Abstract
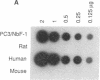
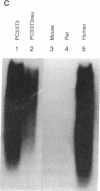
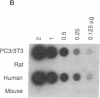
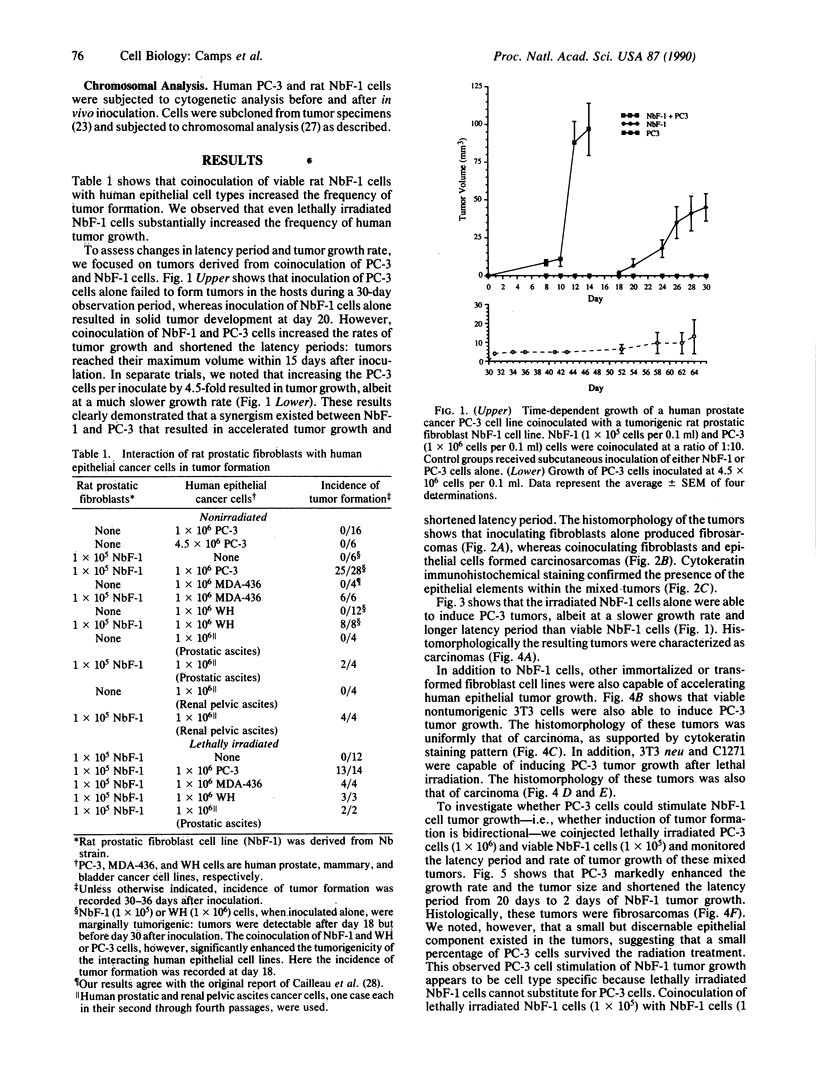
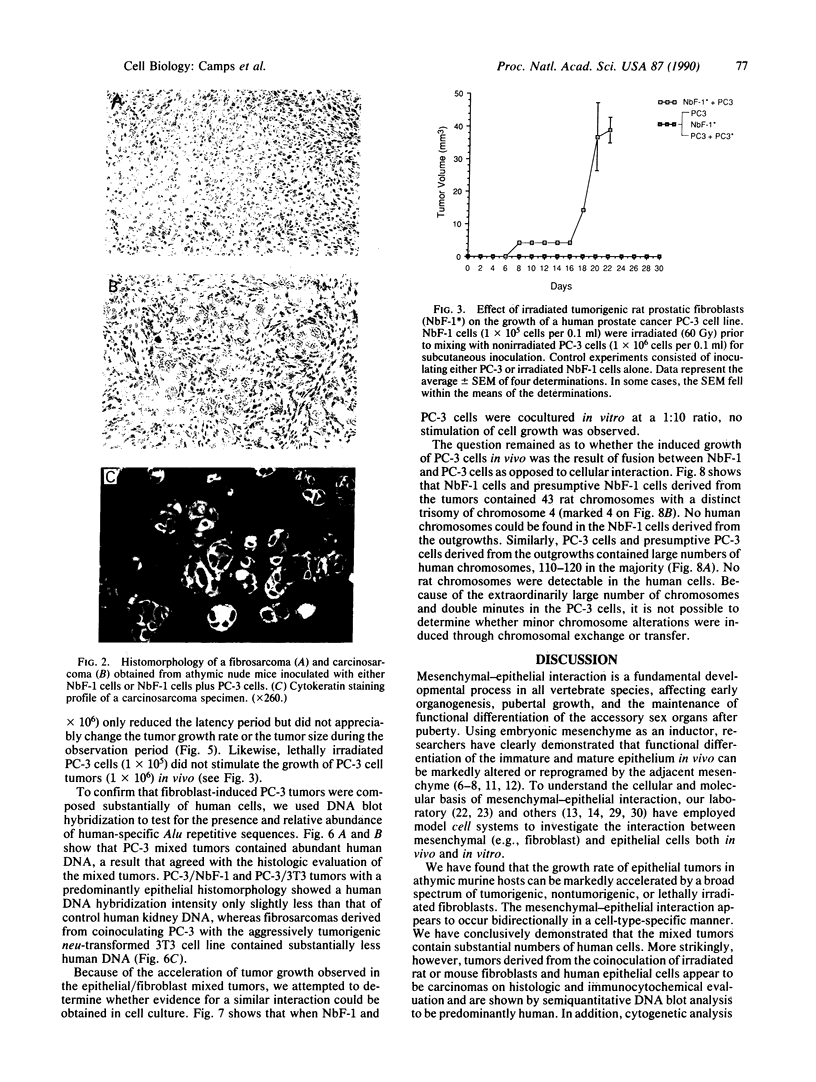
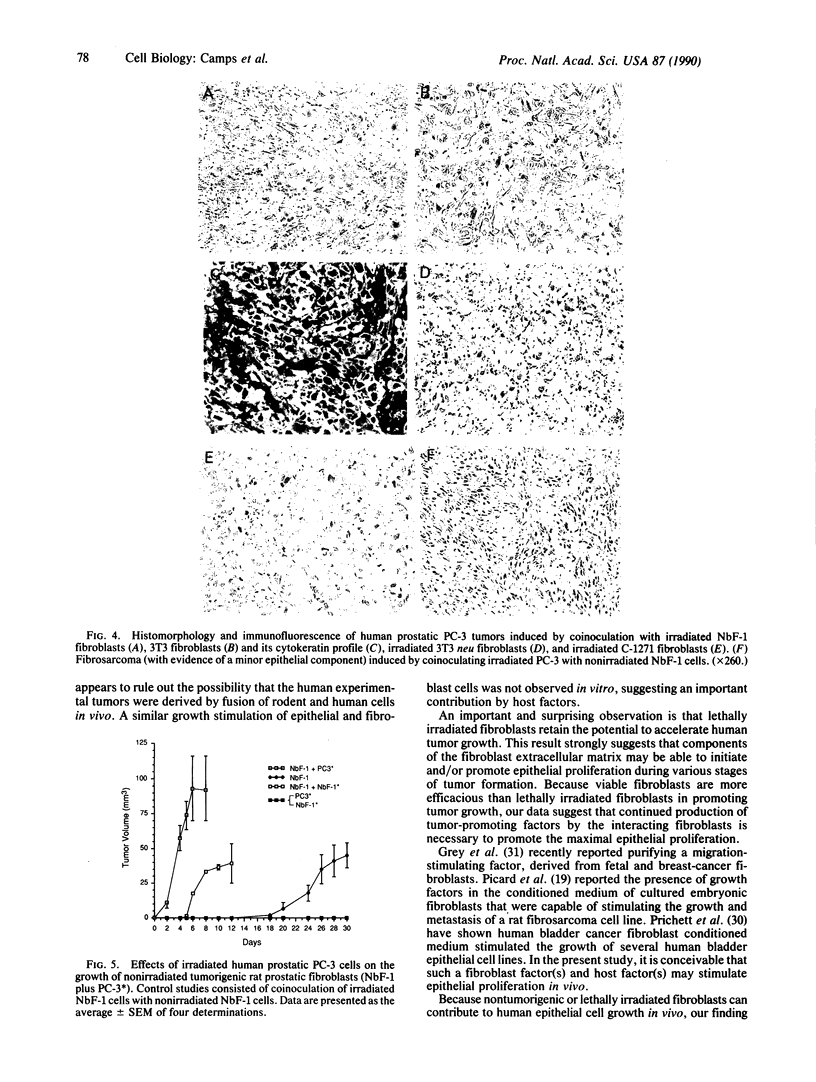
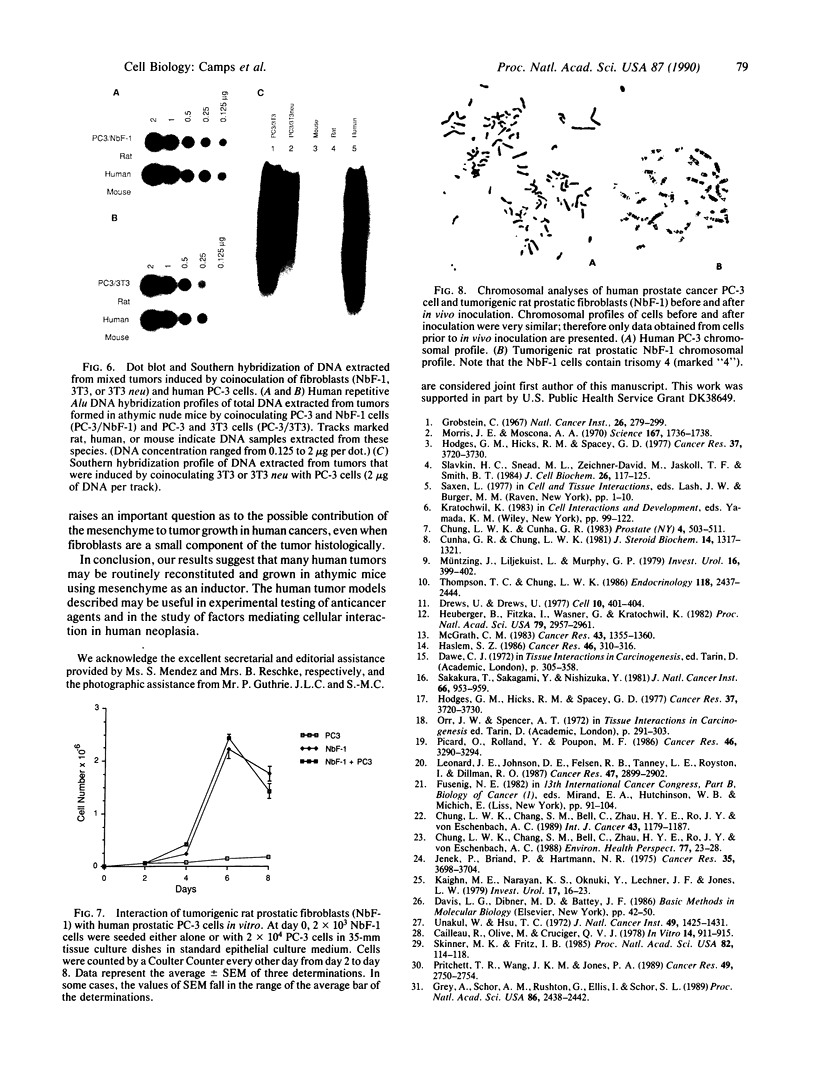
Transformed fibroblasts coinoculated with epithelial cells accelerated the growth and shortened the latency period of human epithelial tumors in athymic mice. Addition of NbF-1 fibroblasts caused epithelial tumors to grow from five marginally tumorigenic or "nontumorigenic" (nontumor-forming) human tumor cell lines or strains: PC-3 (prostate), WH (bladder), MDA-436 (breast), and cells derived from the ascites fluids of patients with metastatic renal pelvic or prostate cancers. Evidence for the human and epithelial nature of these experimental tumors was provided by histologic, immunohistochemical, Southern and dot-blot hybridization, and cytogenetic analyses. Transformed fibroblasts induced predominantly carcinosarcomas, whereas nontumorigenic fibroblasts (NIH 3T3) and lethally irradiated transformed fibroblasts induced exclusively carcinomas. The fibroblast-epithelial interaction appears to occur bidirectionally and does not result from cell fusion. Because coculture experiments in vitro did not demonstrate an increased cell proliferation, it appears that undefined host factors can influence tumor growth. This tumor model may be useful in drug-screening programs and in mechanistic studies of factors regulating human tumor growth and progression.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cailleau R., Olivé M., Cruciger Q. V. