Abstract
Mating in haploid Saccharomyces cerevisiae occurs after activation of the pheromone response pathway. Biochemical components of this pathway are involved in other yeast signal transduction networks. To understand more about the coordination between signaling pathways, we used a “chemical genetic” approach, searching for compounds that would activate the pheromone-responsive gene FUS1 and RLM1, a reporter for the cell integrity pathway. We found that catecholamines (l-3,4-hydroxyphenylalanine [l-dopa], dopamine, adrenaline, and noradrenaline) elevate FUS1 and RLM1 transcription. N-Acetyl-cysteine, a powerful antioxidant in yeast, completely reversed this effect, suggesting that FUS1 and RLM1 activation in response to catecholamines is a result of oxidative stress. The oxidant hydrogen peroxide also was found to activate transcription of an RLM1 reporter. Further genetic analysis combined with immunoblotting revealed that Kss1, one of the mating mitogen-activated protein kinases (MAPKs), and Mpk1, an MAPK of the cell integrity pathway, participated in l-dopa-induced stimulation of FUS1 and RLM1 transcription. We also report that Mpk1 and Hog1, the high osmolarity MAPK, were phosphorylated upon induction by hydrogen peroxide. Together, our results demonstrate that cells respond to oxidative stress via different signal transduction machinery dependent upon the nature of the oxidant.
INTRODUCTION
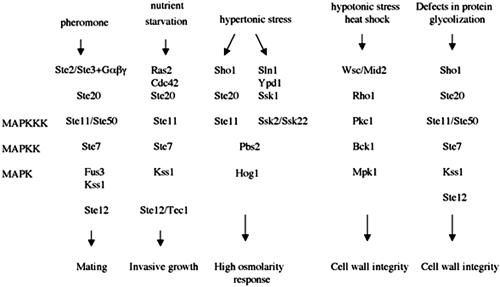
In the haploid cells of the yeast Saccharomyces cerevisiae, four essential MAPK cascades (Figure 1) respond to different external signals to mediate specific responses. The mating pathway is activated by peptide pheromones and induces cell-cycle arrest and the morphological changes required for mating (Elion, 1998; Gustin et al., 1998). The invasive growth pathway is activated by starvation and induces foraging into the agar substratum. The high osmolarity glycerol (HOG) pathway increases intracellular glycerol levels in response to hypertonic stress, whereas the cell integrity pathway is activated by hypotonic stress, heat shock, or impaired cell wall synthesis. Recently, an additional MAPK cascade has been described: the Kss1 vegetative growth pathway (Lee and Elion, 1999). The Kss1 pathway may be activated by cell wall stress or changes in osmolarity (Cullen et al., 2000).
Figure 1.
MAPK cascades which regulate cellular changes in response to external stimuli in haploid S. cerevisae.
The mating pathway is the most studied cellular response to an external signal. As a relatively simple G protein-coupled cascade, it is a widely used model to study mammalian G protein-coupled receptors. The mating cascade includes pheromone receptors (Ste2 and Ste3), G proteins (Gpa1, Ste4, and Ste18), the p21 activating protein kinase Ste20, a mitogen-activated protein kinase kinase kinase (MAPKKK) Ste11, a mitogen-activated protein kinase kinase (MAPKK) Ste7, a scaffolding protein Ste5 and two MAPKs (Fus3 and Kss1) (Gustin et al., 1998; Madhani and Fink, 1998; Farley et al., 1999). Targets of the terminal MAPK include Ste12, a factor required for transcription of pheromone-responsive genes, and Far1, a bifunctional protein required for polarization and G1 arrest (Song et al., 1991; Peter et al., 1993; Tyers and Futcher, 1993; Elion et al., 1993; Roberts et al., 2000). On the other hand, cell cycle arrest and repolarization of cell growth in the form of a mating projection, or “shmoo,” toward the source of the mating signal leads to remodeling of the cell wall, a process that is dependent upon the cell integrity cascade (Buehrer and Errede, 1997; Roberts et al., 2000). The cell integrity pathway regulates cell wall and actin cytoskeleton dynamics (Schmidt and Hall, 1998; Heinisch et al., 1999). It is under the control of protein kinase and is comprised of Bck1 (an MAPKKK), Mkk1 and Mkk2 (an MAPKK), and Mpk1 (an MAPK).
The MAPK cascades in yeast share common components (Hall et al., 1996; Madhani et al., 1997; O'Rourke and Herskowitz, 1998). The specificity of each pathway involves in part the prevention of cross talk between the signaling pathways. Fus3 prevents pheromone-induced activation of the Kss1-dependent pathways at an unknown step (Madhani and Fink, 1998), whereas Hog1 prevents osmolarity-induced activation of the Fus3-Kss1 pathways (O'Rourke and Herskowitz, 1998). The interface between the signaling cascades is not well understood, mainly because of the lack of detectable phenotypes in wild-type strains. To learn more about the cross talk between MAPK cascades in yeast, we used a “chemical genetic” approach and searched for compounds that would activate the expression of FUS1 pheromone response and RLM1 cell integrity reporters.
Here, we demonstrate that treatment of S. cerevisiae cells with catecholamines (adrenaline, noradrenaline, l-3,4-hydroxyphenylalanine [l-dopa], and dopamine) with a propensity for autooxidation activates FUS1 and RLM1 transcription, whereas the well-known oxidant hydrogen peroxide induces only the RLM1 reporter. We also report that treatment of cells with l-dopa results in phosphorylation of Mpk1, an MAPK of cell integrity and Kss1, one of the mating and invasive growth kinases, whereas treatment with hydrogen peroxide induced activation of Mpk1 and Hog1, an MAPK for the general stress response HOG pathway.
MATERIALS AND METHODS
Strains and Plasmids
Standard methods for growth, maintenance, and transformation of yeast and bacteria and for the manipulation of DNA were used throughout (Sherman et al., 1979). S. cerevisiae strains used in this study were in the W303 (ura3-1 leu2-3, 112 trp1-1 his3-11, 15 ade2-1 can1-100 Gal+; Roberts and Fink, 1994) or EG123 strain background (trp1-1 leu2-3, 112 ura3-52 his4 can1; Siliciano and Tatchell, 1984) and are EY957 (W303 wild-type), EY1119 (W303 kss1::HIS3), EY940 (fus3::LEU2), EY966 (kss1::HIS3, fus1::LEU2) (Elaine Elion, Harvard Medical School, Boston, MA; Lee and Elion, 1999), IH 4546 (W303 hog1::TRP1cg), C699-59 (EG123 bck1Δ), SO329 (EG123 MATa FUS1-lacZ::LEU2), SO351 (EG123 FUS1-lacZ::LEU2 sho1::TRP1 (Sean O'Rourke, University of Oregon; O'Rourke and Herskowitz, 1998), DL100 (EG123 wild-type), DL454 (EG123 mpk1::TRP1), DL1985 (EG123 hcs77::LEU2), and DL2278 (EG123 mid2::URA3) (David Levin, John Hopkins University, Baltimore, MD; Lee et al., 1993; Philip and Levin, 2001). Expression plasmids used in this study have been described previously and are plG, px2RLM1 (David Levin, Johns Hopkins University; Jung and Levin, 1999), pFUS1-lacZ (Elaine Elion, Harvard Medical School; Lee and Elion 1999), and pYEpU-FUS1Z (Lee Bardwell, University of California, Irvine, CA; Bardwell et al., 1998a).
