Abstract
Male infertility has become a very serious problem in the human reproduction system, but the molecular mechanism of infertility remains largely unknown. Fertilization is the phenomenon in which a sperm and oocyte find each other, interact, and fuse. Sperm-oocyte fusion-related factors on the sperm side play crucial roles in male infertility. For example, IZUMO1 is well-known as a sperm protein essential for fusion of a sperm and oocyte, but its dysfunction or mutation can result in male infertility. Recent studies showed a novel sperm protein named Bactericidal/permeability-increasing protein (BPI), which takes part in the sperm-oocyte fusion process. The complexity and expected redundancy of the factors involved makes the process intricate, with a still poorly understood mechanism, which is difficult to comprehend in full detail. This review summarizes the known molecules involved in the process of sperm-oocyte fusion, mainly focusing on the relevant factors on the sperm side, whose dysregulation may potentially be associated with male infertility. New insights may come from these molecules in this review, can facilitate the development of new treatments of male infertility, and may have a diagnostic value in infertility.
Keywords: Bactericidal/permeability-increasing protein (BPI), IZUMO1, Male infertility, Sperm-oocyte fusion
Infertility is defined by the World Health Organization (WHO) as the inability to get pregnant over a one-year period of unprotected sexual intercourse [1]. Infertility represents a complex and multi-factorial condition affecting both males and females. Most of all, male infertility has been attracting increasing interest because of the decline in semen quality in young healthy men worldwide [1,2,3]. Many adverse factors affect sperm quality, including lifestyle, diabetes, obesity, hormonal diseases, testicular trauma, cryptorchidism, varicocele, genitourinary infections, ejaculatory disorders, chemo/radiotherapy, or surgical therapies [4,5,6]. It is well-known that genetic disorders of sperm are serious causes of male infertility, e.g., chromosomal abnormalities and gene mutations that cause a disorder of many physiological processes involved in hormonal homeostasis, spermatogenesis, and sperm quality [7, 8]. Sperm gene alterations of several sperm molecules associated with sperm-oocyte fusion on the sperm side were reported to induce infertility; these genetic problems include gene integrity, defective chromatin packaging, and a single-gene mutation. In this review, we tried to highlight those molecules participating in the sperm-oocyte fusion on the sperm side, which are mentioned in recent studies.
Sperm-oocyte Fusion is a Critical Step in Fertilization
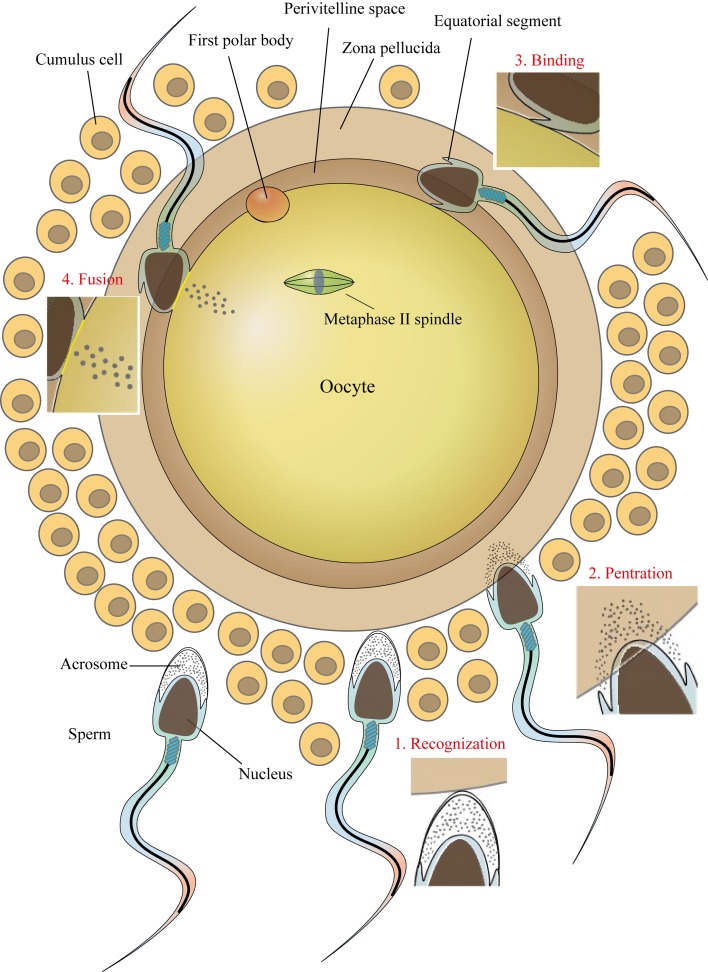
A series of well-orchestrated, highly complex sets of events must occur together in order for a spermatozoon to fertilize an oocyte. For complete fertilization, gametes (especially the sperm) should change their morphology, structure, and function. Sperm go through great transformation in the process of passage through the outside structure of the oocyte with the capacitation and the acrosome reaction changing its motility, physiology, and membrane structure (Fig. 1). It is widely believed that after sperm catches the egg, the acrosome reaction of the sperm is triggered by a component of the zona pellucida (ZP), an extracellular coat surrounding the oocyte. Nonetheless, recently, it was found that most fertilizing sperm began the acrosome reaction before reaching ZP [9]. In fact, the acrosome reaction is already observed in the female reproductive tract, especially at the ampulla [10]. After the acrosome reaction, the spermatozoa are able to penetrate the ZP and enter the perivitelline space. In the perivitelline space, the acrosomal structure of sperm disappears followed by the outer acrosomal membrane′s fusing with the overlying plasma membrane to generate the equatorial segment. The sperm-oocyte plasma membrane fusion has long been known to be initiated in the equatorial segment region [11]. Many proteins in the equatorial segment are reported to participate in the fusion process. Fertilization culminates together with sperm and oocyte finding each other, interacting, and fusing.
