Abstract
Background/Aim
Acetaminophen (APAP)-induced Acute Liver Failure (ALF) is associated with significant mortality. Traditional prognostic scores lack sensitivity. Hypothesis: Serum Liver-type Fatty Acid Binding Protein (FABP1) early (day 1) or late (day 3–5) levels are associated with 21-day mortality in the absence of liver transplant.
Methods
Serum samples from 198 APAP-ALF patients (nested case control study with 99 survivors, 99 non-survivors) were analyzed by ELISA methods and assessed with clinical data from the US Acute Liver Failure Study Group (ALFSG) Registry (1998–2014).
Results
APAP-ALF survivors had significantly lower serum FABP1 levels early (238.6 vs. 690.8 ng/ml, p <0.0001) and late (148.4 vs. 612.3 ng/ml, p <0.0001) compared with non-survivors. FABP1 > 350 ng/ml was associated with significantly higher risk of death at early (p=0.0004) and late (p<0.0001) time points. Increased serum FABP1 early (log FABP1 odds ratio (OR) 1.31, p=0.027) and late (log FABP1 OR 1.50, p =0.005) were associated with significantly increased 21-day mortality after adjusting for significant covariates (MELD, vasopressor use). Areas under the receiver-operating curve (AUROC) for early and late multivariable models were 0.778 and 0.907 respectively. AUROC of the King’s College Criteria (KCC) (Early: 0.552 alone, 0.711 with FABP1; Late: 0.604 alone, 0.797 with FABP1) and ALFSG prognostic index (Early: 0.686 alone, 0.766 with FABP1; Late: 0.711 alone, 0.815 with FABP1) significantly improved with the addition of FABP1 (p <0.002 for all).
Conclusion
In patients with APAP-ALF, FABP1 may have good potential to discriminate survivors from non-survivors and may improve models currently used in clinical practice. Validation of FABP1 as a clinical prediction tool in APAP-ALF warrants further investigation.
Keywords: liver type fatty acid binding protein, Multiorgan failure, prognosis, ALFSG index
INTRODUCTION
Acute liver failure (ALF) is defined by the occurrence of encephalopathy and hepatic synthetic dysfunction within 26 weeks of the first symptoms of liver disease(1). Currently the most common cause of ALF in North America is acetaminophen (APAP) (2, 3). The most widely used prognostic criteria in APAP-ALF patients are the King’s College criteria (KCC) (4, 5). While initially published in 1989, subsequent studies that have utilized KCC have shown relatively poor sensitivity of the APAP criteria, ranging between 25–76% (3, 6–9). More recently, the ALFSG prognostic index also demonstrated limited sensitivity (41%) in APAP-ALF(9).
Liver transplant (LT) in APAP-ALF presents significant challenges due to the rapidity and severity of illness (i.e. high risk of cerebral edema and multiorgan failure), the potential for recovery without LT and the presence of concomitant complex psychosocial issues (contraindications) in several patients (10–12). Given advances in critical care management of ALF, some patients may have a good outcome despite meeting KCC and potentially could avoid unnecessary LT in the presence of improved prognostic markers or scores (7, 13).
Fatty acid binding proteins (FABP) are small (<15 kDa) cytoplasmic proteins that are abundantly expressed in tissues with active fatty acid metabolism, including hepatocytes. The primary function of FABPs is the intracellular transport of long-chain fatty acids(14). The cellular expression of FABPs is responsive to changes in lipid metabolism which can be induced either by pathophysiological conditions, such as ischemia/inflammation(15). Liver-type FABP (FABP1), significantly expressed in hepatocytes, enterocytes and to a lesser degree in renal tubular cells, has been associated with liver injury(16). Levels of FABP1 have been found to be elevated in patients with hepatocyte injury secondary to alcohol or drug toxicity (17). To date there are no published investigations on the role of FABP1 as a biomarker in patients with ALF. FABP1 may have potential relevance in ALF given the severity of hepatic injury, and furthermore the potential for recovery in the absence of transplant, particularly in APAP-ALF.
This nested case control study of randomly selected samples from prospectively enrolled patients from the US Acute Liver Failure Study Group (ALFSG) registry aimed to examine levels of FABP1 in APAP-ALF patients. Specifically, our primary objectives were to test the following hypotheses
Higher FABP1 serum levels are significantly associated with 21-day transplant-free mortality (in the absence of transplant).
A threshold value of FABP1 is significantly associated with 21-day mortality.
Elevated serum levels of FABP1 in APAP-ALF are significantly associated with 21-day mortality after adjusting for other significant covariates.
The addition of FABP1 improves the performance of previously described prognostic models in APAP-ALF (KCC, ALFSG prognostic index).
METHODS
Study Design
This study is a nested case control study of prospectively collected data and biosamples of 198 patients enrolled in the US ALFSG registry/biorepository and is outlined in detail in Supplementary File 1. Between January 1998-December 2014, 1027 APAP-ALF patients were enrolled in the registry. Of these 704 patients were alive at day 21 in the absence of LT. We identified 124 survivors that had early and late serum samples for analysis of which 99 were randomly selected for analysis. Of 224 patients who died in the absence of LT, 87 patients had early and late samples (all were included in this analysis). A further 12 patients with an early sample (of a possible 92) were randomly selected for inclusion in this analysis. Random selection of patients was performed by personnel not involved in the analysis of the samples or statistical analysis for the paper. All enrolling centers were tertiary academic centers and all but one were liver transplant centers. The authors’ Institutional Review Board (IRB)/Health research ethics boards of all enrolling US ALFSG sites have approved all research and all clinical investigation has been conducted according to the principles expressed in the 1975 Declaration of Helsinki. Given patients were unable to provide written consent (critical illness, hepatic encephalopathy ~ HE), written assent was obtained from the next of kin from each patient. Each center implemented monitoring and therapeutic interventions according to institutional standards of care. All authors had access to the study data and reviewed and approved the final manuscript. Reporting of the analysis of this study followed the STROBE Guidelines for reporting case-control studies(18). Consistent with ALFSG studies (19), the primary outcome was 21-day transplant-free survival (no patients received transplant in this analysis).
Participants
Inclusion criteria were: 1) evidence of ALF according to the enrollment criteria for the ALFSG (see operational definitions) AND 2) age =18 years; and 3) HE during the first seven days of study admission (West Haven Criteria)(20, 21). 4) Patients within the ALFSG registry with primary diagnoses of APAP determined by the site investigator. Exclusion criteria were: 1) Cirrhosis/acute on chronic liver failure. 2) Patients without a primary diagnosis of APAP and 3) Patients who received a LT. Serum samples were analyzed on study admission (early; day 1) and late (either day 3, 4 or 5 where available). None of the 198 APAP-ALF patients included received a LT. Patients who received a LT were excluded from our study because listing for transplant is a clinical decision which is not standardized among ALFSG sites. A further 51 healthy controls were analyzed (University of Alberta) for FABP1 only.
