Abstract
Background & Aims
Cytochrome P450 2A5 (CYP2A5) is induced by ethanol, and the ethanol induction of CYP2A5 is regulated by nuclear factor-erythroid 2-related factor 2 (NRF2). Cyp2a5 knockout (Cyp2a5−/−) mice develop more severe alcoholic fatty liver than Cyp2a5+/+ mice. Fibroblast growth factor 21 (FGF21), a PPARα-regulated liver hormone, is involved in hepatic lipid metabolism. Alcoholic and non-alcoholic fatty liver are enhanced in Pparα knockout (Pparα−/−) mice. This study investigates the relationship between the PPARα-FGF21 axis and the enhanced alcoholic fatty liver in Cyp2a5−/− mice.
Methods
Mice were fed the Lieber-Decarli ethanol diet to induce alcoholic fatty liver.
Results
More severe alcoholic fatty liver disease was developed in Cyp2a5−/− mice than in Cyp2a5+/+ mice. Basal FGF21 levels were higher in Cyp2a5−/− mice than in Cyp2a5+/+ mice, but ethanol did not further increase the elevated FGF21 levels in Cyp2a5−/− mice while FGF21 was induced by ethanol in Cyp2a5+/+ mice. Basal levels of serum FGF21 were lower in Pparα−/− mice than in Pparα+/+ mice; ethanol induced FGF21 in Pparα+/+ mice but not in Pparα−/− mice, whereas ethanol induced hypertriglyceridemia in Pparα−/− mice but not in Pparα+/+ mice. Administration of recombinant FGF21 normalized serum FGF21 and triglyceride in Pparα−/− mice. Alcoholic fatty liver was enhanced in liver-specific Fgf21 knockout mice. Pparα and Cyp2a5 double knockout (Pparα−/−/Cyp2a5−/−) mice developed more severe alcoholic fatty liver than Pparα+/+/Cyp2a5−/− mice.
Conclusions
These results suggest that CYP2A5 protects against the development of alcoholic fatty liver disease, and the PPARα-FGF21 axis contributes to the protective effects of CYP2A5 on alcoholic fatty liver disease.
Keywords: CYP2A5, FGF21, PPARα, alcoholic fatty liver
INTRODUCTION
Alcoholic liver disease includes hepatosteatosis (fatty liver), hepatic inflammation, liver fibrosis, cirrhosis, and liver cancer. Fatty liver is a uniform and early response of the liver to alcohol consumption. Alcoholic fatty liver is generally benign, but it is more sensitive to liver injury induced by hepatotoxins such as lipopolysaccharide (Yang et al., 2001). Both cytochromes P450 2A6 (CYP2A6) and CYP2E1 were found to be increased in the liver sections from patients with alcoholic and non-alcoholic fatty liver diseases (Niemela et al., 2000). Using Cyp2e1 knockout (Cyp2e1−/−) mice and Cyp2e1−/− mice reconstituted with human CYP2E1 (humanized CYP2E1 knock-in) mice, we confirmed the important role of CYP2E1 in the development of alcoholic fatty liver disease (Lu et al., 2008, 2010). But it is still unclear whether CYP2A6 also plays an important role in alcoholic fatty liver disease. Recently, we found that CYP2A5, a mouse orthologue of human CYP2A6, was also induced by ethanol in a CYP2E1-dependent manner (Lu et al., 2011). By comparing alcoholic fatty liver disease in Cyp2a5 knockout (Cyp2a5−/−) mice and the corresponding wild-type mice (Cyp2a5+/+), we found that, in contrast to CYP2E1, CYP2A5 does not contribute to the development of alcoholic fatty liver disease but actually can be protective (Hong et al., 2015). CYP2A5 induction by ethanol is regulated by the redox sensitive transcriptional factor nuclear factor-erythroid 2-related factor 2 (NRF2) (Lu et al., 2012). NRF2 signaling usually protects against oxidative injury via regulating a panel of antioxidant genes (Cederbaum, 2009) and NRF2 protects against alcoholic fatty liver disease in mice (Lamlé et al., 2008). Therefore, CYP2A5 might be among the panel of NRF2-regulated antioxidants and its protective effect on alcoholic fatty liver disease might be associated with its antioxidant action. But further study is still needed to address the exact mechanisms by which alcoholic fatty liver disease is enhanced in Cyp2a5−/− mice.
Peroxisome proliferator-activated receptor α (PPARα) is a major regulator for hepatic lipid metabolism. PPARα regulates genes of lipid oxidation by binding to promoters of specific genes (Kersten et al., 1999; Leone et al., 1999). After ethanol feeding, PPARα was upregulated in Cyp2e1−/− mice, and consistently, Cyp2e1−/− mice developed no or little alcoholic fatty liver (Lu et al., 2008). Very interestingly, ethanol feeding did not upregulate PPARα in the Cyp2a5−/− mice although the basal level of PPARα was elevated in the Cyp2a5−/− mice; consistently, alcoholic fatty liver disease was enhanced in the Cyp2a5−/− mice (Hong et al., 2015). These results support the notion that PPARα protects against the development of alcoholic fatty liver. Indeed, Pparα knockout (Pparα−/−) mice develop more severe alcoholic fatty liver disease (Nakajima et al., 2004; Okiyama et al., 2009). PPARα generally regulates expression of lipid metabolism genes encoding acyl-CoA oxidase, carnitine palmitoyltransferase I and short chain acyl-CoA dehydrogenase (Kersten et al., 1999; Leone et al., 1999). After fasting, Pparα−/− mice develop more severe fatty liver due to impaired fat oxidation, but expression of carnitine palmitoyltransferase I and short chain acyl-CoA dehydrogenase is similar in fasted Pparα−/− and wild-type mice (Kersten et al., 1999). Alcoholic fatty liver disease was more pronounced in Pparα−/− mice, however, acyl-CoA oxidase levels were still comparable in Pparα−/− mice and wild-type mice (Okiyama et al., 2009). These results suggest that other PPARα-dependent mechanisms may occur and blunt fatty liver.
Fibroblast growth factor 21 (FGF21) is a novel metabolic regulator (Kharitonenkov et al., 2005). FGF21 exerts its effect via binding to FGF receptor 1 (FR1) (Luo and McKeehan, 2013). FGF21 can lower blood glucose levels and enhance insulin effects on blood glucose levels (Kharitonenkov et al., 2005). FGF21 can also attenuate hepatic steatosis by regulating hepatic lipid metabolism (Badman et al., 2007). FGF21 is mainly produced in liver and liver is a major source of serum FGF21 (Luo et al., 2013). In liver, FGF21 is regulated by PPARα (Badman et al., 2007; Inagaki et al., 2007). In this study, we hypothesize that PPARα protects against alcoholic fatty liver via FGF21; and that alcoholic fatty liver is enhanced in the Cyp2a5−/− mice because the PPARα-FGF21 axis cannot be upregulated in the Cyp2a5−/− mice.
