Abstract
Selective serotonin reuptake inhibitor (SSRI) antidepressants are widely prescribed for depression and other disorders. SSRIs have become one of the most commonly used drugs in the United States, particularly by women. Acute effects on body composition and carbohydrate metabolism have been reported, but little is known regarding the effects of chronic SSRI use. We evaluated the effects of chronic administration of a commonly prescribed SSRI, sertraline HCl, on body weight and composition, fat distribution, carbohydrate metabolism, as well as activity, in adult female depressed and nondepressed cynomolgus monkeys (Macaca fascicularis; n = 42) using a placebo-controlled, longitudinal, randomized study design. Phenotypes were evaluated prior to and after 18 months of oral sertraline (20 mg/kg) or placebo. Over the 18 month treatment period, the placebo group experienced increases in body weight, body fat (visceral and subcutaneous) fasting insulin concentrations, and homeostasis model assessment of insulin resistance scores (HOMA-IR). Sertraline treatment prevented increases in body weight, fat, insulin, and HOMA-IR (all p < 0.05), without significantly altering activity levels. Sertraline treatment altered adiponectin in an unusual way — reducing circulating adiponectin in depressed monkeys without affecting fat mass or body weight. Deleterious effects on adiponectin, a potentially insulin-sensitizing and atheroprotective protein, may result in adverse effects on cardiovascular health despite otherwise beneficial effects on body composition and carbohydrate metabolism.
Keywords: Selective serotonin reuptake inhibitors (SSRIs), Metabolic syndrome, Diabetes mellitus, Depression, Nonhuman primate, Macaque
1. Introduction
Obesity, diabetes mellitus, and metabolic syndrome are all major risk factors for cardiovascular disease (CVD) and stroke. In 2012 more than two-thirds of Americans were overweight, and one-third of adults were classified as obese according the criteria set forth by the American Heart Association (National Center for Health Statistics, 2015). This increasing prevalence of obesity, particularly visceral obesity, is most likely also driving an increased incidence of insulin resistance and type 2 diabetes mellitus (Despres and Lemieux, 2006). The clustering of cardiometabolic risk factors related to visceral obesity includes hypertension and perturbations in lipid and carbohydrate metabolism and is known as the metabolic syndrome (Go et al., 2014).
Metabolic syndrome is associated with depression (Kinder et al., 2004; Pan et al., 2012). Depressive disorders are twice as likely in women as men (Gorman, 2006). One in ten women currently suffers from a depressive disorder and women in their late reproductive years have a higher incidence of depression than any other age/sex group (Pratt and Brody, 2014). Depressive disorders can activate the hypothalamic-pituitary-adrenal axis which in turn promotes visceral fat deposition, inflammatory cytokine secretion, and a cascade of biological changes resulting in elevated blood pressure, dyslipidemia, and impaired carbohydrate metabolism (Kinder et al., 2004; Shively et al., 2009). Likewise, the proinflammatory state subsequent to visceral fat deposition may increase depression risk. Thus, the relationship between metabolic syndrome and depression is likely bidirectional (Dunbar et al., 2008; Gragnoli, 2014; Mansur et al., 2015; Martinac et al., 2014; Pan et al., 2012). Both metabolic syndrome and depression increase CVD risk, including coronary heart disease, the leading cause of death in women (Despres and Lemieux, 2006).
Antidepressant drugs (ADs) are the third most commonly prescribed medication in America. Women are 2.5 times more likely than men to take ADs, and 23% of women aged 40–59 take ADs. Greater than 60% of Americans taking ADs have taken them for at least 2 years and 14% take them for an excess of 10 years (Pratt et al., 2011). SSRIs are the most commonly prescribed class of ADs (Pratt et al., 2011). In addition to depression, SSRIs were recently approved by the Food and Drug Administration for the treatment of hot flushes and have also shown efficacy in treating migraine headaches and premenstrual dysphoric disorder (Orleans et al., 2014; Stone et al., 2003). This widespread use of SSRIs is an important public health issue because the risks and benefits of chronic SSRI treatment on several biologic systems are unknown.
Published effects of SSRIs on metabolic characteristics are mixed. Clinical studies report beneficial as well as deleterious effects of SSRIs on body weight, waist circumference, insulin secretion and fasting blood glucose (Beyazyuz et al., 2013; Ghaeli et al., 2004; Kesim et al., 2011). Observational studies suggest that SSRI use may be associated with increased waist circumference and impaired glucose handling (Raeder et al., 2006; Yoon et al., 2013). Alterations in glucose and lipids could be due to effects of the drug on body weight, body composition, or behavior. Presently, no data exist regarding SSRI effects on body composition.
To date, the effects of these medications have usually been studied in patient populations with diagnoses of depression or anxiety. This adds a level of complexity in interpreting results because of the associations between depression, obesity and the metabolic syndrome, and difficulties in establishing causality. There is a need for longitudinal, controlled, randomized studies to determine how SSRIs influence body composition and carbohydrate metabolism independently of clinical conditions and syndromes; however, long-term trials evaluating SSRI effects are not ethical in healthy human populations given that SSRI use and discontinuation are both associated with a long list of unpleasant and sometimes serious adverse effects (Cascade et al., 2009; Haddad, 2001).
Laboratory-housed nonhuman primates may exhibit behavioral depression (Camus et al., 2014; Hennessy et al., 2014; Shively et al., 1997; Shively et al., 2009; Shively et al., 2008; Shively and Willard, 2012) which resembles human depression in physiological, neurobiological, and behavioral characteristics including reduced body mass, hypothalamic-pituitary-adrenal axis perturbations, autonomic dysfunction, increased cardiovascular disease risk, reduced hippocampal volume, altered serotonergic function, decreased activity levels, and increased mortality (Shively and Willard, 2012; Willard and Shively, 2012). Physiological and neurobiological characteristics of monkeys that exhibit behavioral depression have been well characterized (Shively and Willard, 2012) and include dyslipidemia and exacerbated coronary atherosclerosis (Shively et al., 2009; Shively et al., 2008; Shively et al., 2005). Here we evaluated the effects of SSRIs on body composition and carbohydrate metabolism in depressed and nondepressed female cynomolgus monkeys (Macaca fascicularis). Since the effects of SSRIs were studied in the presence and absence of depression, depression-associated effects on body composition and carbohydrate metabolism could be separated from those due to SSRI treatment. Female cynomolgus monkeys are uniquely well-suited for this study because, in addition to being a useful and well-characterized model of depression (Shively and Willard, 2012; Willard and Shively, 2012), they are also an established model of diet-induced obesity (Mubiru et al., 2011), and type 2 diabetes mellitus (Shively et al., 2009; Wagner et al., 2006).
2. Materials and methods
2.1. Animal subjects
These animals were subjects of a study primarily aimed at evaluating the relationship between depression, SSRI treatment, and coronary artery atherosclerosis. Details about the animals; the diet they were fed; and the methods and results have been previously published with regards to cerebrospinal fluid monoamines (Shively et al., 2014), cardiac function (Groban et al., 2014), cardiovascular risk factors and coronary artery atherosclerosis (Shively et al., 2015), and neural structures (Willard et al., 2015). Forty-five adult, reproductively-aged female cynomolgus monkeys were imported from Indonesia (Institut, Pertanian Bogor, Bogor, Indonesia) and quarantined in single cages for a one-month. Following quarantine, monkeys were randomly assigned to social groups of n = 4–5 and fed a Western-like diet (containing 44% of calories from fat and 0.29 mg/Cal cholesterol) designed to mimic fat and cholesterol content consumed by Northern Americans (Groban et al., 2014). Monkeys were housed in indoor pens (3.05 m × 3.05 m × 3.05m) with 12/12 light/dark and water ad libitum. Monkey ages were estimated from dentition. Monkeys were at least 10 years of age and cycling, thereby approximating premenopausal women in their mid-30s to late-40s. During the 3.5-year study, three animals died of causes unrelated to the experiment resulting in a final sample size of 42. All animal manipulations were performed according to the guidelines of state and federal laws, the US Department of Health and Human Services, and the Animal Care and Use Committee of Wake Forest University School of Medicine. Wake Forest University is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
2.2. Experimental design (Fig. 1)
Fig. 1.
