Abstract
In recent years, the number of people suffering from cancer and multi-resistant infections has increased, such that both diseases are already seen as current and future major causes of death. Moreover, chronic infections are one of the main causes of cancer, due to the instability in the immune system that allows cancer cells to proliferate. Likewise, the physical debility associated with cancer or with anticancer therapy itself often paves the way for opportunistic infections. It is urgent to develop new therapeutic methods, with higher efficiency and lower side effects. Antimicrobial peptides (AMPs) are found in the innate immune system of a wide range of organisms. Identified as the most promising alternative to conventional molecules used nowadays against infections, some of them have been shown to have dual activity, both as antimicrobial and anticancer peptides (ACPs). Highly cationic and amphipathic, they have demonstrated efficacy against both conditions, with the number of nature-driven or synthetically designed peptides increasing year by year. With similar properties, AMPs that can also act as ACPs are viewed as future chemotherapeutic drugs, with the advantage of low propensity to resistance, which started this paradigm in the pharmaceutical market. These peptides have already been described as molecules presenting killing mechanisms at the membrane level, but also acting toward intracellular targets, which increases their success compartively to one-target specific drugs. This review will approach the desirable characteristics of small peptides that demonstrated dual activity against microbial infections and cancer, as well as the peptides engaged in clinical trials.
Keywords: anticancer peptides (ACPs), antimicrobial peptides (AMPs), cancer, multi-resistant infections, bacteria
Introduction
At the beginning of the twenty-first century, the increased appearances of multi-resistant bacterial pathogens have become a worldwide problem (Arias and Murray, 2009). The World Health Organization has already emphasized the urgency in designing new antimicrobial molecules, because conventional antibiotics are increasingly useless as therapeutics, especially against the so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species), which showed a high propensity to develop antibiotic resistance (McKenna, 2013). Another global concern is the rise in the incidence of cancer. Recent data released revealed 12.7 million new cases and 7.6 million deaths, just in 2008 (Ferlay et al., 2010). In Europe alone, 3.45 million new cases were diagnosed and 1.75 million deaths occurred during 2012 (Ferlay et al., 2013). Nowadays, cancer is the second most common cause of death worldwide (Arnold et al., 2015), caused by an abnormal cellular growth, in a uncontrolled manner, with the ability to invade other tissues, leading to the formation of tumor masses, neo-vascularization (angiogenesis), and metastasis (Thundimadathil, 2012). Lung, colorectal, prostate, and breast cancer are the most diagnosed forms of this disease (Domalaon et al., 2016). Considering the numbers revealed, it is urgent to find new anticancer drugs able to control tumor growth with minimal side effects (Dennison et al., 2007). This situation has become worse due to DNA-alkylation, hormone agonists, and antimetabolites, which show insufficient selectivity and unspecific targeting on healthy cells (Smith and White, 1995; Gaspar et al., 2013), contributing to increased resistance to anticancer drugs (Wang K.-r. et al., 2009). Moreover, the intersection between infection and cancer is highlighted by the number of cancer deaths and new occurrences that are related to treatment or chronic infections. Approximately 2 million of the new cancer patients are due to infectious agents like bacteria and viruses (Parkin, 2006; Vedham et al., 2014; Attiê, 2014). Patients that suffer from a chronic infection are more susceptible to cancer due to the weakened immune system, which cannot fight both the pathogen, and the emergence of cancer cells (Rolston, 2001). This weakness can also occur due to cancer treatments that are too aggressive to patient health, such as chemotherapy, radiotherapy, and surgical resection, leaving patients susceptible to infection agents (Fishman, 2011; Xiao et al., 2015). Also, continuous exposure to infection leads to inflammation, contributing to the appearance of cancer (Vedham et al., 20142014).
In recent years, a promising new class of molecules has arisen, and it has different types of advantages against both of the above major world health concerns. Antimicrobial peptides (AMPs) are small peptides essential for the innate immune response of organisms of all branches, presenting activity against a wide range of pathogens, like bacteria, fungi, and viruses (Hancock et al., 2016). More recently, anticancer activity was also described for some of these peptides, termed anticancer peptides (ACPs) (Dennison et al., 2006). Properties like their short time-frame of interaction (which decreases the probability of resistance), low toxicity (which reduces side effects), mode of action, specificity, good solubility, and finally, good tumor penetration, indicate ACPs as a future chemotherapy cancer drug with high potential (Riedl et al., 2011; Figueiredo et al., 2014; Wu et al., 2014; Gaspar et al., 2015; Domalaon et al., 2016).
Peptides with antimicrobial and anticancer activity
Antimicrobial peptides were first identified due to their importance in the innate immunity of a broad number of organisms, gaining interest from the scientific community (Jenssen et al., 2006). From the first identification until today, hundreds of AMPs have been identified and studied, either from natural sources or from in silico designs (Hancock et al., 2016). These peptides are characterized by an amino acid sequence usually from 5 to 50 residues, high hydrophobicity and positive net charge (Melo et al., 2011; Gaspar et al., 2012). These physicochemical properties set the basis for the activity against pathogens (Dennison et al., 2010). Bacteria present negatively charged membranes, promoting AMPs' initial electrostatic interaction. Even knowing that not all AMPs are ACPs, the similarity in terms of action is obvious, due to the phenotype of the membrane surface in cancer cells. In the plasma membrane inner-leaflet of healthy cells there is phosphatidylserine (PS), a negatively charged phospholipid. This asymmetry between inner and outer membrane leaflets is lost in cancer cells, leading to the presence of PS in the outer-leaflet (Bevers et al., 1996). PS exposure, the presence of O-glycosylated mucins, sialylated gangliosides, and heparin sulfate, in conjugation with an increased transmembrane potential, surface area, and membrane fluidity (Schweizer, 2009; Hilchie et al., 2011), promote the specific activity of AMPs toward cancer cells (ACPs), without being affected by tumors' heterogeneity (Kelly et al., 2016).
