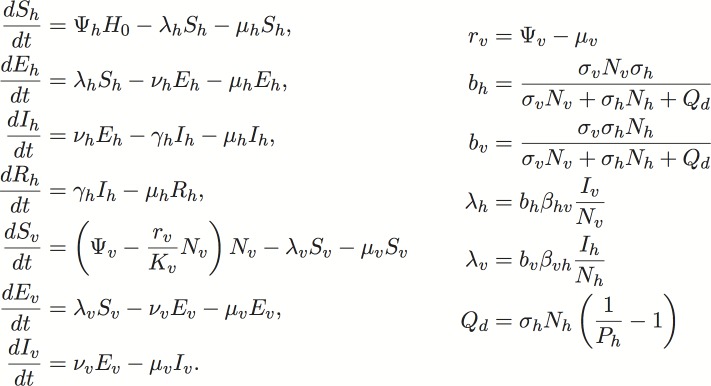Abstract
The recent spread of mosquito-transmitted viruses and associated disease to the Americas motivates a new, data-driven evaluation of risk in temperate population centers. Temperate regions are generally expected to pose low risk for significant mosquito-borne disease; however, the spread of the Asian tiger mosquito (Aedes albopictus) across densely populated urban areas has established a new landscape of risk. We use a model informed by field data to assess the conditions likely to facilitate local transmission of chikungunya and Zika viruses from an infected traveler to Ae. albopictus and then to other humans in USA cities with variable human densities and seasonality. Mosquito-borne disease occurs when specific combinations of conditions maximize virus-to-mosquito and mosquito-to-human contact rates. We develop a mathematical model that captures the epidemiology and is informed by current data on vector ecology from urban sites. The model demonstrates that under specific but realistic conditions, fifty-percent of introductions by infectious travelers to a high human, high mosquito density city could initiate local transmission and 10% of the introductions could result in 100 or more people infected. Despite the propensity for Ae. albopictus to bite non-human vertebrates, we also demonstrate that local virus transmission and human outbreaks may occur when vectors feed from humans even just 40% of the time. Inclusion of human behavioral changes and mitigations were not incorporated into the models and would likely reduce predicted infections. This work demonstrates how a conditional series of non-average events can result in local arbovirus transmission and outbreaks of human disease, even in temperate cities.
Author Summary
Zika and chikungunya viruses are transmitted by Aedes mosquitoes, including Ae. albopictus, which is abundant in many temperate cities. While disease risk is lower in temperate regions where viral amplification cannot build across years, there is significant potential for localized disease outbreaks in urban populations. We use a model informed by field data to assess the conditions likely to facilitate local transmission of virus from an infected traveler to Ae. albopictus and then to other humans in USA cities with variable human densities and seasonality. The model demonstrates that up to 50% of infectious travelers returning to the U.S. could initiate local transmission in temperate cities if are infectious and are exposed to high mosquito densities. This work highlights the need for high-resolution spatial data on Ae. albopictus density, biting behavior, and seasonality to better understand, predict and manage arboviral transmission risk in temperate cities.
Introduction
The Asian tiger mosquito (Aedes albopictus) is a global nuisance, with self-sustaining populations established on nearly every continent. Like its relative, Ae. aegypti, the Asian tiger mosquito is a day-time biter and lays eggs that are resistant to drought. In its native range, the juveniles develop in water-holding tree holes and emerging adult females feed opportunistically on vertebrate species in the surrounding sylvan habitats. Limited vagility of adult mosquitoes restricts natural dispersal distances to a few hundred meters [1,2], but international trade and travel has dispersed the species well beyond its native forests of southeast Asia to urban and peri-urban landscapes throughout the Americas and Europe in the 1980s and Africa in the 1990s [3,4]. Similar to the earlier invasion by Ae. aegypti from Africa, Ae. albopictus has become increasingly associated with urban and peri-urban landscapes as it has expanded its geographic range [5]. Within these landscapes, the species has become increasingly capable of exploiting human-made container habitat and human blood meal hosts.
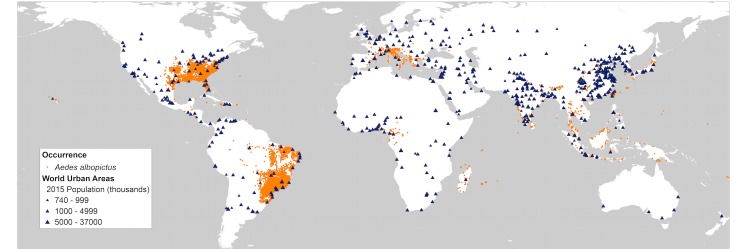
In recent years the introduction of Aedes-transmitted chikungunya and Zika arboviruses to the Western Hemisphere has raised important questions regarding the role that Ae. albopictus might play in arboviral transmission, especially in temperate regions where Ae. aegypti is rare but Ae. albopictus is increasingly abundant. Numerous lab studies indicate that Ae. albopictus can be equally competent (able to acquire and transmit pathogens) as Ae. aegypti for a suite of arboviruses, including chikungunya and Zika [6–11]. Ae. albopictus has also been associated with local arboviral transmission and disease outbreaks, specifically in temperate regions where Ae. aegypti is absent or uncommon[12–17]. However, Ae. albopictus is generally considered less important than Ae. aegypti for transmitting viral infections to humans because it has been shown to feed on a range of vertebrate species beyond human [18–20]. An Ae. aegypti mosquito that bites a human is highly likely to bite another human if it survives to feed more than once, making this species an important vector of arboviruses transmitted between humans [8,21–24]. Ae aegypti is also predominant in tropical regions where transmission cycles and viral amplification can be facilitated by longer seasons and greater opportunity for human-mosquito contacts. By contrast, Ae. albopictus has a far greater capacity than Ae. aegypti for exploiting a range of climates and habitat types, with established Ae. albopictus populations in rural and urban landscapes across both tropical and temperate regions [4,25] (Fig 1). Likewise, while Ae. albopictus host biting behavior is variable across its introduced range, urban regions can be focal areas of predominantly human biting [6,26–30]. In the United States, Ae. albopictus is now widespread throughout the eastern portion of the country, with increasingly urban association as the species has spread northward [5,31,32]. Increases in geographic range, urban occupation, and human biting, would all seem to intensify the potential for this vector to transmit arboviruses to humans. A quantitative evaluation is required to better understand how this behavioral plasticity and variable urban densities influence risk of local outbreaks of arboviral infection in temperate regions, including the densely populated eastern United States.
Fig 1. Global distribution of Aedes albopictus (orange dots) with superimposed major urban areas (blue triangles).
Ae. albopictus occurrence data were from the database provided by [4]. Note in particular the extensive occurrence of cities in the United States within areas inhabited by this mosquito.
Many modeling efforts and risk predictions generate inference based on mean vector densities, human biting rates and other parameters that inform vectorial capacity. There are two limitations in this approach. First, data limitations mean that parameters are derived from data collected across very different landscapes in the species’ native and invasive range. Second, emergent outbreaks like the spreading Zika crisis and the more limited but still alarming human impacts of dengue emergence in Japan or chikungunya in Italy are not the outcome of average conditions–outbreaks occur when a suite of (often extreme or unusual) conditions align. Our goal in this paper is to quantitatively evaluate the potential for Ae. albopictus vectored transmission cycles and local disease outbreaks of emerging Zika and chikungunya viruses in temperate U.S. cities. We define probabilistic parameter distributions that represent mosquito densities, human host-use, and specific vector competencies reported in the literature and employ a mathematical model that explores the full range of observed parameter values to identify conditions that would facilitate local outbreaks in human population centers.
Results
Our model draws on parameter values defined by field data and demonstrates how combinations of realistic parameter distributions can generate significant outbreak potential for chikungunya and Zika viruses in temperate U.S. cities (New York City, Philadelphia, Washington D.C., and Atlanta), where high Ae. albopictus densities are already reported. As expected, a majority of the model runs predicted that no outbreak would occur (R0<1). However, across the scenarios evaluated there is a persistent subset of runs where suites of realistic parameter combinations generate high R0 conditions that result in locally-transmitted human infections, including almost 45% leading to more than 10 new human infections if the mosquitoes bite humans even 40% of the time (Fig 2). For Zika virus, the average value of R0 across all 12 scenarios (encompassing 4 urban densities and 3 season lengths) was 1.1 with a median of 0.82 and a range of 0 to 13.1 (S1 Table). For chikungunya, the average value of R0 was 0.91 with a median of 0.68 and a range of 0 to 7.4 (S1 Table).
