Abstract
The availability of oral direct-acting antiviral (DAAs) has altered the hepatitis C virus (HCV) treatment paradigm for both pre- and post-liver transplant (LT) patients. There is a perceived trade-off between pre- versus post-LT treatment of HCV—treatment may improve liver function but potentially decrease the likelihood of a necessary LT. Our objective was to identify LT-eligible patients with decompensated cirrhosis who would benefit (and not benefit) from pre-LT treatment based on their MELD scores. We simulated a virtual trial comparing long-term outcomes of pre- versus post-LT HCV treatment with oral DAAs for patients having MELD scores between 10 and 40. We developed a Markov-based microsimulation model, SIM-LT (simulation of liver transplant candidates), which simulated the life course of patients on the transplant waiting list and after LT. SIM-LT integrated data from recent trials of oral DAAs (SOLAR 1 and 2), United Network for Organ Sharing (UNOS), and other studies. The outcomes of the model included life expectancy, 1-year and 5-year patient survival, and mortality. Model-predicted patient-survival was validated with UNOS data. We found that, at the national level, treating HCV before LT increased life expectancy if MELD ≤ 27, but could decrease life expectancy at higher MELD scores. Depending on the UNOS region, the threshold MELD score to treat HCV pre-LT varied between 23 and 27, and was lower for UNOS regions 3, 10 and 11, and higher for regions 1, 2, 4, 5, 8 and 9. Sensitivity analysis showed that the thresholds were stable.
Conclusions
Our findings suggest that the optimal MELD threshold below which decompensated cirrhotic patients should receive HCV treatment while awaiting LT is between 23–27, depending on the UNOS region.
INTRODUCTION
Hepatitis C virus (HCV) is the leading indication for liver transplantation in the United States (1). Despite recent advances in HCV treatments, the burden on the transplant waiting list is projected to remain substantial even in the era of oral direct-acting antivirals (DAAs) (2). In patients who develop decompensated cirrhosis, the risk of death in the following year is approximately 15–20%, and liver transplant (LT) generally remains the only life-saving option (3). However, re-infection with HCV after transplantation occurs virtually universally; allograft hepatitis C can be rapidly progressive, and re-transplantation is the only therapeutic option for long-term survival in patients who advance to end-stage liver disease after LT (4, 5).
Until recently, the management of HCV in patients with decompensated cirrhosis and those who underwent LT was challenging because of low efficacy and tolerance of interferon-based therapies (5). However, the availability of oral DAAs has altered the treatment paradigm for both pre- and post-LT patients. Treatment of HCV in these difficult-to-treat patients is feasible, with high success rates, even in those who previously failed interferon-based therapy (6). Their successful use has been associated with improvements in Model for End-stage Liver Disease (MELD) score, and dramatically reduce the rate of HCV recurrence after LT and, by extension, the need for re-transplantation (5, 7). Similarly, successful post-LT antiviral treatment is associated with fibrosis stabilization or regression and improved graft survival, which was difficult to achieve prior to the availability of DAAs (8).
The decision to treat HCV pre- or post-LT has been widely debated (9, 10). There is a tradeoff between pre- versus post-LT treatment of HCV, and it is not clear which patients will benefit from pre-LT treatment and which ones will be better served by waiting until post-transplantation. This is because pre-LT treatment of HCV can improve patients’ MELD score and improve survival on the waiting list, but could delay their liver transplant by moving them further down the waiting list. By improving MELD score by a few points, some patients could also fall below the range for liver transplantation while not significantly improving their health. This situation has been termed as “MELD limbo” or “MELD purgatory” (9). In addition, by eradicating HCV pre-LT, these patients will no longer be eligible to receive an HCV-positive donor organ, which could further reduce their likelihood of receiving a liver transplant. Therefore, some patients may be better off treating HCV after the liver transplant. On the other hand, not providing HCV treatment pre-LT and waiting until the LT could result in worsening of the underlying liver condition (by increasing the patient's MELD score) and increase the likelihood of mortality on the waiting list.
Ideally, a randomized controlled trial comparing HCV treatment pre-LT with post-LT for patients for each MELD score could inform the optimal timing of HCV treatment. However, such a trial will be prohibitively large and time consuming. In such situations, mathematical modeling can inform optimal strategies (11, 12), and has been used to inform clinical decisions in organ transplantation (13-15). The objective of our study was to identify LT-eligible patients with decompensated cirrhosis who will benefit from HCV treatment pre-LT based on their MELD scores (and those who will not), using a mathematical model. We sought to determine a threshold for MELD score below which pre-LT HCV treatment would improve patients’ expected life and vice versa, and also determine the factors that influence this threshold.
METHODS
Model Overview
We simulated a virtual trial comparing long-term outcomes of pre- versus post-LT HCV treatment with oral DAAs for genotype 1 and 4 patients having MELD scores between 10 and 40. For that purpose, we developed a Markov-based microsimulation model (individual-level state transition model) that simulated the life course of patients on the transplant waiting list to evaluate comparative effectiveness of HCV treatment pre- versus post-LT (Figure 1). Our model, SIM-LT (simulation of liver transplant candidates), integrates data from multiple clinical studies including recent trials of oral DAAs, data from United Network for Organ Sharing (UNOS), and other published studies. SIM-LT follows patients on the transplant waiting list, keeps track of their MELD scores, and simulates the natural history of their disease after the transplantation. The outcomes of the model include expected life years, quality-adjusted life years (QALYs), 1-year and 5-year patient survival, need for re-transplantation, and death from background and liver-related causes. The model was developed in the Java programming language from patient perspective with a lifetime time horizon. We used a weekly cycle length to advance time in the model. We ran our model one million times to account for first-order uncertainty.