Operational Definitions
For the purposes of this study, ALF was defined as INR ≥ 1.5 and HE within the first 26 weeks of liver disease in a patient with an acute hepatic insult (22). HE grade was defined by the West Haven Criteria (simplified) as follows; grade 1 ~ any alteration in mentation, grade 2 being somnolent or obtunded but easily rousable or presence of asterixis, grade 3 being rousable with difficulty and, grade 4: unresponsive to deep pain (20, 21). In this study we defined ‘low coma grade’ as grade 1 or 2 and ‘high coma grade’ as grade 3 or 4. The KCC (4) predicts poor outcome (death/transplant) if: a) pH is less than 7.3 or b) if INR is greater than 6.5, creatinine is greater than 3.4 mg/dL, and coma grade is high (3 or 4). The model for end stage liver disease (MELD) is defined as 10*(0.957*log(4)+0.378*log(bilirubin)+1.12*log(INR)) for dialyzed patients and 10*(0.957*log(creatinine)+0.378*log(bilirubin)+1.12*log(INR)) for patients not dialyzed (23). The ALFSG prognostic index calculates the log odds (logit) for 21-day transplant free survival is defined as = 2.67 - 0.95(HE*) + 1.56(Etiology*) - 1.25(Vasopressor Use*) - 0.70 (ln bilirubin) - 1.35 (ln INR)(9). For low coma grade, HE insert 0, for high coma grade insert 1. Acetaminophen is defined by the ALFSG index as a favorable etiology (insert 1). For absence of vasopressor use insert 0, for vasopressor use insert 1.
Laboratory Assays of FABP1
FABP1 was measured in serum samples with a solid-phase enzyme-linked immunosorbent assay (ELISA) following manufacturer’s instructions (Biomatik, USA). Briefly, samples were incubated 2 hours on a monoclonal anti-FABP1 precoated plate. A specific FABP1 biotin-conjugated polyclonal antibody solution was added for 2 hours. After washing plates, avidin conjugated to horseradish peroxidase was added for 30 minutes. Finally, substrate tetramethylbenzidine was added for 15 minutes. Reactions were stopped by addition of sulfuric acid and absorbance was read at 450 nm. Standard curve range from 1.56 to 200 ng/ml. Samples were performed in duplicate and accepted valid with a variation coefficient less than 25%.
Clinical Variables
Clinical, biochemical and outcome data was collected prospectively as part of the U.S. ALFSG registry to be analyzed for the 198 patients in this study. Data assessed in this study from the registry included demographic (age, race, sex), comorbidities, biochemistry early and late (complete blood count, creatinine, transaminases, phosphate, international normalized ratio (INR), bilirubin, ammonia, lactate), hepatic coma grade and requirement for organ support (mechanical ventilation (MV), vasopressors, renal replacement therapy (RRT)).
Statistical Methods
For differences between outcome groups (APAP-ALF survivors, n=99, APAP-ALF non-survivors, n=99), categorical variables were compared using the Chi squared test or Fisher’s exact test (if n<10 in any cell of the two by two table). FABP1 was treated as a continuous variable. Continuous variables were reported as medians with interquartile range (IQR) and compared using the Wilcoxon rank sum test. Survival was defined as the dichotomous outcome, alive or dead at 21 days after enrollment into the registry (no patients received a LT in this analysis). A two-sided p-value of <0.05 was considered statistically significant for all comparisons.
In order to control for variables that may confound the effect of FABP1 on 21-day mortality, logistic regression analysis was performed(24). Aside from FABP1, covariates considered in multivariable modeling included MELD, lactate, vasopressors use, RRT, MV and high coma grade. Separate multivariable (logistic) regression models were derived for FABP1 early (day 1) and late (day 3–5) by including variables which significant on univariate analysis and performing backward elimination with a p-value threshold of 0.05. Model performance was assessed using area under the receiver operating curve (AUROC) and the Hosmer Lemeshow test for goodness of fit. A bootstrap approach was implemented to validate parameter estimates and AUROC from the multivariable regression analysis. Logistic regression was used to assess significance of the addition of FABP1 to existing prognostic scores (KCC, ALFSG prognostic Index)(9). Model performance (early and late) for KCC, ALFSG index, FABP1, FABP1+KCC, and FABP1+ALFSG index were assessed using AUROC statistics. Comparisons of AUROC statistics between models (e.g. KCC vs. FABP1 + KCC) were made using the Delong method(25). A threshold value of FABP1 associated with mortality was established based on maximizing AUROC at both early and late time points. SAS software version 9.3 was used for univariate comparisons and multivariable logistic regression modeling, and R software version 3.1.2 was used for bootstrap analysis and development of graphs(26).
RESULTS
Comparative Analysis of 198 APAP-ALF patients
Demographic and clinical outcomes stratified by mortality (alive at day 21, n=99; deceased, n=99) are shown in Table 1. No patients in this analysis received LT. Comparing APAP-ALF survivors and non-survivors at day 21, there were no significant differences in age (35 vs. 40, p=0.08) or gender (female; 76% vs. 73%, p=0.63). Survivors required significantly less organ support during the 7-days of inpatient study (MV: 65% vs. 93%; vasopressors 12% vs. 70%; RRT 27% vs. 45%). Survivors were less likely to achieve high (3 or 4) HE coma grade (62% vs. 93%, p < 0.0001) and less likely to receive mannitol for intracranial hypertension (22% vs. 46%, p = 0.0003). APAP-ALF survivors were less likely to have complications during the first 7 days of study including seizures (3% vs. 21%, p<0.0001), arrhythmias (25% vs. 38%, p=0.047) or gastrointestinal bleeding (8% vs. 19 %, p=0.037). On admission, 7% of APAP-ALF survivors and 16% of non-survivors met KCC (p = 0.13).
Table 1.