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978 Nov;14(11):911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- Chung L. W., Chang S. M., Bell C., Zhau H. E., Ro J. Y., von Eschenbach A. C. Co-inoculation of tumorigenic rat prostate mesenchymal cells with non-tumorigenic epithelial cells results in the development of carcinosarcoma in syngeneic and athymic animals. Int J Cancer. 1989 Jun 15;43(6):1179–1187. doi: 10.1002/ijc.2910430636. [DOI] [PubMed] [Google Scholar]
- Chung L. W., Chang S. M., Bell C., Zhau H., Ro J. Y., von Eschenbach A. C. Prostatic carcinogenesis evoked by cellular interaction. Environ Health Perspect. 1988 Apr;77:23–28. doi: 10.1289/ehp.887723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L. W., Cunha G. R. Stromal-epithelial interactions: II. Regulation of prostatic growth by embryonic urogenital sinus mesenchyme. Prostate. 1983;4(5):503–511. doi: 10.1002/pros.2990040509. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Chung L. W. Stromal-epithelial interactions--I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981 Dec;14(12):1317–1324. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- Drews U., Drews U. Regression of mouse mammary gland anlagen in recombinants of Tfm and wild-type tissues: testosterone acts via the mesenchyme. Cell. 1977 Mar;10(3):401–404. doi: 10.1016/0092-8674(77)90027-7. [DOI] [PubMed] [Google Scholar]
- Grey A. M., Schor A. M., Rushton G., Ellis I., Schor S. L. Purification of the migration stimulating factor produced by fetal and breast cancer patient fibroblasts. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2438–2442. doi: 10.1073/pnas.86.7.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- Haslam S. Z. Mammary fibroblast influence on normal mouse mammary epithelial cell responses to estrogen in vitro. Cancer Res. 1986 Jan;46(1):310–316. [PubMed] [Google Scholar]