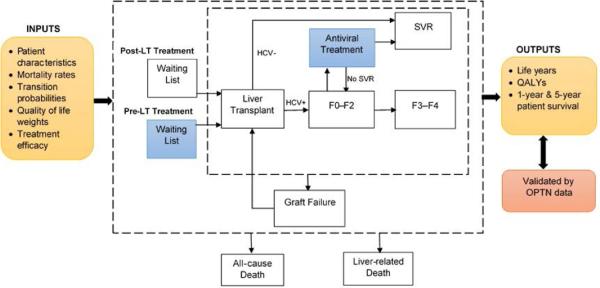
Figure 1.
Model schematic showing the flow of patients pre- and post-liver transplantation (LT). For each patient profile, the model simulated two treatment strategies: (1) pre-LT HCV treatment with DAAs, and (2) post-LT HCV treatment with DAAs. Patients’ likelihood of LT and mortality was associated with MELD score on the waiting list. The MELD score could change while on the waiting list. Patients who were viremic after the LT had a faster progression of liver disease after the LT.
Abbreviations: HCV, hepatitis C virus; QALYs, quality-adjusted life years; SVR, sustained virologic response; OPTN, Organ Procurement Transplant Network.
Baseline population
The baseline population consisted of patients with decompensated cirrhosis having MELD scores in the range of 10–40. The mean age of patients was 50, and we assumed that they have HCV genotypes 1 or 4.
Interventions
For each patient, we simulated two scenarios to compare the long-term outcomes: 1) HCV treatment on the waiting list, and 2) HCV treatment after the LT. Patients were treated with sofosbuvir and ledipasvir plus ribavirin for up to 12 weeks before transplantation. The sustained virologic response (SVR) rates of sofosbuvir plus ledipasvir when treated pre-LT were derived from the SOLAR-1 and SOLAR-2 studies (16-18) (Table 1). Though these studies used sofosbuvir-based therapy, our analysis is not limited to any particular regimen and applies to other regimens that are currently used or will be approved in the near future. We conducted a sensitivity analysis on a wide range of SVR rates.
Table 1.
Liver Transplant Model Variables
| Parameter | Min | Base Case | Max | References |
|---|---|---|---|---|
| Baseline Age | 35 | 50 | 65 | Assumption |
| Sustained virologic response rate | ||||
| Pre-LT SVR rate | 0.700 | 0.840 | 0.930 | (16-18) |
| Post-LT SVR rate | 0.900 | 0.950 | 0.980 | (16-18) |
| Transition probabilities | ||||
| Liver transplant to liver-related death (3 months of 1st LT) | 0.118 | 0.124 | 0.129 | (33) |
| Liver transplant to liver-related death (3 months of repeated LT) | 0.240 | 0.264 | 0.287 | (33) |
| Liver transplant to graft failure (3 months of 1st LT) | 0.161 | 0.167 | 0.173 | (33) |
| Liver transplant to graft failure (3 months of repeated LT) | 0.287 | 0.312 | 0.336 | (33) |
| Sustained virologic response to liver-related death (1st year) | 0.082 | 0.110 | 0.137 | Figure 10, (34) |
| Sustained virologic response to liver-related death (subsequent year) | 0.024 | 0.032 | 0.04 | Figure 10, (34) |
| Sustained virologic response to graft failure | 0.037 | 0.050 | 0.062 | Figure 3, (35) |
| F0-F2 to liver-related death (1st year of 1st LT) | 0.118 | 0.124 | 0.129 | (33) |
| F0-F2 to liver-related death (Subsequent year of 1st LT | 0.040 | 0.041 | 0.042 | (33) |
| F0-F2 to liver-related death (1st year of repeated LT) | 0.240 | 0.264 | 0.287 | (33) |
| F0-F2 to liver-related death (Subsequent year of repeated LT) | 0.070 | 0.072 | 0.075 | (33) |
| F3-F4 to liver-related death (1st year of 1st LT) | 0.118 | 0.124 | 0.129 | (33) |
| F3-F4 to liver-related death (Subsequent year of 1st LT | 0.040 | 0.041 | 0.042 | (33) |
| F3-F4 to liver-related death (1st year of repeated LT) | 0.240 | 0.264 | 0.287 | (33) |
| F3-F4 to liver-related death (Subsequent year of repeated LT) | 0.070 | 0.072 | 0.075 | (33) |
| F0–F2 to graft failure (1st year of 1st LT) | 0.161 | 0.167 | 0.173 | (33) |
| F0–F2 to graft failure (1st year of repeat LT) | 0.287 | 0.312 | 0.336 | (33) |
| F3–F4 to graft failure (1st year of 1st LT) | 0.315 | 0.290 | 0.525 | (36) |
| F3–F4 to graft failure (1st year of repeat LT) | 0.287 | 0.312 | 0.336 | (33) |
| F0–F2 to graft failure (subsequent year of 1st LT) | 0.050 | 0.051 | 0.052 | (33) |
| F0–F2 to graft failure (subsequent year of repeat LT) | 0.093 | 0.095 | 0.098 | (33) |
| F3–F4 to graft failure (subsequent year of 1st LT) | 0.050 | 0.051 | 0.052 | (33) |
| F3–F4 to graft failure (subsequent year of repeat LT) | 0.093 | 0.095 | 0.098 | (33) |
| Graft failure to liver-related death | 0.489 | 0.652 | 0.815 | UNOS data |
| Graft failure to repeat transplant | 0.604 | 0.805 | 1.000 | UNOS data |
| F0–F2 to F3–F4 | 0.040 | 0.044 | 0.055 | (37) |
| Decrease in transplant rate due to achieving SVR | 0.050 | 0.080 | 0.150 | Assumption |
| Health-related quality-of-life weights | ||||
| Transplant waiting list | 0.570 | 0.800 | 0.990 | (38, 39) |
| Liver transplant | 0.370 | 0.600 | 0.730 | (40) |
| F0–F2 | 0.716 | 0.828 | 0.865 | (39, 40) |
| F3–F4 | 0.693 | 0.801 | 0.837 | (39, 40) |
| Antiviral Treatment | 0.770 | 0.890 | 0.930 | (40) |
| Sustained virologic response | 0.770 | 0.890 | 0.930 | (40) |
| Graft failure | 0.570 | 0.800 | 0.990 | (38, 41) |