Demographic and Clinical Parameters in 198 APAP-ALF patients stratified by Day 21 outcome.
| APAP Alive Day 21 (n=99) | APAP Dead Day 21 (n=99) | ||||
|---|---|---|---|---|---|
| N | Number (%) or median (IQR) | N | Number (%) or median (IQR) | P value | |
| Age | 99 | 35 (28–43) | 99 | 40 (30–48) | 0.084 |
| Sex (female) | 99 | 75 (76%) | 99 | 72 (73%) | 0.63 |
| Race | 0.23 | ||||
| White | 99 | 83 (84%) | 99 | 79 (80%) | |
| African-American | 99 | 8 (8%) | 99 | 15 (15%) | |
| Other | 99 | 8 (8%) | 99 | 5 (5%) | |
| Organ support (days 1–7) | |||||
| Mechanical ventilation | 99 | 64 (65%) | 99 | 93 (93%) | <0.0001 |
| Vasopressors | 99 | 12 (12%) | 99 | 69 (70%) | <0.0001 |
| Renal Replacement therapy | 99 | 27 (27%) | 99 | 45 (45%) | 0.0078 |
| KCC | 87 | 7 (7%) | 87 | 16 (16%) | 0.13 |
| Coma Grade 3/4 (Worst days 1–7) | 99 | 61 (62%) | 98 | 91 (93%) | <0.0001 |
| ICP directed therapies (days 1–7) | |||||
| ICP Monitor | 99 | 12 (12%) | 99 | 21 (21%) | 0.086 |
| Mannitol | 99 | 22 (22%) | 99 | 46 (46%) | 0.0003 |
| Hypertonic saline | 99 | 11 (11%) | 99 | 14 (14%) | 0.52 |
| Barbiturates | 99 | 9 (9%) | 99 | 20 (20%) | 0.043 |
| Hypothermia | 99 | 17 (17%) | 99 | 14 (14%) | 0.56 |
| Sedatives | 99 | 70 (71%) | 99 | 88 (89%) | 0.0014 |
| Blood products (days 1–7) | |||||
| Red Blood Cells | 99 | 34 (34%) | 99 | 50 (51%) | 0.021 |
| Fresh Frozen Plasma | 99 | 50 (51%) | 99 | 76 (77%) | 0.0001 |
| Recombinant VIIA | 99 | 3 (3%) | 99 | 5 (5%) | 0.72 |
| Platelets | 99 | 17 (17%) | 99 | 36 (36%) | 0.0023 |
| ICU Complications (days 1–7) | |||||
| Seizures | 99 | 3 (3%) | 99 | 21 (21%) | <0.0001 |
| Arrhythmias | 99 | 25 (25%) | 99 | 38 (38%) | 0.047 |
| GI bleeding | 99 | 8 (8%) | 99 | 19 (19%) | 0.037 |
| Abnormal CT | 15 | 10 (67%) | 12 | 8 (67%) | 1.00 |
| Abnormal CXR | 99 | 88 (89%) | 99 | 83 (84%) | 0.30 |
| Bacteremia/Blood stream infection | 99 | 7 (7%) | 99 | 10 (10%) | 0.61 |
| Cause of death | |||||
| Multiorgan failure | 99 | 51 (52%) | |||
| Cerebral edema | 99 | 38 (39%) | |||
| Unknown | 99 | 9 (9%) | |||
| Listed for transplant | 99 | 15 (15%) | 99 | 23 (23%) | 0.15 |
N: frequency. IQR: interquartile range.
ARDS: acute respiratory syndrome. CT: computed tomography. CXR; chest x-ray.
Amongst the 99 APAP-ALF non-survivors, the most common causes of death reported were multiorgan failure (52%) and neurological complications (39%). Cause of death was unknown in 9% of cases.
Clinical Parameters in 198 APAP-ALF Patients: Admission (Early)
Comparisons of clinical parameters on study admission are shown in Table 2. APAP-ALF survivors had significantly lower serum INR (2.7 vs. 3.4), bilirubin (4.1 vs. 5.0 mg/dl), creatinine (1.4 vs. 2.6 mg/dl) and lactate levels (2.8 vs. 7.0 mmol/L) compared to non-survivors (p<0.003 for all comparisons). Survivors also demonstrated significantly lower MELD scores (23 vs. 29, p<0.0001) than non-survivors on admission. On study admission, survivors were significantly less likely to be on organ support (MV, 58% vs. 80%, p=0.0007; vasopressors, 9% vs. 42%, p<0.0001) or achieve high HE grade (57% vs. 71%, p=0.034).
Table 2.
Biochemical and organ support parameters early (admission) and late (day 3–5) stratified 21 day outcome.
| EARLY (n=198) | APAP Alive Day 21 (n=99) | APAP Dead Day 21 (n=99) | |||
|---|---|---|---|---|---|
| N | Number (%) or median (IQR) | N | Number (%) or median (IQR) | P value | |
| Biochemistry | |||||
| Hemoglobin (g/dL) | 99 | 10.4 (9.2–12.5) | 97 | 10.9 (9.5–12.2) | 0.52 |
| White Blood count (109/L) | 98 | 8.6 (6.4–11.2) | 97 | 10.9 (7.3–17.5) | 0.0008 |
| Platelet count (109/L) | 98 | 132.5 (90.0–195.0) | 97 | 110.0 (67.0–160.0) | 0.0045 |
| INR | 99 | 2.7 (1.8–4.1) | 96 | 3.4 (2.3–4.8) | 0.0023 |
| ALT (IU/L) | 98 | 3380 (1949–6576) | 99 | 3235 (1483–5716) | 0.37 |
| Bilirubin (mg/dL) | 98 | 4.1 (2.5–5.6) | 99 | 5.0 (3.6–7.8) | <0.0001 |
| pH | 88 | 7.4 (7.4–7.5) | 88 | 7.4 (7.3–7.5) | 0.22 |
| Ammonia (venous) (μmol/L) | 51 | 92 (73–140) | 32 | 139 (72–205) | 0.068 |
| Creatinine (mg/dL) | 98 | 1.4 (0.8–3.0) | 98 | 2.6 (1.2–3.8) | 0.0007 |
| Lactate (mmol/L) | 71 | 2.8 (1.7–5.5) | 68 | 7.0 (4.8–11.8) | <0.0001 |
| Phosphate (mg/dL) | 88 | 2.3 (1.7–3.4) | 76 | 3.2 (2.1–4.5) | 0.0061 |
| MELD | 98 | 23.4 (12.7–27.7) | 96 | 29.1 (23.8–34.5) | <0.0001 |