- Heuberger B., Fitzka I., Wasner G., Kratochwil K. Induction of androgen receptor formation by epithelium-mesenchyme interaction in embryonic mouse mammary gland. Proc Natl Acad Sci U S A. 1982 May;79(9):2957–2961. doi: 10.1073/pnas.79.9.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges G. M., Hicks R. M., Spacey G. D. Epithelial-stromal interactions in normal and chemical carcinogen-treated adult bladder. Cancer Res. 1977 Oct;37(10):3720–3730. [PubMed] [Google Scholar]
- Hodges G. M., Hicks R. M., Spacey G. D. Epithelial-stromal interactions in normal and chemical carcinogen-treated adult bladder. Cancer Res. 1977 Oct;37(10):3720–3730. [PubMed] [Google Scholar]
- Janik P., Briand P., Hartmann N. R. The effect of estrone-progesterone treatment on cell proliferation kinetics of hormone-dependent GR mouse mammary tumors. Cancer Res. 1975 Dec;35(12):3698–3704. [PubMed] [Google Scholar]
- Kaighn M. E., Narayan K. S., Ohnuki Y., Lechner J. F., Jones L. W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979 Jul;17(1):16–23. [PubMed] [Google Scholar]
- Leonard J. E., Johnson D. E., Felsen R. B., Tanney L. E., Royston I., Dillman R. O. Establishment of a human B-cell tumor in athymic mice. Cancer Res. 1987 Jun 1;47(11):2899–2902. [PubMed] [Google Scholar]
- McGrath C. M. Augmentation of the response of normal mammary epithelial cells to estradiol by mammary stroma. Cancer Res. 1983 Mar;43(3):1355–1360. [PubMed] [Google Scholar]
- Morris J. E., Moscona A. A. Induction of glutamine synthetase in embryonic retina: its dependence on cell interactions. Science. 1970 Mar 27;167(3926):1736–1738. doi: 10.1126/science.167.3926.1736. [DOI] [PubMed] [Google Scholar]