1. MATERIALS AND METHODS
1.1 Animals and Treatments
The Cyp2a5−/− mice (C57BL/6 background) were created by crossing male Cyp2a5−/− mice (Zhou et al., 2010) (kindly provided by Dr. Xinxin Ding, SUNY College of Nanoscale Science and Engineering, Albany, NY, USA) and female C57BL/6 wild-type mice (purchased from Charles River Laboratory, MA, USA). Littermates (Cyp2a5+/+) were bred as wild-type control. The Pparα−/− mice (B6.129S4 background; strain number 008154) were purchased from Jackson Laboratory. Pparα and Cyp2a5 double knockout mice (Pparα−/−/Cyp2a5−/−) were created by crossing the Pparα−/− mice with Cyp2a5−/− mice. Littermates (Pparα+/+/Cyp2a5−/−) were used as wild-type control mice. The 6th generation of mice were used for the experiments. Liver specific Fgf21 knockout (Fgf21alb-cre) mice and liver specific Fr1 knockout (Fr1alb-cre) mice were created by crossing Fgf21 floxed mice (purchased from Jackson Laboratory; B6.129S6 background; strain number 022361) and Fr1 floxed mice (B6.129S4 background; generously provided by Dr. Philippe Soriano, Department of Development and Regenerative Biology, Icahn School of Medicine at Mount Sinai, NY, USA) with transgenic Alb-Cre recombinase (purchased from Jackson Laboratory; (C57BL/6 x DBA)F2 background; strain number 003574), respectively. The littermates that do not express Alb-Cre recombinase were used as control mice (Fgf21fl/fl and Fr1fl/fl, respectively). The 6th generation of Fgf21alb-cre mice and Fr1alb-cre mice was used for the experiments. All mice were housed in temperature-controlled animal facilities with 12-hour light/12-hour dark cycles and were permitted consumption of tap water and Purina standard chow ad libitum until being fed the experimental diets. The mice received humane care, and experiments were carried out according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals and with approval of the Mount Sinai Animal Care and Use Committee. Nrf2 knockout (Nrf2−/−) mice were fed ethanol in the lab of Dr. Arndt Vogel in the Department of Hepatology, Gastroenterology and Endocrinology, Hannover Medical School, Hannover, Germany as described in (Lamlé et al., 2008). The liver samples from Nrf2−/− mice were generously provided by Dr. Vogel.
In our lab, the mice were initially fed the control liquid dextrose diet (Bio-Serv, Frenchtown, NJ) for 3 days to acclimate them to the liquid diet. Afterward, the mice were fed the liquid ethanol diet (Bio-Serv, Frenchtown, NJ) as described by Lieber and DeCarli (Lieber and DeCarli, 1994). The content of ethanol was gradually increased from 10% of total calories to 20%, 25%, 30%, and finally 35% of total calories (about 6.2% [vol/vol]). The liquid dextrose diet were fed on an iso-energetic basis as a control. The ethanol-fed mice had access to their rations ad libitum. The total feeding duration was 3 weeks (21 days). The amount of food consumed by the different genetically mutated mice used in this study was approximately the same. Some mice were daily injected mouse recombinant FGF21 (rFGF21, purchased from Creative Biomart, Shirley, NY, USA) subcutaneously at 0.25 mg/kg body weight. The mice were fasted for 6 h and then sacrificed by cervical dislocation after blood was collected via the retro-orbital venous sinus under anesthesia by inhalation of isoflurane. The livers were rapidly excised into fragments and washed with cold saline. One piece of liver tissue was put in neutral Formalin solution for paraffin bedding. The other liver tissue aliquots were stored at −80°C for assays.
1.2 Liver Histology
Liver sections were stained with hematoxylin and eosin (H&E) for pathological evaluation as described before (Lu et al., 2010; Hong et al., 2015). Steatosis was quantified as the percentage of cells containing lipid droplets. Five fields (200×) per liver section were examined (one 200× field area contains about 200 cells). Steatosis scoring was based on the following criteria: 0, none; 1, <5%; 2, 5–33%; 3, 34–66%; 4, >67% of cells displaying fat accumulation.
1.3 Quantification of triglyceride (TG) and FGF21
Serum levels of TG were measured using commercially available kits (Pointe Scientific, Canton, MI, USA). Serum FGF21 was measured using a mouse FGF21 kit (BioVendor, Asheville, NC, USA). Liver homogenates were prepared in ice-cold 0.15 M potassium chloride (KCL) and liver TG content was measured as the same manner as serum TG.
1.4 Cytochrome P450 2E1 and 2A5 Activity
Hepatic microsomes were prepared from liver homogenates suspended in 0.15 M KCL. The homogenates were centrifuged at 9,000 × g for 20 min, and then the resulting supernatant fractions were centrifuged at 105,000 × g for 60 min. The resulting pellets (microsomes) were re-suspended in 0.15 M KCL. All procedures were carried out at 4 °C. CYP2E1 activity was measured by the rate of oxidation of 1 mM p-nitrophenol to p-nitrocatechol as described (Lu et al., 2005). Coumarin 7-hydroxylase (COH) is encoded by the mouse Cyp2a5 gene and its human orthologue Cyp2a6 gene, and COH activity is considered as a specific marker for catalytic activities of CYP2A5 and CYP2A6 (Su and Ding, 2004). CYP2A5 activity was measured by assessing COH with 100 μM coumarin as substrate (Kobliakov et al., 1993).
1.5 Western Blotting
Hepatic proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. After 1 h of blocking with 2% fat-free milk, membranes were then incubated for overnight with primary antibodies followed by 1 h incubation with peroxidase secondary anti-rabbit, anti-chicken and anti-goat antibodies (Millipore), respectively. Calnexin or β-actin was detected as a protein loading control. Chemiluminescence was detected by Image Reader LAS-4000 (Fijifilm) after adding Pierce EC Western Blotting Substrate (Pointe Scientific, Canton, MI, USA). Anti-CYP2E1 IgG was a gift from Dr. Jerome Lasker, Hackensack Biomedical Research Institute, Hackensack, NJ, anti-CYP2A5 IgG was a gift from Dr. Risto Juvonen, Department of Pharmacology and Toxicology, University of Kuopio, Kuopio, Finland. Anti-PPARα and anti-FGF21 IgGs were from Abcam, Cambridge, MA, USA. The other IgGs were from Santa Cruz Biotechnology, CA, USA. The bands of proteins were quantified with the Automated Digitizing System (ImageJ gel programs, version 1.34S; National Institutes of Health, Bethesda, MD).
1.6 Statistics
Results are expressed as means ± SEMs. Statistical evaluation was carried out by using one-way analysis of variance (ANOVA) with subsequent the Student-Newman-Keuls post hoc test. P<0.05 was considered as statistical significance.