Fig. 1.
This diagram presents transformation of sperm morphology in terms of gamete fertility. After passing the cumulus cell layer, the acrosome-reacted sperm is capable of penetrating the zona pellucida and entering the perivitelline space. The equatorial segment of the acrosome-reacted sperm interacts with oocyte membrane and fusion begins. Finally, the sperm nucleus is released into the oocyte cytoplasm to accomplish fertilization.
Sperm-oocyte fusion is a critical step in fertilization, which requires a series of proteins from both spermatozoa and oocyte to mediate membrane adhesion and subsequent fusion. Although the mechanisms and molecules in sperm-oocyte fusion on the sperm side are closely related to male infertility, little is still known about the mechanisms or the molecules involved [12]. In recent years, efforts have been made toward the identification of the molecular players and their function, and several molecules on the egg or the sperm side have been found to be essential or nearly essential [13]. Herein, this review mainly focuses on the factors implicated strongly in the sperm-egg fusion process on the sperm side to explain the potential mechanisms of male infertility.
IZUMO1 is Essential for Sperm-oocyte Fusion
The sperm protein IZUMO1 was first discovered through screening of anti-sperm monoclonal antibodies that disrupt the fusion process [14]. Using two-dimensional gel electrophoresis followed by liquid chromatography with tandem mass spectrometry, the antigen was named IZUMO [14]. Mouse IZUMO1 is an approximately 56-kDa protein that appears to be testis specific. Izumo1−/− mice are healthy but males are infertile although they have normal mating behavior and ejaculation, and produce a normal vaginal plug [15]. In vitro fertilization (IVF) assays revealed that after penetration of the ZP, Izumo1-deficient sperm accumulate in the perivitelline space of the oocyte without fusion with oocyte membrane [15]. A knockout of IZUMO1 completely disrupts sperm fusion with an oocyte. There also appears to be an ortholog in humans because antibodies to the putative human IZUMO1 can react with an approximately 37-kDa protein in human sperm lysates, and the antibodies also inhibit fusion of human sperm to a ZP-free oocyte [15]. These results indicated that IZUMO1 is essential for sperm-oocyte fusion on the sperm side.
Furthermore, it was unexpectedly found that IZUMO1 is encoded as a transmembrane protein with an extracellular immunoglobulin-like domain, a single transmembrane region, and a short cytoplasmic tail, but without any fusogenic domain as in other fusion proteins [15]. Therefore, IZUMO1 is likely to combine with other surface proteins participating in the fusion process [11, 16]. It seems probable that IZUMO1 interacts with the associated proteins that directly facilitate the fusion process on the sperm membrane or on the oocyte membrane. Guided by this hypothesis, scientists found that IZUMO1 localization is affected by a testis-specific serine kinase encoded by Tssk6. In Tssk6-deficient sperm, IZUMO1 fails to be localized to the sperm equatorial segment after acrosome exocytosis [17]. Moreover, a knockout of Tssk6 in sperm leads to the inability to fertilize an oocyte in vitro [17, 18]. Thus, the serine kinase Tssk6 mediates localization of IZUMO1 and interacts with IZUMO1 to participate in the sperm-oocyte fusion process. Recent evidence also revealed that an oocyte surface receptor named JUNO interacts with sperm IZUMO1 directly [19]. The oocytes of Juno-deficient mice are completely incapable of being fertilized by acrosome-reacted sperms. Disruption of the interaction should also inhibit the fusion process. Therefore, the interaction between IZUMO1 and Juno seems to be necessary for the adhesion process, thereby promoting the fusion process [19]. Most recently, a new molecular model of IZUMO1-JUNO recognition has been proposed where monomeric IZUMO1 binds JUNO and dimerizes quickly, then an unidentified receptor replaces JUNO to mediate membrane fusion [20]. Several studies revealed the crystal structures of human IZUMO1 and JUNO as well as the IZUMO1-JUNO complex [21,22,23,24,25]. The central β-hairpin region of IZUMO1 is crucial for integrity of JUNO′s binding surface located behind the putative JUNO ligand-binding pocket [22]. Therefore, it was proposed that the interaction of IZUMO1 with JUNO may act as a scaffold (before the beginning of membrane fusion) to juxtapose the two cell membranes in close proximity and to recruit other fusion proteins. Another study also showed that oocyte fusogen CD9 helps the JUNO receptor to interact with sperm IZUMO1 involved in the fusion process [26]. CD9 is well-known as a member of the tetraspanin family [27, 28]. Cd9 knockout females were found to be severely subfertile: a breakthrough for the gamete interaction field [28, 29]. Taken together, these results proved that IZUMO1 is essential for sperm-oocyte fusion on the sperm side through complex mechanisms by interacting with other proteins either on the sperm membrane or on the oocyte membrane.
