Reaction between Nitrosothiols and Thiols: Generation of Nitroxyl (HNO) and Subsequent Chemistry. Biochemistry. 1998;37(16):5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 20.Keceli G, Toscano JP. Reactivity of C-terminal cysteines with HNO. Biochemistry. 2014;53:3689–3698. doi: 10.1021/bi500360x. [DOI] [PubMed] [Google Scholar]
- 21.Ida T, Sawa T, Ihara H, Kasamatsu S, Kunieda K, Tsuchiya Y, Watanabe Y, Kumagai Y, Nishida M, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaiake T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci., USA. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono K, Akaike T, Sawa T, Kumagai Y, Wink DA, Tantillo DJ, Hobbs AJ, Nagy P, Xian M, Lin J, Fukuto JM. The redox chemistry and chemical biology of H2S, hydropersulfides and derived species: Implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014;77:82–94. doi: 10.1016/j.freeradbiomed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saund SS, Sosa V, Henriquez S, Nguyen QNN, Bianco CL, Soeda S, Millikin R, White C, Le H, Tantillo DJ, Kumagai Y, Akaike T, Lin J, Fukuto JM. The chemical biology of hydropersulfides (RSSH): Chemical stability, reactivity and redox roles. Arch. Biochem. Biophys. 2015;588:15–24. doi: 10.1016/j.abb.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millikin R, Bianco CL, White C, Saund SS, Henriquez S, Sosa V, Akaike T, Kumagai Y, Soeda S, Toscano JP, Lin J, Fukuto JM. The chemical biology of protein hydropersulfides: Studies of a possible protective function of biological hydropersulfide generation. Free Rad. Biol. Med. 2016;97:136–147. doi: 10.1016/j.freeradbiomed.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinmann D, Nauser T, Koppenol WH. Selenium and sulfur in exchange reactions: A comparative study. J. Org. Chem. 2010;75:6696–6699. doi: 10.1021/jo1011569. [DOI] [PubMed] [Google Scholar]
- 26.Nauser T, Steinmann D, Grassi G, Koppenol WH. why selenocysteine replaces cysteine in thioredoxin reductase: A radical hypothesis. Biochemistry. 2014;53:5017–5022. doi: 10.1021/bi5003376. [DOI] [PubMed] [Google Scholar]
- 27.Reich HJ, Hondal RJ. Why nature chose selenium. ACS Chem. Biol. 2016;11:821–841. doi: 10.1021/acschembio.6b00031. [DOI] [PubMed] [Google Scholar]
- 28.Jackson MI, Fields HF, Lujan TS, Cantrell MM, Lin J, Fukuto JM. The Effects of Nitroxyl (HNO) on H2O2 Metabolism and Possible Mechanisms of HNO Signaling. Arch. Biochem. Biophys. 2013;538:120–129. doi: 10.1016/j.abb.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianco CL, Moore CD, Fukuto JM, Toscano JP. Selenols are resistant to irreversible modification by HNO. Free Rad. Biol. Med. 2016 doi: 10.1016/j.freeradbiomed.2016.07.008. in press. [DOI] [PubMed] [Google Scholar]
- 30.Dixon RN. The heats of formation of HNO and DNO. J. Chem. Phys. 1996;104:6905–6906. [Google Scholar]
- 31.Lopez BE, Han TH, Shinyashiki M, Fukuto JM. The Antioxidant Actions of Nitroxyl (HNO) Free Radic. Biol. Med. 2007;42:482–491. doi: 10.1016/j.freeradbiomed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Wink DA, Feelisch M, Fukuto J, Christodoulou D, Jourd'heuil D, Grisham MB, Vodovotz Y, Cook JA, Krishna M, DeGraff WG, Kim S, Gamson J, Mitchell JB. The cytotoxicity of nitroxyl: Possible implications for the pathophysiological role of NO. Arch. Biochem. Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 33.Cline MR, Toscano JP. Detection of nitroxyl (HNO) by a prefluorescent probe. J. Phys. Org. Chem. 2011;24:993–998. [Google Scholar]
- 34.Suarez SA, Neuman NI, Munoz M, Alvarez L, Bikiel DE, Brondino CD, Ivanovic-Burmavovic I, Miljkovic JL, Filipovic MR, Marti MA, Doctorovich F. Nitric oxide is reduced to HNO by proton-coupled nucleophilic attack by ascorbate, tyrosine, and other alcohols. A new route to HNO is biological media? J. Am. Chem. Soc. 2015;137:4720–4727. doi: 10.1021/ja512343w. [DOI] [PubMed] [Google Scholar]