| High Coma Grade (3 or 4) | 99 | 56 (57%) | 97 | 69 (71%) | 0.034 |
| Organ support | |||||
| Mechanical ventilation | 99 | 57(58%) | 99 | 79 (80%) | 0.0007 |
| Vasopressors | 99 | 9 (9%) | 99 | 42 (42%) | <0.0001 |
| Renal Replacement therapy | 99 | 19 (19%) | 99 | 24 (24%) | 0.39 |
| FABP1 (ng/ml) | 99 | 238.6 (111.0–552.6) | 99 | 690.8 (258.6–4598.5) | <0.0001 |
| LATE (n=186) | APAP Alive Day 21 (n=99) | APAP Dead Day 21 (n=87) | |||
| N | Number (%) or median (IQR) | N | Number (%) or median (IQR) | P value | |
| Biochemistry | |||||
| Hemoglobin (g/dL) | 94 | 9.9 (9.0–11.1) | 81 | 10.2 (9.2–10.9) | 0.63 |
| White Blood count (109/L) | 94 | 8.1 (5.9–11.6) | 81 | 11.3 (7.1–15.0) | 0.0029 |
| Platelet count (109/L) | 95 | 111.0 (68.0–153.0) | 81 | 66.0 (47.0–100.0) | <0.0001 |
| INR | 94 | 1.5 (1.3–1.8) | 75 | 2.5 (1.8–4.4) | <0.0001 |
| ALT (IU/L) | 94 | 1172 (612–2007) | 78 | 938 (383–1995) | 0.31 |
| Bilirubin (mg/dL) | 92 | 5.5 (2.7–8.2) | 78 | 9.8 (6.7–13.7) | <0.0001 |
| pH | 55 | 7.4 (7.4–7.5) | 76 | 7.4 (7.3–7.5) | 0.042 |
| Ammonia (venous) (μmol/L) | 25 | 62 (44–84) | 17 | 119 (78–133) | 0.0038 |
| Creatinine (mg/dL) | 94 | 1.2 (0.7–2.5) | 82 | 2.4 (1.3–4.0) | <0.0001 |
| Lactate (mmol/L) | 31 | 1.7 (1.0–2.2) | 41 | 3.8 (2.6–6.7) | <0.0001 |
| Phosphate (mg/dL) | 73 | 2.8 (2.3–3.6) | 35 | 3.3 (2.5–4.5) | 0.054 |
| PO2/FiO2 ratio | 48 | 3.3 (2.1–4.5) | 71 | 2.5 (1.4–3.9) | 0.0087 |
| MELD | 87 | 14.2 (5.4–24.6) | 73 | 29.7 (23.5–35.4) | <0.0001 |
| High Coma Grade (3 or 4)* | 59 | 35 (59%) | 82 | 72 (88%) | <0.0001 |
| Organ support | |||||
| Mechanical ventilation | 99 | 49 (49%) | 87 | 74 (85%) | <0.0001 |
| Vasopressors | 99 | 5 (5%) | 87 | 45 (52%) | <0.0001 |
| Renal Replacement therapy | 99 | 10 (19%) | 87 | 27 (31%) | 0.062 |
| FABP1 (ng/ml) | 99 | 148.4 (50.1–290.4) | 87 | 612.3 (292.9–2737.7) | <0.0001 |
N: frequency. IQR: interquartile range. INR: international normalized ratio. AST: aspartate aminotransferase. ALT: alanine aminotransferase. MELD: Model for End-stage Liver Disease.
Hepatic encephalopathy grade according to West-Haven criteria.
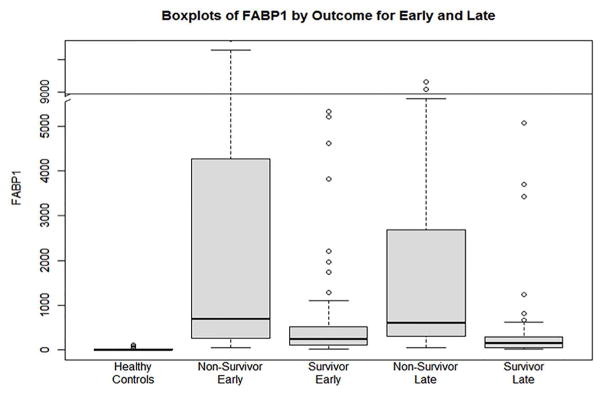
Admission (early) levels of FABP1 are shown in Table 2 and graphically in Figure 1. APAP-ALF survivors had significantly lower admission serum FABP1 levels (238.6 vs. 690.8 ng/ml) compared with non-survivors (p<0.0001). Plots of admission FABP1 and ALT levels are also shown compared in Supplementary File 2.
Figure 1.
Serum levels of FABP1 (ng/ml) in healthy controls, non-survivors (early ~ admission), survivors (early), non-survivors (late ~ day 3–5), survivors (late).
Clinical Parameters in 186 APAP-ALF Patients: Day 3–5 (Late)
Comparisons of clinical parameters on day 3–5 (late) are shown in Table 2. Of the 99 APAP-ALF non-survivors, 87 patients had data available at a late time point (12 died before day 3–5). Late, APAP-ALF survivors (n=99) had significantly lower serum INR (1.5 vs. 2.5), bilirubin (5.5 vs. 9.8 mg/dl), creatinine (1.2 vs. 2.4 mg/dl) and lactate levels (1.7 vs. 3.8 mmol/L) compared to non-survivors (n=87). APAP-ALF survivors also demonstrated significantly lower late MELD scores (14 vs. 30, p<0.0001) than non-survivors. At late time points, survivors were significantly less likely to be on MV (49% vs. 85%, p<0.0001) and vasopressors,(5% vs. 52%, p< 0.0001) or achieve high HE grade (59% vs. 88%, p<0.0001).
Late (days 3–5) levels of FABP1 are shown in Table 2 and graphically in Figure 1. APAP-ALF survivors had significantly lower late serum FABP1 levels (148.4 vs. 612.3 ng/ml) compared with non-survivors (p<0.0001). FABP1 levels were significantly higher in all ALF patients (survivors and non-survivors) compared to healthy controls for both early and late time points (p<0.0001, data not shown).
Multivariable Analysis: Associations with 21-day Mortality
In order to adjust for covariates, multivariable logistic regression for 198 APAP-ALF to determine associations (adjusted) with 21-day mortality were performed (Table 3). Two models were derived; one on admission (early) and one at day 3–5 (late). Values of serum FABP1 were transformed to their natural logarithm (log FABP1) to comply with the linearity assumption in logistic regression.
Table 3.