The physicochemical parameters determining the activity of some AMPs toward cancer cells are still unclear, considering that the characteristics of AMPs/ACPs are very similar. Efforts are being made in order to understand these differences, which would enable an improved design of ACPs (Dennison et al., 2006). Some AMPs can also be ACPs independently of the source of identification or synthetic route of design (Mader and Hoskin, 2006). The number of AMPs encountered in nature that have anticancer activity has increased in recent years. Aurein 1.2 (GLFDIIKKIAESF), a peptide isolated from the frog Litoria aurea, is one example of an AMP with broad-range activity toward bacteria that showed to be highly active toward 55 different cancer cell lines in vitro, without any significant cytotoxic activity (Rozek et al., 2000; Dennison et al., 2007; Giacometti et al., 2007). Another example is the human neutrophil peptide-1 (HNP-1, ACYCRIPACIAGERRYGTCIYQGALWAFCC), an AMP that plays a fundamental role in the defense against pathogens in the innate immune system. Its antimicrobial activity has been fully explored, with a broad spectrum activity against bacteria, but it is the possibility of using this AMP in cancer therapies that attracted attention in recent years (Nishimura et al., 2004; Varkey and Nagaraj, 2005). The full mechanism of action of this peptide against cancer cells has not yet been established, but activity was already confirmed for different cancer cell lines, with very low cytotoxicity against healthy cells (McKeown et al., 2006; Gaspar et al., 2015). Peptides pleuricidin 03 (GRRKRKWLRRIGKGVKIIGGAALDHL) and pleuricidin 07 (RWGKWFKKATHVGKHVGKAALTAYL), AMPs isolated from Atlantic flatfishes, were showed to be highly effective in killing different bacterial strains (Patrzykat et al., 2003). Recently, their anticancer activity was explored and their effectiveness against drug-resistant breast cancer cells confirmed, without toxicity against fibroblasts or erythrocytes, either in in vitro and in vivo models (Hilchie et al., 2011). These are just examples of ACPs that were studied after isolation from different natural sources, like animals, plants, and bacteria. Natural ACPs, even having a high anticancer activity, have normally 30–40 amino acids in their sequence, which increases production costs. Therefore, synthetic routes for ACP design have gained attention. There are different possible approaches available, such as the improvement of natural ACP sequences or the use of in silico methods (Park et al., 1998; Lee et al., 2008). Both strategies take into consideration the improvement of the physicochemical properties, like amphipathicity, hydrophobicity, and overall positive charge, with the objective of better activity toward the target cells (Huang et al., 2011; Melo et al., 2011; Sinthuvanich et al., 2012). Furthermore, other strategies such as hybridizing different ACPs (Hoskin and Ramamoorthy, 2008) or changing the amino acids used for unnatural ones (D-enantiomers or cyclic tetra-substitution of Cα are examples; Hicks, 2016) have also been tested. The possibilities are endless, and depend on what the focus of the improvement is for each case. Bioinformatic algorithms integrated with machine learning, where the design is automatic through the properties chosen, taking into consideration AMP/ACP libraries of existing molecules, are considered the future method for their rational design (Tyagi et al., 2013; Lin et al., 2015).
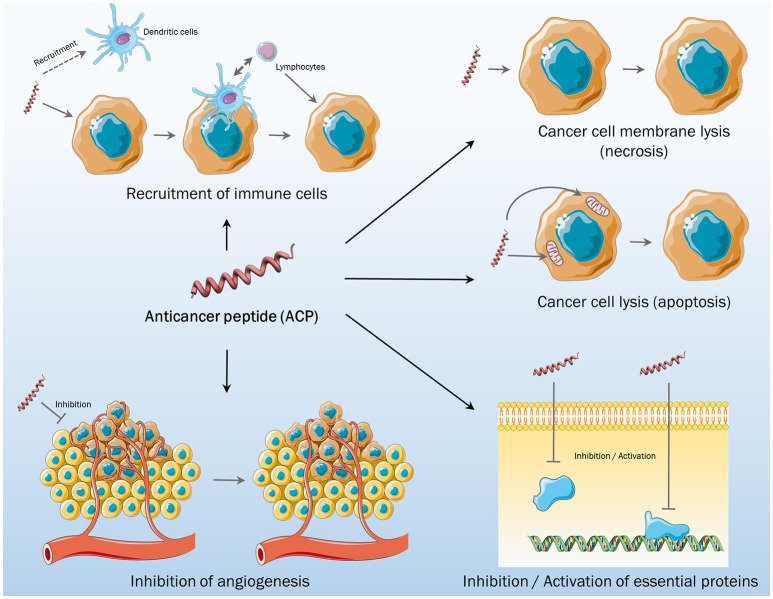
AMPs and ACPs share most of the characteristics, like the physicochemical properties already described. Structure plays a central role in their activity. It is commonly accepted that most AMPs/ACPs do not fold in a well-defined structure when free in solution, but adopt α-helix or β-sheet structure when electrostatic interactions with membranes occur (Hoskin and Ramamoorthy, 2008). Differences in terms of structure were the first method for the classification of ACPs. Examples of some AMPs lately defined as α-ACPs are cecropin, magainin, melittin, and buforin II, with lactoferricin B, HNP-1/3, and gamesin being classified as β-ACPs (Papo and Shai, 2005). More recently, it was noticed that independently of the secondary structure that the peptide adopts, a classification considering the mechanisms of action in the target cancer cells was more suitable (Wu et al., 2014). AMPs were considered membrane-active peptides regarding their primary activity, but over the years, it was clarified that they can also target different processes of the pathogen (namely, metabolism, and cell division) and of the immune system (recruitment of immune cells; Hancock et al., 2016). These aspects were also studied for ACPs, with the identification of cell membrane lytic activity (necrosis), mitochondrial membrane lytic activity (apoptosis), and non-membrane activities (Figure 1; Wu et al., 2014). The first one is the most common anticancer method of targeting, with the electrostatic interactions promoting membrane disruption, leading to necrosis. Polybia-MPI, a natural ACP, and the synthetic BTM-P1 are just two examples (Segura et al., 2007; Wang K.