If patients received less than 6 weeks of antiviral therapy before the transplant, we assumed that the treatment was incomplete and did not result in SVR (16-18). However, if patients received at least 6 weeks of antiviral therapy before the transplant, we assumed that the treatment was complete and patients would achieve SVR as reported by clinical studies. Patients who failed to achieve SVR after the pre-LT antiviral treatment were re-treated again three months after the transplant. We selected the 3-month window as a time point that was sufficiently distanced from the perturbations of the immediate post-LT period but sufficiently early before progressive allograft disease could develop. The SVR rates of post-LT treatment were 95%, as reported by the combined analysis of the SOLAR-1 and SOLAR-2 studies (16-18). As in the pre-LT case, our analysis is also applicable to other DAA combinations that have been used for post-LT antiviral treatment (19).
For the comparator arm, “HCV treatment after the LT,“ we simulated a scenario where patients on the waiting list did not receive HCV treatment until after LT. While on the waiting list, patients’ MELD scores could change naturally. Based on their MELD scores, they could either undergo LT or die due to liver-related mortality or all-cause mortality. Three months after the transplant, all patients were offered antiviral treatment. We used SVR rates of 95% and conducted sensitivity analysis (Table 1).
Disease progression
In the pre-LT setting, we simulated changes in MELD scores. We used a previously published study based on UNOS data to estimate weekly increase or decrease in MELD score, i.e., the natural course of the disease (13, 20). Specifically, data from 1,997 HCV-infected patients on the liver transplant waiting list from UNOS were used to create cubic splines of bilirubin, creatinine, and albumin level and prothrombin time for each patient. The splines were then sampled at regular intervals to obtain a complete longitudinal history of each patient over time. Data was aggregated in groups of paired consecutive MELD scores starting with 10–11, 12–13, etc. We also estimated mortality on the waiting list based on the MELD score as from the same data source (Supplementary Table S1).
We assumed that during the treatment MELD score would follow the natural history, i.e. increase, decrease or remain constant. Four weeks after the end of treatment, we adjusted MELD score based on the treatment response as reported by the combined analysis of SOLAR 1 and 2 trials (16-18). Supplementary Figure S1 shows the probability of increase, decrease, and no change in MELD score 4 weeks after the end of treatment. If the patient achieved SVR, the MELD score was probabilistically updated according to the reported data in Supplementary Figure S1; otherwise, the MELD score continued to follow the natural course of the disease.
After the LT, patients’ natural history was determined by the status of their HCV infection—SVR or viremic. Recurrent HCV infection is universal among patients who are viremic at LT, the majority of whom will have histological evidence of recurrent hepatitis within the first year after LT (21). Therefore, in our model we treated viremic HCV patients 3 months after LT. Patients could die because of liver-related mortality, and the graft could fail due to recurrent allograft HCV or other reasons (22). After graft failure, patients could undergo retransplantation.
Liver Transplantation
We used a published study to estimate weekly probability of getting a liver transplant based on patient's MELD score (23). Supplementary Table S2 provides the 3-month probability of liver transplant that was converted to weekly probability in our model. We accounted for reduction in the likelihood for getting a liver transplant if patient's HCV was treated on the waiting list because this individual will no longer be eligible to receive HCV-positive liver. Specifically, we reduced the probability of undergoing LT by 8% because among all liver transplants in HCV-positive recipients, 8% had HCV-positive donor graft (24). In the sensitivity analysis, we varied this percentage between 5–15%. Patients who needed retransplantation went back on the LT waiting list. Because we did not know their MELD score, we assigned the average probability of liver transplant and liver-related mortality to these patients, which were estimated from UNOS data (Supplementary Section S1).
Health-related quality of life
For each individual in our model, we assigned health-related quality-of-life (QOL) weights, with 0 denoting death and 1 denoting perfect health, and adjusted them for age and sex. We derived EuroQol-5D instrument (Rotterdam, Netherlands) values from a previous study and adjusted them to the US population norm (Supplementary Table S3) (25-27).