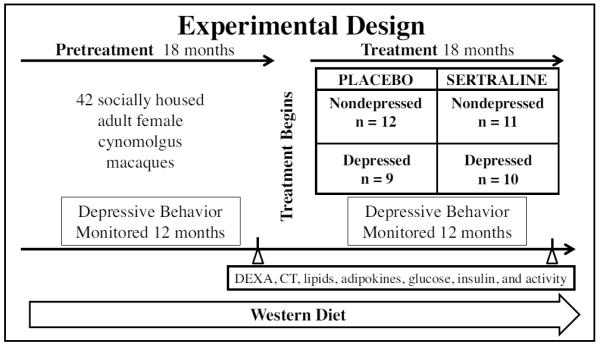
Experimental Design. 42 adult female monkeys consumed a Western diet for an 18 month Pretreatment Phase, during which behavior and physiology were assessed. The monkeys were assigned to sertraline or placebo treatment groups balanced on Pretreatment rates of depression and body weight. The monkeys continued to consume a Western diet during the 18 month Treatment Phase, during which assessments of behavior and physiology were repeated.
The monkeys consumed the Western-like diet for an 18-month Pretreatment Phase during which behavior (including depressive behavior) was recorded. Monkeys were trained to run out of their social group pens into a dosing cage and comply with oral dosing. Stratified randomization was used to assign the monkeys by social group to either placebo (n = 21) or sertraline (n = 21) treatment balanced on Pretreatment rate of depressive behavior and body weight. Thus, depressed and nondepressed monkeys were evenly distributed between the placebo and sertraline groups. Sertraline HCl (Zoloft®) was introduced gradually over a 4-week period to attain a final dose of 20 mg/kg/day; the placebo group received vehicle alone (Shively et al., 2014).
2.3. Circulating levels of sertraline and desmethylsertraline
Cumulative dose-responses to the initiation of SSRI-therapy on circulating levels of sertraline and its metabolite, desmethylsertraline, have been previously described (Shively et al., 2014). In this analysis circulating sertraline and desmethylsertraline were measured in blood samples obtained following 1 week of dosing with placebo and at the end of the Treatment Phase from monkeys sedated with 10–15 mg/kg ketamine HCl 4-h post-dosing. Sertraline and desmethylsertraline were analyzed by gas chromatography (NMS Labs, Willow Grove, PA) as previously described (Shively et al., 2014). During placebo administration, sertraline/desmethylsertraline was undetectable. At the end of the Treatment phase, concentrations of sertraline/desmethylsertraline had reached average levels of 132 ng/ml (range 18–350 ng/ml) and 182 ng/ml (range 21–600 ng/ml), respectively (Willard et al., 2015).
2.4. Cerebrospinal fluid (CSF) levels of 5-hydroxyindoleacetic acid (5-HIAA)
CSF samples were taken to measure 5-HIAA, the major metabolite of serotonin, at the end of the Pretreatment Phase and 15 months after the onset of the Treatment Phase. CSF was obtained by inserting a 22-gauge needle percutaneously into the cisternal space while the animal was sedated and restrained in lateral recumbency. Approximately 1–1.5 cc of CSF was collected and frozen at −70 °C until analysis. 5-HIAA was analyzed using HPLC with electrochemical detection as previously described (Willard et al., 2015). Intraand interassay coefficients of variation were <10%.
Sertraline effects on CSF 5-HIAA have been previously reported (Groban et al., 2014; Shively et al., 2015, 2014; Silverstein et al., 2014; Willard et al., 2015). Sertraline treatment significantly reduced CSF 5-HIAA by approximately 40% (ANOVA treatment × phase interaction F[1,37] = 28.4, p < 0.001).
2.5. Behavior observations
Behavior was recorded during 10 minute focal animal observations, 6–8 times per month, counterbalanced for time of day, for 12 months during both the Pretreatment and Treatment Phases (an average of 33.3 hours/monkey total), using a previously described technique (Shively et al., 2008).
2.5.1. Depressive behavior
As in our previous studies, depressive behavior was defined as a slumped or collapsed body posture, accompanied by a lack of responsiveness to environmental stimuli to which other monkeys are attending, and open eyes to distinguish this behavior from resting or sleeping (Shively et al., 2005). This depressive behavior is easily recognizable, and inter-observer reliability, determined biannually, was r ≥ 0.92 throughout the experiment. The average frequency/hour that the monkeys exhibited depressive behavior was calculated from these observations. Monkeys with pretreatment depressive behavior rates below the mean depression rate were classified as “nondepressed” (n = 23), and those with depressive behavior rates above the mean were classified as “depressed” (n = 19). Sertraline had no effect on depressive behavior (Shively et al., 2015).
2.5.2. Nonstereotypic and stereotypic locomotion
Locomotion was defined as three or more steps. The average frequency/hour that the monkeys exhibited non-stereotypic locomotion, stereotypic (defined as a repetitive motor behavior that occurs at least three times in quick succession), and total (non-stereotypic plus stereotypic locomotion) was calculated from these observations for both the Pretreatment and Treatment Phases (Shively et al., 2008).
2.6. Physical activity by actigraphy
At the end of the Pretreatment and Treatment Phases activity was assessed by recording movement via accelerometry (ActiGraph GT3 × Triaxial Activity Monitor analyzed with ACTILIFE-Desktop Software, Pensacola, FL) (Shively, 1998). Each monkey was outfitted with a nylon mesh protective jacket over a portable actigraphy unit. After a 24-h recovery period, activity was recorded for the next 24 h, and average activity over each hour was calculated for statistical analysis. Activity quantified during nighttime hours only (2400–600 h), was used as a surrogate for sleep disruption.
2.7. Anthropometrics
Body weight and trunk length were measured at the end of the Pretreatment Phase and 16 months after the onset of the Treatment Phase. Body mass index (BMI) was estimated as the ratio of body weight to the square of trunk length measured from the suprasternal notch to the pubic symphysis (in kg/m2) (Shively et al., 2009).
2.8. Body composition and fat distribution
Body composition and fat distribution were determined at the end of the Pretreatment Phase and 16 months after the onset of the Treatment Phase using dual energy X-ray absorptiometry (DEXA) and computer tomography (CT).
2.8.1. Dual energy X-ray absorptiometry (DEXA)
Body composition, including fat mass, lean mass, and bone mass, was determined using DEXA whole-body scans (Hologic Discovery A Dual X-ray Bone Densitometer, Bedford, MA) of anesthetized animals using protocols developed by the manufacturer. Percent fat and lean mass were calculated as a percent of whole body mass.
2.8.2. Computed tomography (CT)
Subcutaneous and visceral abdominal fat volumes were determined from whole body scans conducted using a 32 slice multi-detector CT scanner (Toshiba America Medical Systems Inc. Tustin, CA). Visceral and subcutaneous abdominal tissue areas were measured from cross-sectional images of the abdomen for a 10 cm slab of slices centered on the intervertebral space between lumbar vertebrae L3 and L4. Details regarding CT image analysis have been previously published (Murphy et al., 2014). Briefly, the volumes of adipose tissue within visceral and subcutaneous abdominal tissue areas were recorded with adipose tissue thresholds set at −190 to −30 (Hounsfield Units, HU) using GE Advantage Windows soft ware and a customized version of Medical Image Processing and Visualization (MIPAV, NIH, http://mipav.cit.nih.gov/). Because visceral, rather than subcutaneous, abdominal fat is thought to be a major contributor to metabolic syndrome (Despres and Lemieux, 2006), the ratio of visceral abdominal fat volume to subcutaneous abdominal fat volume was also calculated.