Early (Day 1) and Late (Day 3–5) predictors of 21-day mortality in 198 APAP-ALF Patients
| EARLY | Unadjusted | Multivariable Model (N=194), AUROC=0.778 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | OR | 95% OR CI | P-value | Included in Model | OR | 95% CI | P-value | |
| Log(FABP1) | 198 | 1.603 | (1.311,1.961) | <0.0001 | Yes | 1.305 | (1.031,1.650) | 0.0265 |
| MELD | 194 | 1.803 | (1.047,1.119) | <0.0001 | Yes | 1.042 | (1.004,1.082) | 0.030 |
| Lactate | 132 | 1.205 | (1.095,1.327) | 0.0001 | No | |||
| Vasopressors | 198 | 7.368 | (3.335,16.287) | <0.0001 | Yes | 3.864 | (1.646,9.067) | 0.0019 |
| Renal replacement therapy | 198 | 1.347 | (0.683,2.658) | 0.390 | No | |||
| Mechanical ventilation | 198 | 2.910 | (1.547,5.475) | 0.0009 | No | |||
| High Coma Grade (3 or 4) | 196 | 1.892 | (1.047,3.421) | 0.0348 | No | |||
| LATE (Day 3–5) | Unadjusted | Multivariable Model (N=160), AUROC=0. 907 | ||||||
| N | OR | 95% OR CI | P-value | Included in Model | OR | 95% CI | P-value | |
| Log(FABP1) | 186 | 2.194 | (1.679,2.866) | <0.0001 | Yes | 1.503 | (1.129,1.999) | 0.0052 |
| MELD | 160 | 1.115 | (1.075,1.157) | <0.0001 | Yes | 1.067 | (1.021,1.114) | 0.0039 |
| Lactate | 71 | 6.908 | (2.592,18.406) | 0.0001 | No | |||
| Vasopressors | 186 | 20.143 | (7.462,54.370) | <0.0001 | Yes | 20.735 | (6.164,69.753) | <0.0001 |
| Renal replacement therapy | 186 | 1.895 | (0.964,3.724) | 0.0638 | No | |||
| Mechanical ventilation | 186 | 5.808 | (2.859,11.802) | <0.0001 | No | |||
| High Coma Grade (3 or 4) | 141 | 4.936 | (2.129,11.446) | 0.0002 | No | |||
- EARLY: Renal replacement therapy, mechanical ventilation, high coma grade (p=0.64) and lactate (p=0.55) were not significant on multivariable analysis so not included in the final early model
- LATE: Renal replacement therapy, mechanical ventilation, Coma grade (0.996) not significant on multivariable analysis so not included in the final late model. Lactate (p=0.0137, n=77) was significant on multivariable analysis but reduced sample size due to missing values so not included in the final late model.
Early (Admission) Model
After adjusting for covariates, the multivariable early model included requirement for vasopressors (odds ratio OR 3.86, 95% CI (1.65, 9.07), p=0.0019), MELD (OR 1.042 (1.004, 1.082) per increment, p=0.03) and log FABP1 (OR 1.305 (1.031, 1.650) per unit, p=0.03). This model demonstrated AUROC of 0.778. Plots of the relationship between early FABP1 levels and the adjusted OR of death at 21 days are shown in Supplementary File 3. Early models including log FABP1 adjusting for RRT and creatinine separately demonstrated statistical significance for FABP1 (p <0.0001 and 0.0001 respectively) but not for either RRT (p=0.61) nor creatinine (p=0.48) (Supplementary File 4).
Late (Day 3–5) Model
After adjusting for covariates, the multivariable late (day 3–5) model included requirement for vasopressors (OR 20.74(1.65, 69.07), p<0.0001), MELD (OR 1.067 ((1.021, 1.114) per increment, p=0.0039) and log FABP1 (OR 1.503 (1.129, 1.999) per unit, p=0.005). This model demonstrated AUROC of 0.907. Plots of the relationship between late FABP1 levels and the adjusted OR of death at 21 days are shown in Supplementary file 3. Late models including log FABP1 adjusting for RRT and creatinine separately demonstrated statistical significance for FABP1 (p <0.0001 for both) but not for either RRT (p=0.42) nor creatinine (p=0.54) (Supplementary file 4).
Internal Validation: Bootstrapping
To internally validate the adjusted association between FABP1 and 21-mortality, bootstrapping was performed to derive estimates for the early and late multivariable models presented in Table 3 (vasopressors, MELD, Log FABP). Validation was done using 1000 bootstrapped samples. Performances of both early (AUROC 0.778) and late (0.906) bootstrapped models were consistent with original multivariable models (Supplementary file 5).
Performance of KCC, ALFSG Index and FABP1: Early and Late
To determine the incremental benefit of FABP1 in addition to current scores in predicting 21-day mortality, performance characteristics of KCC, the ALFSG Index (prognostic score) and FABP1 were calculated in early (n=198) and late (n=186). Performance was also assessed for composite logistic regression models (KCC + FABP1, ALFSG Index+ FABP1). Data is shown in Table 4.
Table 4.
Comparison of Model Performance (Early and Late) for 198 APAP-ALF patients.
| Models | EARLY (Day 1) | LATE (Day 3–5) | ||
|---|---|---|---|---|
| Variable | n | AUROC (95% CI) | N | AUROC (95% CI) |
| KCC | 174 | 0.552 (0.502,0.602) | 110 | 0.604 (0.555,0.654) |
| ALFSG Index | 192 | 0.686 (0.624,0.747) | 124 | 0.711 (0.635,0.788) |
| FABP1 | 198 | 0.710 (0.639,0.782) | 186 | 0.820 (0.760,0.881) |
| KCC+FABP1 | 174 | 0.711 (0.635,0.787)1 | 110 | 0.797 (0.712,0.882)3,4 |
| ALFSG Index + FABP1 | 192 | 0.766 (0.699,0.833)2 | 124 | 0.815 (0.736,0.894)5,6 |
| FABP1>350 ng/ml | 198 | 0.626 (0.559,0.694) | 186 | 0.776 (0.715,0.836) |
| KCC+FABP1>350 ng/ml | 174 | 0.651 (0.577,0.726)7 | 110 | 0.794 (0.719,0.869)9,10 |
| ALFSG Index + FABP1>350 ng/ml | 192 | 0.720 (0.652,0.789)8 | 124 | 0.818 (0.746,0.890)11,12 |
ALFSG ~ Acute Liver Failure Study Group Index. AUROC ~ Area under the receiver operator curve. CI ~ Confidence intervals KCC; King’s College Criteria (Acetaminophen). FABP1; Liver-type Fatty acid binding protein.
Early Models (Delong method for comparison of AUROC statistics(25)):
FABP1+KCC vs. KCC: p < 0.0001
FABP1+ALFSG index vs. ALFSG index: p =0.0008
FABP1>350 ng/ml +KCC vs. KCC: p = 0.005
FABP1>350 ng/ml +ALFSG index vs. ALFSG index: p =0.077
Late Models: KCC
FABP1+KCC vs. KCC: p < 0.0001
For LATE model, only 110 patients had complete data to calculate the KCC. For those patients the AUROC of FABP1 alone (n=110) was 0.772 (0.677, 0.866).
FABP1>350 ng/ml +KCC vs. KCC: p < 0.0001
For LATE model, only 110 patients had complete data to calculate the KCC. For those patients the AUROC of FABP1>350 alone (n=110) was 0.760 (0.677, 0.843).