In addition, a recent study indicated that the short cytoplasmic tail of IZUMO1 is highly phosphorylated when it is located in the region from the head/tail region of sperm to the equatorial segment of sperm. In the caput regions of rat epididymis, there are only two phosphorylation sites in the cytoplasmic tail of IZUMO1. Nevertheless, when the sperm pass through the epididymis, the intracellular C-terminal tail of IZUMO1 is phosphorylated at seven sites [13]. In some infertile males, IZUMO1 is present in their sperm as its nonphosphorylated form or the number of phosphorylation sites is reduced compared with those in fertile men. Thus, it could be inferred that strong phosphorylation of IZUMO1 might play an important role in the IZUMO1 involvement in male infertility. Understanding this process requires more research.
A Disintegrin and Metalloprotease 2 (ADAM2) Plays a Role in Sperm-ZP Interaction
Sperm ADAM2 was identified with a fertilization-blocking antibody and characterized as one of the members of a disintegrin and metalloprotease (ADAM) family [30, 31]. ADAMs are known as binding partners for several members of the integrin family [32]. A number of these integrins are expressed on the oocyte surface and perform important functions in sperm-oocyte interaction on the oocyte side. Thus, we will assume that ADAMs may participate in sperm-oocyte interaction via binding to integrins on the oocyte surface. This hypothesis puts specific ADAM-integrin pairs, especially ADAM2 and the integrin α9β1, in a category with abalone sperm lysin and oocyte VERL (vitelline envelope receptor for lysin) as cognate binding partners on the two gametes [33, 34]. Recent studies revealed that ADAM2 can enhance the initial adhesion of sperm to the oolema and increase the sperm attachment rate. The Adam2–/– knockout also has severe defects in sperm membrane interactions and low expression level of several ADAM proteins on the sperm surface [35, 36]. Besides, the Adam2-deficient sperm show impaired migration into the oviduct through the uterotubal junction and fusion to the ZP and the oocyte plasma membrane [37]. Nonetheless, there needs to be more evidence to prove the hypothesis that ADAM2 has a function in the sperm-oocyte fusion through the interaction with the specific integrin on the oocyte membrane.
Sperm Equatorial Segment Protein 1 (SPESP1) is Crucial for Formation of the Equatorial Segment
It is widely accepted that the equatorial segment after the sperm acrosome reaction is important for initiating the fusion with oocyte plasma membrane during fertilization [38]. There are various proteins known to be distributed only in the equatorial segment of sperm. The fusion-related proteins such as IZUMO1 should be localized to the sperm equatorial segment after acrosome endocytosis [3]. A number of sperm equatorial segment proteins (SPESPs) have also been studied regarding their roles in gamete membrane interaction. Among these, SPESP1 has been studied actively because either experiments with anti-SPESP1 antibodies or in vitro assay of Spesp1 knockout mice resulted in severe inhibition of the sperm-oocyte fusion [39,40,41,42]. The sperm equatorial segment can be disrupted completely due to the lack of SPESP1 in Spesp1-deficient males. Furthermore, the disruption of Spesp1 was shown to induce an aberrant distribution of various sperm proteins, such as ADAM family proteins and MN9 antigen, which were found to participate in the fusion process [32, 42]. It has been proposed that SPESP1 may help to restrain the MN9 antigen at the moment of fusion [42]. SPESP1 can interact with these proteins to facilitate the fusion. Although the exact mechanism is unclear, SPESP1 indeed plays an important role in the process of fusion of a sperm with oocyte.