2.9. Carbohydrate metabolism
After an overnight fast, blood samples were collected at the end of the Pretreatment Phase and at the end of the Treatment Phase to determine fasting insulin, glucose, triglyceride, leptin, and adiponectin. Glucose and triglyceride concentrations were determined by colorimetric assay using reagents (ACE-GLU and ACE-TG) and instrumentation (ACE ALERA autoanalyzer) from Alfa Wasserman Diagnostic Technologies (West Caldwell, NJ; Adams et al., 2005). Insulin was determined by enzyme-linked immunosorbent assay (Mercodia, Uppsala, Sweden). Leptin and adiponectin were both determined by radioimmunoassay (Millipore, Billerica, MA). All analyses were performed in the Wake Forest Comparative Medicine Clinical Chemistry and Endocrinology Laboratory and intra- and interassay coefficients of variation were lower than 10% for all analyses. The homeostasis model assessment (HOMA-IR = [mg/dL fasting glucose × mIU/L fasting insulin]/405) was used to determine insulin resistance (Bonora et al., 2000). HOMA-IR correlates with euglycemic glucose clamp results, the reference method for assessing insulin sensitivity, in both humans (Bonora et al., 2000) and macaques (Lee et al., 2011) and has been validated as an estimate of basal insulin resistance in these species.
2.10. Statistical analysis
Statistical analyses were performed using STATISTICA 12.0 for Windows (StatSoft Inc, Tulsa, OK) with significance set at p ≤ 0.05. All variables were evaluated for normality and equality of variances between groups, and log transformations were performed for any variables not meeting the tests for equal variances. Pretreatment phase 5-HIAA concentration for one monkey and Treatment phase subcutaneous abdominal fat volume for another monkey were not available. To verify that groups were balanced prior to treatment, Pretreatment variables were analyzed using 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of variance (ANOVAs). To determine the effect of treatment variables were analyzed by 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) × 2 (Pretreatment phase, Treatment phase) ANOVAs. Results are reported as means ± standard errors of the mean (SEMs). Significant main effects (treatment, depression, and phase) and interaction effects (treatment × phase, depression × phase, and treatment × depression × phase) are reported. In order to determine the best estimate of the magnitude of the treatment effect Treatment values were adjusted for Pretreatment values using 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of covariance (ANCOVAs). All variables had some degree of heterogeneity thus analyses were originally run on transformed data. Analyses were repeated using untransformed data. Transformation had no effect on p-values, therefore significance tests are reported for the untransformed adjusted means ± standard errors of the mean. Significant treatment, depression, and time effects are reported, there were no significant treatment × depression interaction effects. The level of significance was set at p ≤ 0.05. Both untransformed means ± SEMs and adjusted means ± SEMs (covarying for Pretreatment values) are reported in graphs and tables.
3. Results
3.1. Pretreatment characteristics
Pretreatment means, SEMs, main effects of sertraline treatment and depression, and sertraline treatment × depression interaction effects are depicted in Table 1. Prior to treatment, there were no significant differences between the placebo and sertraline groups in body weight and composition, fat distribution, carbohydrate metabolism, depressive behavior and physical activity (all p's >0.05). Compared to their nondepressed counterparts, depressed monkeys displayed increased rates of depressive behavior, had significantly lower mean body weight, BMI, whole body fat mass, percent fat mass, whole body lean mass, abdominal total, visceral, and subcutaneous fat volumes, leptin, and insulin, and higher adiponectin concentrations (all p's < 0.05). While mean lean mass was lower, percent mean lean mass and lean/fat mass ratio were higher in the depressed group. Physical activity did not differ between depressed and nondepressed monkeys.
Table 1.
Characteristics of study population during pretreatment phase.
| Placebo | Sertraline | Effectsa | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Variables | Nondepb n = 12 | Depc n = 9 | Nondepb n = 11 | Depc n = 10 | Depression p= | Sertraline p= | Depression × Sertraline p= |
| Depressive behavior freq/h | 0.01(0.49) | 2.10(0.56) | 0.01(0.51) | 1.37(0.53) | 0.002 | 0.48 | 0.49 |
| Body weight kg | 3.93(0.21) | 2.78(0.24) | 4.01(0.22) | 3.11(0.23) | <0.001 | 0.37 | 0.58 |
| Body Mass Index kg/m2 | 51.7(2.0) | 39.8(2.3) | 44.8(2.1) | 39.7(2.2) | <0.001 | 0.11 | 0.13 |
| Lean mass kg | 2.86(0.10) | 2.18 (0.12) | 3.08(0.11) | 2.51(0.11) | <0.001 | 0.018 | 0.62 |
| Percent lean mass % | 73.3(3.0) | 78.4(1.5) | 76.4(1.9) | 81.1(0.86) | 0.03 | 0.18 | 0.91 |
| Fat mass kg | 1.04(0.14) | 0.50(0.16) | 0.85(0.15) | 0.46(0.15) | 0.004 | 0.46 | 0.62 |
| Percent fat mass % | 23.1(2.1) | 17.6(2.4) | 19.8(2.2) | 14.9(2.3) | 0.03 | 0.18 | 0.89 |
| Abdominal fat volume cm3 | 34.0(5.3) | 12.5(6.2) | 24.0(5.6) | 10.9(5.8) | 0.004 | 0.32 | 0.47 |
| Visceral abdominal fat volume cm3 | 15.9(2.3) | 6.71(2.7) | 12.4(2.4) | 6.79(2.6) | 0.006 | 0.50 | 0.48 |
| Subcutaneous abdominal fat volume cm3 | 15.5(2.8) | 5.34(3.2) | 9.97(2.9) | 4.06(3.0) | 0.01 | 0.26 | 0.48 |
| Visceral: subcutaneous abdominal fat | 1.37(0.31) | 1.47(0.36) | 1.33(0.32) | 1.97(0.34) | 0.50 | 0.28 | 0.43 |
| Leptin ng/ml | 3.00(0.55) | 1.06(0.64) | 2.15(0.58) | 1.52(0.61) | 0.03 | 0.75 | 0.28 |
| Adiponectin ng/ml | 63.1(13) | 73.5(15) | 50.4(14) | 101(15) | 0.04 | 0.61 | 0.17 |
| Triglycerides ng/dl | 39.2(8.1) | 41.4(9.4) | 56.6(8.5) | 43.6(8.9) | 0.54 | 0.27 | 0.39 |
| Glucose mg/dl | 66.5(6.9) | 79.0(8.0) | 70.0(7.2) | 63.4(7.5) | 0.69 | 0.42 | 0.21 |
| Insulin mIU/l | 25.4(5.8) | 12.6(6.6) | 28.6(6.0) | 15.3(6.3) | 0.04 | 0.64 | 0.97 |
| HOMA-IR | 4.73(1.3) | 2.51(1.5) | 5.41(1.3) | 2.47(1.4) | 0.07 | 0.82 | 0.79 |
| 24 h Activity counts | 245969(49812) | 224985(57518) | 329488(52027) | 213456(54566) | 0.21 | 0.51 | 0.38 |
| Nighttime (2400–600 h) activity counts | 13287(3998) | 16856(4617) | 7522(4176) | 9217(4380) | 0.54 | 0.13 | 0.83 |
| Daytime (900–1700 h) activity counts | 147277(31083) | 121885(35892) | 204549(35892) | 127851(34050) | 0.13 | 0.35 | 0.45 |
| Locomotion (frq/h) | 54.1(7.0) | 41.0(8.1) | 51.8(7.4) | 45.8(7.7) | 0.21 | 0.86 | 0.64 |
Values are presented as mean (SEM).