-r. et al., 2009). These ACPs have high selectivity toward cancer cell membranes and develop low resistance, when compared to conventional chemotherapeutic drugs. Activity toward mitochondrial membrane, activating apoptosis signaling, was also observed for some ACPs, such as lactoferricin B and different β-ACPs (Furlong et al., 2006; Paredes-Gamero et al., 2012). After the activity at the membrane level, ACPs can also present other activities, either targeting essential cell proteins, inhibiting angiogenesis, or recruiting immune cells to attack cancer cells (Figure 1; Wu et al., 2014). HNP-1 was shown to be an ACP that recruits and activates dendritic cells in terms of immunomodulatory activity (Wang Y.-s. et al., 2009), but also inhibits angiogenesis, which is essential to the growth and development of tumors (Xu et al., 2008).
Figure 1.
Different mechanisms of action of anticancer peptides.
Potential clinical approaches using ACPs
Although a wide variety of drugs are commercially available, treatments for infections, and cancer have one thing in common: the emergence of resistance against multiple drugs (Baguley, 2010; Theuretzbacher, 2012). Another associated problem is the lack of selectivity of the available drugs, and their consequent undesirable side effects for the patients (Mandell et al., 2001; Baguley, 2010). Thus, there is a need for the development of new antineoplastic and antimicrobial therapies, with higher selectivity, leading to fewer side effects than current ones. It is desirable that these new compounds present different mechanisms of action, without dependence on activity toward a single specific molecule in the target cells, like the ones used nowadays in therapeutics. The main goal is resistance prevention, overcoming the existing mechanisms that cancer and bacterial cells use, being active and diminishing the side effects (Lincke et al., 1990; Arias and Murray, 2009; Kakde et al., 2011).
As described earlier, several AMPs and/or ACPs have become the focus of research by different groups, mainly due to their ability to kill or inhibit the growth of a variety of microorganisms and tumor cells (Wu et al., 2014; Hancock et al., 2016). There are thousands of natural peptides and millions of synthetic peptides obtained by rational design, with a large number presenting antimicrobial and anticancer activity, but only a few being tested (Gordon et al., 2005). Furthermore, from these, unfortunately, only a small number are currently in clinical trials (Table 1). This is mostly due to the numerous challenges associated with the development of these peptides as pharmaceutical drugs, such as synthesis costs, which are higher than the synthesis of organic antibiotic small molecules. Due to this, peptide design has focused on primary structure shortening, accomplishing a lower production cost, and allowing physicochemical properties to be easily changed, which is important for the activity of AMPs/ACPs (Tørfoss et al., 2012a; Domalaon et al., 2016).
Table 1.
Anticancer and antimicrobial peptides in clinical trials, with the indication of the highest phase and the therapeutic condition for which they are being tested.
| Product name | Peptide | Company | Highest phase | Condition treated | Route of administration |
|---|---|---|---|---|---|
| ACPs | |||||
| ANG-4043 | ANG-4043 | AngioChem Co. | Preclinical | Brain metastases | IV |
| CLS-001 | MBI-226 | Cadence Pharmaceuticals Inc. | II | Vulvar intraepithelial neoplasia | IV |
| Carrus Capital Corp. | |||||
| Cutanea Life Sciences Inc. | |||||
| Migenix Inc. | |||||
| GRN-1201 | GRN-1201 | Green Peptide Co. | I | Solid tumors | IV |
| ICT01-2588 | ICT01-2588 | Incanthera Ltd. | I | Vascular disrupting agents | IV |
| University of Bradford | Breast cancer (preclinical) | ||||
| Colorectal cancer (preclinical) | |||||
| Lung cancer (preclinical) | |||||
| Prostate cancer (preclinical) | |||||
| ICT03-Es5 | ICT03-Es5 | Incanthera Ltd. | I | Solid tumors | IV |
| University of Salford | Breast cancer (preclinical) | ||||
| Liver cancer (preclinical) | |||||
| Non-small cell lung cancer (preclinical) | |||||
| ICT04-CYP | ICT04-CYP | Incanthera Ltd. | Preclinical | Bladder cancer | IV |
| University of Bradford | Colorectal cancer | ||||
| ITK-1 | ITK-1 | FUJIFILM Co. | III | Glioblastoma | IV |
| Green Peptide Co. | Prostate cancer | ||||
| Kurume University | |||||
| Oncopore™ | LTX-315 | Lytix Biopharma AS | I | Solid tumors | IV |
| Paclitaxeltrevatide | ANG-1005 | AngioChem Co. | II | Brain metastases | IV |
| Glioblastoma | |||||
| Glioma | |||||
| WT-2725 | WT-2725 | Sumitomo Dainippon Pharma Co. | I | Hematological malignancies | IV |
| Sunovion Pharmaceuticals Inc. | Solid tumors | ||||
| AMPs | |||||
| C16G2 | C16G2 | Chengdu Sen Nuo Wei Biotechnology Co. | II | Dental caries | Topical |
| C3 Jian Inc | |||||
| Cefilavancin® | TD-1792 | GlaxoSmithKline Co. | III | Gram-positive infections | Topical |
| Theravance Biopharma Inc. | Skin and soft tissue infections | ||||
| R-Pharm | |||||