Measurement of lacZ Activity In Vivo
Galactosidase activity from the FUS1 and RLM1 reporter genes was determined by a previously described in vivo assay by using chlorophenol red galactopyranoside (CPRG) as the substrate (Olesnicky et al., 1999). Briefly, freshly saturated cultures of the different yeast transformants were diluted into fresh YNBD media (OD600 of 0.02) containing 0.1 M sodium phosphate, pH 7, and 0.1 mg/ml CPRG (Roche Diagnostics, Indianapolis, IN). The 1-ml cultures were incubated at 30°C in 24-well plates for and monitored after 24 and 48 h, and the amount of CPRG cleaved was determined spectrophometrically at 570 nm. Before addition, the compounds (all from Sigma-Aldrich, St. Louis, MO) were dissolved in 0.1 M sodium phosphate buffer and added in appropriate concentrations. In case of sensitivity of the strain to the tested compound, lower concentrations were used.
Colony-forming Ability and Growth Curve
Sensitivity to the tested drugs was assessed by first allowing the cells to grow to saturation. Cells were then washed and diluted. Identical volumes (10 μl) from serial 1:10 dilutions were spotted onto SC plates with no drug or various concentrations of the drug-containing plates. The colony-forming ability was inspected after 1 and 2 d. Growth curves in the presence of drug were recorded by allowing the cells to grow to saturation, followed by dilution to OD600 = 0.2 and growing for additional 2 h to allow cells to adapt to the medium. The drug was then added to the cultures and the optical density was measured every 2 h.
Western Blot Analysis
Cells were grown to a density of OD600 = 0.1 in appropriate media and then l-dopa was added at 200 μM final concentration. Samples were removed at the indicated times (0, 5, 15, and 30 min) after l-dopa addition, and protein extract was prepared.
Yeast cells were harvested by centrifugation and the cell pellets were washed once with 1 ml of ice-cold buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitors cocktail; Roche Diagnostics). The pellet was suspended in the same buffer, and the cells were broken by vortexing with glass beads at 4°C for 10 min. Glass beads and cell debris were removed by centrifugation, and the supernatant was transferred to separate tubes. Equal amounts of protein (20 μg) were loaded on 10% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride membrane. The anti-phospho-p44/p42 antibody and anti-phospho-p38 antibody, both from New England Biolabs (Beverly, MA) were used at a final dilution 1:2000 in Tris-buffered saline/Tween 20 (TBST) buffer in the presence of 5% dry milk. Anti-Mpk1 and anti-Hog1 antibodies both from Santa Cruz Biotechnology (Santa Cruz, CA) were used at a final dilution 1:1000. Horseradish peroxidase-linked anti-rabbit secondary antibody (Amersham Biosciences, Piscataway, NJ) and anti-goat antibody (Sigma-Aldrich) were used at a dilution 1:1000 in TBST in the presence of 5% nonfat dry milk.
Supplementary Material
The chemical library used in this study is shown as supplementary material.
RESULTS
l-Dopa Activates Mating-responsive FUS1 and Cell Integrity-responsive RLM1 Transcription
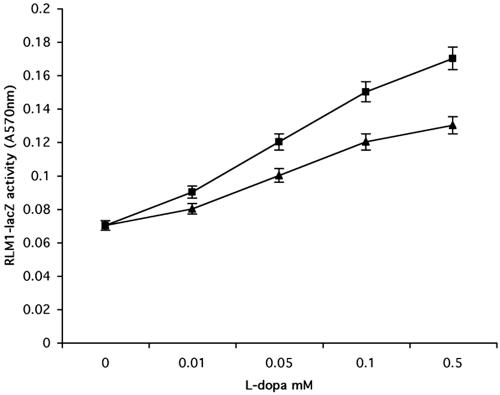
In our study, we used a wild-type yeast strain transformed with a plasmid encoding FUS1 fused to lacZ or a p2 × RLM1 plasmid having two upstream Rlm1 binding sites fused to lacZ. Both plasmids have been used extensively for monitoring the activation of the pheromone response and cell integrity pathways (Jung and Levin, 1999; Lee and Elion, 1999; Cullen et al., 2000). We used a library composed of 100 compounds with different modes of action, including peptides, nucleotides and nucleotide-derivatives, and drug-like small molecules. Of all the compounds tested, only treatment of the yeast cells with catecholamines (adrenaline, noradrenaline, dopamine, and l-dopa) resulted in a dose-dependent activation of FUS1 or RLM1 reporters (Figure 2, A and B). We also tested compounds chemically related to l-dopa, including l-tyrosine, l-tyrosinol, l-phenylalanine, and tyramine. None of these compounds caused any change in the expression of FUS1 compared with the control untreated cells (our unpublished data).
Figure 2.
(A) Catecholamines activate FUS1 reporter in a dose-dependent manner. The wild-type yeast strain (EY957) was plated in 24-well plates on medium containing the β-galactosidase substrate CPRG as described in Materials and Methods. Catecholamines (adrenaline [×], noradrenaline [▴], l-dopa [▪], and dopamine [*]) were added to the media in increasing concentrations (0.01–1 mM) and β-galactosidase activity was measured at 570 nm after 48 h. Results shown are from five independent assays. (B) l-Dopa activates RLM1 reporter in a dose-dependent manner. Wild-type strain (EY957) transformed with px2RLM1 was plated in 24-well plates on media containing the β-galactosidase substrate CPRG as described in Materials and Methods. Catecholamines (adrenaline [×], noradrenaline [▴], l-dopa [▪], and dopamine ([988]) were added to the media in increasing concentrations (0.01–1 mM) and β-galactosidase activity was measured at 570 nm after 48 h. Results shown are from five independent assays.