Effects determined using 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of variance.
Nondep = monkeys exhibiting little or no depressive behavior.
Dep = monkeys exhibiting depressive behavior.
3.2. Treatment phase effects
Treatment unadjusted means ± SEMs, adjusted means ± SEMs (Pretreatment values as covariates), and the percent difference between adjusted sertraline- and placebo-group means are depicted in Table 2.
Table 2.
Charateristics of study populaticon during treatment phase.
| Raw means | Adjusted meansa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Placebo | Sertraline | Placebo | Sertraline | Sertraline v. Placebo | |||||
|
|
|
|
|
||||||
| Variables | Nondepb n = 12 | Depc n = 9 | Nondepb n = 11 | Depc n = 10 | Nondepb n = 12 | Depc n = 9 | Nondepb n = 11 | Depc n = 10 | % Differenced |
| Depressive behavior freq/h | 0.55 (0.91) | 4.60(1.0) | 0.08 (0.95) | 5.85(1.0) | 1.25(0.83) | 3.41(1.0) | 0.78(0.87) | 5.31(0.90) | 30.9 |
| Body weight kg | 4.24 (0.24) | 2.86 (0.27) | 3.96 (0.25) | 3.04 (0.26) | 3.78(0.08) | 3.65(0.09) | 3.42(0.08) | 3.47(0.08) | − 7.23 |
| Body mass index kg/m2 | 53.7(2.3) | 39.9(2.7) | 45.2(2.4) | 39.6(2.5) | 46.4(1.3) | 44.7(1.4) | 44.9(1.2) | 44.5(1.3) | −1.84 |
| Lean mass kg | 2.91 (0.12) | 2.27 (0.14) | 3.07 (0.12) | 2.52 (0.13) | 2.73(0.05) | 2.80(0.07) | 2.66(0.06) | 2.70(0.05) | −2.97 |
| Percent lean mass % | 69.6(2.2) | 77.7(2.5) | 76.7(2.3) | 81.2(2.4) | 73.1(1.2) | 76.4(1.4) | 77.2(1.2) | 77.5(1.3) | 7.19 |
| Fat mass kg | 1.29 (0.16) | 0.55 (0.19) | 0.83 (0.17) | 0.46 (0.18) | 0.97(0.06) | 0.79(0.07) | 0.71(0.06) | 0.75(0.07) | − 17.6 |
| Percent Fat Mass % | 26.8(2.3) | 18.3(2.6) | 19.5(2.4) | 14.6(2.5) | 23.0(1.3) | 19.7(1.4) | 18.9(1.3) | 18.5(1.4) | − 12.4 |
| Abdominal fat volume cm3 | 46.5(6.0) | 17.2(7.0) | 20.0(6.3) | 7.99(6.6) | 33.7(3.0) | 26.1(3.3) | 17.3(2.9) | 18.4(3.2) | − 40.3 |
| Visceral abdominal fat volume cm3 | 20.2(2.7) | 9.22(3.1) | 11.1(2.8) | 4.22(2.9) | 15.9(1.9) | 12.7(2.1) | 9.81(1.9) | 7.64(2.0) | − 38.9 |
| Subcutaneous abdominal fat Volume cm3 | 23.0(4.0) | 7.08(4.6) | 9.37(4.2) | 3.63(4.6) | 14.6(1.5) | 12.4(1.6) | 8.45(1.4) | 10.6(1.7) | − 29.3 |
| Visceral: subcutaneous abdominal fat | 1.14 (0.16) | 1.54 (0.19) | 1.18 (0.17) | 1.25 (0.19) | 1.17(0.15) | 1.55(0.17) | 1.22(0.16) | 1.14(0.18) | − 13.3 |
| Leptin ng/ml | 4.50 (0.67) | 1.14 (0.77) | 2.30 (0.70) | 0.92 (0.73) | 3.67(0.51) | 1.94(0.58) | 2.17(0.51) | 1.33(0.54) | − 37.5 |
| Adiponectin ng/ml | 57.8(10) | 75.7(12) | 61.1(11) | 69.3(11) | 61.3(8.7) | 74.5(10) | 70.2(9.3) | 56.1(10) | −7.01 |
| Triglycerides ng/dl | 54.3(46) | 116(53) | 155(48) | 55.4(50) | 59.0(46) | 119(53) | 146(49) | 56.7(50) | 14.0 |
| Glucose mg/dl | 78.0(10) | 90.9(12) | 77.0(10) | 65.9(11) | 81.3(6.1) | 79.7(7.2) | 76.3(6.4) | 72.8(6.8) | −7.40 |
| Insulin mIU/l | 45.2(9.4) | 49.2(23) | 14.1(3.6) | 16.9(2.1) | 43.3(11) | 52.8(12) | 10.9(11) | 19.4(12) | −68.6 |
| HOMA-IR | 9.91(3.0) | 11.4(3.4) | 2.95(3.1) | 2.81(3.2) | 9.34(2.9) | 12.4(3.4) | 1.93(3.1) | 3.76(3.2) | −73.8 |
| 24 h Activity counts | 196840 (44838) | 211135 (51774) | 251219 (46832) | 165837 (49117) | 202632 (33845) | 229537 (39206) | 206816 (36258) | 191169 (37346) | −7.91 |
| Nighttime (2400–600 h) Activity counts | 12251 (4201) | 19193 (4851) | 7785 (4388) | 11209 (4601) | 10714 (2025) | 14460 (2370) | 11938 (2142) | 12745 (2217) | −1.95 |
| Daytime (900–1700 h) activity counts | 93972 (28315) | 98697 (32696) | 151951 (29574) | 80471 (31018) | 97332 (19059) | 119354 (22206) | 116300 (20569) | 97064 (21011) | −1.53 |
| Locomotion (frq/h) | 47.7(5.3) | 37.1(6.1) | 42.3(5.5) | 35.3(5.8) | 44.7(3.4) | 41.8(4.0) | 40.0(3.5) | 37.2(3.7) | −10.8 |
Values are presented as mean (SEM).
Adjusted means (Pretreatment values as covariates).
Nondep = monkeys exhibiting little or no depressive behavior.
Dep = monkeys exhibiting depressive behavior.
% difference calculated from adjusted sertraline- and placebo-group means, boldface indicates a significant 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of covariance (Pretreatment values as covariate) main effect of treatment (p < 0.05).
3.2.1. Depression Effects on Body Weight and Composition, Fat Distribution, Adipokines, and Carbohydrate Metabolism
Pretreatment differences between depressed and nondepressed monkeys in body weight (Fig. 2A), BMI (Fig. 2B), whole body fat mass (Fig. 2C), abdominal total (Fig. 3A), visceral (Fig. 3B), and subcutaneous fat volume (Fig. 3C), and leptin (Fig. 4A) persisted through the Treatment phase (all p's >0.05). Overall, whole body fat mass increased over time (ANOVA phase effect F[1,38] = 4.37, p = 0.04). Serum leptin concentrations increased only in depressed monkeys (ANOVA depression × phase interaction F[1,38] = 4.15, p = 0.05), resulting in concentrations 75% higher in depressed monkeys than nondepressed monkeys (ANCOVA depression effect F[1,37] = 5.33, p = 0.03). There were no other significant main effects of time or depression × phase interaction effects (all p's > 0.05).