2. Results
2.1 Alcoholic fatty liver is more pronounced in Cyp2a5−/− mice than in Cyp2a5+/+ mice
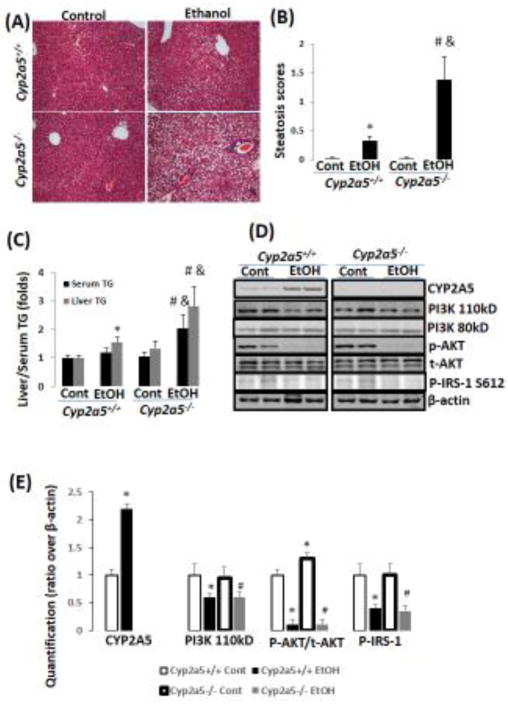
As shown in Fig 1A, ethanol feeding induced formation of lipid droplets in liver of both Cyp2a5+/+ mice and Cyp2a5−/− mice, but the percentage of hepatocytes containing lipid droplets was higher in Cyp2a5−/− mice than in Cyp2a5+/+ mice (Fig. 1B). Consistently, liver TG contents were increased in Cyp2a5−/− mice to a greater extent than in Cyp2a5+/+ mice (Fig 1C grey bars). In a similar manner, serum levels of TG were increased in Cyp2a5−/− mice but not in Cyp2a5+/+ mice (Fig 1C black bars). These results suggest that ablation of CYP2A5 promotes alcoholic fatty liver.
Figure 1.
Chronic ethanol-induced fatty liver is more pronounced in Cyp2a5−/− mice. (A) H&E staining. (B) Steatosis quantification (N=5). (C) Liver and serum TG (N=5). (D) Expression of CYP2A5, PI3K, AKT, and IRS by Western blotting analyses. (E) Quantitation of Western blots (N=4). *P<0.05, compared to Cyp2a5+/+ Control Group; # P<0.05, compared to Cyp2a5−/− Control Group; & P<0.05, compared with Cyp2a5+/+ Ethanol group. Cont, Control; EtOH, Ethanol.
Alcoholic fatty liver is associated with insulin resistance (Zong et al., 2012). Indeed, insulin receptor (IR)-mediated signaling molecules PI3K/AKT and IRS-1 were decreased in response to ethanol feeding (Fig 1D, 1E). However, there were no differences in the decrease in these insulin signaling molecules between Cyp2a5−/− mice and Cyp2a5+/+ mice (Fig 1D, E), suggesting that the ethanol-induced insulin resistance may not explain the difference in development of alcoholic fatty liver between Cyp2a5−/− mice and Cyp2a5+/+ mice.
2.2 Ethanol feeding induces FGF21 in Cyp2a5+/+ mice but not in Cyp2a5−/− mice
FGF21 is a novel metabolic regulator modulating glucose and lipid metabolism (Kharitonenkov et al., 2005). Ethanol feeding caused a 3-fold increase in serum FGF21 in Cyp2a5+/+ mice, however, ethanol feeding had no effect on serum FGF21 in Cyp2a5−/− mice probably because basal levels of serum FGF21 in Cyp2a5−/− mice were already elevated (Fig 2A). Consistently, liver FGF21 expression was also induced by ethanol feeding in Cyp2a5+/+ mice. In Cyp2a5−/− mice, however, ethanol feeding did not induce hepatic FGF21 expression although basal FGF21 expression in liver was already higher compared to Cyp2a5+/+ mice (Fig 2B). Liver FGF21 is regulated by PPARα (Badman et al., 2007; Inagaki et al., 2007). Basal PPARα expression in Cyp2a5−/− mice was higher than that in Cyp2a5+/+ mice although ethanol reduced PPARα expression in both the Cyp2a5−/− mice and Cyp2a5+/+ mice (Fig 2B, C).
Figure 2.
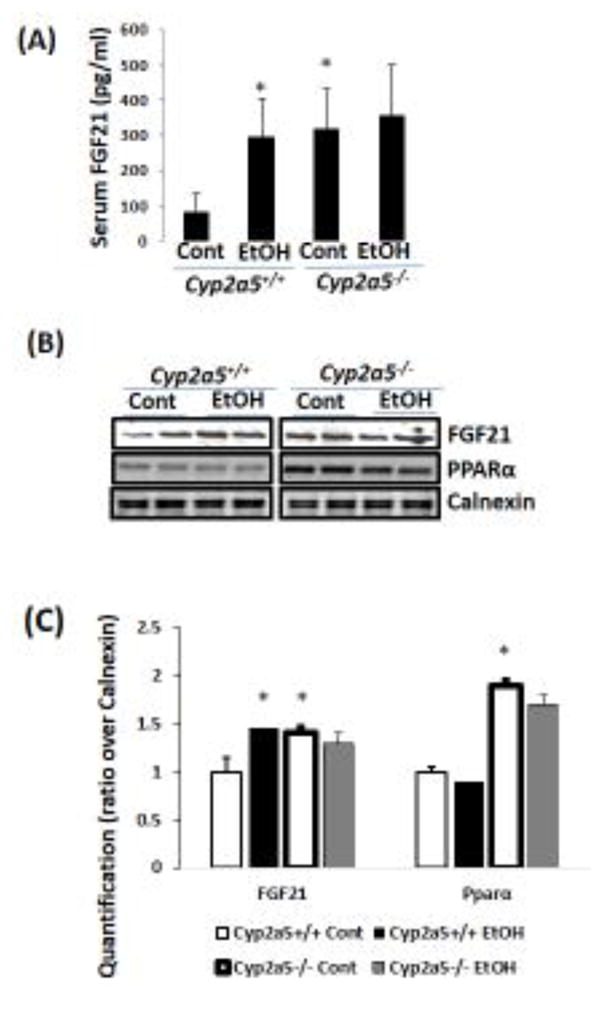
Chronic ethanol induces FGF21 in Cyp2a5+/+ mice but does not in Cyp2a5−/− mice. (A) Serum FGF21 (N=5). (B) Liver expression of FGF21 and PPARα by Western blotting analysis. (C) Quantitation of Western blots (N=4). *P<0.05, compared to Cyp2a5+/+ Control Group. Cont, Control; EtOH, Ethanol.