Outcomes
We projected life years and QALYs for each MELD score (aggregated in groups of two) for pre- and post-LT scenarios. We also projected 1-year and 5-year survival after LT. We further projected these outcomes for each of the 11 UNOS regions. For that purpose, we adjusted the probability of undergoing LT and mortality on the waiting list for each region using region-specific transplantation and death rates (see Supplementary Section S2 and Supplementary Table S4 for details).
Sensitivity Analysis
We performed one-way sensitivity analysis to estimate the effects of all transition probabilities and QOL weights on change in the decision to treat pre- or post-LT. We further conducted probabilistic sensitivity analysis (PSA) by jointly varying all model parameters over 3,000 simulations, then calculating 95% confidence intervals for each model outcome based on the results. We used the recommended statistical distributions to define uncertainty around each input parameter as defined in Supplementary Table S5.
Because of a declining pool of HCV-positive LT recipients on the transplant waiting list, their likelihood of receiving a liver transplant will progressively increase. Therefore, we conducted a sensitivity analysis by increasing the likelihood (from the base case) of receiving a liver transplant by 8%, which is consistent with decrease in likelihood of LT by 8% in HCV-negative patients. We further sought to determine the specific magnitude of the increased likelihood of receiving a transplant that would trigger a change in the threshold for treatment of HCV pre-LT.
RESULTS
We first validated the predicted 1-year and 5-year transplant survival rates with those from UNOS data. Because UNOS data provides survival rates in the pre-DAA era, we simulated the scenario using pre-DAA therapies (Supplementary Section S3), and found that the model's predicted survival rates aligned closely with the rates estimated by the Organ Procurement Transplant Network data (OPTN) (Supplementary Figure S2).
MELD Score Threshold for HCV Treatment
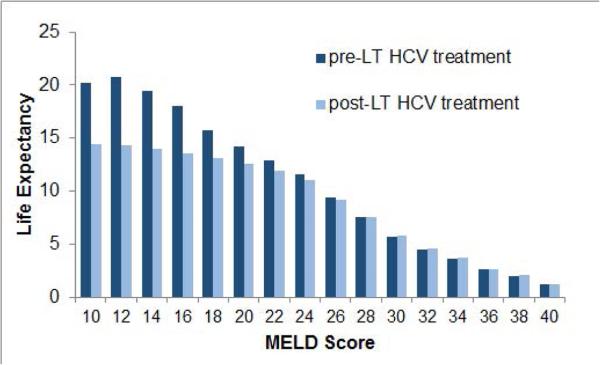
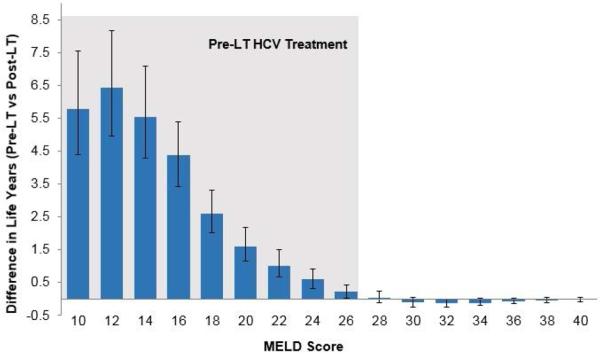
We estimated the expected life years as a function of timing of HCV treatment: pre- versus post-LT. Figure 2 shows that the life expectancy was dependent on patients’ MELD score as well as the timing of HCV treatment. Life expectancy decreased with increases in MELD score on the LT waiting list. For patients with lower MELD scores, the life expectancy was higher when HCV was treated pre-LT and lower if treated post-LT. The trend was reversed for higher MELD scores. We also plotted the difference in life expectancy if HCV was treated pre-LT versus post-LT (Figure 3). The 95% confidence intervals remained positive for MELD ≤ 27, indicating that patients with MELD ≤ 27 would benefit if HCV was treated pre-LT. For patients with MELD > 27, providing HCV treatment pre-LT did not offer benefit, and in fact was associated with decreased life expectancy in some cases. Similar trends were also observed in QALYs under pre- versus post-LT scenarios (Supplementary Figures S3–S4).
Figure 2.
Comparison of life expectancy by MELD score under pre-LT versus post-LT treatment of hepatitis C in decompensated cirrhosis patients on the waiting list.
Abbreviations: LT, liver transplant; MELD, model for end-stage liver disease; HCV, hepatitis C virus.
Figure 3. Difference in life years if HCV is treated pre-LT versus post-LT in patients with decompensated cirrhosis on the transplant waiting list.
The error bars represent 95% confidence interval generated by probabilistic sensitivity analysis. Patients having MELD ≤ 27 will benefit from pre-LT HCV treatment (shown by the shaded region)
Abbreviations: LT, liver transplant; MELD, model for end-stage liver disease; HCV, hepatitis C virus.