The Stimulatory Effect of l-Dopa on FUS1 and RLM1 Transcription Is Due to Oxidative Stress
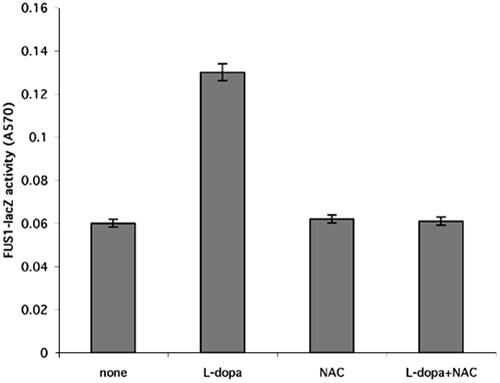
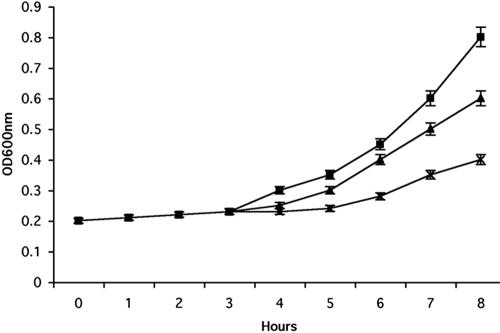
The catecholamines have been shown to be toxic because of their ability to oxidize and produce reactive oxygen species and quinones in contrast to the other tested structural analogues of l-dopa that do not undergo autooxidation (Basma et al., 1995; Han et al., 1996; Mena et al., 1997). We hypothesized that l-dopa and the other catecholamines activated the yeast mating MAPK via autooxidation by forming reactive oxygen species. To test this hypothesis, we simultaneously treated the cells with l-dopa and N-acetyl-cysteine, an antioxidant. The addition of N-acetyl-cysteine abolished the observed l-dopa-induced increase in FUS1-lacZ activity (Figure 3). We observed similar results with the other tested catecholamines. We further test the toxicity of the catecholamines on the yeast cells. We found that concentrations >2 mM are toxic for the cells (our unpublished data). Thus, the induction of the reporters by l-dopa and catecholamines seemed to be caused by their ability to autooxidize. The results in Figure 4A show that sublethal concentrations of l-dopa severely impaired cell growth in liquid culture, whereas the ability of the cells to form colonies remained unchanged (our unpublished data).
Figure 3.
N-Acetyl-cysteine reverses the l-dopa–induced activation of the reporter FUS1. The wild-type yeast strain (EY957) was plated in 24-well plates, treated with l-dopa in different concentrations and N-acetyl-cysteine. Shown are l-dopa (0.02 mM) and N-acetyl-cysteine (2 mM) alone or in combination, and β-galactosidase activity was measured as described in Materials and Methods. Results shown are from five independent assays.
Figure 4.
Growth of wild-type strain (EY957) in the presence of l-dopa. Cells were grown to the early exponential phase, diluted to OD = 0.2 into fresh YEPD medium, and grown for an additional 2 h to allow the cells to adapt to the medium. The culture was split and different concentrations of l-dopa were added (wild-type [▪], 1 mM l-dopa [▴], and 2 mM l-dopa [×]). The optical density of the cultures was monitored by spectrophotometry. Results shown are from five independent assays.
Hydrogen Peroxide Activates RLM1 Transcription
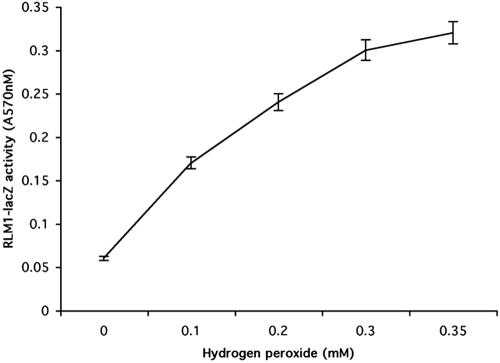
We found that oxidative stress activates both FUS1and RLM1 reporters. To assess whether other oxidants would have the same effect, we tested several widely used oxidants with different modes of action including diamide (a thiol oxidant), menadione (a superoxide-generating agent), hydrogen peroxide, and copper (a redox-active metal). Of all the oxidants tested, only hydrogen peroxide induced RLM1 transcription (Figure 5). We did not observe an induction of FUS1-lacZ activity in the wild-type strain.
Figure 5.
Hydrogen peroxide activates RLM1 reporter in a dose-dependent manner. Wild-type strain (EY957) transformed with px2RLM1 was plated in 24-well plates on media containing the β-galactosidase substrate CPRG as described in Materials and Methods. Hydrogen peroxide was added to the media in increasing concentrations (0.1–1 mM), and β-galactosidase activity was measured at 570 nm after 24 h. Results shown are from five independent assays.
HOG1, KSS1 and MPK1 Mediate Resistance to Oxidants
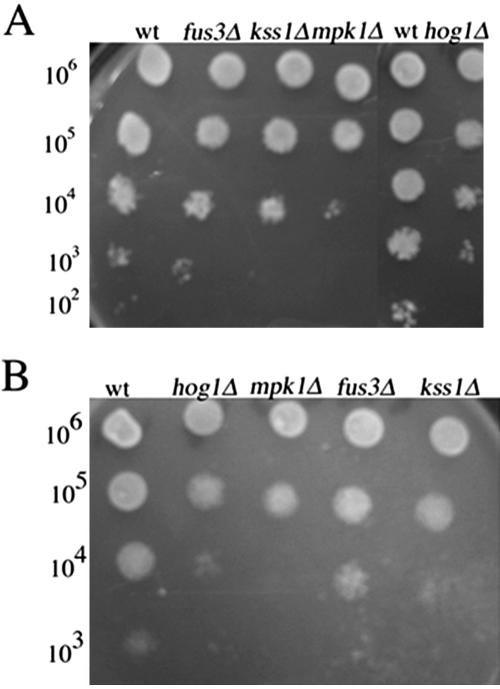
To further examine how the signal generated by hydrogen peroxide or l-dopa is transmitted to the reporters, we tested the sensitivity to hydrogen peroxide of strains disrupted into the four major MAPKs in yeast: HOG1, KSS1, FUS3, and MPK1. The sensitivity of the deletion strain may result from the involvement of MAPK in signaling in the presence of oxidant. To analyze the sensitivity of the strains, we examined the ability of each deletion strain to grow in the presence of hydrogen peroxide (0.1–1 mM) or l-dopa (0.1–1 mM). We found that hog1Δ, mpk1Δ and kss1Δ strains were sensitive to hydrogen peroxide and l-dopa compared with the wild-type strain (Figure 6, A and B), whereas the sensitivity of the fus3Δ strain was similar to that of the wild-type strain. These results demonstrate that Hog1, Kss1, and Mpk1 mediate resistance to oxidative stress.