- 35.Hamer M, Suarez SA, Neuman NI, Alvarez L, Munoz M, Marti MA, Doctorovich F. Discussing endogenous NO/HNO interconversion aided by phenolic drugs and vitamins. Inorg. Chem. 2015;54:9342–9350. doi: 10.1021/acs.inorgchem.5b01347. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X-Q, Xian M, Wanf K, Cheng J-P. Is NO (nitric oxide) an electron acceptor or an electrophile? A detailed tyhermodynamic investigation on the mechanisms of NO-initiated reactions with 3,6-dibromocarbazolide anion and related carbanion. J. Org. Chem. 1999;64:4187–4190. [Google Scholar]
- 37.Zhao Y-L, Bartberger MD, Goto K, Shimada K, Kawashima T, Houk KN. Theoretical evidence for enhanced NO dimerization in aromatic hosts: Implications for the role of the electrophile (NO)2 in nitric oxide chemistry. J. Am. Chem. Soc. 2005;127:7964–7965. doi: 10.1021/ja042247s. [DOI] [PubMed] [Google Scholar]
- 38.Janzen EG, Wilcox AL, Manoharan V. Reactions of nitric oxide with phenolic antioxidants and phenoxyl radicals. J. Org. Chem. 1993;58:3597–3599. [Google Scholar]
- 39.Bazylinski DA, Hollocher TC. Evidence from the reaction between trioxodinitrate(II) and 15NO that trioxodinitrate(II) decomposes into nitrosyl hydride and nitrite in neutral aqueous solution. Inorg. Chem. 1985;24:4285–4288. [Google Scholar]
- 40.Sulc F, Immoos CE, Pervitsky D, Farmer PJ. Efficient trapping of HNO by deoxymyoglobin. J. Am. Chem. Soc. 2004;126:1096–1101. doi: 10.1021/ja0376184. [DOI] [PubMed] [Google Scholar]
- 41.Immoos CE, Sulc F, Farmer PJ, Czarnecki K, Bocian DF, Levina A, Aitkin JB, Armstrong RS, Lay PA. Bonding in HNO-myoglobin as characterized by X-ray absorption and resonance raman Spectroscopies. J. Am. Chem. Soc. 2004;127:814–815. doi: 10.1021/ja0433727. [DOI] [PubMed] [Google Scholar]
- 42.Kumar MR, Pervitsky D, Chen L, Poulos T, Kundu S, Hargrove MS, Rivera EJ, Diaz A, Colon JL, Farmer PJ. Nitrosyl hydride (HNO) as an O2 analogue: long-lived HNO adducts of ferrous globins. Biochemistry. 2009;48:5018–5025. doi: 10.1021/bi900122r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hobbs AJ. Soluble guanylate cyclase: The forgotten sibling. Trends Pharmacol. Sci. 1997;18:484–491. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- 44.Miller TW, Cherney ME, Lee AJ, Francoleon N, Farmer PJ, King SB, Hobbs AJ, Miranda K, Burstyn JN, Fukuto JM. The Effects of Nitroxyl (HNO) on Soluble Guanylate Cyclase Activity: Interactions at Ferrous Heme and Cysteine Thiols. J. Biol. Chem. 2009;284:21788–21796. doi: 10.1074/jbc.M109.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miranda KM, Espey MG, Yamada K, Krishna M, Ludwick N, Kim S, Jourdheuil D, Grisham MB, Feelisch M, Fukuto JM, Wink DA. Unique Oxidative Mechanisms for the Reactive Nitrogen Oxide Species, Nitroxyl Anion. J. Biol. Chem. 2001;276:3, 1720–1727. doi: 10.1074/jbc.M006174200. [DOI] [PubMed] [Google Scholar]
- 46.Miranda KM, Yamada K, Espey MG, Thomas DD, DeGraff W, Mitchell JB, Krishna MC, Colton CA, Wink DA. Further evidence for distinct reactive intermediates from nitroxyl and peroxynitrite: effects of buffer composition on the chemistry of Angeli's salt and synthetic peroxynitrite. Arch. Biochem. Biophys. 2002;401:134–144. doi: 10.1016/S0003-9861(02)00031-0. [DOI] [PubMed] [Google Scholar]
- 47.Nagasawa HT, DeMaster EG, Redfern B, Shirota FN, Goon DJW. Evidence for nitroxyl in the catalasemediated bioactivation of the alcohol deterrent agent cyanamide. J. Med. Chem. 1990;33:3120–3122. doi: 10.1021/jm00174a001. [DOI] [PubMed] [Google Scholar]
- 48.Lee MJC, Nagasawa HT, Elberling JA, DeMaster EG. Prodrugs of nitroxyl as inhibitors of aldehyde dehydrogenase. J. Med. Chem. 1992;35:3648–3652. doi: 10.1021/jm00098a008. [DOI] [PubMed] [Google Scholar]