| CLS-001 | MBI-226 | Cadence Pharmaceuticals Inc. | III | Rosacea | Topical |
| Carrus Capital Corp. | Acne vulgaris (II) | ||||
| Cutanea Life Sciences Inc. | Genital warts (II) | ||||
| Migenix Inc. | |||||
| Dalvance™ | MDL-63,397 | Durata Therapeutics Inc. | II | Osteomyelitis | IV |
| Pfizer Inc. | Osteomyelitis (I) | ||||
| Vicuron Pharmaceuticals Inc. | Pneumonia (preclinical) | ||||
| DPK-060 | DPK-060 | DermaGen AB | II | Atopic dermatitis | Topical |
| Pergamum AB | Otitis externa | ||||
| Karolinska Development AB | |||||
| LL-37 | LL-37 | Pergamum AB | II | Leg ulcer | Topical |
| Karolinska Development AB | |||||
| Locilex® | MSI-78 | Dipexium Pharmaceuticals Inc. | III | Diabetic foot ulcer | Topical |
| Genaera Corp. | Skin and soft tissue infections (I) | ||||
| GlaxoSmithKline Plc. | |||||
| RRD International Inc. | |||||
| Luminaderm® | NP108 | NovaBiotics Ltd. | II | Bovine mastitis | Topical |
| LytixarTM | LTX109 | Lytix Biopharma AS | II | Impetigo | Topical |
| Staphylococcus aureus infections | |||||
| Murepavadin® | POL-7080 | Polyphor Ltd. | II | Pseudomonas aeruginosa infections | IV |
| University of Zurich | Gram-negative infections (I) | ||||
| Novamycin® | NP-339 | NovaBiotics Ltd. | I | Cystic fibrosis | IV |
| Invasive fungal disease | |||||
| Oropharyngeal candidiasis | |||||
| Novarifyn® | NP-432 | NovaBiotics Ltd. | Preclinical | Methicillin-resistant Staphylococcus aureus (MRSA) P. aeruginosa | IV |
| C. difficile infections | |||||
| Novexatin® | NP-213 | NovaBiotics Ltd. | II | Onychomycosis | Topical |
| Taro Pharmaceutical Industries Ltd. | |||||
| NVB302 | NVB302 | Novacta Biosystems Ltd. | I | C. difficile infections | Topical |
| PXL-01 | Lactoferrin | DermaGen AB | III | Post-surgical adhesions | Topical |
| Karolinska Development AB | |||||
| PharmaSurgics AB | |||||
| Promore Pharma | |||||
| Pergamum AB | |||||
| SGX-942 | Dusquetide | Inimex Pharmaceuticals Inc. | Preclinical | Melioidosis | IV |
| SciClone Pharmaceuticals Inc. | |||||
| Soligenix Inc. | |||||
| University of British Columbia | |||||
| Surotomycin | MK-4261 | Cubist Pharmaceuticals Inc. | III | Clostridium difficile infections | IV |
| Merck & Co. Inc. | |||||
| Telavancin® | TD-6424 | Clinigen Group plc | III | Osteomyelitis | IV |
| Innoviva Inc. | Bacterial infections (I) | ||||
| Pendopharm | |||||
| Theravance Biopharma Inc. | |||||
| University of Illinois | |||||
The search was carried out in the investigational drug databases Pharmaprojects (www.pharmaprojects.com), AdisInsight (www.adisinsight.com), Prous DDR (http://www.prous.com), and IDdb3 (http://science.thomsonreuters.com).
In addition, the adverse effects presented by some peptides (high toxicity to mammalian healthy cells and low immune response modification) increase the number of obstacles to applying these molecules to therapy (Hancock, 1997; Andreu and Rivas, 1998; Xiao et al., 2015; Kao et al., 2016). This is not surprising, since the activity of AMPs/ACPs usually depends on membrane-peptide interaction. However, to be commercially useful, it would be necessary to dissociate the toxicity to the mammalian cells from antimicrobial/antitumor activity, which can be achieved by increasing antimicrobial activity, reducing haemolytic activity, or both (Chen et al., 2005; Uggerhøj et al., 2015).
Another obstacle to the applicability of peptides is their susceptibility to proteolysis. Oral administration remains the preferred mode for drug delivery, corresponding to approximately 60% of the administration routes used for drugs (Renukuntla et al., 2013). This occurs due to the advantages that these drugs present, including low production cost and patient compliance in the administration. Even so, peptide drugs usually follow the traditional route of administration, like intramuscular (i.m.) or intravenous (i.v.) injection, due to their poor oral bioavailability, which is expressed by a low resistance to proteases and poor penetration through the intestinal membrane (Hamman et al., 2005). Sensitivity to proteolytic degradation can be mitigated by using rational design to replace naturally occurring amino acids with unnatural ones (Gordon et al., 2005; Uggerhøj et al., 2015). An example is the synthetic design of D-enantiomeric peptides, like DJK-5/6, which show improved activity against bacterial infections in in vivo models, comparable to that of the L-enantiomeric peptides, without showing any cytotoxic activity (de la Fuente-Núñez et al., 2015; Mansour et al., 2016). This type of peptide were also shown to be more actively effective against drug-resistant tuberculosis pathogens, and have already been tested with inhalable spray-dried formulations (Lan et al., 2014; Kwok et al., 2015). In terms of ACPs, SVS-1 was seen to be more effective, compared to its L-isomeric peptide form (Sinthuvanich et al., 2012). β2, 2 amino acids, also unnatural ones, can be another strategy to design AMPs/ACPs that are resistant to proteolysis, with a high effectiveness against the target cells and low toxicity toward healthy cells (Tørfoss et al., 2012a,b).