2.3 FGF21 is induced in Nrf2−/− mice but is not induced in Pparα−/− mice
Previously, we reported that ethanol induction of CYP2A5 was regulated by a NRF2-dependent pathway (Lu et al., 2012). Here, we found that CYP2A5 induction by ethanol was blunted in Nrf2−/− mice while CYP2E1 induction was not affected (Fig 3A, B). Basal FGF21 expression was undetectable in Nrf2−/− mice, which is consistent with the report that Nrf2 regulates FGF21 (Furusawa et al., 2014). However, ethanol was capable of inducing FGF21 in Nrf2−/− mice, although the FGF21 induction by ethanol was weaker compared with Nrf2+/+ mice (Fig 3A, B). Interestingly, basal expression of PPARα, a major regulator of FGF21 in liver, was upregulated in Nrf2−/− mice (Fig 3A, B). To explore the vital role of PPARα in ethanol induction of FGF21, Pparα−/− mice and Pparα+/+ mice were fed ethanol. As shown in Fig 4A, serum FGF21 was almost undetectable in Pparα−/− mice, which is consistent with the notion that FGF21 is regulated by PPARα (Badman et al., 2007; Inagaki et al., 2007). After ethanol feeding, while serum FGF21 was increased in Pparα+/+ mice, serum FGF21 was still undetectable in Pparα−/− mice. Administration of rFGF21 completely restored serum FGF21 in Pparα−/− mice. These results suggest that ethanol induction of FGF21 is regulated by PPARα.
Figure 3.
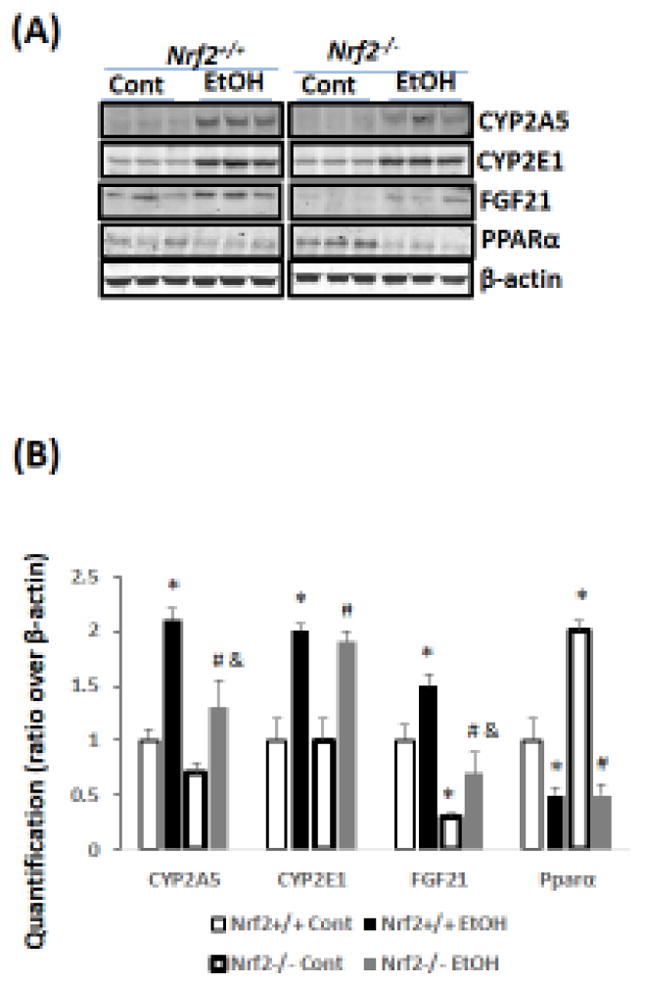
Chronic ethanol induces FGF21 in Nrf2+/+ mice more than in Nrf2−/− mice. (A) Western blots. (B) Quantitation of Western blots (N=3). *P<0.05, compared to Nrf2+/+ Control Group; # P<0.05, compared to Nrf2−/− Control Group; & P<0.05, compared with Nrf2+/+ Ethanol group. Cont, Control; EtOH, Ethanol.
Figure 4.
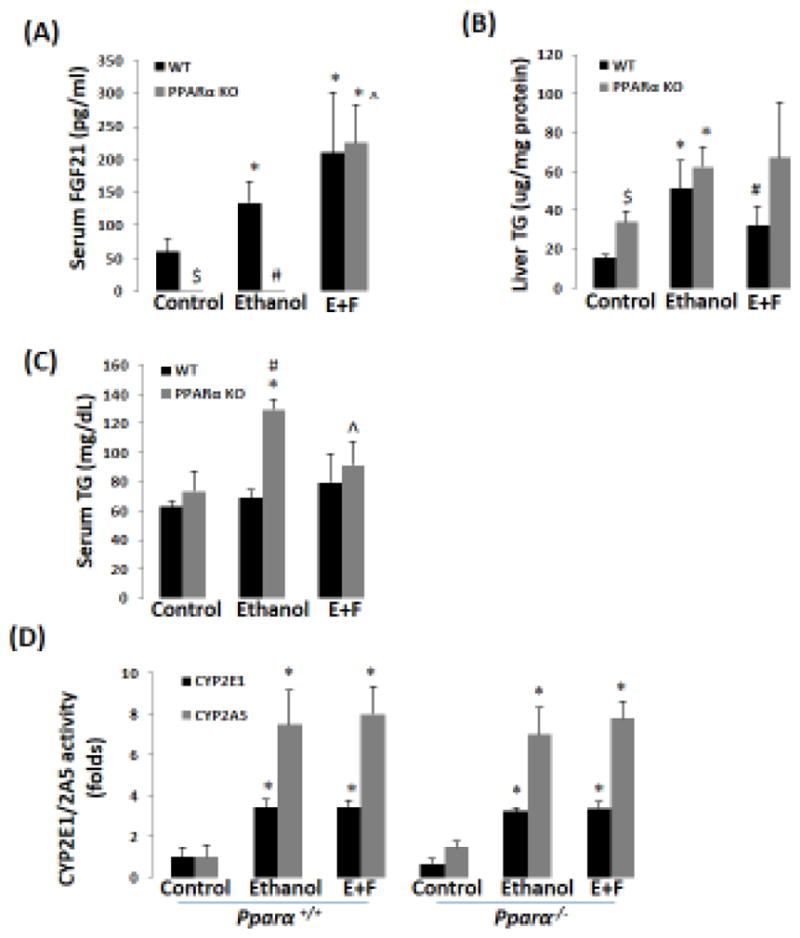
Recombinant FGF21 blunts ethanol-induced hypertriglyceridemia (HTG) in Pparα−/− mice. (A) Ethanol induction of FGF21 was blunted in Pparα−/− mice (N=5). (B) rFGF21 blunted ethanol-induced elevation of liver TG in WT mice but not in Pparα−/− mice (N=5). (C) rFGF21 blunted ethanol-induced HTG in Pparα−/− mice (N=5). (D) Ethanol induction of CYP2E1 and CYP2A5 was not affected in both Pparα+/+ and Pparα−/− mice (N=5). *P<0.05, compared to Control Group. # P<0.05, compared to WT Ethanol Group. $ P<0.05, compared to WT Control Group. ^ P<0.05, compared with PPARα KO Ethanol group. E+F, ethanol plus rFGF21; WT, wild-type; PPARα KO, Pparα−/−.
