Cross-sectional studies have suggested an association between the main female hormone, 17β-estradiol (E2), and the risk of type 2 diabetes (T2D) in postmenopausal women (1,2). Even prospective studies have found that among postmenopausal women not using hormone therapy, higher circulating levels of total and free E2 were strongly and prospectively associated with increased long-term risk of T2D, independently of adiposity or insulin resistance (3,4).
In this issue of Diabetes, Muka et al. (5) examine the association among sex hormone–binding globulin (SHBG)—a protein that binds and carries E2 in the blood and limits its bioavailability—sex hormones, and T2D risk in a large population-based sample of healthy postmenopausal women who were free of T2D at baseline, with a median follow-up of 11 years. They report that lower levels of SHBG (which translate into higher bioavailable E2) and higher concentrations of total E2 are associated with increased risk of T2D, independently of established risk factors, including BMI and insulin resistance. In contrast, they observed no association between testosterone and T2D risk. Their findings are reinforced by a systematic meta-analysis of 13 studies with 14,902 participants who included 1,912 case subjects with T2D, confirming that lower SHBG and increased total E2 are robust risk markers of T2D in postmenopausal women.
Of course, one limitation of this study is the lack of reliability of the immuno-based steroid assays used. Mass spectrometry is clearly the most reliable technology available for steroid assays, especially in postmenopausal women with low E2 levels (6). However, the generalizability of the finding makes it unlikely that the results were an assay artifact.
This study raises new questions regarding the pathogenesis of T2D in women. The obvious first one is whether circulating E2 is instrumental in predisposing postmenopausal women to T2D. The answer is probably no, as large randomized controlled trials have consistently shown decreased T2D incidence in women assigned to menopausal treatment with estrogens (7–9). In addition, the accumulation of decades of mechanistic studies in preclinical models conclusively demonstrated that therapeutic doses of E2 and other estrogens improve glucose homeostasis (10). A false perception exists that estrogens impair carbohydrate metabolism. This belief stems from old studies using high doses of oral contraceptives, which produced glucose intolerance in women (11). Indeed, no beneficial effect of estrogens on glucose homeostasis is observed outside a narrow therapeutic or physiological window (12,13), and high doses of oral estrogens or high serum concentrations of endogenous E2, such as are seen during pregnancy or in transsexuals, produce insulin resistance (10). This was not the case in the current study, which focused on untreated postmenopausal women with low circulating E2 concentrations. Furthermore, no association between E2 and T2D has been found in premenopausal women, who exhibit higher circulating E2 concentrations. Finally, and consistent with a beneficial role for endogenous E2 in glucose homeostasis in women, a prospective cohort study with a follow-up of 11 years concluded that early menopause—leading to more prolonged E2 deficiency—is associated with a greater risk of T2D (14).
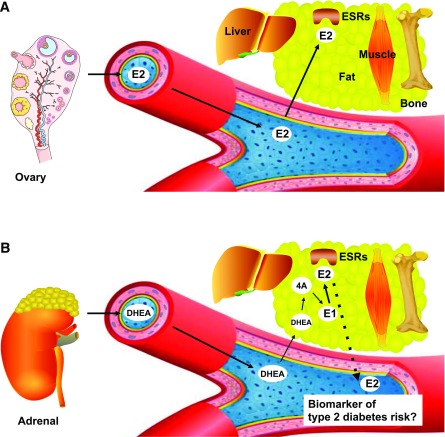
The remaining question is whether increased E2 is a new indirect biomarker of a pathological process predisposing postmenopausal women to T2D. A fundamental concept in the interpretation of relationships between circulating E2 levels and disease in epidemiological studies is that, unlike in premenopausal women, E2 does not function as a circulating hormone in postmenopausal women because its ovarian production is suppressed. Rather, in postmenopausal women E2 is produced locally in extraovarian tissues such as breast, brain, adipose tissue, muscle, and bone from circulating sources of adrenal androgens, mainly dehydroepiandrosterone, which is converted to androstenedione, which in turn undergoes aromatization to estrone (E1) (15). In fact, nearly the only means of E2 production after menopause is E1 formation. E2 is produced locally in extraovarian tissues and acts locally as a paracrine and intracrine factor (16). E2 is also inactivated in the same cells where its synthesis takes place before being released as inactive (Fig. 1). Therefore, it is difficult to see how one can readily equate circulating levels of E2 to the concentrations that are present in target cells and thus to E2 action in those cells. In postmenopausal women, circulating E2 is not the driver of estrogen action; there is little physiological relationship between serum E2 concentrations (free or total) and cellular estrogen output. The consequence of this biological paradigm is that the association between higher circulating E2 and T2D in postmenopausal women is unlikely to reflect causality between increased E2 signaling and the development of T2D. Rather, E2 concentrations may reflect a leakage from increased local E2 biosynthesis or decreased E2 elimination from extraovarian sites as a compensatory adaptive mechanism (Fig. 1). Because E2 favors metabolic homeostasis (10), high E2 concentrations may also reflect a form of estrogen resistance as a biomarker for the pathological process that predisposes to T2D. In fact, there is an elevation in circulating E2 in clinical situations of estrogen resistance (17), just as there is an increase in circulating insulin in insulin resistance. Current standard measurement of serum E2 levels poorly reflects cellular E2 concentrations, biosynthesis, catabolism, and elimination. Studies are needed to develop novels assays that will provide accurate measures of cellular estrogen biosynthesis and sensitivity and that will be informative in epidemiological studies associating E2 with chronic diseases.