- 49.DeMaster EG, Redfern B, Nagasawa HT. Mechanisms of inhibition of aldehyde dehydrogenase by nitroxyl, the active metabolite of the alcohol deterrent cyanamide. Biochem. Pharmacol. 1998;56:2007–2015. doi: 10.1016/s0006-2952(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 50.Fukuto JM, Chiang K, Hszieh R, Wong P, Chaudhuri G. The pharmacological properties of nitroxyl (HNO): A potent vasodilator with activity similar to nitric oxide (NO) and/ or endothelium-derived relaxing factor (EDRF) J. Pharmacol. Exp. Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- 51.Zeller A, Wenzl MV, Beretta M, Stesse H, Russwurm M, Koesling D, Schmidt K, Mayer B. Mechanisms underlying activation of soluble guanylate cyclase by the nitroxyl donor Angeli's salt. Mol. Pharm. 2009;76:1115–1122. doi: 10.1124/mol.109.059915. [DOI] [PubMed] [Google Scholar]
- 52.Lin EQ, Irvine JC, Cao AH, Alexander AE, Love JE, Patel R, McMullen JR, Kaye DM, Kemp-Harper BK, Ritchie RH. Nitroxyl stimulates soluble guanylyl cyclase to suppress cardiomyocyte hypertrophy and superoxide generation. PLoS ONE. 2012;7(4):e34892. doi: 10.1371/journal.pone.0034892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arcaro A, Lembo G, Tocchetti CG. Nitroxyl (HNO) for treatment of acute heart failure. Curr. Heart Fail. Rep. 2014;11:227–235. doi: 10.1007/s11897-014-0210-z. [DOI] [PubMed] [Google Scholar]
- 54.Sabbah HN, Tocchetti CG, Wang M, Daya S, Gupta RC, Tunin RS, Mazhari R, Takimoto E, Paolocci N, Cowart D, Colucci WS, Kass DA. A novel approach for the acute treatment of heart failure. Circ. Heart Fail. 2013;6:1250–1258. doi: 10.1161/CIRCHEARTFAILURE.113.000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge Y, Moss RL. Nitroxyl, redox switches, cardiac myofilaments and heart failure: A prequel to novel therapeutics? Circ. Res. 2012;111:954–956. doi: 10.1161/CIRCRESAHA.112.278416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mi T, Xiao Z, Guo W, Tang Y, Hiess F, Xiao J, Wang Y, Zhang JZ, Zhang L, Wang R, Jones PP, Chen SRW. Role of Cys3602 in the function and regulation of the cardiac ryanodine receptor. Biochem. J. 2015;467:177–190. doi: 10.1042/BJ20141263. [DOI] [PubMed] [Google Scholar]
- 57.Hildago C, Donoso P, Carrasco MA. The ryanodine receptors Ca2+ release channels: cellular redox sensors? IUBMB Life. 2005;57:315–322. doi: 10.1080/15216540500092328. [DOI] [PubMed] [Google Scholar]
- 58.Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, DiBenedetto G, O'Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng H, Kass DA, Paolocci N. Nitroxyl improves cellular heart finction by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ, Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Froehlich JP, Mahaney JE, Keceli G, Pavlos CM, Goldstein R, Redwood AJ, Sumbilla C, Lee DI, Tocchetti CG, Kass DA, Paolocci N, Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- 60.Sivakumaran V, Stanley BA, Tocchetti CG, Ballin JD, Caceres V, Zhou L, Keceli G, Rainer PP, Lee DI, Huke S, Ziolo MT, Kranias EG, Toscano JP, Wilson GM, O'Rourke B, Kass DA, Mahaney JE, Paolocci N. HNO Enhances SERCA2a Activity and Cardiomyocyte Function by Promoting Redox-Dependent Phospholamban Oligomerization. Antioxid. Redox Signaling. 2013;19:1185–1197. doi: 10.1089/ars.2012.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keceli G, Majumdar A, Thorpe CN, Mahaney JE, Paolocci N, Toscano JP. Determination of HNO-derived modifications on the cardiac protein phosphlamban. FASEB J. 2016;30:1092.7. [Google Scholar]