2.4 rFGF21 blunts ethanol-induced hypertriglyceridemia in Pparα−/− mice
PPARα plays an important role in the development of alcoholic fatty liver disease (Nakajima et al., 2004; Okiyama et al., 2009). Although a comparable increase in liver TG was observed in Pparα−/− mice and Pparα+/+ mice (Fig 4B), ethanol-induced hypertriglyceridemia was observed in Pparα−/− mice rather than in Pparα+/+ mice (Fig 4C). Administration of rFGF21 blunted the increase in liver TG in Pparα+/+ mice (Fig 4B) and the increase in serum TG in Pparα−/− mice (Fig 4C). PPARα and rFGF21 had no effects on liver CYP2E1 and CYP2A5 activities (Fig 4D). These results suggest that the enhanced alcoholic fatty liver found in Pparα−/− mice is associated with the lower FGF21 levels.
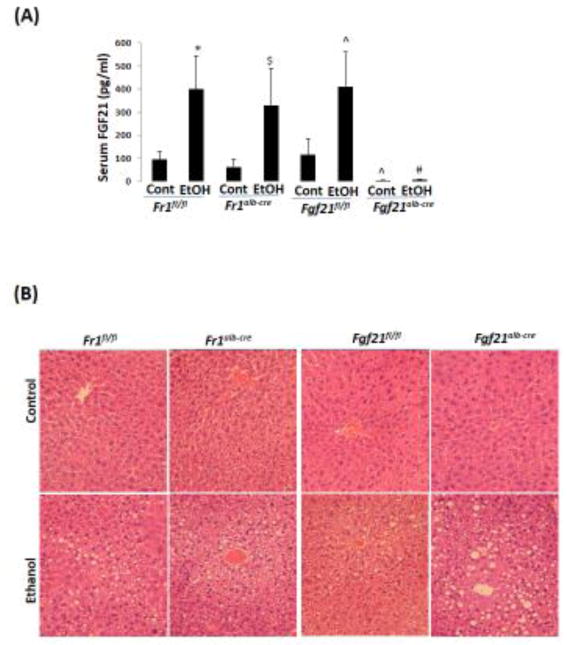
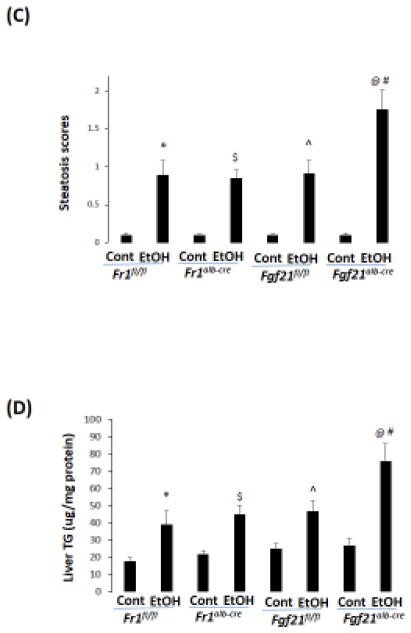
2.5 Chronic ethanol-induced fatty liver is comparable in Fr1alb-cre mice but is more severe in Fgf21alb-cre mice
To further test the role of FGF21 in the development of alcoholic fatty liver, we created liver specific FGF21 knockout (Fgf21alb-cre) mice and liver specific FR1 knockout (Fr1alb-cre) mice and compared the effects of ethanol in these mice with the corresponding control mice, Fgf21fl/fl mice and Fr1fl/fl mice, respectively. Serum FGF21 was almost undetectable in the Fgf21alb-cre mice while it was unchanged in the Fr1alb-cre mice compared with the Fr1fl/fl mice; after ethanol feeding, the serum FGF21 was increased by 4-fold in the Fr1alb-cre mice but it was not induced in the Fgf21alb-cre mice (Fig 5A). Correspondingly, Fgf21alb-cre mice developed more severe alcoholic fatty liver than Fgf21fl/fl mice (Fig 5B, C), and liver TG accumulation was higher in Fgf21alb-cre mice than in Fgf21fl/fl mice (Fig 5D), suggesting that FGF21 plays an indispensible role in the development of alcoholic fatty liver. However, alcoholic fatty liver was comparable in the Fr1alb-cre mice and Fr1fl/fl mice, suggesting that FGF21 does not exert its effects through liver FR1.
Figure 5.
Alcoholic fatty liver is enhanced in Fgf21alb-cre mice but is not in Fr1alb-cre mice. (A) Ethanol induction of FGF21 was blunted in Fgf21alb-cre mice but was not in Fr1alb-cre mice (N=5). (B) H&E staining in liver sections shows that alcoholic fatty liver was enhanced in Fgf21alb-cre mice but not in Fr1alb-cre mice. (C) Steatosis scores (N=5). (D) Liver TG contents (N=5). *P<0.05, compared to Fr1fl/fl Control Group. # P<0.05, compared to Fgf21fl/fl Ethanol Group. ^ P<0.05, compared with Fgf21fl/fl control group. $ P<0.05, compared to Fr1alb-cre Control Group. @ P<0.05, compared to Fr1alb-cre Control Group. Cont, Control; EtOH, Ethanol.
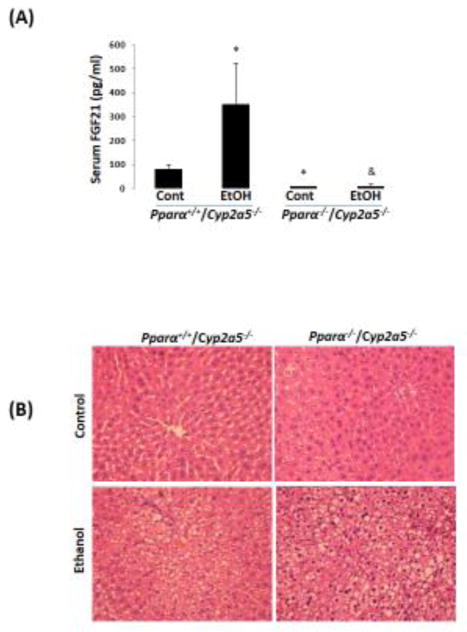
2.6 Pparα−/−/Cyp2a5−/− mice develop more severe alcoholic fatty liver than Pparα+/+/Cyp2a5−/− mice
To investigate whether the basal upregulation of PPARα-FGF21 in Cyp2a5−/− mice protects against alcoholic fatty liver, we designed a model to abrogate the upregulation of PPARα in Cyp2a5−/− mice by creating Pparα−/−/Cyp2a5−/− mice. A comparison between Pparα−/−/Cyp2a5−/− mice and Pparα+/+/Cyp2a5−/− mice would help to define the role of PPARα-FGF21 in the enhanced alcoholic fatty liver in Cyp2a5−/− mice. As shown in Fig 6A, serum FGF21 was much lower in Pparα−/−/Cyp2a5−/− mice than in Pparα+/+/Cyp2a5−/− mice; ethanol feeding increased serum FGF21 in Pparα+/+/Cyp2a5−/− mice but did not in Pparα−/−/Cyp2a5−/− mice. Alcoholic fatty liver was more pronounced in Pparα−/−/Cyp2a5−/− mice than in Pparα+/+/Cyp2a5−/− mice (Fig 6B, C), and liver TG accumulation was also higher in Pparα−/−/Cyp2a5−/− mice than in Pparα+/+/Cyp2a5−/− mice (Fig 6D), suggesting that abrogating the upregulation of the PPARα-FGF21 axis enhances alcoholic fatty liver; and the difference in alcoholic fatty liver between Cyp2a5−/− mice and Cyp2a5+/+ mice might be due to the inability of ethanol to upregulate the PPARα-FGF21 axis in Cyp2a5−/− mice.