Together with proteolysis comes the limitation of pharmacokinetics and pharmacodynamics, because it is difficult to evaluate the direct action of the peptide against the pathogen in vivo and relate to a specific mode of action (Drusano, 2004). Moreover, the time of circulation, which is essential for a drug to be efficient, is not easy to determine (Kelly et al., 2016). Different strategies have been proposed for this problem, like the use of drug carriers, such as bacteriophages (Dąbrowska et al., 2014). Using a natural bacterial phage, displaying ACPs on their surface, increases the targeting (dynamics of action) and allows for improved dual activity. Conjugating the peptide with cell-penetrating peptides (CPPs) can be another interesting strategy to improve the specificity of the targeting. Some authors have used TAT protein from HIV virus as the CPP, conjugated to an AMP/ACP (HPRP-A1) in order to increase the specificity toward cancer cells (Hao et al., 2015). Coating or conjugation of peptides with polymers, like polyethylene glycol (PEG), can also increase circulation and improve pharmacokinetics/dynamics, independently of the polymer used, by allowing a higher time of circulation and improving their penetration toward the target cancer cells (Kelly et al., 2016).
In conclusion, these modifications may promote changes in amphipathic/hydrophobic properties, leading to the reduced cytotoxicity of peptides toward mammalian cells, without jeopardizing antimicrobial/anticancer efficiency, rendering peptides more impervious to proteolysis, and thus bestowing on them improved therapeutic activity and pharmaceutic design (Chen et al., 2005; Uggerhøj et al., 2015; Kang et al., 2017).
Conclusion and future directions
In conclusion, AMPs and ACPs have been known for several decades, but only in the last one an increasing number of publications on thier in vivo activities has arisen. Consequently, few peptides are used in medical practice. However, we believe that in the upcoming years peptides will have a major impact on the treatment of infectious diseases and cancer, two of the world's greatest healthcare concerns. As shown here, different microbial infections and/or cancer-targeting peptides are in clinical trials, with approval for clinical application expected for the next few years (at least 10 in the next 5 years). Moreover, that number should tend to increase due to advances in the rational design of peptides, minimizing or eliminating cytotoxic effects. In addition, advances in the large-scale synthesis of peptides has made this process cheaper, thus making peptide-based therapies likely to become more accessible to patients. Another strategy that has gained attention is the combined use of peptides with conventional drugs, which reduces costs per treatment, minimizing the problem of resistance and preventing recurrence. Thus, AMPs and ACPs have great potential, both alone and in combination with conventional drugs, to be used in infection and cancer therapies, mostly due to their effective mechanisms of action on the target cells.
Author contributions
MF, OS, SG, and NS wrote the article. SG, NS, and OF reviewed the article.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia – Ministério da Ciência, Tecnologia e Ensino Superior (FCT-MCTES, Portugal), by Brazilian funding agencies CNPq, CAPES, FADPDF, FINEP, and FUNDECT, and by Marie Skłodowska-Curie, Research, and Innovation Staff Exchange (MSCA-RISE, European Union) project INPACT (call H2020-MSCA-RISE-2014, grant agreement 644167). MF acknowledges FCT-MCTES fellowship SPRH/BD/100517/2014. OS holds a postdoctoral scholarship from the National Council of Technological and Scientific Development (CNPq) and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT; 300583/2016-8).
References
- Andreu D., Rivas L. (1998). Animal antimicrobial peptides: an overview. Biopolymers 47, 415–433. [DOI] [PubMed] [Google Scholar]
- Arias C. A., Murray B. E. (2009). Antibiotic-resistant bugs in the 21st Century — a clinical super-challenge. N.Engl. J. Med. 360, 439–443. 10.1056/NEJMp0804651 [DOI] [PubMed] [Google Scholar]
- Arnold M., Karim-Kos H. E., Coebergh J. W., Byrnes G., Antilla A., Ferlay J., et al. (2015). Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European Cancer Observatory. Eur. J. Cancer 51, 1164–1187. 10.1016/j.ejca.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Attiê R. (2014). Acute bacterial infection negatively impacts cancer specific survival of colorectal cancer patients. World J. Gastroenterol. 20:13930. 10.3748/wjg.v20.i38.13930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley B. C. (2010). Multiple drug resistance mechanisms in Cancer. Mol. Biotechnol. 46, 308–316. 10.1007/s12033-010-9321-2 [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Confurius P., Zwaal R. F. (1996). Regulatory mechanisms in maintenance and modulation of transmembrane lipid asymmetry: pathophysiological implicationspub.Com/. Lupus 5, 480–487. [DOI] [PubMed] [Google Scholar]