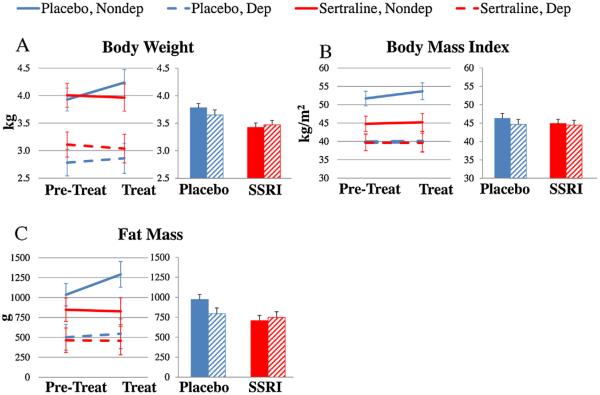
Fig. 2.
The Effects of Sertraline Treatment on Body Weight and Composition. The data were analyzed using 2 (Pretreatment, Treatment) × 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of variance (ANOVAs). In order to adjust for Pretreatment differences the data were also analyzed by 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of covariance (ANCOVAs) adjusted for Pretreatment values. (A) Body weight increased during the treatment phase in placebo-treated, but not sertraline-treated monkeys (ANOVA treatment × phase interaction F[1,38] = 10.2, p = 0.003; ANCOVA treatment effect F[1,37] = 11.9, p = 0.001). (B) Sertraline had no effect on body mass index (ANOVA treatment × phase interaction F[1,38] = 0.54, p = 0.47; ANCOVA treatment effect F[1,37] = 0.45, p = 0.51). C. Whole body fat mass increased during the treatment phase in placebo-treated, but not sertraline-treated monkeys (ANOVA treatment × phase interaction) F[1,38] = 6.22, p = 0.017; ANCOVA treatment effect F[1,37] = 5.56, p = 0.02 Placebo treatment: blue; Sertraline treatment: red. Nondepressed: Solid lines or bars; Depressed: dotted lines or hatched bars (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
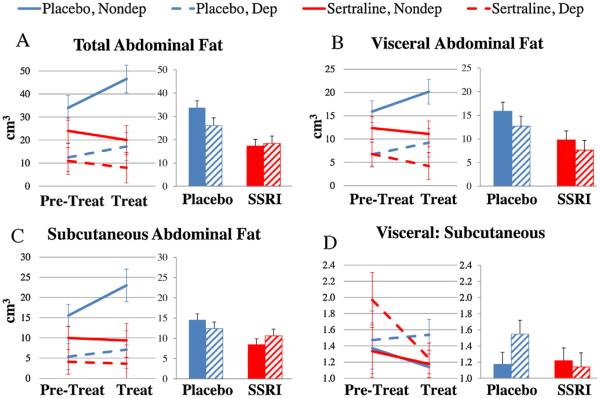
Fig. 3.
The Effects of Sertraline Treatment on Fat Distribution. The data were analyzed using 2 (Pretreatment, Treatment) × 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of variance (ANOVAs). In order to adjust for Pretreatment differences the data were also analyzed by 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of covariance (ANCOVAs) adjusted for Pretreatment values. (A) Total abdominal fat increased during the treatment phase in placebo-treated, but not sertraline-treated monkeys (ANOVA treatment × phase interaction p < 0.001; ANCOVA treatment effect p < 0.001). (B) Visceral abdominal fat volume increased during the treatment phase in placebo-treated, but not sertraline-treated monkeys (ANOVA treatment × phase interaction p = 0.01; ANCOVA treatment effect, p = 0.01). (C) Subcutaneous abdominal fat volume increased during the treatment phase in placebo-treated, but not sertraline-treated monkeys (ANOVA treatment × phase interaction p = 0.01; ANCOVA treatment effect p = 0.01). (D) Sertraline treatment had no effect on the ratio of visceral to subcutaneous fat volumes (ANOVA treatment × phase interaction p = 0.25; ANCOVA treatment effect p = 0.28). Placebo treatment: blue; Sertraline treatment: red. Nondepressed: Solid lines or bars; Depressed: dotted lines or hatched bars (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
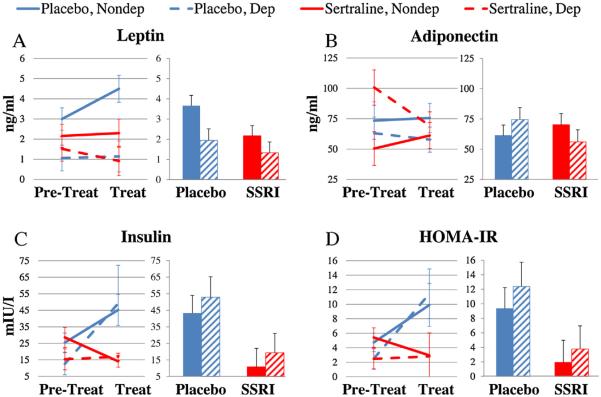
Fig. 4.
The Effects of Sertraline Treatment on Adipokines and Carbohydrate Metabolism. The data were analyzed using 2 (Pretreatment, Treatment) × 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) mixed-models analyses of variance (ANOVAs). In order to adjust for Pretreatment differences the data were also analyzed by 2 (Placebo, Sertraline) × 2 (Nondepressed, Depressed) analyses of covariance (ANCOVAs) adjusted for Pretreatment values. (A) Serum leptin concentrations tended to increase in placebo-treated monkeys, but not in sertraline-treated monkeys (ANOVA treatment × phase interaction F[1,38] = 3.78, p = 0.06; ANCOVA treatment effect F[1,37] = 4.02, p = 0.05). (B) There was a significant treatment × depression × phase interaction effect on adiponectin (ANOVA F[1,38] = 4.15, p = 0.05) but no significant treatment effects (ANOVA phase × treatment interaction F[1,38] = 0.53, p = 0.47; ANCOVA treatment effect F[1,37] = 0.26. p = 0.61). (C) Sertraline treatment reduced insulin (ANOVA treatment × phase interaction F[1,38] = 8.77, p = 0.005; ANCOVA treatment effect F[1,37] = 8.46, p = 0.006). (D) Sertraline treatment reduced HOMA-IR (ANOVA treatment × phase interaction F[1,38] = 6.94, p = 0.01; ANCOVA F[1,37] = 6.71, p = 0.01). Placebo-treatment: blue; Sertraline-treatment: red. Nondepressed: Solid lines or bars; Depressed: dotted lines or hatched bars (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2. Sertraline Effects on Body Weight and Composition in Depressed and Nondepressed Female Macaques (Fig. 2)
Placebo-treated monkeys gained body weight whereas sertraline-treated monkeys did not (Fig. 2A; ANOVA treatment × phase interaction F[1,38] = 10.2, p < 0.01). ANCOVA revealed that sertraline prevented the 8% weight gain observed in the placebo group (ANCOVA treatment effect F[1,37] = 11.9, p = 0.001). Sertraline treatment had no effect on BMI (Fig. 2B; ANOVA treatment × phase interaction F[1,38] = 0.54, p = 0.47; ANCOVA treatment effect F[1,37] = 0.45, p = 0.51). Placebo-treated monkeys also gained body fat whereas sertraline-treated monkeys did not (Fig. 2C; ANOVA treatment × phase interaction F[1,38] = 6.22, p = 0.02). Whole body fat volume was about 18% lower in sertraline-treated monkeys (ANCOVA treatment effect F[1,37] = 5.56, p = 0.02). Sertraline treatment did not affect percent body fat over time (Not shown; ANOVA treatment × phase interaction F[1,38] = 3.52, p = 0.07), but ANCOVA revealed that percent body fat in placebo-treated monkeys was about 12% higher than in the sertraline treatment group (Not shown; ANCOVA treatment effect F[1,37] = 3.96, p = 0.05). Sertraline did not significantly alter lean mass (Not shown; ANOVA treatment × phase interaction F[1,38] = 1.81, p = 0.19).