Analysis by UNOS Regions
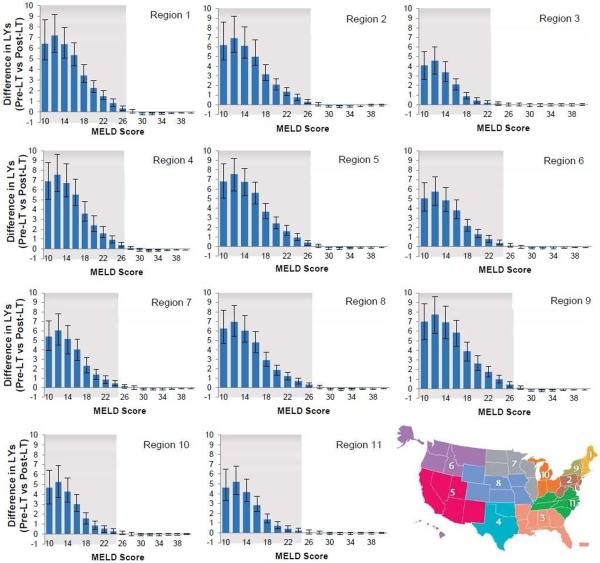
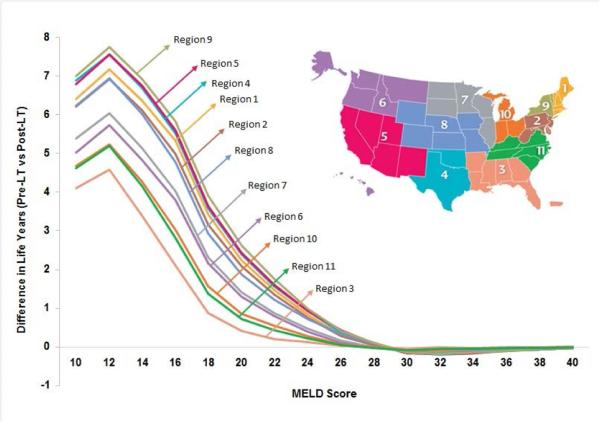
We further analyzed the results for each UNOS region to account for differences in waiting time for LT (Figure 4). We found that the threshold MELD score below which treating HCV pre-LT increased life expectancy compared to waiting until after the LT varied by region. The threshold was higher in regions with longer time on the LT waiting list and lower in regions with shorter time on the LT waiting list. For instance, Region 3, which has the shortest waiting time on LT waiting list, had a MELD threshold of 23, and Region 9, which has the longest waiting time, had a threshold of 27. We also observed that the benefits (i.e. difference in life expectancy) of antiviral treatment pre-LT were higher in regions having longer time on the LT waiting list (Figure 5). For instance, a patient in region 3 having MELD score 20–21 on the waiting list would gain 0.42 life years with pre-LT (versus post-LT) HCV treatment, whereas the corresponding patient in region 9 would gain 2.62 life years.
Figure 4. Difference in life years if hepatitis C virus is treated pre- versus post-liver transplant in 11 UNOS regions.
The error bars represent 95% confidence interval generated by probabilistic sensitivity analysis. Patients having MELD score in the shaded region will benefit from pre-LT HCV treatment. The map (insert) shows the 11 UNOS regions.
Abbreviations: LT, liver transplant; MELD, model for end-stage liver disease; United Network for Organ Sharing.
Figure 5. Comparison of life years gained (or lost) by treating HCV pre-LT versus post-LT in different UNOS regions.
The benefit in pre-LT treatment is higher in regions with longer time on the LT waiting list and vice versa.
Abbreviations: LT, liver transplant; MELD, model for end-stage liver disease; United Network for Organ Sharing.
Sensitivity Analysis
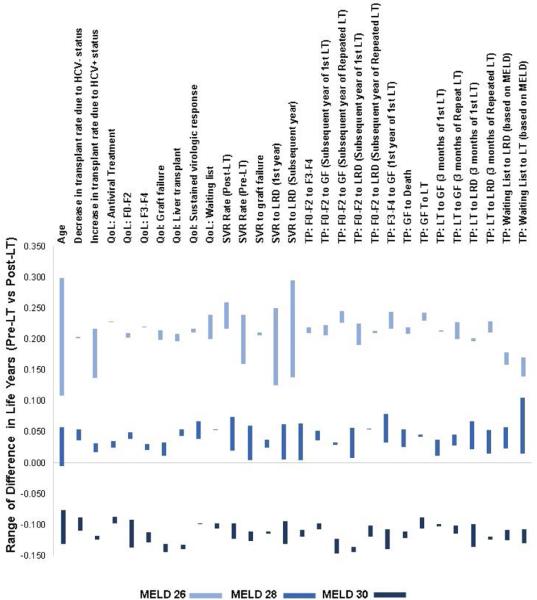
We conducted a 1-way sensitivity analysis to identify parameters that exerted the strongest influence on the MELD score threshold, i.e. which parameters could change the decision to treat HCV pre versus post-LT. For this purpose, we simulated change in life expectancy in patients with MELD scores 26–27, 28–29 and 30–31 by using the upper and lower range of each model input. Each bar in Figure 6 represents the lower and upper value of difference in life years by providing HCV treatment pre- versus post-LT for MELD scores 26–27, 28–29 and 30–31. For patients having a MELD score 26–27, the life expectancy always increased (shown by positive life years on Y-axis) by treating pre-LT, irrespective of the value of the input variable (higher or lower), i.e., the optimal decision was to treat HCV pre-LT if MELD ≤ 27. Similarly, for patients having MELD score 30–31, the life expectancy always decreased by treating pre-LT, irrespective of the value of the input variable. This implies that the optimal decision was to treat post-LT if MELD ≥ 30. For patients having MELD score 28–29, the difference in life expectancy with pre- versus post-LT treatment remained positive but close to 0, irrespective of the value of input variables.
Figure 6. One-way sensitivity analysis showing change in MELD score threshold to treat hepatitis C virus pre- versus post-LT.