Figure 6.
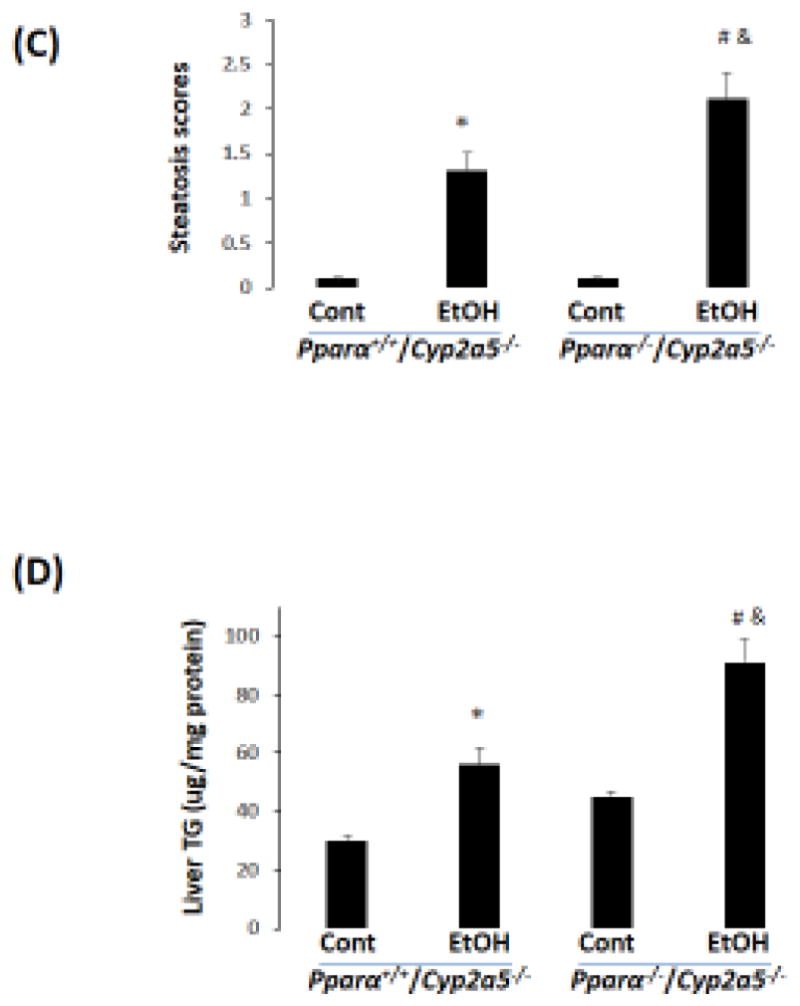
Alcoholic fatty liver is more pronounced in Pparα−/−/Cyp2a5−/− mice than in Pparα+/+/Cyp2a5−/− mice. (A) Ethanol induction of FGF21 was blunted in Pparα−/−/Cyp2a5−/− mice (N=4). (B) H&E staining in liver sections shows that alcoholic fatty liver was more pronounced in Pparα−/−/Cyp2a5−/− mice than in Pparα+/+/Cyp2a5−/− mice. (C) Steatosis scores (N=5). (D) Liver TG contents (N=5). *P<0.05, compared to Pparα+/+/Cyp2a5−/− Control Group. # P<0.05, compared to Pparα−/−/Cyp2a5−/− Control Group. & P<0.05, compared to Pparα+/+/Cyp2a5−/− Ethanol Group. Cont, Control; EtOH, Ethanol.
3. Discussion
Kirby et al first suggested that CYP2A5 may modulate hepatic lipid metabolism (Kirby et al., 2011). CYP2A6 upregulation was observed in patients with alcoholic and non-alcoholic fatty liver, and CYP2A6 co-localized with lipid droplets (Niemela et al., 2000). Previously, we reported that CYP2A5 protected against alcoholic liver injury (Hong et al., 2015), but the molecular mechanism for this was not clear. In the present study, we found that alcoholic fatty liver was more pronounced in Cyp2a5−/− mice than in Cyp2a5+/+ mice, and that the alcoholic fatty liver was more pronounced in Pparα−/−/Cyp2a5−/− mice than in Pparα+/+/Cyp2a5−/− mice, suggesting that CYP2A5, together with PPARα, may participate in regulation of lipid metabolism and in the development of alcoholic fatty liver.
FGF21 is a recently-described lipid metabolism regulator (Kharitonenkov et al., 2005). Basal levels of serum FGF21 and liver FGF21 expression were elevated in Cyp2a5−/− mice compared to Cyp2a5+/+ mice. However, ethanol did not further increase serum FGF21 or liver FGF21 expression in Cyp2a5−/− mice while ethanol did elevate FGF21 in Cyp2a5+/+ mice. In liver, FGF21 is regulated by PPARα, a lipid metabolism regulator (Badman et al., 2007; Inagaki et al., 2007). Indeed, we found that serum FGF21 was almost undetectable in Pparα−/− mice and ethanol feeding did not increase serum FGF21 but ethanol did induce hypertriglyceridemia in Pparα−/− mice. In contrast, ethanol feeding induced serum FGF21 but did not cause an elevation of serum TG in Pparα+/+ mice. Administration of rFGF21 restored the blood FGF21 and normalized serum TG levels in ethanol-fed Pparα−/− mice. These results suggest that FGF21 may act as a downstream mediator of the PPARα signaling pathway, resulting in a formation of a PPARα-FGF21 axis which protecte against the development of alcoholic fatty liver. The treatment with rFGF21 led to a decrease in liver TG in Pparα+/+ mice but did not in Pparα−/− mice. Alcoholic fatty liver is known to be enhanced in Pparα−/− mice (Nakajima et al., 2004). In our study, liver TG accumulation was not higher in Pparα−/− mice as compared with Pparα+/+ mice, but hypertriglyceridemia was observed in the Pparα−/− mice but was not in the Pparα+/+ mice. Administration of rFGF21 blunted the elevation of serum TG in the Pparα−/− mice, indicating that FGF21 is still protective in Pparα−/− mice. Although serum TG levels were lowered by rFGF21, liver TG accumulation was not decreased by rFGF21 in Pparα−/− mice. Possible reasons for why rFGF21 did not ameliorate liver fat accumulation in Pparα−/− mice may be: 1. PPARα regulates a panel of lipid metabolism genes, and FGF21 may need to cooperate with other PPARα-regulated genes to exert hepatic lowering effects; 2. While the rFGF21 dose (0.25mg/kg) was sufficient to exert actions in the Pparα+/+ mice (where endogenous FGF21 is also produced), it may not be sufficient for Pparα−/− mice (where no or little endogenous FGF21 is produced); 3. The rFGF21 may be lowering non-hepatic secretion of TG into the serum e.g. adipose tissue lipolysis, thereby lowering hypertriglyceridemia but not hepatic TGF levels.