- Chen Y., Mant C. T., Farmer S. W., Hancock R. E. W., Vasil M. L., Hodges R. S. (2005). Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J. Biol. Chem. 280, 12316–12329. 10.1074/jbc.M413406200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dąbrowska K., Kaźmierczak Z., Majewska J., Miernikiewicz P., Piotrowicz A., Wietrzyk J., et al. (2014). Bacteriophages displaying anticancer peptides in combined antibacterial and anticancer treatment. Future Microbiol. 9, 861–869. 10.2217/fmb.14.50 [DOI] [PubMed] [Google Scholar]
- de la Fuente-Núñez C., Reffuveille F., Mansour S. C., Reckseidler-Zenteno S. L., Hernández D., Brackman G., et al. (2015). D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 22, 196–205. 10.1016/j.chembiol.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison S. R., Harris F., Bhatt T., Singh J., Phoenix D. A. (2010). A theoretical analysis of secondary structural characteristics of anticancer peptides. Mol. Cell. Biochem. 333, 129–135. 10.1007/s11010-009-0213-3 [DOI] [PubMed] [Google Scholar]
- Dennison S. R., Harris F., Phoenix D. A. (2007). The interactions of aurein 1.2 with cancer cell membranes. Biophys. Chem. 127, 78–83. 10.1016/j.bpc.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Dennison S., Whittaker M., Harris F., Phoenix D. (2006). Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr. Protein Pept. Sci. 7, 487–499. 10.2174/138920306779025611 [DOI] [PubMed] [Google Scholar]
- Domalaon R., Findlay B., Ogunsina M., Arthur G., Schweizer F. (2016). Ultrashort cationic lipopeptides and lipopeptoids: evaluation and mechanistic insights against epithelial cancer cells. Peptides 84, 58–67. 10.1016/j.peptides.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Drusano G. L. (2004). Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat. Rev. Microbiol. 2, 289–300. 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H.-R., Bray F., Forman D., Mathers C., Parkin D. M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J., Rosso S., Coebergh J. W. W., Comber H., et al. (2013). Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer 49, 1374–1403. 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- Figueiredo C. R., Matsuo A. L., Massaoka M. H., Polonelli L., Travassos L. R. (2014). Anti-tumor activities of peptides corresponding to conserved complementary determining regions from different immunoglobulins. Peptides 59, 14–19. 10.1016/j.peptides.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Fishman J. A. (2011). Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transplant. 17, S34–S37. 10.1002/lt.22378 [DOI] [PubMed] [Google Scholar]
- Furlong S., Mader J., Hoskin D. (2006). Lactoferricin-induced apoptosis in estrogen-nonresponsive MDA-MB-435 breast cancer cells is enhanced by C6 ceramide or tamoxifen. Oncol. Rep. 15, 1385–1390. 10.3892/or.15.5.1385 [DOI] [PubMed] [Google Scholar]
- Gaspar D., Freire J. M., Pacheco T. R., Barata J. T., Castanho M. A. R. B. (2015). Apoptotic human neutrophil peptide-1 anti-tumor activity revealed by cellular biomechanics. Biochim. Biophys. Acta Mol. Cell Res. 1853, 308–316. 10.1016/j.bbamcr.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Gaspar D., Veiga A. S., Castanho M. A. (2013). From antimicrobial to anticancer peptides. A review. Front. Microbiol. 4:294. 10.3389/fmicb.2013.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar D., Veiga A. S., Sinthuvanich C., Schneider J. P., Castanho M. A. R. B. (2012). Anticancer peptide SVS-1: efficacy precedes membrane neutralization. Biochemistry 51, 6263–6265. 10.1021/bi300836r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti A., Cirioni O., Riva A., Kamysz W., Silvestri C., Nadolski P., et al. (2007). In vitro activity of aurein 1.2 alone and in combination with antibiotics against gram-positive nosocomial cocci. Antimicrob. Agents Chemother. 51, 1494–1496. 10.1128/AAC.00666-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Romanowski E. G., McDermott A. M. (2005). A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 30, 505–515. 10.1080/02713680590968637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman J. H., Enslin G. M., Kotzé A. F. (2005). Oral delivery of peptide drugs: barriers and developments. BioDrugs 19, 165–177. 10.2165/00063030-200519030-00003 [DOI] [PubMed] [Google Scholar]
- Hancock R. E. (1997). Peptide antibiotics. Lancet 349, 418–422. 10.1016/S0140-6736(97)80051-7 [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Haney E. F., Gill E. E. (2016). The immunology of host defence peptides: beyond antimicrobial activity. Nat. Rev. Immunol. 16, 321–334. 10.1038/nri.2016.29 [DOI] [PubMed] [Google Scholar]
- Hao X., Yan Q., Zhao J., Wang W., Huang Y., Chen Y. (2015). TAT modification of alpha-helical anticancer peptides to improve specificity and efficacy. PLoS ONE 10:e0138911. 10.1371/journal.pone.0138911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks R. P. (2016). Antibacterial and anticancer activity of a series of novel peptides incorporating cyclic tetra-substituted Cα amino acids. Bioorg. Med. Chem. 24, 4056–4065. 10.1016/j.bmc.2016.06.048 [DOI] [PubMed] [Google Scholar]