Late Models: ALFSG Index
FABP1+ALFSG index vs. ALFSG index: p =0.0012
For LATE model, only 124 patients had complete data to calculate the ALFSG Index. For those patients the AUROC of FABP1 alone (n=124) was 0.757 (0.668, 0.845).
FABP1>350 ng/ml +ALFSG index vs. ALFSG index: p =0.0048
For LATE model, only 124 patients had complete data to calculate the ALFSG Index. For those patients the AUROC of FABP>350 ng/ml alone (n=124) was 0.746 (0.669, 0.823).
On admission, AUROC were for KCC (0.552), ALFSG Index (0.686) and FABP1 (0.710). The AUROC for KCC+FABP1 was 0.711 and was significantly improved over KCC alone (p<0.0001). The AUROC of ALFSG Index+FABP1 was 0.766 and was significantly improved over ALFSG Index alone (p=0.0008).
At late time points (Day 3–5), AUROC were for KCC (0.604), ALFSG Index (0.711) and FABP1 (0.820). At late time points, 124 patients had complete data to calculate the ALFSG Index. For those 124 patients, the AUROC of FABP1 alone (n=124) was 0.757 whereas the AUROC ALFSG Index+FABP1 was 0.815, which was significantly improved over ALFSG index alone (p=0.0012). For 110 patients who had complete data to calculate the KCC, the AUROC of FABP1 alone (n=110) was 0.772. The AUROC for KCC+ FABP1 was 0.797 and was significantly improved over KCC alone (p<0.0001).
Performance of KCC, ALFSG Index and FABP1 Threshold: Early and Late
Based on maximizing the AUROC for both early and late time points, FABP1 values greater than 350 ng/ml were determined to be significantly associated with mortality. Performance was also assessed for composite logistic regression models using this threshold (KCC + FABP1>350 ng/ml, ALFSG Index+ FABP1>350 ng/ml). Data is shown in Table 4.
On admission, the AUROC for KCC+FABP1>350 ng/ml was 0.651 and was significantly improved over KCC alone (p=0.005). The AUROC of ALFSG Index+FABP1>350 ng/ml was 0.720 and was not significantly improved over ALFSG Index alone (p=0.077).
At late time points, 124 patients had complete data to calculate the ALFSG Index. For those 124 patients, the AUROC of FABP1>350 ng/ml alone (n=124) was 0.746 whereas the AUROC ALFSG Index+FABP1>350 ng/ml was 0.818, which was significantly improved over ALFSG index alone (p=0.0048). For 110 patients who had complete data to calculate the KCC, the AUROC of FABP1>350 ng/ml alone (n=110) was 0.760. The AUROC for KCC+ FABP1>350 ng/ml was 0.794 and was significantly improved over KCC alone (p<0.0001). Accuracy, sensitivity, and specificity and their 95% Binomial confidence intervals for KCC, ALFSG Index, and FABP1>350 ng/ml are presented in Supplementary file 6.
DISCUSSION
Key Results
In this nested case-control study of 198 APAP-ALF patients, we report the first published analysis of FABP1 in a large series of well-characterized APAP-ALF patients. Serum levels of FABP1 were significantly higher at early and late time points in APAP-ALF non-survivors. After adjusting for significant covariates reflecting severity of illness (MELD, vasopressor dependence), serum FABP1 (log) was significantly associated with 21-day mortality measured at both early (admission) and late (day 3–5) time points. Finally, FABP1 improved performance (AUROC) by using in combination with existing prognostic scores (KCC, ALFSG prognostic index). Serum FABP1 greater than 350 ng/ml was associated with significantly higher risk of death at early (p=0.0004) and late (p<0.0001) time points. We specifically excluded patients who received LT to avoid the inevitable difficulty of assigning outcome in those rescued by transplantation and because protocols for deciding whether or not to transplant varied between enrolling ALFSG sites.
Comparison with literature
APAP-ALF represents the severest form of hepatic injury resulting in massive necrosis. In the presence of hepatocyte injury, cytoplasmic proteins pass through large clefts embedded within a single endothelial cell layer and into the circulation. Hepatocyte damage results in FABP1 detection in blood (27) and has been proposed as a sensitive serum marker of hepatocellular damage in liver transplant recipients(28). Recently FABP1 has been shown to be a diagnostic marker of liver injury in patients with hepatitis C infection(29) and associated with drug-induced liver injury(30). However, to date FABP1 has not been evaluated as a prognostic marker in these conditions or ALF.
FABP1 has physiological properties that could potentially be advantageous as a prognostic biomarker. It is present in high abundance in hepatocytes (approximately 2.7mg/g of liver tissue), has a lower molecular mass (14kDa) and shorter plasma half-life (11min) compared to other cytosolic proteins (e.g. ALT)(31). This suggests FABP1 as a significantly more sensitive biomarker of hepatocyte necrosis compared to ALT (96kDa and half-life of 40–60 minutes); an alternate marker of hepatocyte damage but with poor prognostic value in APAP-ALF (see Supplementary File 2)(27). Furthermore, alpha-glutathione s-transferase (α-GST) has also been proposed as potential biomarker of hepatocyte damage however its larger size (26kDa) and relatively higher expression in kidney and intestine reduce its sensitivity and specificity(28).
Due to its low molecular mass, FABP1 levels in the serum are influenced by both renal function and RRT. FABP1 is present in significantly smaller quantities in the kidney (proximal tubules)(17, 32). It has been demonstrated in cirrhotic patients that elevated levels of urinary FABP1 (but not serum) discriminate between patients with acute tubular necrosis and hepatorenal syndrome (33). For this reason, it is important to adjust for renal dysfunction and renal support when assessing the prognostic discrimination of FABP1 in APAP-ALF patients. Multivariable analysis including the MELD score, which incorporates renal function (creatinine and presence of RRT) at both early and late time points, and was significantly associated with 21-day mortality. RRT was not significantly associated with mortality (both at early or late time points) after adjusting for covariates and therefore was excluded from final models due to collinearity with MELD. After adjusting for MELD, FABP1 was nonetheless still significantly associated with 21-day mortality at both early and late time points. Furthermore, elevated FABP1 levels (early and late) were significantly associated with 21-day mortality after adjusting for the KCC, which also incorporates renal function (creatinine). Hence the specificity of FABP1 for hepatic injury compared to the kidney and intestine may contribute to its potential discriminatory ability.