Cysteine-rich Secretory Protein 1 (CRISP1) is an Epididymal Protein Participating in the Fusion Process
The epididymal protein CRISP1, which is a member of the cysteine-rich secretory proteins (CRISPs) family, was identified as a sperm surface protein. Once the acrosome reaction occurs, CRISP1 migrates to the equatorial segment, where the sperm fuses with the oocyte plasma membrane [43]. At a structural level, CRISP1 contains a CAP domain, which has been implicated in cell-cell interactions. Coincubation of peptides derived from the CAP domain (amino acid residues 114–158) of rat CRISP1 reduced sperm-oocyte fusion during IVF [44]. IVF results indicated that Crisp1-deficient sperm show significant difficulty with penetration of the ZP and fusion with the oocyte plasma membrane [45]. These results suggest that CRISP1 participates in both ZP interaction and the sperm fusion with the oocyte. A similar epididymal protein was observed on human sperm and named AEG-related protein (ARP) [46,47,48]. It is also reported that human ARP plays a role in gamete fusion through complementary sites on the surface of the human oocyte.
Bactericidal/Permeability-increasing Protein (BPI) is a Novel Sperm Protein Involved in Sperm-oocyte Fusion
Recently, a novel sperm protein named bactericidal/permeability-increasing protein (BPI) has received a lot of attention. BPI is a 55–60 kDa single-chain cationic protein that belongs to a conserved family of lipid-transfer proteins. BPI can inhibit all the proinflammatory activities of lipopolysaccharides (LPS), including neutrophil oxidase enzyme activation, cytokine release, and nitric oxide formation [49]. In the male murine reproductive system, BPI was reported to be selectively expressed in testes and in the epididymis, not in the seminal vesicles, prostate, or solidification glands [50]. In our colleagues′ previous study, they discovered that mouse BPI is secreted by the epididymal epithelium and then localized to the surface of the sperm plasma membrane [51]. BPI is not expressed in the organs closer to the external environment; this finding suggests that BPI may have multiple functions in the male reproductive system not only the antimicrobial function in other organs. Furthermore, they found that BPI is enriched in the equatorial segment [51]. Thus, our colleagues and we are interested in the function of BPI in male infertility. Recent studies also implied that BPI may take part in the sperm-oocyte fusion process because incubation with anti-BPI antiserum reduces the number of sperm fused with oocytes significantly in an IVF assay [52, 53].
Taken together, these pieces of evidence indicate a dual origin of the BPI that is associated with mouse spermatozoa. This expression pattern of BPI is similar to that of some antimicrobial proteins, such as hCAP18/SOB3 localized both in the epididymal epithelia and within human spermatozoa acrosomes, potentially displaying zona pellucida-binding activity [54, 55]. Additionally, cystatin-related epididymal spermatogenic protein (CRESP) is reported to have both an antimicrobial function and a role in the sperm-oocyte fusion process [56, 57]. Due to the mutualism function of BPI in the interaction between proteins and microorganisms, it can be speculated that BPI on the sperm surface may not directly interact with the oocyte membrane but interacts with or regulates the function of some fusion-related proteins on the sperm surface such as CRESP, playing the role of a bridge molecule in the sperm-oocyte fusion process. This hypothesis requires more evidence and further testing.
Other Molecular Mechanisms Involved in Sperm-oocyte Fusion
Cell-cell fusion
Although this review concentrates on molecules participating in gamete fusion, knowledge of the molecular mechanism underlying general cell-cell fusion could be truly useful. The cell-cell fusion mechanism remains poorly understood despite its physiological importance in the entire biological process. Recent related studies were focused on the discovery of fusogens: cell fusion proteins that bring the membranes closer together and mediate the mixing of bilayer membranes. In mammals, one family named syncytins has been reported as a well-defined fusogen. This family includes proteins that originate from endogenous retroviruses related to the HIV Gp41 envelope glycoprotein and function in the syncytial trophoblasts and in viral fusion [58-60]. For example, syncytin-1 was found to be expressed in the equatorial segment or acrosomal region of spermatozoa, while its receptor (ASCT-2) is expressed on oocytes; they together possibly participate in gamete fusion [61]. In a synaptic system, a family named SNAREs is also discovered as fusogens playing an important role in synaptic vesicles fusion process [62, 63]. In a sexual fusion system, it is hypothesized that sperm-oocyte fusion-related proteins, such as sperm IZUMO1, interact with fusogenic proteins on the sperm membrane or on the oocyte membrane to participate in the sperm-oocyte fusion. Sperm fusogens are thus expected to be located in the reproductive system and can function in cooperation with IZUMO1 or other molecules.