X-axis (on top) shows parameters used in the model, and Y-axis estimates sensitivity of the MELD score threshold based on the input value of each parameter. The model results were analyzed with upper and lower values of each parameter (shown in Table 1) and difference in life years were plotted. Each bar represents the lower and upper value of difference in life years by providing HCV treatment pre- versus post-LT for MELD scores 26, 28 and 30. For example, model results were analyzed using patient's baseline age of 35 and 65. For patients with MELD 26, difference in life years was 0.299 if age as 35 and 0.101 if age was 65. Similarly, the corresponding values were 0.056 and −0.005 for patients with MELD 28, and −0.129 and −0.077 for patients with MELD 30. In general, we found that for patients having MELD score 26, the life expectancy always increased (shown by positive life years on Y-axis) by treating pre-LT, irrespective of the value of the input variable (higher or lower). Similarly, for patients having MELD score 30, the life expectancy always decreased (shown by negative life years on Y-axis) by treating pre-LT, irrespective of the value of the input variable. For patients having MELD score 28, the difference in life expectancy with pre- versus post-LT treatment remained positive but close to 0, irrespective of the value of input variables.
Abbreviations: HCV, hepatitis C virus; MELD, Model for End-stage Liver Disease; QoL, quality of life; SVR, sustained virologic response; GF, graft failure; TP, transition probability; LT, liver transplant; LRD, liver-related death.
When we increased the likelihood of getting a liver transplant in HCV-positive patients on the transplant waiting list, the threshold MELD score to treat HCV pre-LT fell below 26 when the rate of LT in HCV+ patients increases by 10% (Supplementary Figure S5 shows). Similarly, we found that the threshold to treat HCV pre-LT changed to a MELD score < 24 when the rate of LT in HCV+ patients increased by 40% (Supplementary Figure S6).
DISCUSSION
The availability of DAAs has changed the HCV treatment paradigm in liver transplant candidates as well as recipients. In this study, we evaluated the tradeoff between pre- versus post-LT treatment of HCV with DAAs—treatment can improve liver function but potentially decrease the likelihood of transplant. Our findings suggest that the optimal threshold to treat HCV in decompensated cirrhosis patients on the transplant waiting list is between MELD scores 23–27, depending on the UNOS region. Patients below those MELD thresholds could benefit from HCV treatment before the transplant; however, treating HCV above these thresholds may be associated with decreased life expectancy. Our results may help inform clinicians about the timing of HCV treatment in liver transplant candidates.
In this study, we determined the optimal timing of HCV treatment in LT-eligible patients that has been raised in recent studies (9, 10). Our results align with the opinion expressed in these studies that in some patients clearing HCV infection before transplantation may have paradoxical consequences, in that their decreased odds of undergoing transplantation will not be offset by their improved liver function while on the waiting list. To our knowledge, our study is the first to evaluate (and quantify) such tradeoffs from the clinical and patient perspective. Other modeling-based studies have addressed this topic. However, those studies evaluated the cost-effectiveness of HCV treatment pre- versus post-LT (28, 29), which should not preempt the comparative effectiveness analysis from the patient perspective, the focus of the current study. The question of cost-effectiveness, while important, may not be used to inform the clinical decision of pre- versus post-LT treatment of HCV. Instead, such an analysis could inform the choice of drugs among multiple options that could be cost-effective from payer's perspective. Another study evaluated the timing of HCV treatment with peginterferon and ribavirin, whose results may not be applicable in the era of DAAs (30).
Our study has numerous strengths. First, we conducted a comprehensive analysis of the tradeoffs of HCV treatment pre- versus post- liver transplant by simulating a virtual trial that compared the long-term outcomes in patients under the two scenarios. Conducting such a clinical trial in real life could be prohibitively expensive and time consuming. Second, our SIM-LT model incorporated data from recently published clinical trials, OPTN and other sources, and has been validated with UNOS-reported patient outcomes. Therefore, our analysis provides strong evidence to inform clinical decision-making. Third, our study highlights that the timing of HCV treatment is dependent on patients MELD score as well the UNOS region.
While the guidance offered by this model is useful, it is not meant to be used an algorithm for HCV treatment for each patient. There are several factors (such as refractory complications of portal hypertension) that determine the patient's urgency for LT that are not reflected in MELD alone. Therefore, our results may not apply to these situations. In addition, our analysis did not include decompensated cirrhosis patients having HCC, a considerable portion of the patients on the waiting list. Such patients will have a different natural history of the disease and are outside the scope of the current model.
Our study has a number of limitations. First, long-term data on transplant recipients who achieved SVR is extremely limited, especially when considering different MELD groups. Therefore, we assumed that the patient and graft survival in these patients would be similar to non-HCV transplant recipients. In addition, long-term data on MELD score changes after HCV treatment was based on the two SOLAR studies only. We will need to re-analyze our results as more data becomes available in future (31). Second, we we did not explicitly model Child Turcotte-Pugh class in our analysis. Third, we assumed that patients would be treated only once after the transplant. Although the SVR rates are very high, around 5% of patients will fail post-LT treatment with existing DAAs, and we assumed that these patients will not be eligible for retreatment. However, we expect that the majority of these patients could be successfully treated in the near future. Fourth, we did not model patient dropout from the waiting list resulting from MELD improvement because limited data is available to inform such a decision. In our opinion, this exclusion will not affect our primary analysis of optimal MELD threshold for HCV treatment pre- versus post-LT. Fifth, our model did not adjust for survival based on MELD score at transplant, and adjusting for survival could have resulted in higher MELD threshold to provide post-LT HCV treatment. Finally, our model did not account for potential reduction in the LT waiting list because of delisting of HCV patients after successful antiviral therapy and decreasing need for LT in HCV patients because of the availability of DAAs (32). Their inclusion could have resulted in a lower MELD score threshold to provide post-LT HCV treatment.