Fig 2. Proportion of the human population in a neighborhood infected with Zika virus at the end of the 90-day season in Atlanta (colorbar) as a function of R0 and Ph, the proportion of blood meals that are human.
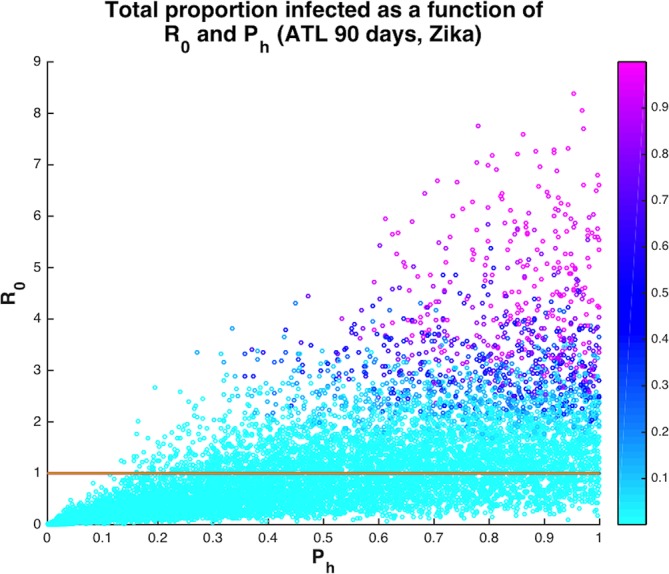
The solid line is at R0 = 1. When Ph> = 0.4, then 62.5% runs have R0>1 and 44.8% of runs result in at least 10 people infected after a single introduction. On the other hand, when Ph <0.4, then only 10.8% of runs have R0>1 and 4.0% of runs result in at least 10 people infected.
We specifically evaluated how duration of active mosquito season following the arrival of an infectious traveler and propensity for biting diverse vertebrate species, where every non-human bite slows the transmission process, influence outbreak potential for different urban densities. As might be expected, higher probability of human host-use is associated with greater R0 (Fig 3). For a given seasonal duration and human population density, increasing the proportion of bites on humans in the mosquito population above 40% resulted in more model runs that returned R0 >1, signifying increased potential for local transmission and human disease even when a significant proportion of blood meals are from non-human animals (Fig 2). The average number of times a human was bitten per day in the model ranges from 0 to 4 bites. Even for number of bites per person per day below 1, there were several scenarios with significant onward transmission (Fig 4).
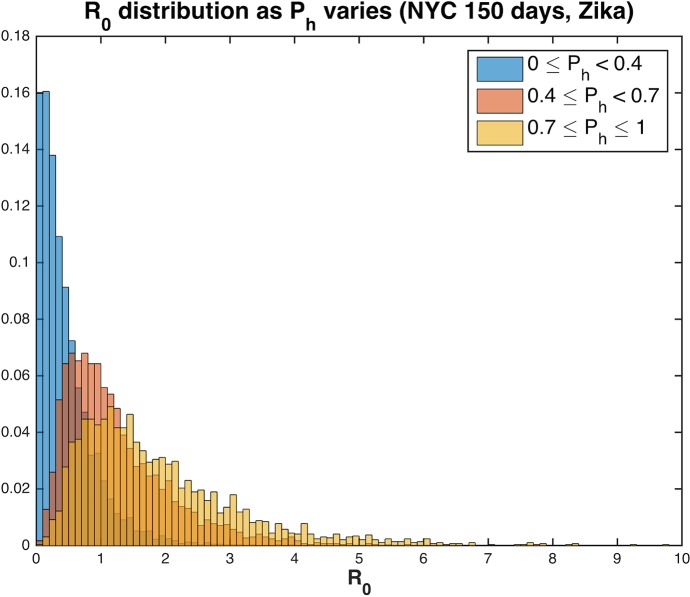
Fig 3. Distribution of R0 for Zika virus across ranges of human feeding rates, Ph, for New York City.
With Ph≥0.4 probability of an outbreak increases significantly, resulting in 62.7% of runs with R0>1. However, when Ph < 0.4, the percent of runs with R0>1 decreases to 10.1% (for Ph≥0.8, 76.3% of runs have R0>1). When Ph < 0.4, the mean value of R0 is 0.46, while for Ph≥0.4, the mean value of R0 is 1.55 and if Ph≥0.8, the mean value of R0 jumps to 1.97.
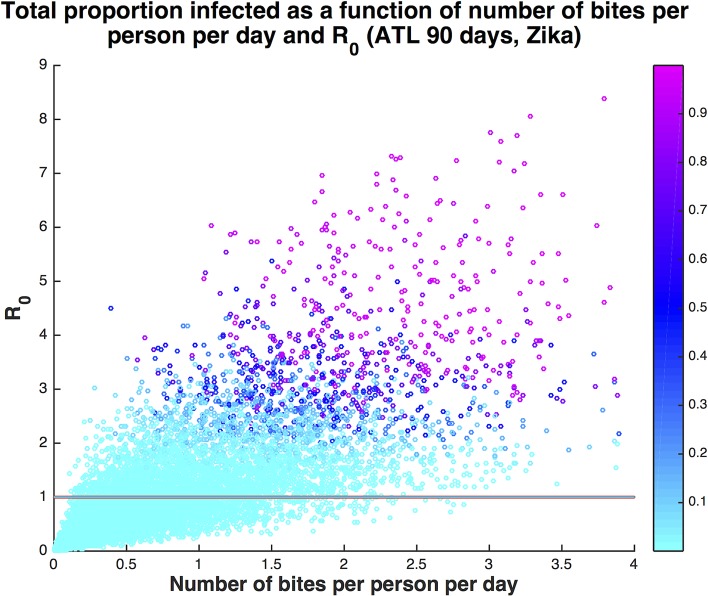
Fig 4. Proportion humans in a neighborhood infected with Zika virus (colorbar) as a function of number of bites per person per day and R0.
The solid line is at R0 = 1. Even when the average number of bites per person per day is less than 1 (68% of all runs), many runs result in autochthonous transmission. Of the runs with number of bites less than 1, 34% result in at least one new infection, 10% result in at least 10 infections, and 2% result in at least 100 infections. If the season is extended to 120 days, that increases to 34%, 14%, and 4%, respectively.
Potential human infection was positively associated with seasonal duration representing the length of time with active, high-density mosquito populations following the introduction of an infectious traveler. For example, the 90-day scenario for Zika in Philadelphia resulted in 51.8% of runs with at least one new human infection from a single primary introduction and 14.4% resulted in more than 100 people infected. Across all scenarios, the 90-day season results in 14.4% of runs with greater than 100 people infected, 120-day season in 20.4% of runs with greater than 100 people infected and 150-day season in 24.8% of runs with greater than 100 people infected (S1 Table, Fig 5). So, while on average there is only one new infection generated following a single primary introduction during a season, the chance of a relatively large outbreak increases substantially with season length as more mosquitoes become infected. Extending the season also reduced the value of Ph needed to result in potentially severe outbreaks (S1 Fig). Although season length varies from year to year in the cities we investigated, the climate in Atlanta and Washington D.C. tends to support longer mosquito seasons of at least 4–5 months, corresponding to our 120 and 150-day seasons. On the other hand, New York City and Philadelphia would be more likely to have 90-day peak mosquito seasons, on average. However, anomalously warm (or cold) years could change mosquito season length and thus, risk.
Fig 5.