Besides the possibility of fusogenic proteins, it has been demonstrated that various adhesion molecules and enzymatic activities related to cell fusion are also involved in sperm-oocyte fusion because this process is in the category of cell-cell fusion processes. For adhesion molecules, cadherin is known as a human sperm protein. Cadherin is a transmembrane glycoprotein involved in calcium-dependent cell-cell adhesion events. An IVF assay indicated that anticadherin antibodies reduce the fusion of human sperm to a ZP-free hamster oocyte [64, 65]. Cadherin′s participation in gamete interaction has not been fully investigated. In addition, zinc metalloproteases are necessary for some intercellular fusion processes, such as cell-cell fusion in yeast. Inhibitors of zinc metalloproteases and zinc chelators are both found to reduce sperm-oocyte fusion. These observations indicate that a zinc metalloprotease may take part in the sperm-oocyte fusion process [66]. Research into the mechanisms on the basis of the studies of cell-cell fusion should bring new insights into the sperm-oocyte fusion process.
MicroRNAs
MicroRNAs (miRNAs) are small noncoding single-stranded RNA molecules that are physiologically produced in eukaryotic cells to regulate or mostly downregulate genes by pairing with their complementary base sequence in relevant mRNA molecules in the cytoplasm. It has been reported that a dysfunction in miRNA processing such as the use of the Cre-LoxP system to create a specific mutant of sperm, Dorsha and Dicer, can result in azoospermia and infertility [67, 68]. Because of the necessity of single miRNA in target mRNA expression, targeted deletion of miRNA leads to a perceptible infertility phenotype in mice. Furthermore, in order to characterize the involvement of specific miRNAs that are highly expressed in the male reproductive system, several studies have been focused on miRNA function in this system. Double disruption of miR-34b/c and miR-449 miRNA clusters, which are highly expressed in testes, can cause dysregulation of more than 200 molecules and may lead to serious male and female infertility [69, 70]. In addition, recent evidence showed that miR-27b could negatively regulate the expression of CRISP2, which is involved in asthenozoospermia [71]. IVF studies showed that CRISP2 knockout sperm have a deficiency in penetration of the egg vestments (i.e., cumulus cells and ZP) and problems with fusion with the egg [72]. Based on these results, there is a greater possibility for miRNAs to take part in the sperm-oocyte fusion through regulation of the related proteins such as CRISP1, even though the exact function of miRNAs in the fusion needs further research. Reinforcing the role of miRNAs is identification of these molecules as potential therapeutic targets for the diagnosis and treatment of male infertility.
Due to the complex and important process of sperm-oocyte fusion in the sexual reproduction system, lots of molecules (that have the ability to participate in male infertility and were not elaborated exactly in this paper) have been summarized well in other reviews [73]. For example, sperm lysozyme-like protein (SLLP1), endoplasmic reticulum protein 29 (ERp29), and prostate and testis expression (PATE)-like proteins were reported to be involved in the sperm-oocyte interaction [74,75,76]. Disruption of these molecules can lead to a perceptible infertility phenotype in mice.
Conclusion
Sperm-oocyte fusion is one of the most impressive events in sexual reproduction, culminating in the merger of plasmatic membranes; the molecules involved in sperm-oocyte fusion on the sperm side are closely related to male infertility [77]. The elucidation of its molecular mechanism has confused scientists for a long time. This review highlighted the molecules participating in the sperm-oocyte fusion on the sperm side (summarized in Table 1). Although many mechanisms for some molecules are to be refined and verified, this paper focused on the potential molecular mechanisms that may provide new ideas for clinical treatment of male infertility. Besides the molecules mentioned above, molecular mechanisms of the cell-cell fusion process such as formation of myotubes, placenta, multinucleated osteoclasts, and macrophages might be involved in the sperm reproduction system. This hypothesis needs to be tested by more experiments. Therefore, the focus on the role of sperm-oocyte fusion in male reproductive disorders can further elucidate the molecular mechanisms of male infertility and holds promise for identification of efficient biomarkers and therapeutic agents for these disorders. This study conclusively provides a novel insight into some of the mechanisms leading to sperm-oocyte fusion on the sperm side, offering a possible therapeutic target for treatment of male infertility or even for male contraception. We strongly believe that a combination of genetic, biochemical, and biophysical approaches will eventually identify and characterize these elusive proteins required for fertilization.
Table 1. Sperm molecules involved in sperm-oocyte fusion.
| Molecule | Method | Fusion | Reference |
| ADAM2 | Knockout | ~ 50% | [35, 36] |
| α-L-Fucosidase | Antibodies against α-L-Fucosidase | Reduced | [78, 79] |
| BPI | Antibodies against BPI | Reduced | [51] |
| Calpains | Antibodies against Calpains | Reduced | [80] |
| CRISP1 | Knockout | Reduced | [45] |
| CRISP2 | Knockout | Reduced | [72] |
| ERp29 | Antibodies against ERp29 | Reduced | [75] |
| IZUMO1 | Knockout | Failed | [15] |
| SLLP1 | Antibodies against SLLP1 | Reduced | [76] |
| SPACA6 | Knockout | Failed | [81] |
| SPESP1 | Knockout | Severely reduced | [39,40,41,42] |
| TSSK6 | Knockout | Failed | [17] |
Conflict of Interest: The authors declare that they have no conflicts of interest.