Sensitivity analysis suggested that our model results remain robust, as changes to model input variables did not appreciably change the model findings. None of the variables had a significant influence on the MELD threshold. We also accounted for overall uncertainty by conducting probabilistic sensitivity analysis and generated 95% confidence intervals.
We observed differences in HCV treatment recommendations by UNOS regions. Because liver transplant wait times vary across regions, patient-level decisions to treat HCV pre- versus post-LT should be dependent on UNOS region. In future, rezoning of UNOS regions could reduce disparities in time spent on the waiting list; our analysis will therefore need to be evaluated in this context. The reduction of these disparities can be expected to narrow the threshold range, which could simplify decision-making.
Conclusion
Our study evaluated the trade-off of providing HCV treatment before and after liver transplantation in decompensated patients. The optimal MELD score threshold below which to treat HCV pre versus post liver transplant in these patients is between 23–27, depending on the UNOS region. Patients below the threshold will benefit from initiating treatment before liver transplantation (LT), and those above the threshold will benefit from waiting until after LT for treatment. These data should be useful in informing decision-making for patients with HCV infection who are awaiting liver transplantation.
Supplementary Material
Two-sentence summary of the manuscript.
There is a trade-off between pre- versus post-LT treatment of HCV in decompensated cirrhosis patients on the transplant waiting list. We found that the optimal MELD threshold below which these patients should receive HCV treatment is between 23–27, depending on the UNOS region.
Acknowledgement
Funding/Support: This study was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR000146 and by Health Resources and Services Administration contract 234-2005-37011C. Dr. Chung was supported in part by NIH DK078772 and the MGH Research Scholars Program. Dr. Kanwal's effort was supported in part by the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413). Dr. Ayer's effort was supported in part by the National Science Foundation under award number 1452999. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Author contribution:
Study concept and design: Chhatwal, Samur, Kues, Ayer, Kanwal, Chung Drafting of manuscript: Chhatwal
Critical revision of the manuscript for important intellectual content: Chhatwal, Samur, Kues, Ayer, Roberts, Kanwal, Hur, Donnell, Chung
Statistical analysis: Chhatwal, Samur, Kues, Donnell.
Interpretation of data: Chhatwal, Samur, Kues, Ayer, Roberts, Kanwal, Hur, Chung
REFERENCES
- 1.Rosen HR. Chronic Hepatitis C Infection. New England Journal of Medicine. 2011;364:2429–2438. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 2.Chhatwal J, Wang X, Ayer T, Kabiri M, Chung RT, Hur C, Donohue JM, et al. Hepatitis C disease burden in the United States in the era of oral direct-acting antivirals. Hepatology. 2016 doi: 10.1002/hep.28571. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunchorntavakul C, Reddy KR. Management of Hepatitis C Before and After Liver Transplantation in the Era of Rapidly Evolving Therapeutic Advances. Journal of Clinical and Translational Hepatology. 2014;2:124–133. doi: 10.14218/JCTH.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011 doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roche B, Samuel D. Hepatitis C virus treatment pre- and post-liver transplantation. Liver International. 2012;32:120–128. doi: 10.1111/j.1478-3231.2011.02714.x. [DOI] [PubMed] [Google Scholar]
- 6.Curry MP, O'Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. New England Journal of Medicine. 2015;373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 7.Saxena V, Terrault N. Current Management of Hepatitis C Virus: Regimens for Peri-Liver Transplant Patients. Clin Liver Dis. 2015;19:669–688, vi. doi: 10.1016/j.cld.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, Rosen CB, et al. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008;8:2426–2433. doi: 10.1111/j.1600-6143.2008.02362.x. [DOI] [PubMed] [Google Scholar]
- 9.Carrion AF, Khaderi SA, Sussman NL. Model for end-stage liver disease limbo, model for end-stage liver disease purgatory, and the dilemma of treating hepatitis C in patients awaiting liver transplantation. Liver Transplantation. 2016;22:279–280. doi: 10.1002/lt.24383. [DOI] [PubMed] [Google Scholar]
- 10.Bunchorntavakul C, Reddy KR. Treat chronic hepatitis C virus infection in decompensated cirrhosis – pre- or post-liver transplantation? the ironic conundrum in the era of effective and well-tolerated therapy. Journal of Viral Hepatitis. 2016;23:408–418. doi: 10.1111/jvh.12534. [DOI] [PubMed] [Google Scholar]
- 11.Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, Kuntz KM. State-transition modeling: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force. Medical Decision Making. 2012;32:690–700. doi: 10.1177/0272989X12455463. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenberg FA, Beck JR. Markov models in medical decision making. Medical decision making. 1993;13:322. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 13.Alagoz O, Maillart LM, Schaefer AJ, Roberts MS. The optimal timing of living-donor liver transplantation. Management Science. 2004;50:1420–1430. [Google Scholar]