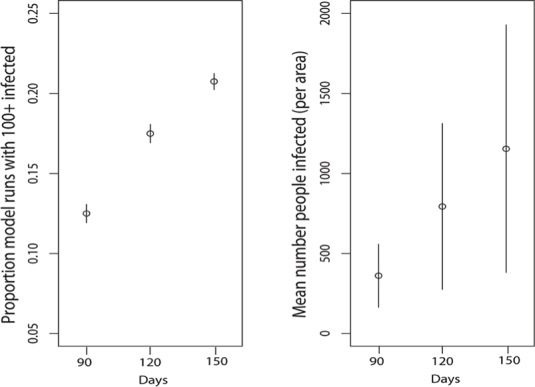
Season length (days, x axes) is positively associated with a) the proportion of model runs that resulted in 100 or more human infections with chikungunya virus and b) potential mean numbers of infected humans per square mile. Significant human infection is possible at even 90 days and uncertainty shown captures variability across cities, as well as human biting propensity and other parameter states. The mean number of people infected moves from 396 to 892 to 1376 and the median from 2.1 to 2.4 to 2.5 as season increases from 90 to 150 days.
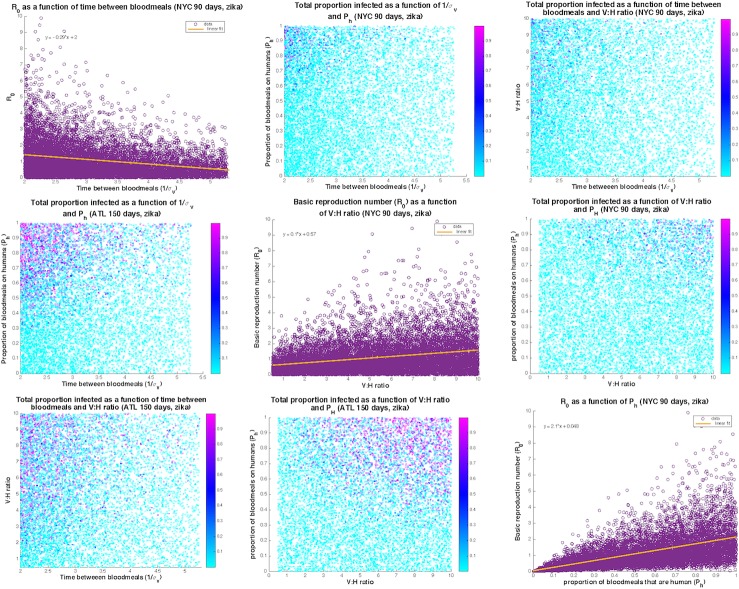
To quantify sensitivity of output to specific parameter combinations and inform targets for surveillance and mitigation, partial rank correlation coefficients were calculated separately for Zika and chikungunya. Values of R0 for Zika were most sensitive to variation in the percent of bites on humans, initial mosquito density, and mosquito biting frequency (Table 1). Chikungunya’s R0 was also highly sensitive to percent of bites on humans versus dead-end hosts and had similar sensitivies to the other parameters as Zika. For New York City, with the shorter 90-day mosquito season, large outbreaks are generally characterized by a vector to host ratio larger than 2, time between bloodmeals less than 4.5 days and proportion of bites on humans greater than 0.5 (upper right triangle of Fig 6). If one of these parameters is on the high end of its range, then the other two can be in mid-range and still result in a large outbreak. As the mosquito season lengthens, the range over which large absolute numbers of people may be infected increases. For Atlanta with a 150-day peak mosquito season, large outbreaks are occurring with vector to host ratios as low as 1, time between bloodmeals as high as 5 days and proportion of bites on humans as low as 0.3 (lower left triangle of Fig 6). The highest R0 values are seen for frequent biting (less than 3 days between bloodmeals) and high proportion of bites on humans (diagonal, Fig 6).
Table 1. Sensitivity Analysis.
PRCC values computed for R0 and proportion of the human population infected at the end of season for Zika and chikungunya (Philadelphia, 90-day season). Absolute values close to zero indicate low sensitivity and absolute values close to one indicate high sensitivity. Negative values indicate an inverse relationship between the parameter and the quantity of interest (output).
| Parameter | nu_v | mu_v | beta_hv | K_v | sigma_v | sigma_h | nu_h | beta_vh | gamma_h | P_h |
|---|---|---|---|---|---|---|---|---|---|---|
| PRCC value R0 ZIKA | 0.1575 | -0.5235 | 0.5668 | 0.6132 | 0.5514 | 0.2267 | -0.0061 | 0.559 | -0.4739 | 0.8871 |
| PRCC value proportion infected ZIKA | 0.2027 | -0.4182 | 0.5804 | 0.6263 | 0.5669 | 0.2382 | 0.0269 | 0.5716 | -0.4066 | 0.8918 |
| PRCC value R0 CHIK | 0.1158 | -0.4793 | 0.6956 | 0.6148 | 0.5469 | 0.215 | -0.0034 | 0.3039 | -0.281 | 0.8847 |
| PRCC value proportion infected CHIK | 0.148 | -0.3891 | 0.702 | 0.6227 | 0.5541 | 0.2203 | 0.008 | 0.3115 | -0.2543 | 0.8873 |
Fig 6. Plots of the top three most sensitive parameters for Zika as they relate to each other and to the percent of the population infected by the end of the outbreak for New York City (upper right triangle) and Atlanta (lower left triangle) and to R0 (diagonal).
The range of parameters resulting in large absolute numbers infected is larger for longer mosquito seasons (Atlanta). Large outbreaks tend to be restricted to high biting rates (time between bloodmeals) and high proportions of bites on humans.
While variable human density across the representative cities does not influence the mean R0 values or percent of runs with more than 100 human infections, the absolute size of the outbreaks and mean percent of the population infected are associated with human density. For very low human density regions, outbreaks with more than 100 infections would become highly improbable or even impossible, but these results hold for human densities seen in urban areas. For example, the mean number of people infected for a 90-day season in Atlanta (lowest human density) is 175, while for New York (highest human density) it is 676 (S1 Table). Note that we are considering local transmission within a square mile plot, so the percent infected is the percent of people living in or spending significant time in that local area (Table S4 in the S1 Text gives number of people per square mile).
Discussion
Our model indicates that risk of local transmission of Zika and chikungunya viruses and human disease outbreaks in temperate U.S. cities is considerable. Regardless of season length, there is a greater than 50% chance of some onward transmission if a human case is introduced to a temperate, urban landscape with high Ae. albopictus population density. This means that one of every two infectious travelers could initiate local transmission under the right conditions. This is, of course, not a prediction that we expect to be validated by documented human outbreaks. For one thing, both viruses can be predominantly asymptomatic in humans. But perhaps more importantly, the suite of parameters that is necessary to achieve this R0 represents a pretty specific chain of events and conditions and while the parameter values are each realistic, we actually don’t have the data to assess how frequently the suite of conditions occur, in real space, that support mosquito exposure to an infectious traveler, extrinsic incubation and then subsequent transmission to a second human host. The first necessary condition is high population abundance of Ae. albopictus. Studies confirm high densities and growing populations of this species across the eastern U.S. and as far north as New York [31–33]. A second necessary condition is that the female Ae. albopictus must bite humans at least 40% of the time. The Asian tiger mosquito’s vectorial capacity is persistently questioned because the propensity for biting humans versus other vertebrates varies widely, as the species appears to opportunistically bite the most available vertebrates [19,20,26,28–30,34–37]. We show that while a higher probability of human host-use is associated with greater R0, increasing the proportion of bites from humans above 40% increased potential for local transmission and resulting human disease. This percentage threshold of human biting is frequently exceeded in studies within urban landscapes [26,28–30,37]. A third condition that our model confirms is the importance of seasonal duration. When mosquito density and biting activity remains high for a longer period of time there is greater potential for local transmission. This duration is influenced by seasonal temperatures as well as the timing of when the first infectious traveler is accessible to mosquito bites.
The ability to manage mosquito population growth and associated arboviral transmission to humans requires early recognition of conditions that facilitate high vector population density and human biting behavior. When these conditions are favorable, transmission following the arrival of an infectious traveler can progress rapidly, as demonstrated in the 2014 urban dengue outbreak vectored by Ae. albopictus in Tokyo, Japan [38,39]. Although some researchers consider non-zoonotic arboviruses (e.g., Zika, chikungunya, and dengue viruses) unlikely to become endemic in temperate regions where seasonality is a strong filter on transmission, we demonstrate that a conditional series of non-average events can result in local pathogen transmission and outbreaks of disease in humans. This study confirms that non-average conditions likely to facilitate transmission after the introduction of an infectious traveler include years with particularly long, warm seasons in regions with high densities of competent vectors, high biting rates, and higher proportions of bites on humans, which often corresponds to high human density (Fig 6).