Acknowledgments
This study was funded by grants from the National Natural Science Foundation of China (grant number 81200465), Guangdong Natural Science Foundation (grant number 2014A030313785, 2016A030313029), Guangdong Foundation of Science and Technology (grant number 2014A020212038), Medical Scientific Research Foundation of Guangdong Province (grant number A2015408), Shenzhen Foundation of Science and Technology (grant number GJHZ20140414170821192 and JCYJ20140414170821337, JCYJ20160229204849975, GJHZ20160301163138685, JCYJ20150330102720122), Sanming Project of Medicine in Shenzhen, Fund for High Level Medical Discipline Construction of Shenzhen (2016031638), the Project of Shenzhen Engineering Center (GCZX2015043017281705), Clinical Doctor-Basic Scientist Combination Foundation of Shenzhen Second People′s Hospital and Key Laboratory Project of Shenzhen Second People′s Hospital.
References
- 1.Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLCζ): oocyte activation and clinical links to male factor infertility. Adv Biol Regul 2013; 53: 292–308. [DOI] [PubMed] [Google Scholar]
- 2.Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am 2014; 41: 195–204. [DOI] [PubMed] [Google Scholar]
- 3.Klinovska K, Sebkova N, Dvorakova-Hortova K. Sperm-egg fusion: a molecular enigma of mammalian reproduction. Int J Mol Sci 2014; 15: 10652–10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Practice Committee of American Society for Reproductive Medicine. Report on varicocele and infertility. Fertil Steril 2008; 90(Suppl): S247–S249. [DOI] [PubMed] [Google Scholar]
- 5.Quinn GP, Vadaparampil ST, Lee JH, Jacobsen PB, Bepler G, Lancaster J, Keefe DL, Albrecht TL. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol 2009; 27: 5952–5957. [DOI] [PubMed] [Google Scholar]
- 6.Gaur DS, Talekar MS, Pathak VP. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol 2010; 53: 35–40. [DOI] [PubMed] [Google Scholar]
- 7.Ferlin A. New genetic markers for male fertility. Asian J Androl 2012; 14: 807–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Flynn O'Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril 2010; 93: 1–12. [DOI] [PubMed] [Google Scholar]
- 9.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA 2011; 108: 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Spina FA, Puga Molina LC, Romarowski A, Vitale AM, Falzone TL, Krapf D, Hirohashi N, Buffone MG. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev Biol 2016; 411: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans JP. Sperm-egg interaction. Annu Rev Physiol 2012; 74: 477–502. [DOI] [PubMed] [Google Scholar]
- 12.Kaji K, Kudo A. The mechanism of sperm-oocyte fusion in mammals. Reproduction 2004; 127: 423–429. [DOI] [PubMed] [Google Scholar]
- 13.Young SA, Aitken J, Baker MA. Phosphorylation of Izumo1 and Its Role in Male Infertility. Asian J Androl 2015; 17: 708–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okabe M, Yagasaki M, Oda H, Matzno S, Kohama Y, Mimura T. Effect of a monoclonal anti-mouse sperm antibody (OBF13) on the interaction of mouse sperm with zona-free mouse and hamster eggs. J Reprod Immunol 1988; 13: 211–219. [DOI] [PubMed] [Google Scholar]
- 15.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005; 434: 234–238. [DOI] [PubMed] [Google Scholar]
- 16.Ellerman DA, Pei J, Gupta S, Snell WJ, Myles D, Primakoff P. Izumo is part of a multiprotein family whose members form large complexes on mammalian sperm. Mol Reprod Dev 2009; 76: 1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosnik J, Miranda PV, Spiridonov NA, Yoon SY, Fissore RA, Johnson GR, Visconti PE. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci 2009; 122: 2741–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiridonov NA, Wong L, Zerfas PM, Starost MF, Pack SD, Paweletz CP, Johnson GR. Identification and characterization of SSTK, a serine/threonine protein kinase essential for male fertility. Mol Cell Biol 2005; 25: 4250–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014; 508: 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue N, Hagihara Y, Wright D, Suzuki T, Wada I. Oocyte-triggered dimerization of sperm IZUMO1 promotes sperm-egg fusion in mice. Nat Commun 2015; 6: 8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydin H, Sultana A, Li S, Thavalingam A, Lee JE. Molecular architecture of the human sperm IZUMO1 and egg JUNO fertilization complex. Nature 2016; 534: 562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohto U, Ishida H, Krayukhina E, Uchiyama S, Inoue N, Shimizu T. Structure of IZUMO1-JUNO reveals sperm-oocyte recognition during mammalian fertilization. Nature 2016; 534: 566–569. [DOI] [PubMed] [Google Scholar]