3.2.3. Sertraline effects on abdominal fat distribution in depressed and nondepressed female macaques (Fig. 3)
Abdominal fat volume (total, visceral, and subcutaneous; Fig. 3A–C) increased in placebo-treated, but not sertraline-treated monkeys (total abdominal fat ANOVA phase × treatment interaction F[1,38] = 17.0, p < 0.001). Total abdominal fat volume in placebo-treated monkeys increased by 38% compared to a 20% decrease in the sertraline treatment group (total abdominal fat ANCOVA treatment effect F[1,37] = 16.0, p < 0.001). Similar results were observed in both the visceral (ANOVA phase × treatment interaction F[1,38] = 7.45, p = 0.01; ANCOVA treatment effect F[1,37] = 8.21, p = 0.01) and subcutaneous (ANOVA phase × treatment interaction F[1,37] = 7.87, p = 0.01; ANCOVA treatment effect F[1,36] = 6.64, p = 0.01) abdominal fat depots measured by CT. Sertraline treatment did not affect the ratio of visceral to subcutaneous abdominal fat volumes (Fig. 3D; ANOVA phase × treatment interaction F[1,37] = 1.38, p = 0.25; ANCOVA treatment effect F[1,36] = 1.23, p = 0.28).
3.2.4. Sertraline effects on adipokines in depressed and nondepressed female macaques (Fig. 4A,B)
Serum leptin concentrations tended to increase in placebo-treated monkeys, but not in sertraline-treated monkeys; this effect did not reach statistical significance (ANOVA phase × treatment interaction F[1,38] = 3.78, p = 0.06). ANCOVA revealed that leptin concentrations in placebo-treated monkeys were about 60% higher than those in the sertraline treatment group (ANCOVA treatment effect F[1,37] = 4.02, p = 0.05). There was a significant treatment × depression × phase interaction effect on adiponectin (Fig. 4B; ANOVA F[1,38] = 4.15, p = 0.05); no other effects reached significance (all p's > 0.05). There was no main effect of sertraline treatment on adiponectin (ANCOVA F[1,37] = 0.26. p = 0.61).
3.2.5. Sertraline effects on carbohydrate metabolism in depressed and nondepressed female macaques (Fig. 4C)
Fasting insulin concentrations increased significantly in the placebo-treated but not the sertraline-treated monkeys (Fig. 4C; ANOVA phase × treatment interaction F[1,38] = 8.77, p = 0.005). ANCOVA revealed that insulin values in placebo-treated monkeys were about 200% higher, on average, than those in the sertraline treatment group (ANCOVA treatment effect F[1,37] = 8.46, p = 0.01). HOMA-IR also increased in the placebo-treated, but not the sertraline-treated monkeys (Fig. 4D; ANOVA phase × treatment interaction F[1,38] = 6.94, p = 0.01). HOMA-IR was nearly 300% higher, on average, in placebo- versus sertraline-treated monkeys (ANCOVA treatment effect F[1,37] = 6.71, p = 0.01). Sertraline had no effect on fasting glucose concentrations (Not shown; ANOVA phase × treatment interaction F[1,38] = 1.09, p = 0.30; ANCOVA treatment effect F[1,37] = 0.80, p = 0.37) or circulating triglycerides (Not shown; ANOVA phase × treatment interaction F[1,38] = 0.044, p = 0.84; ANCOVA treatment effect F[1,37] = 0.06, p = 0.80).
3.3. Sertraline effects on activity in depressed and nondepressed macaques
There were no significant effects of sertraline or depression, and no interactions on accelerometry or locomotive phenotypes in behavior observations (all p's >0.05).
4. Discussion
This is the first published study examining the effects of long-term SSRI treatment on body composition and carbohydrate metabolism in a placebo-controlled, longitudinal, randomized pre-clinical trial. Previously we reported that sertraline had no effect on plasma lipid concentrations (total plasma cholesterol, high density lipoprotein cholesterol, or their ratio). Nevertheless, sertraline-treated monkeys had more extensive coronary artery atherosclerosis than placebo controls (Shively et al., 2015). This suggests that lipid independent risk factors mediate sertraline effects on coronary artery atherosclerosis. The current findings suggest these are not related to adverse effects of sertraline on body weight, adiposity, or insulin sensitivity.
The results of this study are compelling because a stratified randomization design was used to assign individuals to antidepressant treatment groups on the basis of body weight and depressive behavior, thus preventing confounders in clinical trials. This is an important strength, as sertraline and other SSRIs are prescribed for a variety of non-depressive disorders which could bias the outcomes of clinical trials. Other strengths include the use of subjects naïve to antidepressant treatment prior to onset of the study. This study controlled for a number of other characteristics including, sex, diet, light/day cycles, housing, and treatment compliance. Plasma concentrations of sertraline and metabolite desmethylsertraline demonstrated the drug was successfully delivered via oral dosing, producing plasma concentrations similar to those measured in patients (Reis et al., 2004; Shively et al., 2014). CSF monoamine metabolite changes were also similar to those observed in human patients, indicating a clinically relevant oral dose of sertraline (Sheline et al., 1997; Shively et al., 2014). Sertraline had no effect on depressive behavior (Shively et al., 2015). Similarly in patients, SSRI effects on depressive symptoms are inconsistent (nearly 50% of patients fail to respond to first-line SSRI treatment) and specific drug effects only mildly improve upon placebo-expectancy effects (Arroll et al., 2005).
This trial used a well-established model of depression which shares many physiological and neurobiological characteristics of depression with human beings (Shively and Willard, 2012). Depressed monkeys are generally dyslipidemic, have lower body weight and BMI, and develop more coronary artery atherosclerosis than their non-depressed counterparts (Shively et al., 2009, 2008, 2005) as replicated in the current study (Shively et al., 2015). Here we report that, compared to their nondepressed counterparts, depressed monkeys have lower lean mass, fat mass, percent fat mass, leptin and insulin along with higher adiponectin levels. In depressed patients, weight gain is one of the most commonly reported side-effects; however weight loss has also been recognized as a diagnostic criterion for depressive disorders (Polivy and Herman, 1976; Zung, 1965). It seems that the relationship between body mass and depression in humans is actually U-shaped, with the heaviest and leanest patients being the most depressed (de Wit et al., 2009). The metabolic profile observed in the depressed monkeys is consistent with a subgroup of depressed human patients and reflects lower overall total and percent fat mass, suggesting multi-system differences between depressed and nondepressed monkeys, and potentially patients, in energy metabolism.
Throughout the Pretreatment and Treatment phases the animals were fed a western-like diet, previously shown to increase body weight, adiposity, insulin concentrations, and HOMA-IR scores (Mubiru et al., 2011; Wagner et al., 2009). This weight gain was observed in placebo-treated animals, while sertraline prevented significant increases in body weight and adiposity. The prevention of an 8% increase in body weight by sertraline treatment translates to a noticeable and clinically relevant difference of about 12 lbs for a 130 lb woman, and could be responsible for the reduced leptin, HOMA IR, and increased adiponectin.
The effects of sertraline on body composition and carbohydrate metabolism observed in this reverse-translational study were similar to those observed in the few published clinical trials. Sertraline associated reductions in fasting insulin concentration independent of systemic glucose have been reported in depressed human patients after 12 weeks treatment (Kesim et al., 2011). In another clinical trial of similar duration (3–4 months) in patients with generalized anxiety disorder; the SSRI fluoxetine was associated with significant decreases in body weight, BMI, waist circumference and triglycerides (Beyazyuz et al., 2013). Large epidemiologic studies noted that SSRI use was associated with increased rates of obesity and diabetes mellitus. Unlike the epidemiologic studies and clinical trials conducted, this study was tightly controlled and represents the best estimate of a sertraline effect on metabolic outcomes.