Recent introductions of both chikungunya virus and Zika virus to the Western Hemisphere have been followed by rapid intensification of human disease and/or broad geographical spread, particularly in and near urban centers [40,41]. Public health officials need validated assessments of how likely these viruses will be locally transmitted, even in temperate regions where Ae. albopictus populations are abundant and introduction of an infected traveler is likely. There has been repeated documentation of return and visiting travelers infected with chikungunya and more recently, Zika, over the past four years (Fig 7). For example, in the first six months of 2016 alone, 182 (5%) of 3605 residents of New York City who had returned from an area with ongoing Zika virus transmission were infected with Zika virus, as confirmed by RT-PCR or serologic testing [42]. These travelers can serve as sources of local transmission particularly if they are asymptomatic.
Fig 7. A map of 2016 introductions of Zika virus to the United States (CDC Zika website, http://www.cdc.gov/zika/geo/united-states.html, accessed January 3, 2017).
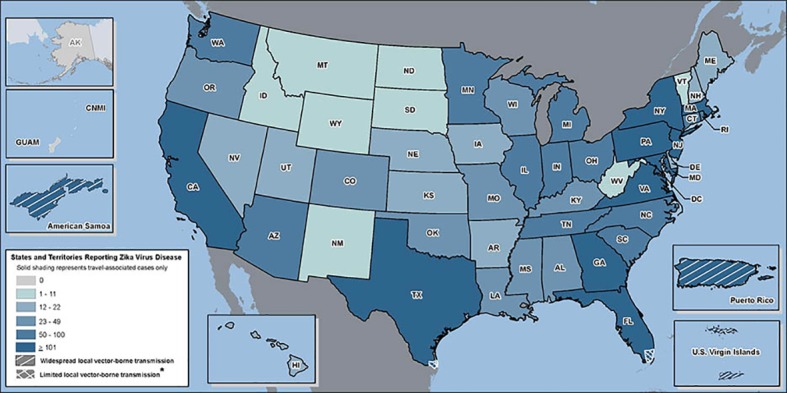
As of January 1, 2017 there were 4,592 travel-associated Zika cases and 216 locally acquired Zika cases reported in the United States in 2015–2016. In 2015, a total of 896 travel-related chikungunya cases and 1 locally acquired case were reported in the United States.
The R0 models quantify the probability of at least one local transmission event for each infected individual entering one of the cities at the beginning of the transmission season. Arrivals later in the season would lead to lower outbreak sizes, but multiple infected individuals arriving at once would increase the probability of a larger outbreak. While local chikungunya transmission has not yet led to significant human disease in the contiguous United States, our results suggest that the chance for local Zika transmission is greater. Our predictions show that the risk of local transmission and human infection with Zika is, on average, slightly higher than for chikungunya virus in temperate cities, which is consistent with differences in human infection rates reported on Yap island (73% human population with Zika infection [43]) versus chikungunya prevalence on Reunion Island (35% population prevalence[44]). Number, timing, and location of introductions will affect probability of successful introduction as well. In 2015, there were 38 chikungunya introductions to the state of New York, 3 introductions to Pennsylvania, 0 to Washington D.C., 9 to Maryland, and 4 to Georgia from June to August (http://diseasemaps.usgs.gov/mapviewer/). Greater certainty in specific parameter values, particularly vector competence of Ae. albopictus for Zika transmission, will increase the precision of our model predictions.
We chose to focus on urban areas dominated by Ae. albopictus, which has a much larger distribution in the temperate zone worldwide than does Ae. aegypti. Consequently, we did not include Gulf States such as Florida, Louisiana, and Texas in our analysis. High-density urban areas in these states are often dominated by Ae. aegypti or a mixture of Ae. aegypti and Ae. albopictus. For example, Miami’s urban regions (and the Florida Keys), where local transmission of dengue, chikungunya, and Zika have taken place, have high densities of Ae. aegypti [45–49]. Although published data are sparse for Houston, where local dengue transmission has occurred, there is evidence for similar mosquito distribution patterns there [50]. Adapting the model to include Ae. aegypti and mixtures of Ae. aegypti and Ae. albopictus would be an important next step. Based on our model results, we predict that the much longer peak mosquito seasons in the Gulf States along with higher mosquito feeding rates and very high proportion of bites on humans by Ae. aegypti would result in even higher risk of arboviral outbreaks (see, e.g. Fig 6).
As with any modeling effort, the results presented are contingent on the assumptions made in defining structure and parameterization. Our model assumes that all parameters are independent. However, it is likely that some are correlated; for instance temperature may simultaneously influence vector competence, biting rate, and vector life history [51,52]. To our knowledge, there have been no lab or field studies examining the effects of temperature on Zika replication in mosquitoes and there is very little on the affect of temperature on chikungunya’s EIP [9,52,53]. Although for dengue, increased temperature tends to decrease the EIP up to a point [54,55], increased temperature also tends to decrease mosquito lifespan, leading to nonlinear and complex relationships between temperature and transmission rates[9]. Moreover, most studies have been done in the lab and at constant temperatures for mosquito populations from one location. How this translates to field conditions and different mosquito populations is unclear since there is evidence for mosquito adaptation to local climate [9] and different dengue EIP responses to daily temperature fluctuations [51,54]. More data, including relationships between temperature regimes common to temperate regions and EIP and mosquito lifespan, are needed to better understand co-variation in mosquito and pathogen dynamics in real field conditions.
Likewise, current studies demonstrate considerable variation in Ae. albopictus density and human biting within a city and across land-use types [21,39,56]. More field data and behavioral evaluation are needed to refine model assumptions and parameters regarding when and where mosquito density and percent human feeding is likely to facilitate onward human transmission. For dengue and Ae. aegypti, mosquito density and virus transmission are closely related, but at different spatial scales [57], with the scale to best consider dengue transmission still unclear. Although there has been some success correlating vector abundance or environmental factors with risk for dengue[58], other studies have shown poor correlation [59], particularly for urban areas with mosquitoes that breed in human-created habitat and thus depend less on environmental factors such as rainfall[60]. Percent human feeding and mosquito density data are needed to rigorously assess the thresholds and scales at which mitigations of mosquito abundance and human biting rates might be effective.
The model assumes that mosquito population density (and vector:human ratio) are at the carrying capacity (and vector:human ratio) used to initialize the model throughout the specified seasonal duration (i.e., 90, 120 or 150 days). This density level defines the vector to human ratio and strongly influences R0 and numbers of additional human infections within a season. Our parameters represent ranges that capture real observed densities, but due to limitations in available field data, do not necessarily represent the relative duration of how long peak densities are generally maintained. Ongoing work in Maryland supports the assumption that a 90 day season of high Ae. albopictus density is likely in most years in that region [32]. It takes mosquito populations several weeks to ramp up to high densities. The beginning of our season is assumed to be when mosquitoes are at the high densities that can persist throughout the summer and early fall across the range of cities included. Likewise, the model does not incorporate mitigations or behavior changes, so it represents potential outbreak size rather than probable outbreak size since once autochthonous transmission is detected, significant mitigation efforts are likely. However, it should be noted that because 80% of Zika infections are asymptomatic[43], time to detection of an outbreak and response could be longer than for other diseases. Chikungunya, on the other hand, is highly symptomatic (around 80–90% of those infected exhibit symptoms, [61,62]), so it is more likely to be detected and motivate mitigation efforts.
Finally, the model assumes that within the square-mile area considered, mosquitoes and humans are homogeneous in space and can be characterized by mean densities. The scale is consistent with CDC’s assessment of risk in Miami (one sq mile around area with known transmission, https://www.cdc.gov/zika/intheus/florida-update.html). However, both mosquito and human densities can vary across space, resulting in varying risk of human-mosquito contact. Depending on human movement, daily activities, and vector exposure (which will depend upon socioeconomic factors, among other things), our model may under- or over-estimate risk, as has been illustrated by data and in individual-based models for mosquito-borne disease that incorporate more heterogeneity [63–66]. However, models that assume even mixing have performed relatively well and been useful in understanding risk of urban mosquito-borne disease transmission [67–69].