- 23.Han L, Nishimura K, Sadat Al Hosseini H, Bianchi E, Wright GJ, Jovine L. Divergent evolution of vitamin B9 binding underlies Juno-mediated adhesion of mammalian gametes. Curr Biol 2016; 26: R100–R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura K, Han L, Bianchi E, Wright GJ, de Sanctis D, Jovine L. The structure of sperm Izumo1 reveals unexpected similarities with Plasmodium invasion proteins. Curr Biol 2016; 26: R661–R662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato K, Satouh Y, Nishimasu H, Kurabayashi A, Morita J, Fujihara Y, Oji A, Ishitani R, Ikawa M, Nureki O. Structural and functional insights into IZUMO1 recognition by JUNO in mammalian fertilization. Nat Commun 2016; 7: 12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassarman PM. Reproductive biology: Sperm protein finds its mate. Nature 2014; 508: 466–467. [DOI] [PubMed] [Google Scholar]
- 27.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005; 6: 801–811. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein E. The complexity of tetraspanins. Biochem Soc Trans 2011; 39: 501–505. [DOI] [PubMed] [Google Scholar]
- 29.Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 2000; 24: 279–282. [DOI] [PubMed] [Google Scholar]
- 30.Primakoff P, Hyatt H, Tredick-Kline J. Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J Cell Biol 1987; 104: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blobel CP, Wolfsberg TG, Turck CW, Myles DG, Primakoff P, White JM. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 1992; 356: 248–252. [DOI] [PubMed] [Google Scholar]
- 32.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med 2008; 29: 258–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eto K, Huet C, Tarui T, Kupriyanov S, Liu HZ, Puzon-McLaughlin W, Zhang XP, Sheppard D, Engvall E, Takada Y. Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1: implications for sperm-egg binding and other cell interactions. J Biol Chem 2002; 277: 17804–17810. [DOI] [PubMed] [Google Scholar]
- 34.Tomczuk M, Takahashi Y, Huang J, Murase S, Mistretta M, Klaffky E, Sutherland A, Bolling L, Coonrod S, Marcinkiewicz C, Sheppard D, Stepp MA, White JM. Role of multiple beta1 integrins in cell adhesion to the disintegrin domains of ADAMs 2 and 3. Exp Cell Res 2003; 290: 68–81. [DOI] [PubMed] [Google Scholar]
- 35.Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG. Fertilization defects in sperm from mice lacking fertilin beta. Science 1998; 281: 1857–1859. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura H, Cho C, Branciforte DR, Myles DG, Primakoff P. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev Biol 2001; 233: 204–213. [DOI] [PubMed] [Google Scholar]
- 37.Kim T, Oh J, Woo JM, Choi E, Im SH, Yoo YJ, Kim DH, Nishimura H, Cho C. Expression and relationship of male reproductive ADAMs in mouse. Biol Reprod 2006; 74: 744–750. [DOI] [PubMed] [Google Scholar]
- 38.Yanagimachi R. Fertility of mammalian spermatozoa: its development and relativity. Zygote 1994; 2: 371–372. [DOI] [PubMed] [Google Scholar]
- 39.Wolkowicz MJ, Shetty J, Westbrook A, Klotz K, Jayes F, Mandal A, Flickinger CJ, Herr JC. Equatorial segment protein defines a discrete acrosomal subcompartment persisting throughout acrosomal biogenesis. Biol Reprod 2003; 69: 735–745. [DOI] [PubMed] [Google Scholar]
- 40.Wolkowicz MJ, Digilio L, Klotz K, Shetty J, Flickinger CJ, Herr JC. Equatorial segment protein (ESP) is a human alloantigen involved in sperm-egg binding and fusion. J Androl 2008; 29: 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv ZM, Wang M, Xu C. Antifertility characteristics of the N-terminal region of mouse equatorial segment protein. Anat Rec (Hoboken) 2010; 293: 171–181. [DOI] [PubMed] [Google Scholar]
- 42.Fujihara Y, Murakami M, Inoue N, Satouh Y, Kaseda K, Ikawa M, Okabe M. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J Cell Sci 2010; 123: 1531–1536. [DOI] [PubMed] [Google Scholar]
- 43.Rochwerger L, Cuasnicu PS. Redistribution of a rat sperm epididymal glycoprotein after in vitro and in vivo capacitation. Mol Reprod Dev 1992; 31: 34–41. [DOI] [PubMed] [Google Scholar]