- 62.Gao WD, Murray CI, Tian Y, Zhing X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE, Paolocci N. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ. Res. 2012;111:1002–1011. doi: 10.1161/CIRCRESAHA.112.270827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaananen AJ, Kankuri E, Rauhala P. Nitric oxide-related species-induced protein oxidation: reversible, irreversible and protective effects on enzyme function of papain. Free. Radic. Biol. Med. 2005;38:1102–1111. doi: 10.1016/j.freeradbiomed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Vaananen AJ, Salmenpera P, Hukkanen M, Rauhala P, Kankuri E. Cathepsin B is a differentiation-resistant target for nitroxyl (HNO) in THP-1 monocyte/macrophages. Free Radic. Biol. Med. 2006;41:120–131. doi: 10.1016/j.freeradbiomed.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 65.Lopez BE, Wink DA, Fukuto JM. The Inhibition of Glyceraldehyde-3-Phosphate Dehydrogenase by Nitroxyl (HNO) Arch. Biochem. Biophys. 2007;465:430–436. doi: 10.1016/j.abb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 66.Lopez BE, Rodriguez CE, Pribadi M, Cook N, Shinyashiki M, Fukuto JM. Selective Inhibition of Yeast Glycolysis by Nitroxyl (HNO): Implications to HNO Pharmacology/Physiology. Arch. Biochem. Biophys. 2005;442:140–148. doi: 10.1016/j.abb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 67.Shen B, English AM. Mass spectrometric analysis of nitroxyl-mediated protein modification: comparison of products formed with free and protein-based cysteines. Biochemistry. 2005;44:14030–14044. doi: 10.1021/bi0507478. [DOI] [PubMed] [Google Scholar]
- 68.Cook NM, Shinyashiki M, Jackson MI, Leal FA, Fukuto JM. Nitroxyl (HNO)-Mediated Disruption of Thiol Proteins: Inhibition of the Yeast Transcription Factor Ace1. Arch. Biochem. Biophys. 2003;410:89–95. doi: 10.1016/s0003-9861(02)00656-2. [DOI] [PubMed] [Google Scholar]
- 69.Winterbourne CC. The biological chemistry of hydrogen peroxide. Meth. Enzymol. 2013;528:3–25. doi: 10.1016/B978-0-12-405881-1.00001-X. [DOI] [PubMed] [Google Scholar]
- 70.Zarpelon AC, Souza GR, Cunha TM, Schivo IRS, MArchesi M, Casagrande R, Pinge-Filho P, Cunha FQ, Ferreira SH, Miranda KM, Verri WA., Jr. The nitroxyl donor, Angeli's salt, inhibits inflammatory hyperalgesia in rats. Neuropharmacology. 2013;71:1–9. doi: 10.1016/j.neuropharm.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Longhi-Balbinot DT, Rossaneis AC, Pinho-Ribeiro FA, Bertozzi MM, Cunha FQ, Alves-Filho JC, Cunha TM, Peron JPS, Miranda KM, Casagrande R, Verri WA., Jr. The nitroxyl donor, Angeli's salt, reduces chronic constriction injury-induced neuropathic pain. Chemico-Biological Interact. 2016;256:1–8. doi: 10.1016/j.cbi.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zgheib C, Kurdi M, Zouein FA, Gunter BW, Stanley BA, Zgheib J, Romero DG, King SB, Paolocci N, Booz GW. Acyloxy nitroso compounds inhibit LIF signaling in endothelial cells and cardiac myocytes: Evidence that STAT3 signaling in redox-sensitive. PLoS ONE. 2012;7:e4313. doi: 10.1371/journal.pone.0043313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salie MJ, Oram DS, Kuipers DP, Scripture JP, Cheng J, MacDonald GJ, Louters LL. Nitroxyl (HNO) acutely activates the glucose uptake activity of GLUT1. Biochimie. 2012;94:864–869. doi: 10.1016/j.biochi.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda KM, Feelisch M, Wink DA, Kass DA, Paolooci N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic. Biol. Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. 2003. [DOI] [PubMed] [Google Scholar]
- 75.Fukuto JM, Cisneros CJ, Kinkade RL. A comparison of the chemistry associated with the biological signaling and actions of nitroxyl (HNO) and nitric oxide (NO) J. Inorg. Biochem. 2013;118:201–208. doi: 10.1016/j.jinorgbio.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 76.Irvine JC, Kemp-Harper BK, Widdop RE. Chronic administration of the HNO donor Angeli's salt does not lead to tolerance, cross tolerance or endothelial dysfunction: Comparison with GTN and DEA/NO. Antiox. Redox Sig. 2011;14:1615–1624. doi: 10.1089/ars.2010.3269. [DOI] [PubMed] [Google Scholar]
- 77.Ignarro LJ. Nitric oxide: A unique endogenous signaling molecule in vascular biology. Biosci. Reports. 1998;19:51–71. doi: 10.1023/a:1020150124721. [DOI] [PubMed] [Google Scholar]