Scientists and public health officials involved with arbovirus transmission have had limited ability to make credible predictions, in part based on limited information about conditions that permit an outbreak and the likelihood those conditions will be met. Our model provides quantitative assessments of the probability of an outbreak (R0) and the potential numbers of human victims when key parameter values can be specified. Guided by published data on virus and mosquito vital rates, the model indicates that outbreaks can plausibly occur in major cities in the eastern United States, with hundreds of potential victims in localized areas, under conditions that are not atypical. The model suggests that outbreaks are more likely in urban areas with higher human and mosquito population densities, in years and cities with longer growing seasons, when infected travelers arrive early in the growing season, and when Ae. albopictus have fewer non-human hosts that result in wasted bites. These conditions are most likely met in urban landscapes where social, structural and environmental inequities facilitate human-mosquito contact and potentially limit early detection and mitigation of local transmission. Climate change, urban wildlife ecology, and human behavior all would appear to strongly influence the probability of new outbreaks in major U.S. cities.
Methods
Ethics Statement
The published literature we used does not reveal confidential information regarding human participants, so this study did not require IRB approval and no confidential human data has been revealed here.
We used a compartmental mathematical transmission model adapted from [70] to evaluate the potential for Ae. albopictus transmission of Zika and chikungunya virus to humans following the introduction of an infectious traveler. The model follows standard epidemiological model structure and assumes that all humans are susceptible (Sh), exposed and incubating (Eh), infectious (Ih), or recovered and immune (Rh). Likewise, mosquitoes are also assumed to be susceptible (Sh), exposed and incubating (Eh), or infectious (Ih). The model includes population dynamics for mosquitoes with density-dependent emergence of adult female mosquitoes and a carrying capacity, Kv. We adapted the Manore et al. 2014 model to sample from literature-informed variation in parameter space, and account for variability in use of human blood meal hosts (see Fig 8 for equations and parameter definitions). Studies demonstrate that propensity for human biting by Ae. albopictus across its invasive range varies widely and that the species appears to opportunistically bite whatever birds or mammals most readily available [18,20,26,28,30,34,35,71], although some studies indicate a human preference [37]. We assumed that of the total number of mosquito bites per day a certain proportion, Ph, are on humans and 1- Ph are on alternate hosts. We assumed that the non-human alternate hosts are not susceptible to the pathogen and thus, when an infected mosquito bites a non-human animal, the bite is “wasted” in the sense that the virus is not passed on to the animal. However, if the infected mosquito survives to bite again and the next bite is on a susceptible human, then the infected mosquito could pass on the virus to the human. The model does not consider other modes of transmission such as male to female sexual transmission of Zika in humans.
Fig 8. Model equations.
The model follows standard epidemiological model structure and assumes that all humans are susceptible (Sh), exposed and incubating (Eh), infectious (Ih), or recovered and immune (Rh). Likewise, mosquitoes are assumed to be susceptible (Sh), exposed and incubating (Eh), or infectious (Ih). The force of infection terms, λv and λh, depend on total number of mosquitoes, Nv, and total number of humans, Nh, along with the mosquito biting rates, σv, human bite availability, σh, and bites on alternate hosts, Qd. All parameter definitions and values can be found in the Supplementary Text.
A number of epidemiological models have considered arboviral transmissions (particularly dengue and chikungunya) focusing on different aspects of disease transmission [67,70,72–76] and characteristics such as seasonality, temperature dependence, cross-immunity with multiple strains, and control measures [77–85]. Several recently published modeling studies for Zika transmission have focused on fitting models to current transmission in the South Pacific and South and Central America [68,86–88] with Ae. aegypti dominating transmission. Only one, to our knowledge, has considered risk in temperate regions of Europe with transmission by Ae. albopictus [89]. Our model assesses risk in temperate regions of the United States dominated by Ae. albopictus and with no current local transmission of Zika or chikungunya, but with frequent introductions from other regions. We explicitly include human and mosquito dynamics and determine risk by computing both R0 and absolute size of a potential outbreak.
We used parameter values for chikungunya from [70] with an updated baseline and range for chikungunya’s extrinsic incubation period (EIP) based on meta-analysis in [91]. Zika parameters for human incubation and infectious periods (ranges are wide due to uncertainty, 3–12 days and 3–14 days, respectively), transmission probabilities given an infected contact (again, ranges slightly larger than for chikungunya based on the few current models and high uncertainty, 0.1–0.75 for Zika as opposed to 0–0.54 for chikungunya), and the EIP (higher than chikungunya), were based on the most up-to-date Zika field and modeling literature (see the S1 Text and S2 Table for references and data used).
In the model, mosquitoes bite infected or susceptible humans at a rate defined by the per-human vector density and the propensity for biting humans versus other animals. Mosquitoes become infectious and transmit virus to susceptible humans as a function of this biting rate, the number of infected humans, and vector competence (S1 Text). Vector competence integrates mosquito survival and EIP for the specific virus along with transmission probability given a bite on a susceptible human. Parameter values informing Ae. albopictus life history and specific vector competence for Zika and chikungunya virus transmission were estimated from published studies (S1 Text and S2 Table). To inform parameters related to Ae. albopictus population dynamics and vector competence, separate searches for Aedes albopictus survival, death, emergence and egg-laying rates and for Zika and chikungunya and Ae. albopictus were performed to supplement the studies and parameter values used in [70]. Details of the data and studies chosen are in included in the S1 Text description of the model.
Vector densities were varied from 0.5 to 10 times the human density in a square mile (2.59 square kilometers). Vector density was assumed to be at carrying capacity, Kv, for the duration of the season-length specified (90 to 150 days). We are thus assuming that fluctuations in vector abundance are minor within the time frame of our predictions and that those fluctuations remain within our vector-to-host ratio range. We also used human biting rate experiments to verify that the ratio we used resulted in a realistic number of bites per person per day (see model description in S1 Text and S2 Table for a more detailed outline of our rationale). Carrying capacity was drawn randomly from a uniform distribution bounded by values representing 0.5 to 10 mosquitoes per human host. A uniform distribution was chosen due to lack of more informative data. We considered representative human density per square mile representing four eastern U.S. cities with high to low urban residential densities: New York City (NY), Philadelphia (PA), Washington (DC), and Atlanta (GA). The vector density range captures large variability in published (S1 Text and S2 Table, mean vector-to-host ratios ranging from 3.1 to 10) and current data on Ae. albopictus populations in urban regions [25].
We varied the peak mosquito season lengths from 90 to 150 days to capture the effect of season length on risk. The short, 90-day season could represent either a later seasonal introduction of an infectious traveler or a shorter northeastern season (i.e., June-August), while a 150-day season represents a potential mid-May to mid-October season with early viral introductions. In general, Atlanta and Washington D.C./Baltimore would be expected to have longer mosquito seasons corresponding to the 150-day season in the model and New York and Philadelphia would be expected to have shorter mosquito seasons corresponding to the 90-day season in the model. In addition to human and vector density, percent of human blood meals, and season length, we varied human and mosquito incubation periods, mosquito biting rate, human biting tolerance, human infectious period, and transmission probabilities given an infected contact, across ranges based on the literature (see S1 and S2 Tables and the S1 Text for parameter and variable definitions and parameter values).
The quantities of interest computed from the model were the basic reproduction number (R0) and the cumulative absolute number of people infected at the end of the season given an introduction at the beginning of the season. The basic reproduction number is the expected number of secondary cases from one introduced case in a fully susceptible population. We used the next generation method to compute the basic reproduction number [90], which in that framework is the geometric mean of the expected number of transmissions to mosquitoes from one infected human and the expected number of transmissions to humans from one infected mosquito in fully susceptible populations (S1 Text). The cumulative number of people infected was computed by running numerical simulations of the model in MATLAB for the given seasonal duration. The model was run for local transmission in a square mile using each city’s specific human population density. The model was initialized with mosquitoes at carrying capacity and fully susceptible and one infected human introduced on day 1.