- Hilchie A. L., Doucette C. D., Pinto D. M., Patrzykat A., Douglas S., Hoskin D. W. (2011). Pleurocidin-family cationic antimicrobial peptides are cytolytic for breast carcinoma cells and prevent growth of tumor xenografts. Breast Cancer Res. 13, R102. 10.1186/bcr3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin D. W., Ramamoorthy A. (2008). Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 1778, 357–375. 10.1016/j.bbamem.2007.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. B., Wang X. F., Wang H. Y., Liu Y., Chen Y. (2011). Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol. Cancer Ther. 10, 416–426. 10.1158/1535-7163.MCT-10-0811 [DOI] [PubMed] [Google Scholar]
- Jenssen H., Hamill P., Hancock R. E. W. (2006). Peptide antimicrobial agents. Clin. Microbiol. Rev. 19, 491–511. 10.1128/CMR.00056-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakde D., Jain D., Shrivastava V., Kakde R., Patil A. T. (2011). Cancer therapeutics-opportunities, challenges and advances in drug delivery. J. Appl. Pharm. Sci. 1, 1–10. [Google Scholar]
- Kang H. K., Kim C., Seo C. H., Park Y. (2017). The therapeutic applications of antimicrobial peptides (AMPs): a patent review. J. Microbiol. 55, 1–12. 10.1007/s12275-017-6452-1 [DOI] [PubMed] [Google Scholar]
- Kao C., Lin X., Yi G., Zhang Y., Rowe-Magnus D. A., Bush K. (2016). Cathelicidin antimicrobial peptides with reduced activation of toll-like receptor signaling have potent bactericidal activity against colistin-resistant bacteria. MBio 7, e01418–16. 10.1128/mBio.01418-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Kia A. F.-A., Hassan F., O'Grady S., Morgan M. P., Creaven B. S., et al. (2016). Polymeric prodrug combination to exploit the therapeutic potential of antimicrobial peptides against cancer cells. Org. Biomol. Chem. 14, 9278–9286. 10.1039/C6OB01815G [DOI] [PubMed] [Google Scholar]
- Kwok P. C., Grabarek A., Chow M. Y., Lan Y., Li J. C., Casettari L., et al. (2015). Inhalable spray-dried formulation of D-LAK antimicrobial peptides targeting tuberculosis. Int. J. Pharm. 491, 367–374. 10.1016/j.ijpharm.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Lan Y., Lam J. T., Siu G. K., Yam W. C., Mason A. J., Lam J. K. (2014). Cationic amphipathic D-enantiomeric antimicrobial peptides with in vitro and ex vivo activity against drug-resistant Mycobacterium tuberculosis. Tuberculosis 94, 678–689. 10.1016/j.tube.2014.08.001 [DOI] [PubMed] [Google Scholar]
- Lee H. S., Park C. B., Kim J. M., Jang S. A., Park I. Y., Kim M. S., et al. (2008). Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett. 271, 47–55. 10.1016/j.canlet.2008.05.041 [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Lim Y. F., Russo E., Schneider P., Bolliger L., Edenharter A., et al. (2015). Multidimensional design of anticancer peptides. Angew. Chemie Int. Ed. 54, 10370–10374. 10.1002/anie.201504018 [DOI] [PubMed] [Google Scholar]
- Lincke C. R., van der Bliek A. M., Schuurhuis G. J., van der Velde-Koerts T., Smit J. J., Borst P. (1990). Multidrug resistance phenotype of human BRO melanoma cells transfected with a wild-type human mdr1 complementary DNA. Cancer Res. 50, 1779–1785. [PubMed] [Google Scholar]
- Mader J. S., Hoskin D. W. (2006). Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 15, 933–946. 10.1517/13543784.15.8.933 [DOI] [PubMed] [Google Scholar]
- Mandell L. A., Ball P., Tillotson G. (2001). Antimicrobial safety and tolerability: differences and dilemmas. Clin. Infect. Dis. 32, S72–S79. 10.1086/319379 [DOI] [PubMed] [Google Scholar]
- Mansour S. C., Pletzer D., de la Fuente-Núñez C., Kim P., Cheung G. Y., Joo H.-S., et al. (2016). Bacterial abscess formation is controlled by the stringent stress response and can be targeted therapeutically. EBioMedicine 12, 219–226. 10.1016/j.ebiom.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M. (2013). Antibiotic resistance: the last resort. Nature 499, 394–396. 10.1038/499394a [DOI] [PubMed] [Google Scholar]
- McKeown S. T. W., Lundy F. T., Nelson J., Lockhart D., Irwin C. R., Cowan C. G., et al. (2006). The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral Oncol. 42, 685–690. 10.1016/j.oraloncology.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Melo M. N., Ferre R., Feliu L., Bardají E., Planas M., Castanho M. A. R. B. (2011). Prediction of antibacterial activity from physicochemical properties of antimicrobial peptides. PLoS ONE 6:e28549. 10.1371/journal.pone.0028549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Abiko Y., Kurashige Y., Takeshima M., Yamazaki M., Kusano K., et al. (2004). Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J. Dermatol. Sci. 36, 87–95. 10.1016/j.jdermsci.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Papo N., Shai Y. (2005). Host defense peptides as new weapons in cancer treatment. Cell. Mol. Life Sci. 62, 784–790. 10.1007/s00018-005-4560-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Gamero E. J., Martins M. N. C., Cappabianco F. A. M., Ide J. S., Miranda A. (2012). Characterization of dual effects induced by antimicrobial peptides: regulated cell death or membrane disruption. Biochim. Biophys. Acta 1820, 1062–1072. 10.1016/j.bbagen.2012.02.015 [DOI] [PubMed] [Google Scholar]
- Park C. B., Kim H. S., Kim S. C. (1998). Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 244, 253–257. 10.1006/bbrc.1998.8159 [DOI] [PubMed] [Google Scholar]
- Parkin D. M. (2006). The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 118, 3030–3044. 10.1002/ijc.21731 [DOI] [PubMed] [Google Scholar]