Figure 6.
(A and B) Deletion in HOG1, KSS1, or MPK1 lead to sensitivity to l-dopa and hydrogen peroxide. Identical volumes (10 μl) of 10-fold serial dilutions of exponentially growing wild-type strain (EY957) and the isogenic kss1Δ, fus3Δ, hog1Δ strains were spotted onto YEPD plates containing various concentrations of l-dopa and hydrogen peroxide and then incubated for 48 h at 30°C. Shown are representative examples of the plates incubated with 1 mM l-dopa (A) and 1 mM hydrogen peroxide (B).
Kss1 and Mpk1 Are Phosphorylated upon Treatment with l-Dopa
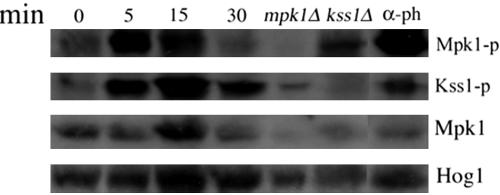
To further examine the direct involvement of the four MAPKs in l-dopa signaling, we used Western blot analysis. We used an antibody against anti-phospho-42/44 that has been used successfully to identify doubly phosphorylated forms of the mating MAPKs (Kss1 and Fus3) as well as Mpk1 in the cell integrity pathway (Verna et al., 1997; Bardwell et al., 1998; Martin et al., 2000; Sabbagh et al., 2001). Treatment with l-dopa caused a rapid (within 5 min) and transient (up to 15 min) increase in the phosphorylated form of Mpk1 (Figure 7). Stimulation with α-pheromone, known to induce the cell integrity and mating pathways (Zarzov et al., 1996; Madhani and Fink, 1998; Farley et al., 1999), served as a positive control. As expected, phosphorylated Mpk1 was not detected in cells lacking MPK1. Reprobing with anti-Mpk1 revealed that the increase in the phosphorylated form of Mpk1 is not due to an increase in the abundance of the protein. We also observed a transient increase (within 5 min) in the phosphorylated form of Kss1 (Figure 7). The effect was more prolonged (up to 30 min) than Mpk1 induction. In keeping with the results detailed above, no increase in the active form of Fus3 MAPK was seen.
Figure 7.
l-Dopa stimulates Mpk1and Kss1 MAPK. Mid-log cultures of wild-type strain cells (EY957) were treated with l-dopa at 2 mM final concentration and at 50 nM α-pheromone used as positive control. Samples were taken at the indicated times (0, 15, and 30 min). The α-pheromone was added for 60 min. The isogenic mpk1Δ and kss1Δ, used as controls, were treated with l-dopa for 15 min. Western blot analysis was performed as described in Materials and Methods by using anti-phospho-p44/p42 antibody to detect the phosphorylated Mpk1, Kss1 and Fus3, anti-Mpk1 to detect the total amount of Mpk1 protein and anti-Hog1 as a loading control.
To further confirm our results, we also performed genetic analyses. We measured FUS1 activation by l-dopa in fus3Δkss1Δ strains. The double mutant strain has a lower basal level of FUS1 transcription compared with the single mutant kss1Δ, which allows a clearer interpretation of the results (Bardwell et al., 1998b). We did not find induction of FUS1 transcription after treatment with l-dopa compared with an l-dopa–induced increase observed in the fus3Δ strain and the wild-type strain (our unpublished data), suggesting that Kss1 is the main MAPK involved in l-dopa induction of FUS1 transcription. Surprisingly, mpk1Δ cells still showed increased RLM1 transcription, even though significantly reduced upon l-dopa treatment compared with the wild-type strain (Figure 8). This result suggests that additional components are involved in RLM1 reporter induction by l-dopa.
Figure 8.
l-Dopa activation of RLM1 reporter in mpk1Δ strain. Wild-type strain (DL100) (▪) and the isogenic strain mpk1Δ (▴) were transformed with px2RLM1 and plated in 24-well plates on media containing the β-galactosidase substrate CPRG as described in Materials and Methods. l-Dopa was added to the media in increasing concentrations (0.1–0.5 mM), and β-galactosidase activity was measured at 570 nm after 48 h. Results shown are from five independent assays.
We found that in addition to kss1Δ and mpk1Δ cells, hog1Δ cells are also sensitive to catecholamines. To determine whether Hog1 is phosphorylated upon treatment with l-dopa, we performed Western blot analysis by using phospho-p38 antibody. The antibody recognizes the TGY motif characteristic of stress-activated mitogen-activated protein kinases activated by phosphorylation of threonine and tyrosine (Cano and Mahadevan, 1995). It has been used for detecting activated Hog1 (Maeda et al., 1994). Treatment with l-dopa did not cause activation of Hog1p (our unpublished data). Therefore, Hog1 must mediate resistance to l-dopa via an alternative mechanism. We also checked whether the disruption of HOG1 would alter the induction of FUS1 or RLM1 transcription by l-dopa. We could not detect any change in FUS1-lacZ or RLM1 transcription activation in the wild-type strain and in the hog1 mutant (our unpublished data).
Pheromone Response Pathway and Cell Integrity Pathway Act in Parallel
We further examined how the two pathways cross-regulate one another by measuring FUS1 activation in an mpk1Δ strain and RLM1 reporter induction in a kss1Δfus3Δ strain upon treatment with l-dopa. We observed a higher basal level of RLM1 reporter in kss1Δfus3Δ and a higher basal level of FUS1-lacZ in mpk1, respectively, suggesting that the two pathways cross-regulate each other. Treatment of the cells with l-dopa resulted in a decrease of RLM1 transcription in kss1Δfus3Δ strain and a decrease in FUS1-lacZ transcription in mpk1Δ cells (Figure 9). However, additional immunoblotting analysis revealed that Mpk1 and Kss1 are still phosphorylated upon l-dopa treatment in kss1Δ and mpk1Δ strains, respectively, suggesting that these pathways may act in parallel (our unpublished data).
Figure 9.