In order to fully explore the variation in parameter values and risk, we sampled from the given parameter ranges (S1 Text) and computed our quantities of interest using 10,000 randomly selected parameter combinations for each of the four human densities and three seasonal duration scenarios. We varied all but the two least sensitive parameters of the model (human death rate and mosquito emergence rate, which does not affect mosquito densities once the population is at carrying capacity). The model’s ability to generate realistic values for bites per human per day and other derived statistics was confirmed. Model validation was done previously using baseline parameters for chikungunya and dengue and compared favorably to observed outbreaks [70]. We did not have access to data to validate the model’s ability to predict observed Zika case data where Ae. albopictus transmission has been confirmed.
Supporting Information
From left to right, 90-day, 120-day, and 150-day peak mosquito seasons are shown. As season length increases, the percent of serious outbreaks increases and the needed percent of human feeding to result in a serious outbreak decreases.
(TIFF)
The red line is at R0 = 1.
(TIFF)
(TIFF)
The solid line is at R0 = 1.
(TIFF)
From left to right, 90-day, 120-day, and 150-day peak mosquito seasons are shown.
(TIFF)
(XLSX)
(XLSX)
(PDF)
Acknowledgments
We thank Jennifer Pendleton and Alexander Wolf for assisting with Fig 1.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was conducted as a part of the Climate Change and Vector-borne Diseases Working Group at the National Institute for Mathematical and Biological Synthesis, by National Science Foundation through NSF Award #DBI-1300426, with additional support from The University of Tennessee, Knoxville. CM was supported by NSF SEES grant CHE-1314029, NSF RAPID (DEB 1641130), a Los Alamos National Laboratory Director’s Postdoctoral Fellowship, and NIH-MIDAS grant U01-GM097661. SL was supported by NSF CHNS grant DEB 1211797. FA was supported by KU grant NFGRF 2302100. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marini F, Caputo B, Pombi M, Tarsitani G, Della Torre A (2010) Study of Aedes albopictus dispersal in Rome, Italy, using sticky traps in mark-release-recapture experiments. Medical and Veterinary Entomology 24: 361–368. 10.1111/j.1365-2915.2010.00898.x [DOI] [PubMed] [Google Scholar]
- 2.David MR, Lourenco-de-Oliveira R, de Freitas RM (2009) Container productivity, daily survival rates and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighbourhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Memorias Do Instituto Oswaldo Cruz 104: 927–932. [DOI] [PubMed] [Google Scholar]
- 3.Benedict MQ, Levine RS, Hawley WA, Lounibos LP (2007) Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector-Borne and Zoonotic Diseases 7: 76–85. 10.1089/vbz.2006.0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, et al. (2015) The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 4: e08347 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochlin I, Ninivaggi DV, Hutchinson ML, Farajollahi A (2013) Climate Change and Range Expansion of the Asian Tiger Mosquito (Aedes albopictus) in Northeastern USA: Implications for Public Health Practitioners. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen CC, Williams CR, van den Hurk AF (2015) The Usual Suspects: Comparison of the Relative Roles of Potential Urban Chikungunya Virus Vectors in Australia. Plos One 10: e0134975 10.1371/journal.pone.0134975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards SL, Anderson SL, Smartt CT (2010) Vector competence of Florida mosquitoes for chikungunya virus. Journal of Vector Ecology 35: 439–443. 10.1111/j.1948-7134.2010.00105.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vega-Rua A, Zouache K, Girod R, Failloux AB, Lourenco-de-Oliveira R (2014) High Level of Vector Competence of Aedes aegypti and Aedes albopictus from Ten American Countries as a Crucial Factor in the Spread of Chikungunya Virus. Journal of Virology 88: 6294–6306. 10.1128/JVI.00370-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge J-M, et al. (2014) Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proceedings of the Royal Society B: Biological Sciences 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong PSJ, Li MZI, Chong CS, Ng LC, Tan CH (2013) Aedes (Stegomyia) albopictus (Skuse): A Potential Vector of Zika Virus in Singapore. Plos Neglected Tropical Diseases 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckner EA, Alto BW, Lounibos LP (2013) Vertical Transmission of Key West Dengue-1 Virus by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) Mosquitoes From Florida. Journal of Medical Entomology 50: 1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, et al. (2008) Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: Biology and control. Parasite-Journal De La Societe Francaise De Parasitologie 15: 3–13. [DOI] [PubMed] [Google Scholar]
- 13.Paupy C, Kassa FK, Caron M, Nkoghe D, Leroy EM (2012) A Chikungunya Outbreak Associated with the Vector Aedes albopictus in Remote Villages of Gabon. Vector-Borne and Zoonotic Diseases 12: 167–169. 10.1089/vbz.2011.0736 [DOI] [PubMed] [Google Scholar]
- 14.Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, et al. (2010) Comparative Role of Aedes albopictus and Aedes aegypti in the Emergence of Dengue and Chikungunya in Central Africa. Vector-Borne and Zoonotic Diseases 10: 259–266. 10.1089/vbz.2009.0005 [DOI] [PubMed] [Google Scholar]
- 15.Roiz D, Bousses P, Simard F, Paupy C, Fontenille D (2015) Autochthonous Chikungunya Transmission and Extreme Climate Events in Southern France. Plos Neglected Tropical Diseases 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L'Ambert G, et al. (2015) Chikungunya outbreak in Montpellier, France, September to October 2014. Eurosurveillance 20: 8–13. [DOI] [PubMed] [Google Scholar]
- 17.Furuya H (2015) Estimation of reproduction number and probable vector density of the first autochthonous dengue outbreak in Japan in the last 70 years. Environmental Health and Preventive Medicine 20: 466–471. 10.1007/s12199-015-0488-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delatte H, Desvars A, Bouetard A, Bord S, Gimonneau G, et al. (2010) Blood-Feeding Behavior of Aedes albopictus, a Vector of Chikungunya on La Reunion. Vector-Borne and Zoonotic Diseases 10: 249–258. 10.1089/vbz.2009.0026 [DOI] [PubMed] [Google Scholar]
- 19.Kek R, Hapuarachchi HC, Chung C-Y, Bin Humaidi M, Razak MABA, et al. (2014) Feeding Host Range of Aedes albopictus (Diptera: Culicidae) Demonstrates Its Opportunistic Host-Seeking Behavior in Rural Singapore. Journal of Medical Entomology 51: 880–884. [DOI] [PubMed] [Google Scholar]
- 20.Sivan A, Shriram AN, Sunish IP, Vidhya PT (2015) Host-feeding pattern of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in heterogeneous landscapes of South Andaman, Andaman and Nicobar Islands, India. Parasitology Research 114: 3539–3546. 10.1007/s00436-015-4634-5 [DOI] [PubMed] [Google Scholar]
- 21.Almeida APG, Baptista S, Sousa C, Novo M, Ramos HC, et al. (2005) Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to dengue virus transmission. Journal of Medical Entomology 42: 419–428. [DOI] [PubMed] [Google Scholar]
- 22.Delatte H, Bagny L, Brengue C, Bouetard A, Paupy C, et al. (2011) The invaders: Phylogeography of dengue and chikungunya viruses Aedes vectors, on the South West islands of the Indian Ocean. Infection Genetics and Evolution 11: 1769–1781. [DOI] [PubMed] [Google Scholar]
- 23.Reiskind MH, Westbrook CJ, Lounibos LP (2010) Exposure to chikungunya virus and adult longevity in Aedes aegypti (L.) and Aedes albopictus (Skuse). Journal of Vector Ecology 35: 61–68. 10.1111/j.1948-7134.2010.00029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vega-Rua A, Zouache K, Caro V, Diancourt L, Delaunay P, et al. (2013) High Efficiency of Temperate Aedes albopictus to Transmit Chikungunya and Dengue Viruses in the Southeast of France. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaDeau SL, Allan BF, Leisnham PT, Levy MZ (2015) The ecological foundations of transmission potential and vector-borne disease in urban landscapes. Functional Ecology 29: 889–901. 10.1111/1365-2435.12487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faraji A, Egizi A, Fonseca DM, Unlu I, Crepeau T, et al. (2014) Comparative Host Feeding Patterns of the Asian Tiger Mosquito, Aedes albopictus, in Urban and Suburban Northeastern USA and Implications for Disease Transmission. PLoS Negl Trop Dis 8: e3037 10.1371/journal.pntd.0003037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roche B, Leger L, L'Ambert G, Lacour G, Foussadier R, et al. (2015) The Spread of Aedes albopictus in Metropolitan France: Contribution of Environmental Drivers and Human Activities and Predictions for a Near Future. Plos One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz J, Eritja R, Alcaide M, Montalvo T, Soriguer RC, et al. (2011) Host-Feeding Patterns of Native Culex pipiens and Invasive Aedes albopictus Mosquitoes (Diptera: Culicidae) in Urban Zones From Barcelona, Spain. Journal of Medical Entomology 48: 956–960. [DOI] [PubMed] [Google Scholar]