FABP1 is involved in uptake, transport and metabolism of long-chain fatty acids. In addition, FABP1 has multiple biologic functions including roles in fatty acid transport, storage and metabolism(34). FABP1 also has an important role in facilitating hepatic fatty acid oxidation and is involved in trafficking and delivery of various ligands to cellular destinations such as enzymes, membranes and nucleus(35). FABP1 also acts as a cellular antioxidant as it contains a cysteine group and has been found to influence cell growth, since it has been demonstrated to be elevated during stages of mitosis(36). Hepatic long chain fatty acid uptake is directly associated with FABP1 expression levels(37). Therefore, an increased expression of hepatocyte FABP1 could be a compensatory mechanism in attempt to rescue energy-deprived hepatocytes undergoing necrosis. A failed attempt elicits necrosis, resulting in up-regulated levels of FABP1 being released into circulation. Studies have demonstrated that increased hepatocyte necrosis is associated with higher mortality in ALF(38). Therefore we speculate massive necrosis of hepatocytes containing over-expressed levels of FABP1 could justify the association with non-spontaneous hepatic recovery and explain the higher FABP1 levels found in non-survivors.
This study demonstrated that the addition of FABP1 to both the KCC and the ALFSG prognostic index significantly improved discrimination between 21-day survivors and non-survivors when measured at both early and late time points. Use of KCC alone in this study yielded an AUROC of 0.552 on admission and 0.604 at later time points. This is similar to results published recently for the entire ALFSG cohort (AUROC 0.56 on admission)(5, 9). McPhail and colleagues recently demonstrated in a meta-analysis that the pooled sensitivity of KCC in APAP-ALF was 58%(39). Adding FABP1 to KCC significantly improved prognostic discrimination both at early (AUROC: KCC alone 0.552, 0.711 with FABP1) and late time points (0.604, 0.815 with FABP1). Similar improvements were noted when adding FABP1 to the ALFSG prognostic index (ALFSG Index: AUROC early alone: 0.686, 0.766 with FABP1; late alone 0.711, 0.815 with FABP1). KCC and ALFSG prognostic scores rely on markers of synthetic hepatic function (INR, Bilirubin), hepatic encephalopathy (ALFSG Index), renal dysfunction (MELD) and organ support (vasopressors, ALFSG Index). Potentially FABP1 may add discriminatory and prognostic information regarding hepatic injury or recovery that is not captured by these other covariates.
Limitations
The following limitations of this study warrant consideration. It is a nested case control study and as such the event rate of the primary outcome (21-day mortality) was 50%, higher than published in cohort series. Although patients were enrolled and samples were collected prospectively, analysis was done retrospectively and therefore can comment on association and discrimination (between survivors and non-survivors) and not on the absolute risks of death according to serum FABP1 levels. To account for potential confounding in the study design, we performed multivariable analysis to adjust for other significant covariates (MELD, vasopressors, KCC, ALFSG prognostic index), which demonstrated FABP1 as a statistically significant discriminatory biomarker after adjusting for covariates. To avoid confounding related to LT since transplant listing decisions for APAP-ALF and the organ availability were not consistent between study centers (Simmons et al, ALFSG unpublished data), samples from patients who received a LT were not evaluated in this study. The case-control design of the study may have introduced selection bias, as the primary outcome of survival is automatically unbalanced within the clinical profile of the groups. However in an attempt to reduce observation bias, data was collected prospectively and within this specific study design, researchers measuring FABP1 were blinded to the clinical and outcome data of patients at the time of patient selection and sample analysis. We did not have access to a control group with critical illness but not APAP-ALF as a comparator to elucidate the association between FABP1 and critical illness. Validation was performed with bootstrapping, as independent external samples were unavailable. The relationship of FABP1 and outcomes in ALF should be validated by external samples. Nonetheless despite these limitations, we believe these results are robust as they include APAP-ALF cases from across 16 tertiary liver transplant centers comprising the US ALFSG and the statistically significant associations between FABP1 with mortality after adjusting for significant covariates (MELD, vasopressors) and known prognostic scores (KCC, ALFSG Index).
Conclusions
In patients with APAP-ALF, FABP1 may have good potential to discriminate survivors from non-survivors and may improve models currently used in clinical practice. Validation of FABP1 as a clinical prediction tool in APAP-ALF warrants further investigation.
Supplementary Material
Study Summary/Highlights.
“Acetaminophen-induced ALF survivors had significantly lower serum FABP1 levels early at serial time points compared with non-survivors after adjusting for covariates. FABP1 could potentially improve discrimination between survivors from non-survivors and may improve models currently used in clinical practice.”
Acknowledgments
Members and institutions participating in the Acute Liver Failure Study Group 1998–2016 are as follows: W.M. Lee, M.D. (Principal Investigator); Anne M. Larson, M.D., Iris Liou, M.D., University of Washington, Seattle, WA; Oren Fix, M.D., Swedish Medical Center, Seattle, WA; Michael Schilsky, M.D., Yale University, New Haven, CT; Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL (deceased), Daniel Ganger, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H.B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA; Constantine J. Karvellas MD, University of Alberta, Edmonton, AB; Jodi Olson MD, University of Kansas, Kansas City, KA; Ram Subramanian MD, Emory, Atlanta, GA; James Hanje MD, Ohio State University, Columbus,OH; Bilal Hameed MD, University of California San Francisco, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, Ph.D., Nahid Attar, Linda S. Hynan, Ph.D., and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, Ph.D., Wenle Zhao, Ph.D., Jaime Speiser, Catherine Dillon, Holly Battenhouse and Michelle Gottfried. Special thanks to Dr. Elaine Leslie for study advice.
Financial support: The study was sponsored by NIH grant U-01 58369 (from NIDDK) and a grant from the University of Alberta Hospital Foundation (UHF).
Nonstandard abbreviations
- ALF
acute liver failure
- ALFSG
Acute Liver Failure Study Group
- APAP
acetaminophen
- FABP1
Liver-type fatty acid binding protein
- HE
hepatic encephalopathy
- ICU
Intensive Care Unit
- INR
international normalized ratio
- IQR
Interquartile range
- KCC
King’s College Criteria
- LT
Liver transplantation
- MAP
mean arterial pressure (mm Hg)
- MELD
model for end stage liver disease score
- MV
mechanical ventilation
- OR
Odds Ratio
- RRT
renal replacement therapy
Footnotes
Format: This paper followed the STROBE guideline for reporting cohort studies (BMJ 2007): See Supplementary File #7.
Author Contributions
CJK: Conceived the study concept and design, performed analysis and interpretation of the data, drafted the final manuscript.
JLS: Performed statistical analysis and interpretation of data. Critically revised the final manuscript.
MT: Performed laboratory analysis and revised the final manuscript
WML: Supervisor of entire US Acute Liver Failure Study Group (U-01 Grant). Critically revised the manuscript for important intellectual content.