The results of this study suggests that sertraline may have beneficial effects on adiposity, although adiponectin, a potentially insulin-sensitizing and atheroprotective protein, was altered in an unusual way. Adiponectin was the only variable for which a significant phase × treatment × depression interaction was observed, an effect driven by decreases in plasma adiponectin in the sertraline-treated depressed monkeys. These monkeys also expressed the greatest degree of coronary artery atherosclerosis (Shively et al., 2015).
Adiponectin is thought to have beneficial effects on cardiovascular health. Adiponectin is released by adipose tissue, although circulating adiponectin concentration generally decrease with obesity and weight gain and increase with weight loss. It may protects cardiovascular health through vasodilatory, anti-apoptotic, anti-inflammatory, and anti-oxidative activities in cardiac and vascular cells (Ebrahimi-Mamaeghani et al., 2015; Hui et al., 2012). Antiatherogenic properties of adiponectin have been demonstrated in both preclinical (Fantuzzi, 2013; Rubio-Guerra et al., 2013) and animal model studies (Cai et al., 2015; Ebrahimi-Mamaeghani et al., 2015). Across the entire cohort, coronary artery atherosclerosis was not significantly correlated with adiponectin (data not shown: r = 0.06, p = 0.71), nor were the two associated specifically in the sertraline-treated depressed monkeys (data not shown: r = 0.13, p = 0.73) Adiponectin exists in three oligomeric forms, each possessing distinct biological activities, which were not differentiated in this study (Hui et al., 2012). The potential mechanisms underlying reduction in adiponectin by sertraline are not known.
The results of this nonhuman primate study suggest that sertraline may have beneficial effects on body composition and carbohydrate metabolism but deleterious effects on the cardiovascular risk factor adiponectin. The mechanisms by which sertraline might influence body composition, carbohydrate metabolism, and adiponectin regulation are not known. We hypothesize that sertraline may affect body composition and carbohydrate metabolism by blocking serotonin reuptake and thereby increasing signaling at the central serotonin receptors, specifically 5-HT2C, which appear to regulate food intake, body weight insulin sensitivity, and glucose homeostasis (Berglund et al., 2013). Higher activity levels were not observed, but reduced food intake in sertraline-treated monkeys may explain the observed differences in body weight, fat mass, and insulin resistance compared to placebo-controls. Food intake was not measured in individual animals in the current study. Altered gastrointestinal motility might also affect nutrient absorption and is known to be regulated by serotonin interactions with receptors and transporters (Gershon, 2004). Mechanistic studies will be useful to understand and confirm the effects of sertraline treatment on body composition and carbohydrate metabolism observed in this study. Attention to sertraline effects on adiponectin regulation at the cellular level are especially indicated and may explain adverse effects of SSRIs on atherosclerosis despite otherwise beneficial effects on body composition and carbohydrate metabolism. Finally, there is also a need for long-term clinical trials to assess the benefits of sertraline treatment on metabolic syndrome in patient populations.
5. Conclusion
The data presented here suggest that sertraline may have effects on body composition and carbohydrate metabolism, both components of metabolic syndrome. Results of this study are compelling due to the use of a clinically translational nonhuman primate model allowing for placebo-controlled, longitudinal, randomized study design. Long-term clinical trials are needed to confirm these findings in patient populations.
Acknowledgements
We acknowledge the following people for their technical support: Beth Uberseder, Dana Morgan, Amanda Gogolak, Stephen Loiacono, Stephanie Willard, JD Bottoms, and Maryanne Post.
Role of the funding source This work was supported in part by NIH grants ROIHL87103, R21MH86731, T32OD10957, and the Pepper Older Americans for Independence Center (P30 AG21332). The contents are solely the responsibility of the authors and do not necessarily represent the view of the NIH.
Footnotes
Conflict of interest None
Contributors MGSM performed the data analysis and contributed to data interpretation. CAS designed and directed the study and contributed to data interpretation. TBC oversaw the measurement of atherosclerosis extent and contributed to data interpretation. SEA monitored the diet, provided veterinary expertise, performed the iliac artery biopsies, and contributed to data interpretation. JJC oversaw the measurement of abdominal fat deposition. SBK provided funding for the body composition measures. SRJ performed the monoamine measurements. TCR supervised biochemical measurements, and aided in data interpretation.
References
- Adams MR, Golden DL, Williams JK, Franke AA, Register TC, Kaplan JR. Soy protein containing isoflavones reduces the size of atherosclerotic plaques without affecting coronary artery reactivity in adult male monkeys. J. Nutr. 2005;135:2852–2856. doi: 10.1093/jn/135.12.2852. [DOI] [PubMed] [Google Scholar]
- Arroll B, Macgillivray S, Ogston S, Reid I, Sullivan F, Williams B, Crombie I. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann. Fam. Med. 2005;3:449–456. doi: 10.1370/afm.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, Vianna CR, Williams KW, Xu Y, Elmquist JK. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Invest. 2013;123:5061–5070. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyazyuz M, Albayrak Y, Egilmez OB, Albayrak N, Beyazyuz E. Relationship between ssris and metabolic syndrome abnormalities in patients with generalized anxiety disorder: a prospective study. Psychiatry Invest. 2013;10:148–154. doi: 10.4306/pi.2013.10.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- Cai X, Li X, Li L, Huang XZ, Liu YS, Chen L, Zhang K, Wang L, Li X, Song J, Li S, Zhang Y, Zhang M. Adiponectin reduces carotid atherosclerotic plaque formation in ApoE−/− mice: roles of oxidative and nitrosative stress and inducible nitric oxide synthase. Mol. Med. Rep. 2015;11:1715–1721. doi: 10.3892/mmr.2014.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus SM, Rochais C, Blois-Heulin C, Li Q, Hausberger M, Bezard E. Depressive-like behavioral profiles in captive-bred single- and socially-housed rhesus and cynomolgus macaques: a species comparison. Front. Behav. Neurosci. 2014;8:47. doi: 10.3389/fnbeh.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascade E, Kalali AH, Kennedy SH. Real-world data on SSRI antidepressant side effects. Psychiatry (Edgmont) 2009;6:16. [PMC free article] [PubMed] [Google Scholar]
- de Wit LM, van Straten A, van Herten M, Penninx BW, Cuijpers P. Depression and body mass index, a u-shaped association. BMC Public Health. 2009;9:14. doi: 10.1186/1471-2458-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Dunbar JA, Reddy P, Davis-Lameloise N, Philpot B, Laatikainen T, Kilkkinen A, Bunker SJ, Best JD, Vartiainen E, Kai Lo S, Janus ED. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care. 2008;31:2368–2373. doi: 10.2337/dc08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Mamaeghani M, Mohammadi S, Arefhosseini SR, Fallah P, Bazi Z. Adiponectin as a potential biomarker of vascular disease. Vasc. Health Risk Manage. 2015;11:55–70. doi: 10.2147/VHRM.S48753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64:1–10. doi: 10.1016/j.cyto.2013.06.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Review article: serotonin receptors and transporters — roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 2004;20(Suppl. 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- Ghaeli P, Shahsavand E, Mesbahi M, Kamkar M-Z, Sadeghi M, Dashti-Khavidaki S. Comparing the effects of 8-week treatment with fluoxetine and imipramine on fasting blood glucose of patients with major depressive disorder. J. Clin. Psychopharmacol. 