Figure 1.
Schematic representation of the origin of E2 in women. A: In premenopausal women, E2 is produced by the ovary and acts as a circulating hormone. Therefore, circulating E2 is the driver of E2 action in peripheral tissues. B: In postmenopausal women, ovarian E2 production is abolished. E2 is synthesized locally in extraovarian tissues from an adrenal source of dehydroepiandrosterone (DHEA) and androstenedione (4A). The local precursor of E2 is E1 after aromatization from 4A. E2 is synthesized, acts, and is metabolized locally, and therefore circulating E2 is not a driver of E2 action. E2 could be a indirect biomarker of T2D risk in postmenopausal women. ESR, estrogen receptor.
Article Information
Funding. This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-107444).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 577.
References
- 1.Golden SH, Dobs AS, Vaidya D, et al. . Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 2007;92:1289–1295 [DOI] [PubMed] [Google Scholar]
- 2.Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI; Postmenopausal Estrogen/Progestin Intervention Trial . Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab 2003;88:1646–1652 [DOI] [PubMed] [Google Scholar]
- 3.Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 2007;50:2076–2084 [DOI] [PubMed] [Google Scholar]
- 4.Kalyani RR, Franco M, Dobs AS, et al. . The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab 2009;94:4127–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muka T, Nano J, Jaspers L, et al. Associations of steroid sex hormones and sex hormone–binding globulin with the risk of type 2 diabetes in women: a population-based cohort study and meta-analysis. Diabetes 2017;66:577–586 [DOI] [PubMed] [Google Scholar]
- 6.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev 2007;16:1713–1719 [DOI] [PubMed] [Google Scholar]
- 7.Bonds DE, Lasser N, Qi L, et al. . The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia 2006;49:459–468 [DOI] [PubMed] [Google Scholar]
- 8.Kanaya AM, Herrington D, Vittinghoff E, et al.; Heart and Estrogen/progestin Replacement Study . Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2003;138:1–9 [DOI] [PubMed] [Google Scholar]
- 9.Margolis KL, Bonds DE, Rodabough RJ, et al.; Women’s Health Initiative Investigators . Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004;47:1175–1187 [DOI] [PubMed] [Google Scholar]
- 10.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013;34:309–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wynn V, Doar JW. Some effects of oral contraceptives on carbohydrate metabolism. Lancet 1966;2:715–719 [DOI] [PubMed] [Google Scholar]
- 12.Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci (Lond) 2002;102:151–166 [DOI] [PubMed] [Google Scholar]
- 13.Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 2011;22:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand JS, van der Schouw YT, Onland-Moret NC, et al.; InterAct Consortium . Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care 2013;36:1012–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol 2015;145:133–138 [DOI] [PubMed] [Google Scholar]
- 16.Simpson ER, Misso M, Hewitt KN, et al. . Estrogen—the good, the bad, and the unexpected. Endocr Rev 2005;26:322–330 [DOI] [PubMed] [Google Scholar]
- 17.Smith EP, Boyd J, Frank GR, et al. . Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994;331:1056–1061 [DOI] [PubMed] [Google Scholar]