FGF21 exerts its effect via binding to and activating FR1 (Potthoff et al., 2012). Adipose tissues have comparatively high expression of FR1 (Fisher et al., 2011); FR1 expression in liver is only about 10% the level of expression detected in adipose tissue (Fisher et al., 2011), which leads to an expectation that FGF21 acts on adipose tissue to a greater extent than liver. We found that alcoholic fatty liver was comparable in the Fr1alb-cre mice and Fr1fl/fl mice, suggesting that FGF21 does not exert its lipid modulating effects through liver FR1. About 60% of hepatic fat is derived from adipose tissues (Donnelly et al., 2005), and adipose tissues play a very important role in the development of alcoholic fatty liver disease (Poggi and Di Luzio, 1964; Horning et al., 1960; Zhong et al., 2012). We plan to create adipose specific Fr1 knockout mice to address whether FGF21 exerts its effects through adipose FR1.
Both mouse CYP2A5 and human CYP2A6 are regulated by NRF2 (Lu et al., 2012; Yokota et al., 2011). Recently, we found that ethanol induction of CYP2A5 was lower in Nrf2−/− mice than in Nrf2+/+ mice, suggesting that ethanol induction of CYP2A5 was also regulated, at least in part, by NRF2-dependent pathway (Lu et al., 2012). Redox sensitive NRF2 usually up-regulates a panel of antioxidant enzymes to antagonize oxidative stress (Cederbaum, 2009) and NRF2 deficiency enhanced alcoholic liver injury in mice (Lamlé et al., 2008), suggesting that NRF2 protects against alcoholic liver injury via its antioxidant promoting effects. Whether Cyp2a5 is among the panel of NRF2-regulated antioxidant genes is still unclear, but CYP2A5 may act as an antioxidant to protect against alcohol-induced oxidative liver injury (Hong et al., 2015). NRF2 can also regulate FGF21 expression (Furusawa et al., 2014). Does NRF2 also exert effects via FGF21? Here we found that ethanol induction of FGF21 was also lower in Nrf2−/− mice, which is consistent with the more severe alcoholic liver injury in Nrf2−/− mice (Lamlé et al., 2008). Thus, in addition to its antioxidant promoting effects, NRF2 may also regulate hepatic lipid metabolism through FGF21.
To directly address whether the PPARα-FGF21 axis is related to the enhanced fatty liver in Cyp2a5−/− mice, we developed Pparα−/−/Cyp2a5−/− mice. In addition to liver, adipose tissue also produce FGF21. In liver, FGF21 is regulated by PPARα, but in adipose tissues, FGF21 is regulated by PPARγ but not by PPARα (Dutchak et al., 2012). Thus, adipose FGF21 should not be affected in Pparα−/−/Cyp2a5−/− mice which maintain PPARγ signaling. Similarly, to exclude the influence of adipose FGF21, liver-specific Fgf21 knockout (Fgf21alb-cre) mice were applied instead of global FGF21 knockout mice to examine whether FGF21 plays a role in alcoholic fatty liver. Fgf21alb-cre mice developed more pronounced alcoholic fatty liver than Fgf21fl/fl mice, and most importantly, Pparα−/−/Cyp2a5−/− mice developed a more severe alcoholic fatty liver than Pparα+/+/Cyp2a5−/− mice. Serum FGF21 was much lower in Pparα−/−/Cyp2a5−/− mice than in Pparα+/+/Cyp2a5−/− mice. These results suggest that the PPARα-FGF21 axis regulates hepatic lipid metabolism, and it is due to the failed induction of the PPARα-FGF21 axis that Cyp2a5−/− mice develop more severe alcoholic fatty liver. In Cyp2a5+/+ mice, ethanol induced a compensatory upregulation of the PPARα-FGF21 axis, which regulates hepatic lipid metabolism and blunts the development of fatty liver disease. When the PPARα-FGF21 axis was not upregulated as compensation, the fatty liver was more pronounced as seen in the Cyp2a5−/− mice.
In this study, we used several colonies of genetically mutated mice with different background. Alcohol-induced fatty liver in mice may be susceptible to genetic background. Therefore, the comparison was made among those mice with the same genetic background, and the comparison between those mice with different background was avoided. For example, Pparα+/+/Cyp2a5−/− mice and Pparα−/−/Cyp2a5−/− mice were litter mates sharing the same background, thus, the comparison between them leads to a conclusion that the enhanced alcoholic fatty liver in the Cyp2a5−/− mice is due to the failed upregulation of the PPARα-FGF21 axis by ethanol in Cyp2a5−/− mice. However, due to the different background, Fgf21alb-cre mice and Fr1alb-cre mice are not comparable. Although liver TG levels and steatosis scores were statistically higher in Fgf21alb-cre mice than in Fr1alb-cre mice (Fig. 5C, 5D), a conclusion that liver FGF21 plays a more important role in alcoholic fatty liver than liver FR1 was not from a direct comparison between Fgf21alb-cre mice and Fr1alb-cre mice. Instead, by comparing Fgf21alb-cre mice and Fgf21fl/fl mice, we know that liver FGF21 plays an important role in alcoholic fatty liver and by comparing Fr1alb-cre mice and Fr1fl/fl mice, we conclude that liver FR1 has no effect on alcoholic fatty liver. Thus, liver FGF21 is more important than liver FR1 in alcoholic fatty liver.
Mouse Cyp2a5 is an ortholog of human Cyp2a6, and the findings in this study may be extrapolated from mouse to human being. Cyp2a6 gene, which is highly polymorphic, has approximately 40 annotated allelic variants, and homozygosis for some of these alleles leads to a total lack of CYP2A6 activity. Individual difference in CYP2A6 expression and activity is due primarily to genetic polymorphisms in the Cyp2a6 gene (Raunio and Rahnasto-Rilla, 2012). There have been near 300 single nucleotide polymorphisms (SNPs) found in the Cyp2a6 upstream sequence, and polymorphism of CYP2A6 has been associated with smoking behavior, drug clearance and lung cancer risk (Di et al., 2009). Whether the polymorphisms of human Cyp2a6 affect sensitivity of patients to alcoholic fatty liver disease needs further studies.