CFR: Conceived the idea of the study. Assisted in developing study design and interpretation of data, critically revised the final manuscript for important intellectual content.
Disclosures/Conflict of interest: All authors (CK, JLS, MT, WML, and CFR) have no personal or funding conflicts of interest.
References
- 1.O’Grady JG, Williams R. Classification of acute liver failure. Lancet. 1993;342:743. [PubMed] [Google Scholar]
- 2.Fagan E, Wannan G. Reducing paracetamol overdoses. BMJ. 1996;313:1417–1418. doi: 10.1136/bmj.313.7070.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 4.O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 5.Speiser JL, Lee WM, Karvellas CJ Group USALFS. Predicting outcome on admission and post-admission for acetaminophen-induced acute liver failure using classification and regression tree models. PLoS One. 2015;10:e0122929. doi: 10.1371/journal.pone.0122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology. 2002;36:659–665. doi: 10.1053/jhep.2002.35069. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology. 2007;45:789–796. doi: 10.1002/hep.21503. [DOI] [PubMed] [Google Scholar]
- 8.Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558–563. doi: 10.1016/S0140-6736(02)07743-7. [DOI] [PubMed] [Google Scholar]
- 9.Koch DG, Tillman H, Durkalski V, Lee WM, Reuben A. Development of a Model to Predict Transplant-free Survival of Patients with Acute Liver Failure. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karvellas CJ, Safinia N, Auzinger G, Heaton N, Muiesan P, O’Grady J, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30:826–833. doi: 10.1111/j.1478-3231.2010.02243.x. [DOI] [PubMed] [Google Scholar]
- 11.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 12.Reddy KR, Schilsky M, Stravitz RT, Eberle C, Durkalski V, Fontana RJ, et al. Liver transplantation for Acute Liver Failure: Results from the NIH Acute Liver Failure Study Group. Hepatology. 2012;56:246A. [Google Scholar]
- 13.Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163–169. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 14.Pelsers MM, Glatz JF. Detection of brain injury by fatty acid-binding proteins. Clin Chem Lab Med. 2005;43:802–809. doi: 10.1515/CCLM.2005.135. [DOI] [PubMed] [Google Scholar]
- 15.Bass NM, Barker ME, Manning JA, Jones AL, Ockner RK. Acinar heterogeneity of fatty acid binding protein expression in the livers of male, female and clofibrate-treated rats. Hepatology. 1989;9:12–21. doi: 10.1002/hep.1840090104. [DOI] [PubMed] [Google Scholar]
- 16.Pelsers M, Hermens W, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clinica Chimica Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529–535. doi: 10.1016/s0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, et al. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med. 2016;164:724–732. doi: 10.7326/M15-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conn HO, Lieberthal MM, editors. The hepatic coma syndromes and lactulose. Baltimore: Williams & Wilkins; 1979. [Google Scholar]
- 21.Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. The American journal of digestive diseases. 1978;23:398–406. doi: 10.1007/BF01072921. [DOI] [PubMed] [Google Scholar]
- 22.O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 23.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Song X, Gray RH. Comparison of the missing-indicator method and conditional logistic regression in 1:m matched case-control studies with missing exposure values. Am J Epidemiol. 2004;159:603–610. doi: 10.1093/aje/kwh075. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 26.Angelo C, Ripley B. Bootstrap R (S-Plus) functions. 2012. [Google Scholar]
- 27.van den Broek MA, Bloemen JG, Dello SA, van de Poll MC, Olde Damink SW, Dejong CH. Randomized controlled trial analyzing the effect of 15 or 30 min intermittent Pringle maneuver on hepatocellular damage during liver surgery. J Hepatol. 2011;55:337–345. doi: 10.1016/j.jhep.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Pelsers MM, Morovat A, Alexander GJ, Hermens WT, Trull AK, Glatz JF. Liver fatty acid-binding protein as a sensitive serum marker of acute hepatocellular damage in liver transplant recipients. Clin Chem. 2002;48:2055–2057. [PubMed] [Google Scholar]
- 29.Akbal E, Koklu S, Kocak E, Cakal B, Gunes F, Basar O, et al. Liver fatty acid-binding protein is a diagnostic marker to detect liver injury due to chronic hepatitis C infection. Arch Med Res. 2013;44:34–38. doi: 10.1016/j.arcmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Mikus M, Drobin K, Gry M, Bachmann J, Lindberg J, Yimer G, et al. Elevated levels of circulating CDH5 and FABP1 in association with human drug-induced liver injury. Liver Int. 2016 doi: 10.1111/liv.13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergani L, Fanin M, Martinuzzi A, Galassi A, Appi A, Carrozzo R, et al. Liver fatty acid-binding protein in two cases of human lipid storage. Mol Cell Biochem. 1990;98:225–230. doi: 10.1007/BF00231388. [DOI] [PubMed] [Google Scholar]
- 32.Negishi K, Noiri E, Doi K, Maeda-Mamiya R, Sugaya T, Portilla D, et al. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am J Pathol. 2009;174:1154–1159. doi: 10.2353/ajpath.2009.080644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622–632. doi: 10.1002/hep.26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bass NM. The cellular fatty acid binding proteins: aspects of structure, regulation, and function. Int Rev Cytol. 1988;111:143–184. doi: 10.1016/s0074-7696(08)61733-7. [DOI] [PubMed] [Google Scholar]
- 35.Veerkamp JH, van Moerkerk HT. Fatty acid-binding protein and its relation to fatty acid oxidation. Mol Cell Biochem. 1993;123:101–106. doi: 10.1007/BF01076480. [DOI] [PubMed] [Google Scholar]
- 36.Custer RP, Sorof S. Mitosis in hepatocytes is generally associated with elevated levels of the target polypeptide of a liver carcinogen. Differentiation. 1985;30:176–181. doi: 10.1111/j.1432-0436.1985.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang G, Chen QM, Minuk GY, Gong Y, Burczynski FJ. Enhanced expression of cytosolic fatty acid binding protein and fatty acid uptake during liver regeneration in rats. Mol Cell Biochem. 2004;262:41–49. doi: 10.1023/b:mcbi.0000038214.52184.82. [DOI] [PubMed] [Google Scholar]
- 38.Donaldson BW, Gopinath R, Wanless IR, Phillips MJ, Cameron R, Roberts EA, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology. 1993;18:1370–1376. [PubMed] [Google Scholar]
- 39.McPhail MJ, Farne H, Senvar N, Wendon JA, Bernal W. Ability of King’s College Criteria and Model for End-Stage Liver Disease Scores to Predict Mortality of Patients With Acute Liver Failure: A Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:516–525. e515. doi: 10.1016/j.cgh.2015.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.