- Patrzykat A., Gallant J. W., Seo J., Pytyck J., Douglas S. E. (2003). Novel antimicrobial peptides derived from flatfish genes. Antimicrob. Agents Chemother. 47, 2464–2470. 10.1128/AAC.47.8.2464-2470.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renukuntla J., Vadlapudi A. D., Patel A., Boddu S. H., Mitra A. K. (2013). Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 447, 75–93. 10.1016/j.ijpharm.2013.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S., Zweytick D., Lohner K. (2011). Membrane-active host defense peptides – challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 164, 766–781. 10.1016/j.chemphyslip.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston K. V. (2001). The spectrum of pulmonary infections in cancer patients. Curr. Opin. Oncol. 13, 218–223. 10.1097/00001622-200107000-00002 [DOI] [PubMed] [Google Scholar]
- Rozek T., Wegener K. L., Bowie J. H., Olver I. N., Carver J. A., Wallace J. C., et al. (2000). The antibiotic and anticancer active aurein peptides from the Australian bell frogs Litoria aurea and Litoria raniformis. Eur. J. Biochem. 267, 5330–5341. 10.1046/j.1432-1327.2000.01536.x [DOI] [PubMed] [Google Scholar]
- Schweizer F. (2009). Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 625, 190–194. 10.1016/j.ejphar.2009.08.043 [DOI] [PubMed] [Google Scholar]
- Segura C., Guzmán F., Salazar L. M., Patarroyo M. E., Orduz S., Lemeshko V. (2007). btm-p1 polycationic peptide biological activity and 3D-dimensional structure. Biochem. Biophys. Res. Commun. 353, 908–914. 10.1016/j.bbrc.2006.12.113 [DOI] [PubMed] [Google Scholar]
- Sinthuvanich C., Veiga A. S., Gupta K., Gaspar D., Blumenthal R., Schneider J. P. (2012). Anticancer β-hairpin peptides: membrane-induced folding triggers activity. J. Am. Chem. Soc. 134, 6210–6217. 10.1021/ja210569f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. L., White I. N. H. (1995). Chemoprevention of breast cancer by tamoxifen: risks and opportunities. Toxicol. Lett. 82–83, 181–186. 10.1016/0378-4274(95)03476-5 [DOI] [PubMed] [Google Scholar]
- Theuretzbacher U. (2012). Accelerating resistance, inadequate antibacterial drug pipelines and international responses. Int. J. Antimicrob. Agents 39, 295–299. 10.1016/j.ijantimicag.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Thundimadathil J. (2012). Cancer treatment using peptides: current therapies and future prospects. J. Amino Acids 2012, 1–13. 10.1155/2012/967347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tørfoss V., Ausbacher D., de Cavalcanti-Jacobsen C. A., Hansen T., Brandsdal B. O., Havelkova M., et al. (2012a). Synthesis of anticancer heptapeptides containing a unique lipophilic β2,2-amino acid building block. J. Pept. Sci. 18, 170–176. 10.1002/psc.1434 [DOI] [PubMed] [Google Scholar]
- Tørfoss V., Isaksson J., Ausbacher D., Brandsdal B.-O., Flaten G. E., Anderssen T., et al. (2012b). Improved anticancer potency by head-to-tail cyclization of short cationic anticancer peptides containing a lipophilic β 2,2 -amino acid. J. Pept. Sci. 18, 609–619. 10.1002/psc.2441 [DOI] [PubMed] [Google Scholar]
- Tyagi A., Kapoor P., Kumar R., Chaudhary K., Gautam A., Raghava G. P. S. (2013). In silico models for designing and discovering novel anticancer peptides. Sci. Rep. 3:2984. 10.1038/srep02984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggerhøj L. E., Poulsen T. J., Munk J. K., Fredborg M., Sondergaard T. E., Frimodt-Moller N., et al. (2015). Rational design of alpha-helical antimicrobial peptides: do's and don'ts. ChemBioChem 16, 242–253. 10.1002/cbic.201402581 [DOI] [PubMed] [Google Scholar]
- Varkey J., Nagaraj R. (2005). Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines. Antimicrob. Agents Chemother. 49, 4561–4566. 10.1128/AAC.49.11.4561-4566.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedham V., Divi R. L., Starks V. L., Verma M. (2014). Multiple infections and cancer: implications in epidemiology. Technol. Cancer Res. Treat. 13, 177–194. 10.7785/tcrt.2012.500366 [DOI] [PubMed] [Google Scholar]
- Wang K.-r., Yan J. X., Zhang B. Z., Song J. J., Jia P. F., Wang R. (2009). Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett. 278, 65–72. 10.1016/j.canlet.2008.12.027 [DOI] [PubMed] [Google Scholar]
- Wang Y.-s., Li D., Shi H. S., Wen Y. J., Yang L., Xu N., et al. (2009). Intratumoral expression of mature human neutrophil peptide-1 mediates antitumor immunity in mice. Clin. Cancer Res. 15, 6901–6911. 10.1158/1078-0432.CCR-09-0484 [DOI] [PubMed] [Google Scholar]
- Wu D., Gao Y., Qi Y., Chen L., Ma Y., Li Y. (2014). Peptide-based cancer therapy: opportunity and challenge. Cancer Lett. 351, 13–22. 10.1016/j.canlet.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Xiao Y. F., Jie M. M., Li B. S., Hu C. J., Xie R., Tang B., et al. (2015). Peptide-based treatment: a promising cancer therapy. J. Immunol. Res. 2015, 1–13. 10.1155/2015/761820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Wang Y. S., Pan W. B., Xiao B., Wen Y. J., Chen X. C., et al. (2008). Human alpha-defensin-1 inhibits growth of human lung adenocarcinoma xenograft in nude mice. Mol. Cancer Ther. 7, 1588–1597. 10.1158/1535-7163.MCT-08-0010 [DOI] [PubMed] [Google Scholar]