2004;24:386–388. doi: 10.1097/01.jcp.0000132441.27854.0d. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics, Stroke, C., Statistics, S. Heart disease and stroke statistics-2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM. Gender differences in depression and response to psychotropic medication. Gend. Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- Gragnoli C. Hypothesis of the neuroendocrine cortisol pathway gene role in the comorbidity of depression, type 2 diabetes, and metabolic syndrome. Appl. Clin. Genet. 2014;7:43–53. doi: 10.2147/TACG.S39993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groban L, Kitzman DW, Register TC, Shively CA. Effect of depression and sertraline treatment on cardiac function in female nonhuman primates. Psychosom. Med. 2014;76:137–146. doi: 10.1097/PSY.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24:183–197. doi: 10.2165/00002018-200124030-00003. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, McCowan B, Jiang J, Capitanio JP. Depressive-like behavioral response of adult male rhesus monkeys during routine animal husbandry procedure. Front. Behav. Neurosci. 2014;8:309. doi: 10.3389/fnbeh.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br. J. Pharmacol. 2012;165:574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesim M, Tiryaki A, Kadioglu M, Muci E, Kalyoncu NI, Yaris E. The effects of sertraline on blood lipids, glucose, insulin and HBA1C levels: a prospective clinical trial on depressive patients. J. Res. Med. Sci. 2011;16:1525–1531. [PMC free article] [PubMed] [Google Scholar]
- Kinder LS, Carnethon MR, Palaniappan LP, King AC, Fortmann SP. Depression and the metabolic syndrome in young adults: findings from the third national health and nutrition examination survey. Psychosom. Med. 2004;66:316–322. doi: 10.1097/01.psy.0000124755.91880.f4. [DOI] [PubMed] [Google Scholar]
- Lee HW, Muniyappa R, Yan X, Yue LQ, Linden EH, Chen H, Hansen BC, Quon MJ. Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic glucose clamps in rhesus monkeys. Endocrinology. 2011;152:414–423. doi: 10.1210/en.2010-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur RB, Brietzke E, McIntyre RS. Is there a metabolic-mood syndrome? A review of the relationship between obesity and mood disorders. Neurosci. Biobehav. Rev. 2015;52:89–104. doi: 10.1016/j.neubiorev.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Martinac M, Pehar D, Karlovic D, Babic D, Marcinko D, Jakovljevic M. Metabolic syndrome, activity of the hypothalamic-pituitary-adrenal axis and inflammatory mediators in depressive disorder. Acta Clin. Croat. 2014;53:55–71. [PubMed] [Google Scholar]
- Mubiru JN, Garcia-Forey M, Higgins PB, Hemmat P, Cavazos NE, Dick EJ, Jr., Owston MA, Bauer CA, Shade RE, Comuzzie AG, Rogers J. A preliminary report on the feeding of cynomolgus monkeys (Macaca fascicularis) with a high-sugar high-fat diet for 33 weeks. J. Med. Primatol. 2011;40:335–341. doi: 10.1111/j.1600-0684.2011.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RA, Register TC, Shively CA, Carr JJ, Ge Y, Heilbrun ME, Cummings SR, Koster A, Nevitt MC, Satterfield S, Tylvasky FA, Strotmeyer ES, Newman AB, Simonsick EM, Scherzinger A, Goodpaster BH, Launer LJ, Eiriksdottir G, Sigurdsson S, Sigurdsson G, Gudnason V, Lang TF, Kritchevsky SB, Harris TB. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:109–117. doi: 10.1093/gerona/glt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics . Health, United States, 2014: With Special Feature on Adults Aged 55–64. 2015. [PubMed] [Google Scholar]
- Orleans RJ, Li L, Kim MJ, Guo J, Sobhan M, Soule L, Joffe HV. FDA approval of paroxetine for menopausal hot flushes. N. Engl J. Med. 2014;370:1777–1779. doi: 10.1056/NEJMp1402080. [DOI] [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, Hu FB. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Clinical depression and weight change: a complex relation. J. Abnorm. Psychol. 1976;85:338. doi: 10.1037//0021-843x.85.3.338. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States: United States, 2005–2008 NCHS data brief, no. 76. National Center for Health Statistics; Hyattsville, MD: 2011. [PubMed] [Google Scholar]
- Pratt LA, Brody DJ. Depression in the U.S. household population, 2009–2012. NCHS data brief, no. 172. National Center for Health Statistics; Hyattsville, MD: 2014. [PubMed] [Google Scholar]
- Raeder MB, Bjelland I, Emil Vollset S, Steen VM. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland health study. J. Clin. Psychiatry. 2006;67:1974–1982. doi: 10.4088/jcp.v67n1219. [DOI] [PubMed] [Google Scholar]
- Reis M, Aberg-Wistedt A, Agren H, Hoglund P, Akerblad AC, Bengtsson F. Serum disposition of sertraline, N-desmethylsertraline and paroxetine: a pharmacokinetic evaluation of repeated drug concentration measurements during 6 months of treatment for major depression. Hum. Psychopharmacol. 2004;19:283–291. doi: 10.1002/hup.599. [DOI] [PubMed] [Google Scholar]
- Rubio-Guerra AF, Cabrera-Miranda LJ, Vargas-Robles H, Maceda-Serrano A, Lozano-Nuevo JJ, Escalante-Acosta BA. Correlation between levels of circulating adipokines and adiponectin/resistin index with carotid intima-media thickness in hypertensive type 2 diabetic patients. Cardiology. 2013;125:150–153. doi: 10.1159/000348651. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J. Clin. Psychopharmacol. 1997;17:11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior and central monoaminergic function in female cynomolgus monkeys. Biol. Psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol. Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci. Biobehav. Rev. 2009;33:133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom. Med. 2008;70:637–645. doi: 10.1097/PSY.0b013e31817eaf0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Appt SE, Clarkson TB. Effects of long-term sertraline treatment and depression on coronary artery atherosclerosis in premenopausal female primates. Psychosom. Med. 2015;77:267–278. doi: 10.1097/PSY.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol. Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Higley JD, Willard SL. Sertraline effects on cerebrospinal fluid monoamines and species-typical socioemotional behavior of female cynomolgus monkeys. Psychopharmacology (Berl.) 2014;231:1409–1416. doi: 10.1007/s00213-013-3329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp. Neurol. 2012;233:87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein MG, El-Amin CK, Shively CA. Selective serotonin reuptake inhibitor and bleeding in a cynomolgus macaque (Macaca fascicularis) Comp. Med. 2014;64:221–223. [PMC free article] [PubMed] [Google Scholar]
- Stone KJ, Viera AJ, Parman CL. Off-label applications for SSRIs. Am. Fam. Physician. 2003;68:498–504. [PubMed] [Google Scholar]
- Wagner JD, Jorgensen MJ, Cline JM, Lees CJ, Franke AA, Zhang L, Ayers MR, Schultz C, Kaplan JR. Effects of soy vs casein protein on body weight and glycemic control in female monkeys and their offspring. Am. J. Primatol. 2009;71:802–811. doi: 10.1002/ajp.20716. [DOI] [PubMed] [Google Scholar]
- Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, Jr., Kaplan JR. Old world nonhuman primate models of type 2 diabetes mellitus. ILAR J. 2006;47:259–271. doi: 10.1093/ilar.47.3.259. [DOI] [PubMed] [Google Scholar]
- Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) Am. J. Primatol. 2012;74:528–542. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- Willard SL, Uberseder B, Clark A, Daunais JB, Johnston WD, Neely D, Massey A, Williamson JD, Kraft RA, Bourland JD, Jones SR, Shively CA. Long term sertraline effects on neural structures in depressed and nondepressed adult female nonhuman primates. Neuropharmacology. 2015;99:369–378. doi: 10.1016/j.neuropharm.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JM, Cho EG, Lee HK, Park SM. Antidepressant use and diabetes mellitus risk: a meta-analysis. Korean J. Fam. Med. 2013;34:228–240. doi: 10.4082/kjfm.2013.34.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung WW. A self-rating depression scale. Arch. Gen. Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]