- Müntzing J., Liljekvist J., Murphy G. P. Chalones and stroma as possible growth-limiting factors in the rat ventral prostate. Invest Urol. 1979 Mar;16(5):399–402. [PubMed] [Google Scholar]
- Picard O., Rolland Y., Poupon M. F. Fibroblast-dependent tumorigenicity of cells in nude mice: implication for implantation of metastases. Cancer Res. 1986 Jul;46(7):3290–3294. [PubMed] [Google Scholar]
- Pritchett T. R., Wang J. K., Jones P. A. Mesenchymal-epithelial interactions between normal and transformed human bladder cells. Cancer Res. 1989 May 15;49(10):2750–2754. [PubMed] [Google Scholar]
- Sakakura T., Sakagami Y., Nishizuka Y. Accelerated mammary cancer development by fetal salivary mesenchyma isografted to adult mouse mammary epithelium. J Natl Cancer Inst. 1981 May;66(5):953–959. [PubMed] [Google Scholar]
- Skinner M. K., Fritz I. B. Testicular peritubular cells secrete a protein under androgen control that modulates Sertoli cell functions. Proc Natl Acad Sci U S A. 1985 Jan;82(1):114–118. doi: 10.1073/pnas.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavkin H. C., Snead M. L., Zeichner-David M., Jaskoll T. F., Smith B. T. Concepts of epithelial-mesenchymal interactions during development: tooth and lung organogenesis. J Cell Biochem. 1984;26(2):117–125. doi: 10.1002/jcb.240260207. [DOI] [PubMed] [Google Scholar]
- Thompson T. C., Chung L. W. Regulation of overgrowth and expression of prostatic binding protein in rat chimeric prostate gland. Endocrinology. 1986 Jun;118(6):2437–2444. doi: 10.1210/endo-118-6-2437. [DOI] [PubMed] [Google Scholar]
- Unakul W., Hsu T. C. The C- and G-banding patterns of Rattus norvegicus chromosomes. J Natl Cancer Inst. 1972 Nov;49(5):1425–1431. [PubMed] [Google Scholar]