- 29.Sawabe K, Isawa H, Hoshin K, Sasaki T, Roychoudhury S, et al. (2010) Host-Feeding Habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) Collected at the Urban and Suburban Residential Areas of Japan. Journal of Medical Entomology 47: 442–450. 10.1603/ME09256 [DOI] [PubMed] [Google Scholar]
- 30.Valerio L, Marini F, Bongiorno G, Facchinelli L, Pombi M, et al. (2010) Host-Feeding Patterns of Aedes albopictus (Diptera: Culicidae) in Urban and Rural Contexts within Rome Province, Italy. Vector-Borne and Zoonotic Diseases 10: 291–294. 10.1089/vbz.2009.0007 [DOI] [PubMed] [Google Scholar]
- 31.Bartlett-Healy K, Unlu I, Obenauer P, Hughes T, Healy S, et al. (2012) Larval Mosquito Habitat Utilization and Community Dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). Journal of Medical Entomology 49: 813–824. [DOI] [PubMed] [Google Scholar]
- 32.LaDeau SL, Leisnham PT, Biehler D, Bodner D (2013) Higher Mosquito Production in Low-Income Neighborhoods of Baltimore and Washington, DC: Understanding Ecological Drivers and Mosquito-Borne Disease Risk in Temperate Cities. International Journal of Environmental Research and Public Health 10: 1505–1526. 10.3390/ijerph10041505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowling Z, Ladeau SL, Armbruster P, Biehler D, Leisnham PT (2013) Socioeconomic Status Affects Mosquito (Diptera: Culicidae) Larval Habitat Type Availability and Infestation Level. Journal of Medical Entomology 50: 764–772. [DOI] [PubMed] [Google Scholar]
- 34.Farjana T, Tuno N (2013) Multiple Blood Feeding and Host-Seeking Behavior in Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology 50: 838–846. [DOI] [PubMed] [Google Scholar]
- 35.Savage HM, Niebylski ML, Smith GC, Mitchell CJ, Craig GB (1993) Host-feeding patterns of Aedes albopictus (Diptera, Culicidae) at a temperate North-American site. Journal of Medical Entomology 30: 27–34. [DOI] [PubMed] [Google Scholar]
- 36.Niebylski ML, Savage HM, Nasci RS, Craig GB (1994) Blood Hosts of Aedes albopictus in the United States. Journal of the American Mosquito Control Association 10: 447–450. [PubMed] [Google Scholar]
- 37.Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS (2006) Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central north Carolina. Journal of Medical Entomology 43: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quam MB, Sessions O, Kamaraj US, Rocklöv J, Wilder-Smith A (2015) Dissecting Japan's Dengue Outbreak in 2014. The American Journal of Tropical Medicine and Hygiene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuda Y, Maekawa Y, Ogawa K, Itokawa K, Komagata O, et al. (2016) Biting Density and Distribution of Aedes albopictus during the September 2014 Outbreak of Dengue Fever in Yoyogi Park and the Vicinity of Tokyo Metropolis, Japan. Japanese Journal of Infectious Diseases 69: 1–5. 10.7883/yoken.JJID.2014.576 [DOI] [PubMed] [Google Scholar]
- 40.Cauchemez S, Ledrans M, Poletto C, Quenel P, de Valk H, et al. (2014) Local and regional spread of chikungunya fever in the Americas. Eurosurveillance 19: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staples JE, Breiman RF, Powers AM (2009) Chikungunya Fever: An Epidemiological Review of a Re-Emerging Infectious Disease. Clinical Infectious Diseases 49: 942–948. 10.1086/605496 [DOI] [PubMed] [Google Scholar]
- 42.Lee C, Vora N, Bajwa WI (2016) Zika Surveillance and Preparedness-New York City, 2015–2016. MMWR Morb Mortal Wkly Rep 65. [DOI] [PubMed] [Google Scholar]
- 43.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, et al. (2009) Zika Virus Outbreak on Yap Island, Federated States of Micronesia. New England Journal of Medicine 360: 2536–2543. 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 44.Gerardin P, Guernier V, Perrau J, Fianu A, Le RK, et al. (2008) Estimating Chikungunya prevalence in La Reunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis 8: 1471–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lounibos LP, O'Meara GF, Juliano SA, Nishimura N, Escher RL, et al. (2010) Differential Survivorship of Invasive Mosquito Species in South Florida Cemeteries: Do Site-Specific Microclimates Explain Patterns of Coexistence and Exclusion? Annals of the Entomological Society of America 103: 757–770. 10.1603/AN09142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rey JR, Lounibos P (2015) Ecology of Aedes aegypti and Aedes albopictus in the Americas and disease transmission. Biomedica 35: 177–185. [DOI] [PubMed] [Google Scholar]
- 47.Leisnham PT, Juliano SA (2009) Spatial and temporal patterns of coexistence between competing Aedes mosquitoes in urban Florida. Oecologia 160: 343–352. 10.1007/s00442-009-1305-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monaghan AJ, et al. (2016) On the Seasonal Occurrence and Abundance of the Zika Virus Vector Mosquito Aedes Aegypti in the Contiguous United States. PLoS Currents 8: ecurrents.outbreaks.50dfc57f46798675fc46798663e46798677d46798677da46798563da46798676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robert M, Christofferson R, Silva N, Vasquez C, Mores C, et al. (2016) Modeling Mosquito-Borne Disease Spread in U.S. Urbanized Areas: The Case of Dengue in Miami PLoS ONE 11: e0161365 10.1371/journal.pone.0161365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Champion SR, Vitek CJ (2014) Aedes Aegypti and Aedes Albopictus Habitat Preferences in South Texas, USA. Environmental Health Insights 8: 35–42. 10.4137/EHI.S16004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrington LB, Armijos MV, Lambrechts L, Scott TW (2013) Fluctuations at a Low Mean Temperature Accelerate Dengue Virus Transmission by Aedes aegypti. Plos Neglected Tropical Diseases 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westbrook CJ, Reiskind MH, Pesko KN, Greene KE, Lounibos LP (2010) Larval Environmental Temperature and the Susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to Chikungunya Virus. Vector-Borne and Zoonotic Diseases 10: 241–247. 10.1089/vbz.2009.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waldock J, Chandra NL, Lelieveld J, Proestos Y, Michael E, et al. (2013) The role of environmental variables on Aedes albopictus biology and chikungunya epidemiology. Pathogens and Global Health 107: 224–241. 10.1179/2047773213Y.0000000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, et al. (2011) Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America 108: 7460–7465. 10.1073/pnas.1101377108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tjaden NB, Thomas SM, Fischer D, Beierkuhnlein C (2013) Extrinsic Incubation Period of Dengue: Knowledge, Backlog, and Applications of Temperature Dependence. Plos Neglected Tropical Diseases 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen CD, Lee HL, Lau KW, Abdullah AG, Tan SB, et al. (2014) Biting behavior of Malaysian mosquitoes, Aedes albopictus Skuse, Armigeres kesseli Ramalingam, Culex quinquefasciatus Say, and Culex vishnui Theobald obtained from urban residential areas in Kuala Lumpur. Asian Biomedicine 8: 315–321. [Google Scholar]
- 57.Scott TW, Morrison AC (2010) Vector Dynamics and Transmission of Dengue Virus: Implications for Dengue Surveillance and Prevention Strategies Vector Dynamics and Dengue Prevention In: Rothman AL, editor. Dengue Virus. Berlin: Springer-Verlag Berlin; pp. 115–128. [DOI] [PubMed] [Google Scholar]
- 58.Eisen RJ, Eisen L (2008) Spatial Modeling of human risk of exposure to vector-borne pathogens based on epidemiological versus arthropod vector data. Journal of Medical Entomology 45: 181–192. [DOI] [PubMed] [Google Scholar]