(A) RLM1 transcription in treated with l-dopa kss1Δfus3Δ strain. Wild-type strain (EY957) (▪) and the isogenic kss1Δfus3Δ (♦) strain were transformed with px2RLM1 and then plated in 24-well plates on media containing the β-galactosidase substrate CPRG as described in Materials and Methods. l-Dopa was added to the media in increasing concentrations (0.01–0.5 mM), and β-galactosidase activity was measured at 570 nm after 24 h. Results shown are from five independent assays. (B) FUS1 transcription in mpk1Δ strain. Wild-type strain (DL100) (▪) and the isogenic mpk1Δ (▴) strain were transformed with px2RLM1 and then plated in 24-well plates on media containing the β-galactosidase substrate CPRG as described in Materials and Methods. l-Dopa was added to the media in increasing concentrations (0.01–0.5 mM), and β-galactosidase activity was measured at 570 nm after 24 h. Results shown are from five independent assays
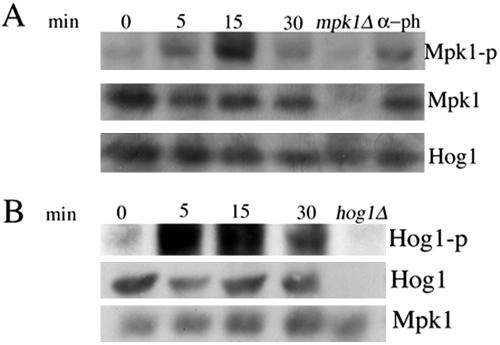
Hog1 and Mpk Are Phosphorylated upon Treatment of Cells with Hydrogen Peroxide
We also analyzed the involvement of the signaling MAPK in hydrogen peroxide activation of RLM1 reporter. Western blot with anti-phosphop-44/p42 showed rapid and transient (within 5 min) phosphorylation after hydrogen peroxide treatment (Figure 10A). An increase in phosphorylated forms of Kss1 or Fus3 was not detected (our unpublished data). Additional genetic analysis showed an increase in RLM1 transcription in mpk1Δ strain, even though significantly reduced compared with the wild-type strain (Figure 11A), suggesting that similar to the data obtained with l-dopa, additional components are involved in RLM1 transcription induction by oxidants. Because hog1Δ was found to be sensitive to hydrogen peroxide, we also examined for activation of Hog1 by using anti-phospho-p38 antibody. As shown in Figure 10B, Hog1 is phosphorylated upon treatment with hydrogen peroxide. Hog1 was the only band that did not occur in the hog1Δ mutant under stress conditions. The increase in the phosphorylated form of Hog1 is not due to an increase in the endogenous protein as shown by reprobing with anti-Hog1 antibody. Phosphorylation was detected in 5 min after induction with 10 mM hydrogen peroxide and remained high after 30 min of treatment. We observed similar kinetics of Hog1 phosphorylation in response to 1.2 mM NaCl, which has been shown to activate the HOG pathway (Brewster et al., 1993; our unpublished data). Additional genetic analysis showed that induction of RLM1 reporter by hydrogen peroxide was significantly reduced in hog1 mutant cells (Figure 11B), suggesting the involvement of Hog1 in the activation of RLM1 transcription by the oxidant. Interestingly, the disruption of HOG1 did not abolish the phosphorylation of Mpk1, suggesting that Hog1 influences the activation of RLM1 reporter by an as yet unknown mechanism (our unpublished data).
Figure 10.
(A) Mpk1 is phosphorylated on exposure of cells to hydrogen peroxide. Mid-log cultures of wild-type strain cells (EY957) were treated with hydrogen peroxide at 5 mM final concentration with 50 nM α-pheromone as a positive control. Samples were taken at the indicated times (0, 15, and 30 min). Wild-type cells were treated with α-pheromone for 60 min. Hydrogen peroxide was added to mpk1Δ strain for 15 min. Western blot analysis was performed as described in Materials and Methods by using anti-phospho-p44/p42 antibody to detect the phosphorylated Mpk1, Kss1 and Fus3, anti-Mpk1 to detect the total protein and anti-Hog1 as a loading control. (B) Hydrogen peroxide activates Hog1 MAPK. Mid-log cultures of wild-type strain cells (EY957) were treated with hydrogen peroxide at 5 mM final concentration. Samples were taken at the indicated times (0, 15, and 30 min). Strain disrupted in HOG1 gene, used as negative control, was treated with hydrogen peroxide for 15 min. Western blot analysis was performed as described in Materials and Methods by using anti-phospho-p38 antibody to detect the phosphorylated Hog1, with anti-Hog1 to detect the total protein and anti-Mpk1 as a loading control.
Figure 11.
(A) Hydrogen peroxide activation of RLM1 transcription in mpk1Δ strain. Wild-type strain (Dl100) (▪) and the isogenic mpk1Δ (▴) strain were transformed with px2RLM1 and then plated in 24-well plates on media containing the β-galactosidase substrate CPRG as described in Materials and Methods. Hydrogen peroxide was added to the media in increasing concentrations (0.1–0.5 mM), and β-galactosidase activity was measured at 570 nm after 24 h. Results shown are from five independent assays. (B) RLM1 transcription induction in hog1Δ strain. Wild-type strain (EY957) (▪) and the isogenic hog1Δ strain (•) were transformed with px2RLM1 and then plated in 24-well plates on media containing the β-galactosidase substrate CPRG as described in Materials and Methods. Hydrogen peroxide was added to the media in increasing concentrations (0.1–0.5 mM), and β-galactosidase activity was measured at 570 nm after 24 h. Results shown are from five independent assays.
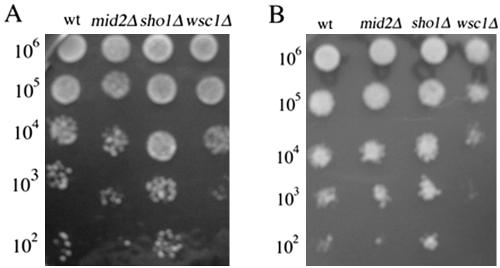
Wsc1 Mutant Cells Are Sensitive to Hydrogen Peroxide and l-Dopa
To further analyze how the signal generated by l-dopa and hydrogen peroxide is transmitted to the RLM1 reporter, we tested the sensitivity of strains disrupted in Wsc1, Mid2, plasma membrane sensor proteins of the cell integrity pathway to l-dopa, and hydrogen peroxide (Ketela et al., 1999; Philip and Levin, 2001). We also tested the sensitivity of the sho1Δ strain, another membrane sensor protein, which has been implicated in HOG pathway and “kss1 pathway” (Posas et al., 1996; Cullen et al., 2000). As shown in Figure 12, A and B, we found that the wsc1Δ strain is the most sensitive to both compounds compared with mid2Δ, sho1Δ, and the wild-type strains. These results suggest that Wsc1 play a role in oxidative stress response. The data are in agreement with the published results by Zu et al., 2001. However, the wsc1Δ strain still showed the same dose-dependent increase in RLM1 transcription after treatment with both oxidants (our unpublished data). The most plausible explanation is that Wsc1 mediates resistance to oxidants via a parallel mechanism.