In conclusion, CYP2A5 protect against the development of alcoholic fatty liver disease, and FGF21 acts as a downstream molecule of PPARα signaling pathway to regulate liver lipid metabolism and contribute to the CYP2A5 protective effects on alcoholic fatty liver disease.
Acknowledgments
Financial support: These studies were supported by USPHS grants AA020877 and AA024723 from the National Institute on Alcohol Abuse and Alcoholism.
We thank Dr. Xinxin Ding for Cyp2a5−/− mice, Dr. Philippe Soriano for Fr1 floxed mice, Dr. Jerome Lasker for CYP2E1 antibody, Dr. Risto Juvonen for CYP2A5 antibody, Drs. Arndt Vogel and Jutta Lamlé for liver samples from Nrf2+/+ and Nrf2−/− mice fed with ethanol.
Abbreviation
- CYP2A6
Cytochrome P450 2A6
- CYP2E1
Cytochrome P450 2E1
- Cyp2e1−/− mice
Cyp2e1 knockout mice
- CYP2A5
Cytochrome P450 2A5
- Cyp2a5−/− mice
Cyp2a5 knockout mice
- NRF2
nuclear factor-erythroid 2-related factor 2
- Nrf2−/− mice
Nrf2 knockout mice
- PPARα
peroxisome proliferator-activated receptor α
- Pparα−/− mice
Pparα knockout mice
- FGF21
fibroblast growth factor 21
- Pparα−/−/Cyp2a5−/− mice
Pparα and Cyp2a5 double knockout mice
- Fgf21alb-cre mice
liver specific Fgf21 knockout mice
- FR1
FGF receptor 1
- Fr1alb-cre mice
liver specific Fr1 knockout mice
- rFGF21
mouse recombinant FGF21
- TG
triglyceride
- KCL
potassium chloride
- H&E
hematoxylin and eosin
- COH
coumarin 7-hydroxylase
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- PI3K/AKT
phosphatidylinositol 3-kinase/Protein Kinase B
- IRS-1
insulin receptor substrate 1
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. Nrf2 and antioxidant defense against CYP2E1 toxicity. Expert Opin Drug Metab Toxicol. 2009;5:1223–44. doi: 10.1517/17425250903143769. [DOI] [PubMed] [Google Scholar]
- Di YM, Chow VD, Yang LP, Zhou SF. Structure, function, regulation and polymorphism of human cytochrome P450 2A6. Curr Drug Metab. 2009;10:754–80. doi: 10.2174/138920009789895507. [DOI] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, Kharitonenkov A, Spiegelman BM, Maratos-Flier E. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Uruno A, Yagishita Y, Higashi C, Yamamoto M. Nrf2 induces fibroblast growth factor 21 in diabetic mice. Genes Cells. 2014;19:864–78. doi: 10.1111/gtc.12186. [DOI] [PubMed] [Google Scholar]
- Hong F, Liu X, Ward SC, Xiong H, Cederbaum AI, Lu Y. Absence of cytochrome P450 2A5 enhances alcohol-induced liver injury in mice. Dig Liver Dis. 2015;47:470–7. doi: 10.1016/j.dld.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning MG, Williams EA, Maling HM, Brodie BB. Depot fat as source of increased liver triglycerides after ethanol. Biochem Biophys Res Commun. 1960;3:635–40. doi: 10.1016/0006-291x(60)90077-2. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby GM, Nichols KD, Antenos M. CYP2A5 induction and hepatocellular stress: an adaptive response to perturbations of heme homeostasis. Curr Drug Metab. 2011;12:186–97. doi: 10.2174/138920011795016845. [DOI] [PubMed] [Google Scholar]
- Kobliakov V, Kulikova L, Samoilov D, Lang MA. High expression of cytochrome P4502a5 (coumarin 7-hydroxylase) in mouse hepatomas. Mol Carcinog. 1993;7:276–280. doi: 10.1002/mc.2940070411. [DOI] [PubMed] [Google Scholar]
- Lamlé J, Marhenke S, Borlak J, et al. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159–68. doi: 10.1053/j.gastro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. Animal models of chronic ethanol toxicity. Methods Enzymol. 1994;233:585–594. doi: 10.1016/s0076-6879(94)33061-1. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med. 2010;49:1406–16. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang XH, Cederbaum A. Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway. Toxicol Sci. 2012;128:427–38. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–94. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wu D. Ethanol induction of CYP2A5: permissive role for CYP2E1. Drug Metab Dispos. 2011;39:330–6. doi: 10.1124/dmd.110.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, McKeehan WL. Stressed Liver and Muscle Call on Adipocytes with FGF21. Front Endocrinol (Lausanne) 2013;4:194. doi: 10.3389/fendo.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, Fukushima Y, Peters JM, Gonzalez FJ, Aoyama T. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology. 2004;40:972–80. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- Niemela O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P4502A6, 2E1 and 3A and production of protein aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ, Aoyama T. Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. J Hepatol. 2009;50:1236–46. doi: 10.1016/j.jhep.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi M, Di Luzio NR. The role of liver and adipose tissue in the pathogenesis of the ethanol-induced fatty liver. J Lipid Res. 1964;5:437–41. [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–24. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunio H, Rahnasto-Rilla M. CYP2A6: genetics, structure, regulation, and function. Drug Metabol Drug Interact. 2012;27:73–88. doi: 10.1515/dmdi-2012-0001. [DOI] [PubMed] [Google Scholar]
- Su T, Ding X. Regulation of the cytochrome P4502A genes. Toxicol Appl Pharmacol. 2004;199:285–294. doi: 10.1016/j.taap.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Yang S, Lin H, Diehl AM. Fatty liver vulnerability to endotoxin-induced damage despite NF-kappaB induction and inhibited caspase 3 activation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G382–G392. doi: 10.1152/ajpgi.2001.281.2.G382. [DOI] [PubMed] [Google Scholar]
- Yokota S, Higashi E, Fukami T, et al. Human CYP2A6 is regulated by nuclear factor-erythroid 2 related factor 2. Biochem Pharmacol. 2011;81:289–94. doi: 10.1016/j.bcp.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180:998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhuo X, Xie F, et al. Role of CYP2A5 in the clearance of nicotine and cotinine: insights from studies on a CYP2a5-null mouse model. J Pharmacol Exp Ther. 2010;332:578–587. doi: 10.1124/jpet.109.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Armoni M, Harel C, Karnieli E, Pessin JE. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2012;302:E532–9. doi: 10.1152/ajpendo.00258.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]