- 14.Van Arendonk KJ, Chow EKH, James NT, Orandi BJ, Ellison TA, Smith JM, Colombani PM, et al. Choosing the Order of Deceased Donor and Living Donor Kidney Transplantation in Pediatric Recipients: A Markov Decision Process Model. Transplantation. 2015;99:360–366. doi: 10.1097/TP.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow EK, Massie AB, Muzaale AD, Singer AL, Kucirka LM, Montgomery RA, Lehmann HP, et al. Identifying appropriate recipients for CDC infectious risk donor kidneys. Am J Transplant. 2013;13:1227–1234. doi: 10.1111/ajt.12206. [DOI] [PubMed] [Google Scholar]
- 16.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr., Fried MW, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. The Lancet Infectious Diseases. 16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 18.Gane E, Manns M, McCaughan G, Curry MP, Peck-Radosavljevic M, Vlierberghe HV, Arterburn S, et al. Ledipasvir/sofosbuvir with ribavirin in patients with decompensated cirrhosis or liver transplantation and HCV infection: SOLAR-1 and -2 trials. Vol. 2015. AASLD; 2015. Abstract 1049. [Google Scholar]
- 19.Gutierrez JA, Carrion AF, Avalos D, O'Brien C, Martin P, Bhamidimarri KR, Peyton A. Sofosbuvir and simeprevir for treatment of hepatitis C virus infection in liver transplant recipients. Liver Transpl. 2015;21:823–830. doi: 10.1002/lt.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shechter SM, Bryce CL, Alagoz O, Kreke JE, Stahl JE, Schaefer AJ, Angus DC, et al. A clinically based discrete-event simulation of end-stage liver disease and the organ allocation process. Medical Decision Making. 2005;25:199–209. doi: 10.1177/0272989X04268956. [DOI] [PubMed] [Google Scholar]
- 21.Terrault NA. Hepatitis C therapy before and after liver transplantation. Liver Transplantation. 2008;14:S58–S66. doi: 10.1002/lt.21624. [DOI] [PubMed] [Google Scholar]
- 22.Kalambokis G, Manousou P, Samonakis D, Grillo F, Dhillon AP, Patch D, O’Beirne J, et al. Clinical outcome of HCV-related graft cirrhosis and prognostic value of hepatic venous pressure gradient. Transplant International. 2009;22:172–181. doi: 10.1111/j.1432-2277.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 23.Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, Schnitzler MA, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011;11:2362–2371. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai JC, O'Leary JG, Trotter JF, Verna EC, Brown RS, Jr., Stravitz RT, Duman JD, et al. Risk of advanced fibrosis with grafts from hepatitis C antibody-positive donors: a multicenter cohort study. Liver Transpl. 2012;18:532–538. doi: 10.1002/lt.23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong CAKY, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, Krahn M. Health-state utilities and quality of life in hepatitis C patients. The American Journal of Gastroenterology. 2003;98:630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 26.Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth B, Manns M, et al. Cost effectiveness of peginterferon-2b plus ribavirin versus interferon -2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52:425. doi: 10.1136/gut.52.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Medical Decision Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 28.Tapper EB, Hughes M, Buti M, Dufour J-F, Flamm S, Curry M, Afdhal NH. The Optimal Timing of Hepatitis C Therapy in Transplant Eligible Patients With Child B and C Cirrhosis: A Cost-Effectiveness Analysis. AASLD; 2015. Abstract 1663. [DOI] [PubMed] [Google Scholar]
- 29.Njei B, McCarty TR, Ditah IC, Lim JK, Fortune BE. 504 Optimal Timing for Hepatitis C Treatment in Cirrhotic Patients Awaiting Liver Transplantation: A Cost-Effectiveness Analysis. Vol. 2015. AASLD; 2015. [DOI] [PubMed] [Google Scholar]
- 30.Saab S, Hunt DR, Stone MA, McClune A, Tong MJ. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: A decision analysis model. Liver Transplantation. 2010;16:748–759. doi: 10.1002/lt.22072. [DOI] [PubMed] [Google Scholar]
- 31.Cheung M, Foster G, Irving W, McLauchlan J, Walker A, Hudson B, Verma S, et al. Antiviral treatment in patients with advanced hepatitis C virus cirrhosis with sofosbuvir and either ledipasvir or daclatasvir, with or without ribavirin: observational cohort study. The Lancet. 2016;387:S26. [Google Scholar]
- 32.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C in the United States: Model-based predictions. Annals of Internal Medicine. 2014;161:170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Based on OPTN data as of March 4, 2016. 2016 Retrieved from http://optn.transplant.hrsa.gov/.
- 34.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 35.Berenguer M, Prieto M, San Juan F, Rayon JM, Martinez F, Carrasco D, Moya A, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;36:202–210. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 36.Berenguer M, Prieto M, Rayon JM, Mora J, Pastor M, Ortiz V, Carrasco D, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852–858. doi: 10.1053/jhep.2000.17924. [DOI] [PubMed] [Google Scholar]
- 37.Samuel D, Feray C. Recurrent hepatitis C after liver transplantation: clinical and therapeutical issues. J Viral Hepat. 2000;7:87–92. doi: 10.1046/j.1365-2893.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 38.Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, Krahn M. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 39.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, Ryder S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer. 2008;98:1166–1175. doi: 10.1038/sj.bjc.6604301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chhatwal J, Ferrante SA, Brass C, El Khoury AC, Burroughs M, Bacon B, Esteban-Mur R, et al. Cost-Effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 Infection in the United States. Value in Health. 2013;16:973–986. doi: 10.1016/j.jval.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.