Figure 12.
(A and B) Cells lacking WSC1 are the most sensitive to l-dopa and hydrogen peroxide. Identical volumes (10 μl) of 10-fold serial dilutions of exponentially growing wild-type strain (DL100) and the isogenic wsc1Δ, sho1Δ and mid2Δ were spotted onto YEPD plates containing various concentrations of l-dopa and hydrogen peroxide and then incubated for 48 h at 30°C. Shown are the representative examples of the plates incubated with 1 mM l-dopa (A) and 1 mM hydrogen peroxide (B).
DISCUSSION
How the yeast MAPK signaling cascades cross-regulate each other has been difficult to study because of the lack of visible cellular phenotypes under normal conditions. One of the genetic approaches used previously is based on selection for mutants having altered expression of a FUS1 gene. Recently published data using this method revealed a new signaling pathway, “kss1 pathway,” which is activated in mutants compromised for protein glycosylation (Cullen et al., 2000). By using a “chemical genetic” approach, we found that l-dopa, as well as the related catecholamines adrenaline, noradrenaline, and dopamine, stimulate FUS1 and an RLM1 reporters constructs. The effects of l-dopa on pheromone response gene and cell integrity pathways seemed to be due to autooxidation. Testing other oxidants with different modes of action showed that hydrogen peroxide also induces the RLM1 reporter, but not FUS1 transcription.
The activation of the cell integrity pathway was also apparent from the phosphorylation status of the MAPK kinase Mpk1. Although evidences exist that hydrogen peroxide activates p44/p42 MAPK in mammalian cells, our results show for the first time activation of p44/p42 MAPK by hydrogen peroxide in the yeast S. cerevisiae (Hannken et al., 2000; Nguyen et al., 2004; van Rossum et al., 2004). Interestingly Mkp1, a Pneumocystis carinii homologue of Mpk1, has been found to be activated by the same oxidant (Fox and Smulian, 1999).
We did not observe an induction of RLM1 transcription by diamide, menadione, or copper. It is possible that the mechanism for activation of the cell integrity pathway by oxidative stress depends on the nature of the oxidant. It has been shown that strains having mutations in electron transport chain functions are very sensitive to hydrogen peroxide (Thorpe et al., 2004). On the other hand, the oxidation products of catecholamine oxidation target mitochondria (Berman and Hastings, 1999). In this respect, l-dopa and hydrogen peroxide share a common mechanism. Genetic analysis performed in an mpk1Δ strain surprisingly revealed that cells lacking Mpk1 are still able to activate RLM1 reporter, although the induction was attenuated. Two-hybrid analysis has shown that RLM1 interacts with two MAPKs: Mpk1 and its homologue Mlp1 (Watanabe et al., 1997). Interestingly, cells lacking mitochondrial glutaredoxin exhibit a high induction of Mlp1 as revealed by transcriptome analysis (Belli et al., 2004). It is possible that Mlp1 might play a significant role in RLM1 reporter induction by oxidants. The observed rapid phosphorylation of Mpk1 also suggests a very rapid response. Oxidant-induced alteration in the cellular redox may be the trigger that activates the cell integrity pathway. Hydrogen peroxide has been found to rapidly activate the Yap transcription factor by oxidation of the Cys residues involved in the formation of critical disulfide bonds (Delaunay et al., 2000). Interestingly, this method of oxidant sensing is restricted to distinct oxidants because diamide was not found to exert the same effect. Pkc1 has Cys residues forming the Zn finger in the regulatory (C1) domain (Levin et al., 1990) that may render it a suitable target for redox-oxidation regulation.
Western blot analysis also revealed that the addition of l-dopa leads to an increase in the activated doubly phosphorylated form of Kss1, an MAPK of the mating and invasive growth pathways. However, cells treated with l-dopa did not exhibit physiological changes associated with invasive growth such as elongated cell morphology (our unpublished data). We suggest a role for Kss1 independent of mating or invasive growth in the stimulation of the pheromone responsive gene FUS1 by l-dopa. Kss1 has been implicated in cell integrity protection, resulting in FUS1-lacZ induction in mutants having impaired mannosylation of glycoproteins (Cullen et al., 2000). The putative sensor for this pathway has been found to be Sho1. We did not observe sensitivity of a sho1Δ strain to l-dopa compared with the wild-type strain. However, we do not exclude the possibility that oxidative stress-induced disturbances in glycosylation may trigger the activation of FUS1 transcription. In this respect, the signal could be sensed by another protein.
Our results also suggest cross talk between the two pathways activated by l-dopa, even though they might not act identically. The decrease in RLM1 transcription in kss1Δfus3Δ could suggest that Kss1 might be an upstream regulator of Mpk1 induction, an assumption supported by the observed increase in FUS1 transcription in the mpk1Δ strain. The latter could be result of a feedback mechanism. Additionally, treatment with l-dopa did not affect the survival of bck1Δ cells (our unpublished data). These results support the contention that the two pathways are not identical. Bck1 likely influences FUS1 transcription by other means.
We also found that Hog1 is phosphorylated upon treatment with hydrogen peroxide. This finding is consistent with the results published by Haghnazari and Heyer (2004), whereas another study (Alonco-Monge et al., 2003) failed to observe Hog1 phosphorylation after 10-min treatment with hydrogen peroxide (Singh, 2000). The significant reduction of RLM1 transcription in hog1Δ mutants implicates the involvement of Hog1 in RLM1 reporter activation by hydrogen peroxide. It has been suggested that Hog1 can regulate Rlm1 activity by unknown mechanisms under hyperosmotic stress conditions (Hahn and Thiele, 2002). On the other hand, we did not observe a difference in the phosphorylation of Mpk1 in hog1 mutant cells, suggesting that Hog1 regulation of Rlm1 and Mpk1 activation by hydrogen peroxide have different upstream regulators.
Our results suggest that different signaling mechanisms are induced in an oxidant-specific manner. Hog1 phosphorylation may be involved in protecting the cells from strong oxidants such as hydrogen peroxide, whereas prooxidants such as l-dopa may act via an alternative mechanism or that the different nature of the autooxidation products results in different stimuli
The effect of catecholamines on survival has been studied so far only in mammalian cells (Han et al., 1996; Mena et al., 1997; Varella et al., 1999) However, it has been shown that apomorphine, a dopamine agonist, and its oxidation product 8-oxo-seimiquinone are toxic for the cells, but sublethal concentrations enhance survival when cells are pretreated with other oxidants (Picada et al., 2003). It also has been suggested that l-dopa as well as dopamine stimulate the MAPK activity of the classical extracellular signal-regulated kinase (ERK) pathway in neuronally derived cultured PC12 cells (Yan et al., 1999; Koshimura et al., 2000). The yeast pheromone pathway has been suggested to be an orthologue of the classical mammalian ERK pathway (Caffrey et al., 1999). In this respect, our study also may shed light on the mechanisms underlying the neuron-protective effects of l-dopa in mammalian cells.