- 59.Pepin KM, Leach CB, Marques-Toledo C, Laass KH, Paixao KS, et al. (2015) Utility of mosquito surveillance data for spatial prioritization of vector control against dengue viruses in three Brazilian cities. Parasites & Vectors 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang FS, Tseng YT, Hsu PS, Chen CD, Lian IB, et al. (2015) Re-assess Vector Indices Threshold as an Early Warning Tool for Predicting Dengue Epidemic in a Dengue Non-endemic Country. Plos Neglected Tropical Diseases 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K (2013) Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion 53: 2567–2574. 10.1111/j.1537-2995.2012.03960.x [DOI] [PubMed] [Google Scholar]
- 62.Moro ML, Grilli E, Corvetta A, Silvi G, Angelini R, et al. (2012) Long-term chikungunya infection clinical manifestations after an outbreak in Italy: A prognostic cohort study. Journal of Infection 65: 165–172. 10.1016/j.jinf.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 63.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, et al. (2013) House-to-house human movement drives dengue virus transmission. Proceedings of the National Academy of Sciences of the United States of America 110: 994–999. 10.1073/pnas.1213349110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manore CA, Hickmann KS, Hyman JM, Foppa IM, Davis JK, et al. (2015) A network-patch methodology for adapting agent-based models for directly transmitted disease to mosquito-borne disease. Journal of Biological Dynamics 9: 52–72. 10.1080/17513758.2015.1005698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vazquez-Prokopec GM, Bisanzio D, Stoddard ST, Paz-Soldan V, Morrison AC, et al. (2013) Using GPS Technology to Quantify Human Mobility, Dynamic Contacts and Infectious Disease Dynamics in a Resource-Poor Urban Environment. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kraemer MUG, Perkins TA, Cummings DAT, Zakar R, Hay SI, et al. (2015) Big city, small world: density, contact rates, and transmission of dengue across Pakistan. Journal of the Royal Society Interface 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poletti P, Messeri G, Ajellii M, Vallorani R, Rizzo C, et al. (2011) Transmission Potential of Chikungunya Virus and Control Measures: The Case of Italy. Plos One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kucharski AJ, Funk S, Eggo RM, Mallet HP, Edmunds WJ, et al. (2016) Transmission Dynamics of Zika Virus in Island Populations: A Modelling Analysis of the 2013–14 French Polynesia Outbreak. Plos Neglected Tropical Diseases 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yakob L, Clements ACA (2013) A Mathematical Model of Chikungunya Dynamics and Control: The Major Epidemic on Reunion Island. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manore CA, Hickmann KS, Xu S, Wearing HJ, Hyman JM (2014) Comparing dengue and chikungunya emergence and endemic transmission in A. aegypti and A. albopictus. Journal of Theoretical Biology 356: 174–191. 10.1016/j.jtbi.2014.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamgang B, Nchoutpouen E, Simard F, Paupy C (2012) Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasites & Vectors 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Focks DA, Daniels E, Haile DG, Keesling JE (1995) A simulation-model of the epidemiology of urban dengue fever—literature analysis, model development, preliminary validation, and samples of simulation results. American Journal of Tropical Medicine and Hygiene 53: 489–506. [DOI] [PubMed] [Google Scholar]
- 73.Esteva L, Vargas C (1999) A model for dengue disease with variable human population. Journal of Mathematical Biology 38: 220–240. [DOI] [PubMed] [Google Scholar]
- 74.Favier C, Degallier N, Rosa-Freitas MG, Boulanger JP, Costa Lima JR, et al. (2006) Early determination of the reproductive number for vector-borne diseases: the case of dengue in Brazil. Tropical Medicine & International Health 11: 332–340. [DOI] [PubMed] [Google Scholar]
- 75.Ferguson NM, Donnelly CA, Anderson RM (1999) Transmission dynamics and epidemiology of dengue: insights from age-stratified sero-prevalence surveys. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 354: 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chowell G, Diaz-Duenas P, Miller JC, Alcazar-Velazco A, Hyman JM, et al. (2007) Estimation of the reproduction number of dengue fever from spatial epidemic data. Mathematical Biosciences 208: 571–589. 10.1016/j.mbs.2006.11.011 [DOI] [PubMed] [Google Scholar]
- 77.Bartley LM, Donnelly CA, Garnett GP (2002) The seasonal pattern of dengue in endemic areas: mathematical models of mechanisms. Transactions of the Royal Society of Tropical Medicine and Hygiene 96: 387–397. [DOI] [PubMed] [Google Scholar]
- 78.Feng ZL, VelascoHernandez JX (1997) Competitive exclusion in a vector-host model for the Dengue fever. Journal of Mathematical Biology 35: 523–544. [DOI] [PubMed] [Google Scholar]
- 79.Dumont Y, Chiroleu F (2010) Vector control for the chikungunya disease. Mathematical Biosciences and Engineering 7: 313–345. 10.3934/mbe.2010.7.313 [DOI] [PubMed] [Google Scholar]
- 80.Dumont Y, Chiroleu F, Domerg C (2008) On a temporal model for the Chikungunya disease: Modeling, theory and numerics. Mathematical Biosciences 213: 80–91. 10.1016/j.mbs.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 81.Wearing HJ, Rohani P, Keeling MJ (2005) Appropriate models for the management of infectious diseases. Plos Medicine 2: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moulay D, Aziz-Alaoui MA, Kwon HD (2012) Optimal control of chikungunya disease: larvae reduction, treatment and prevention. Mathematical Biosciences and Engineering 9: 369–392. 10.3934/mbe.2012.9.369 [DOI] [PubMed] [Google Scholar]
- 83.Moulay D, Pigne Y (2013) A metapopulation model for chikungunya including populations mobility on a large-scale network. Journal of Theoretical Biology 318: 129–139. 10.1016/j.jtbi.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 84.Chao DL, Halstead SB, Halloran ME, Longini IM (2012) Controlling Dengue with Vaccines in Thailand. Plos Neglected Tropical Diseases 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adams B, Holmes EC, Zhang C, Mammen MP, Nimmannitya S, et al. (2006) Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proceedings of the National Academy of Sciences of the United States of America 103: 14234–14239. 10.1073/pnas.0602768103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishiura H, Kinoshita R, Mizumoto K, Yasuda Y, Nah K (2016) Transmission potential of Zika virus infection in the South Pacific. International Journal of Infectious Diseases 45: 95–97. 10.1016/j.ijid.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 87.Chowell G, Hincapie-Palacio D, Ospina J, Pell B, Tariq A, et al. (2016) Using Phenomenological Models to Characterize Transmissibility and Forecast Patterns and Final Burden of Zika Epidemics. PLOS Currents Outbreaks 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Majumder MS, Santillana M, Mekaru SR, McGinnis DP, Khan K, et al. (2016) Utilizing Nontraditional Data Sources for Near Real-Time Estimation of Transmission Dynamics During the 2015–2016 Colombian Zika Virus Disease Outbreak. JMIR Public Health Surveill 2: e30 10.2196/publichealth.5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rocklöv J, Quam MB, Sudre B, German M, Kraemer MUG, et al. Assessing Seasonal Risks for the Introduction and Mosquito-borne Spread of Zika Virus in Europe. EBioMedicine 9: 250–256. 10.1016/j.ebiom.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Mathematical Biosciences 180: 29–48. [DOI] [PubMed] [Google Scholar]
- 91.Rebecca C Christofferson, Daniel M Chisenhall, Helen J Wearing, and Christopher N Mores. Chikungunya viral fitness measures within the vector and subsequent transmission potential. PloS one, 9(10):e110538, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
From left to right, 90-day, 120-day, and 150-day peak mosquito seasons are shown. As season length increases, the percent of serious outbreaks increases and the needed percent of human feeding to result in a serious outbreak decreases.
(TIFF)
The red line is at R0 = 1.
(TIFF)
(TIFF)
The solid line is at R0 = 1.
(TIFF)
From left to right, 90-day, 120-day, and 150-day peak mosquito seasons are shown.
(TIFF)
(XLSX)
(XLSX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.