Supplementary Material
Acknowledgments
We thank Elaine Elion, David Levin, Ira Herskowitz's laboratory, and Lee Bardwell for generously providing yeast strains and plasmids. This work was supported by Public Health Service grant EY10223 to S.J.O.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–02–0142. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–02–0142.
Abbreviations used: ERK, extracellular signal-regulated kinase; HOG, high osmolarity glycerol; l-dopa, l-3,4-hydroxyphenylalanine; MAPK, mitogen-activated protein kinase.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Alonco-Monge, R., Navarro-Garcia, F., Roman, E., Negredo, A.I., Eisman, B., Nombela, C., and Pla, J. (2003). The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eucaryot. Cell 2, 351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell, L., Cook, J.G., Zhu-Shimoni, J.X., Voora, D., and Thorner, J. (1998a). Differential regulation of transcription: repression by unactivated mitogen-activated protein kinase Kss1 requires the Dig1 and Dig2 proteins. Proc. Natl. Acad. Sci. USA 95, 15400-15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell, L., Cook, J.G., Voora, D., Baggott, D.M., Martinez, A.R., and Thorner, J. (1998b). Repression of yeast Ste12 transcription factor by direct binding of unphosphorylated Kss1 MAPK and its regulation by STE7 MEK. Genes Dev. 12, 2887-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basma, A.N., Nicklas, W.J., and Geller, H.M.,. (1995). L-dopa cytotoxicity to PC12 cells in culture is via its autooxidation. J. Neurochem. 64, 825-832. [DOI] [PubMed] [Google Scholar]
- Buehrer, B., and Errede, B. (1997). Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol. Cell. Biol. 17, 6517-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli, G., Molina, M.M., Garcia-Martinez, J., Perez-Ortin, J.E., and Herrero, E. (2004). Saccharomyces cerevisiae glutaredoxin 5-deficient cells subjected to continuous conditions are affected in the expression of specific sets of genes. J. Biol. Chem. 12386-12395. [DOI] [PubMed]
- Berman, S.B. and Hastings, T.G. (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J. Neurochem. 3, 1127-1137. [DOI] [PubMed] [Google Scholar]
- Brewster, J.L., de Valoir, T., Dwyer, N.D., Winter, E., and Gustin, M.C. (1993). An osmosensing signal transduction pathway in yeast. Science 259, 1760-1763. [DOI] [PubMed] [Google Scholar]
- Cano, E., and Mahadevan, L.C.,. (1995). Parallel signal processing among mammalian MAPKs. Trends Biochem. Sci. 20, 117-122. [DOI] [PubMed] [Google Scholar]
- Caffrey, D.R., O'Neill, L.A.J., and Schields, D.C. (1999). The evolution of the MAP kinase pathways: coduplication of interacting proteins leads to new signaling cascades. J. Mol. Evol. 49, 567-582. [DOI] [PubMed] [Google Scholar]
- Cullen, P.J., Schultz, J., Horecka, J., Stevenson, B.J., Jigami, Y., and Sprague, G.F. (2000). Defects in protein glycosylation cause SHO1-dependent activation of a STE12 signaling pathway in yeast. Genetics 155, 1005-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay, A., Isnard, A.D., and Toledano, M.B. (2000). H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 19, 5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion, E.A., Satterberg, B., and Kranz, J.E. (1993). FUS3 phosphorylates multiple components of the mating signal transduction cascade: evidence for STE12 and FAR1. Mol. Biol. Cell 4, 495-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion, E.A. (1998). Routing MAP kinase cascades. Science 281, 1625-1626. [DOI] [PubMed] [Google Scholar]
- Farley, F.W., Satterberg, G. Goldsmith, E.A., and Elion, E.A. (1999). Relative dependence of different outputs of the S. cerevisiae pheromone response pathway on the MAP kinase Fus3p. Genetics 151, 1425-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, D., and Smulian, A.G. (1999). Mitogen-activated protein kinase Mkp1 of Pneumocystis carinii complements the slt2Delta defect in the cell integrity pathway of Saccharomyces cerevisiae. Mol. Microbiol. 34, 451-462. [DOI] [PubMed] [Google Scholar]
- Gustin, M.C., Albertyn, J., Alexander, M.R., and Davenport, K. (1998). MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62, 1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, C.K., Mytilineou, C., and Cohen, G. (1996). L-DOPA up-regulates glutathione and protects mesencephalic cultures against oxidative stress. J. Neurochem. 66, 501-510. [DOI] [PubMed] [Google Scholar]
- Hahn, J., and Thiele, D.J. (2002). Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. 24, 21278-21284. [DOI] [PubMed] [Google Scholar]
- Hall, J.P., Cherkasova, V., Elion, E.A., Gustin, M.C., and Winter, E. (1996). The osmoregulatory pathway represses mating pathway activity in Saccharomyces cerevisiae: isolation of a FUS3 mutant that is insensitive to the repression mechanism. Mol. Cell. Biol. 16, 6715-6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannken, T., Schroeder, R., Zahner, G., Stahl, R.A., and Wolf, G. (2000). Reactive oxygen species stimulate p44/42 mitogen-activated protein kinase and induce p27(Kip1): role in angiotensin II-mediated hypertrophy of proximal tubular cells. J. Am. Soc. Nephrol. 11, 1387-1397. [DOI] [PubMed] [Google Scholar]
- Haghnazari, E., and Heyer W.D. (2004). The Hog1 MAP kinase pathway and the Mec1 DNA damage checkpoint pathway independently control the cellular responses to hydrogen peroxide. DNA Repair 3, 769-776. [DOI] [PubMed] [Google Scholar]
- Heinisch, J.J., Lorberg, A., Schmitz, H.P., and Jacoby, J.J. (1999). The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32, 671-680. [DOI] [PubMed] [Google Scholar]
- Jung, U.S., and Levin, D.E. (1999). Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34, 1048, 1057 [DOI] [PubMed] [Google Scholar]
- Ketela, T., Green, R., and Bussey, H. (1999). Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181, 3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimura, K., Tanaka, J., Murakami, Y., and Kato, Y. (2000). Effects of dopamine and L-DOPA on survival of PC12 cells. J. Neurosci. Res. 62, 112-119. [DOI] [PubMed] [Google Scholar]
- Lee, B.N., and Elion, E.A. (1999). The MAPKKK Ste11 regulates vegetative growth through a kinase cascade of shared signaling components. Proc. Natl. Acad. Sci. USA 96, 12679-12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.S., Irie, K., Gotoh, Y., Watanabe, Y., Araki, H., Nishida, E., Matsumoto, K., and Levin, D.E. (1993). A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol. Cell. Biol. 13, 3067-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, D., Fields, F.O., Kunisawa, R., Bishop, J.M., and Thorner, J. (1990). A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 62, 213-224. [DOI] [PubMed] [Google Scholar]
- Maeda, T., Wurgler-Murphy, S.M., and Saito, S.M.,. (1994). A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369, 242-245. [DOI] [PubMed] [Google Scholar]
- Martin, H., Rodriguez-Pachon, J.M., Ruiz, C., Nombela, C., and Molina, M.,. (2000). Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275, 1511-1519. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D., and Fink, G.R. (1998). The riddle of MAP kinase signaling specificity. Trends Genet. 14, 151-155. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D., Styles, C.A., and Fink, G.R. (1997). MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 91, 673-684. [DOI] [PubMed] [Google Scholar]
- Mena, M.A., Davila, V., and Sulzer, D. (1997). Neurotrophic effects of L-DOPA in postnatal midbrain dopamine neuron/cortical astrocyte cocultures. J. Neurochem. 69, 1398-1408. [DOI] [PubMed] [Google Scholar]
- Nguyen, A., Chen, P., and Cai, H. (2004). Role of CaMKII in hydrogen peroxide activation of ERK1/2, p38 MAPK, HSP27 and actin reorganization in endothelial cells. FEBS Lett. 572, 307-313. [DOI] [PubMed] [Google Scholar]
- Olesnicky, N.S., Brown, A.J., Dowell, S.J., and Casselton, L.A. (1999). A constitutively active G-protein-coupled receptor causes mating self-compatibility in the mushroom Coprinus. EMBO J. 18, 275, 2756-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picada, J.N., Maris, A.F., Ckless, K., Salvador, M., Khromov-Borisov, N.N., and Henriques, J.A. (2003). Differential mutagenic, antimutagenic and cytotoxic responses induced by apomorphine and its oxidation product, 8-oxo-apomorphine-semiquinone, in bacteria and yeast. EMBO J. 19, 5157-5166. [DOI] [PubMed] [Google Scholar]
- Peter, M., Gartner, A., Horecka, J., Ammerer, G., and Herskowitz, I. (1993). FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell 73, 747-760. [DOI] [PubMed] [Google Scholar]
- Philips, J., and Herskowitz, I. (1998). Osmotic balance regulates cell fusion during mating in Saccharomyces cerevisiae. J. Cell Biol. 143, 375-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, B., and Levin, D.E. (2001). Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21, 271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas, F., Wurgler-Murphy, S.M., Maeda, T., Witten, E.A., Thai, T.C., and Saito, H. (1996). Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 86, 865-875. [DOI] [PubMed] [Google Scholar]
- O'Rourke, S.M., and Herskowitz, I. (1998). The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 12, 2874-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R.L., and Fink, G.R. (1994). Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8, 2974-2985. [DOI] [PubMed] [Google Scholar]
- Roberts, C.J., et al. (2000). Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287, 873-880. [DOI] [PubMed] [Google Scholar]
- van Rossum, G.S., Drummen, G.P., Verkleij, A.J., Post, J.A., and Boonstra, J. (2004). Activation of cytosolic phospholipase A2 in Her14 fibroblasts by hydrogen peroxide: a p42/44(MAPK)-dependent and phosphorylation-independent mechanism. Biochim. Biophys. Acta 1636, 183-195. [DOI] [PubMed] [Google Scholar]
- Sabbagh, W., Jr. Flatauer, L.J., Bardwell, A.J., and Bardwell, L. (2001). Mol. Cell 8, 683-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A., and Hall, M.N. (1998). Signaling to the actin cytoskeleton. Annu. Rev. Cell. Dev. Biol. 14, 305-338. [DOI] [PubMed] [Google Scholar]
- Sherman, F., Fink, G.R., and Hicks, J.B. (1979). Methods in Yeast Genetics, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Singh, K.,. (2000). The sln1p-sskp1 two-component system mediates response to oxidative stress and in an oxidant-specific fashion. Free Radic. Biol. Med. 10, 1043-1050. [DOI] [PubMed] [Google Scholar]
- Siliciano, P.G., and Tatchell, K. (1984). Transcription and regulatory signals at the mating type locus in yeast. Cell. 37, 969-978. [DOI] [PubMed] [Google Scholar]
- Song, D., Dolan, J.W., Yuan, Y.L., and Fields, S. (1991). Pheromone-dependent phosphorylation of the yeast STE12 protein correlates with transcriptional activation. Genes Dev. 5, 741-750. [DOI] [PubMed] [Google Scholar]
- Thorpe, G.W., Fong, C.S., Alic, N., Higgins, V.J., and Dawes, I.W. (2004). Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 101, 6564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers, M., and Futcher, B. (1993). Far1 and Fus3 link the mating pheromone signal transduction pathway to three G1-phase Cdc28 kinase complexes. Mol. Cell. Biol. 13, 5659-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varella, M.H., de Mello, F.G., and Linden, R. (1999). Evidence for an anti-apoptotic role of dopamine in developing retinal tissue. J. Neurochem. 73, 485-492. [DOI] [PubMed] [Google Scholar]
- Verna, J., Lodder, A., Lee, K., Vagts, A., and Ballester, R. (1997). A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94, 13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, Y., Takaesu, G., Hagiwara, M., Irie, K., and Matsumoto, K. (1997). Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17, 2615-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Z., Feng, J., Fienberg, A.A., and Greengard, P. (1999). D(2) dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc. Natl. Acad. Sci. USA 96, 11607-11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzov, C., Jung, U.S., Garrett-Engele, P., Roe, T., Cyert, M.S., and Levin, D. (1996). The SLT2 (MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15, 83-91. [PMC free article] [PubMed] [Google Scholar]
- Zu, T., Verna, J., and Ballester, R.,. (2001). Mutations in WSC genes for putative stress receptors result in sensitivity to multiple stress conditions and impairment of Rlm1-dependent gene expression in Saccharomyces cerevisiae. Mol. Genet. Genomics 266, 142-155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.