- 44.Koppers AJ, Reddy T, OBryan MK. The role of cysteine-rich secretory proteins in male fertility. Asian J Androl 2011; 13: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, Rubinstein M, Eddy EM, Cuasnicu PS. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev Biol 2008; 320: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai J, Fu SH, Cai LL, Sun L, Cong YL. [Identification of differential proteins in the seminal plasma of healthy fertile and non-obstructive azoospermia men by shotgun proteomic strategy]. Zhonghua Nan Ke Xue 2010; 16: 887–896. (in Chinese) [PubMed] [Google Scholar]
- 47.Hayashi M, Fujimoto S, Takano H, Ushiki T, Abe K, Ishikura H, Yoshida MC, Kirchhoff C, Ishibashi T, Kasahara M. Characterization of a human glycoprotein with a potential role in sperm-egg fusion: cDNA cloning, immunohistochemical localization, and chromosomal assignment of the gene (AEGL1). Genomics 1996; 32: 367–374. [DOI] [PubMed] [Google Scholar]
- 48.Krätzschmar J, Haendler B, Eberspaecher U, Roosterman D, Donner P, Schleuning WD. The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. Eur J Biochem 1996; 236: 827–836. [DOI] [PubMed] [Google Scholar]
- 49.Ciornei CD, Egesten A, Engström M, Törnebrandt K, Bodelsson M. Bactericidal/permeability-increasing protein inhibits endotoxin-induced vascular nitric oxide synthesis. Acta Anaesthesiol Scand 2002; 46: 1111–1118. [DOI] [PubMed] [Google Scholar]
- 50.Lennartsson A, Pieters K, Vidovic K, Gullberg U. A murine antibacterial ortholog to human bactericidal/permeability-increasing protein (BPI) is expressed in testis, epididymis, and bone marrow. J Leukoc Biol 2005; 77: 369–377. [DOI] [PubMed] [Google Scholar]
- 51.Zhou ZP, Xia XY, Guo QS, Xu C. Bactericidal/permeability-increasing protein originates in both the testis and the epididymis and localizes in mouse spermatozoa. Asian J Androl 2014; 16: 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li K, Liu Y, Xia X, Wang L, Lu M, Hu Y, Xu C. Bactericidal/permeability-increasing protein in the reproductive system of male mice may be involved in the sperm-oocyte fusion. Reproduction 2013; 146: 135–144. [DOI] [PubMed] [Google Scholar]
- 53.Yano R, Matsuyama T, Kaneko T, Kurio H, Murayama E, Toshimori K, Iida H. Bactericidal/Permeability-increasing protein is associated with the acrosome region of rodent epididymal spermatozoa. J Androl 2010; 31: 201–214. [DOI] [PubMed] [Google Scholar]
- 54.Doussau M, Lasserre A, Hammami-Hamza S, Massaad C, Gasc JM, Finaz C. Testicular and epididymal dual origin of hCAP-18/SOB3, a human sperm protein. Fertil Steril 2008; 90: 853–856. [DOI] [PubMed] [Google Scholar]
- 55.Hammami-Hamza S, Doussau M, Bernard J, Rogier E, Duquenne C, Richard Y, Lefèvre A, Finaz C. Cloning and sequencing of SOB3, a human gene coding for a sperm protein homologous to an antimicrobial protein and potentially involved in zona pellucida binding. Mol Hum Reprod 2001; 7: 625–632. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Yuan Q, Chen S, Cai H, Lu M, Liu Y, Xu C. Antimicrobial activity and molecular mechanism of the CRES protein. PLoS One 2012; 7: e48368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chau KM, Cornwall GA. Reduced fertility in vitro in mice lacking the cystatin CRES (cystatin-related epididymal spermatogenic): rescue by exposure of spermatozoa to dibutyryl cAMP and isobutylmethylxanthine. Biol Reprod 2011; 84: 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan DC, Kim PS. HIV entry and its inhibition. Cell 1998; 93: 681–684. [DOI] [PubMed] [Google Scholar]
- 59.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol 2008; 15: 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dupressoir A, Marceau G, Vernochet C, Bénit L, Kanellopoulos C, Sapin V, Heidmann T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc Natl Acad Sci USA 2005; 102: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjerregaard B, Lemmen JG, Petersen MR, Østrup E, Iversen LH, Almstrup K, Larsson LI, Ziebe S. Syncytin-1 and its receptor is present in human gametes. J Assist Reprod Genet 2014; 31: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol 2008; 15: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell 1998; 92: 759–772. [DOI] [PubMed] [Google Scholar]
- 64.Marín-Briggiler CI, Veiga MF, Matos ML, Echeverría MF, Furlong LI, Vazquez-Levin MH. Expression of epithelial cadherin in the human male reproductive tract and gametes and evidence of its participation in fertilization. Mol Hum Reprod 2008; 14: 561–571. [DOI] [PubMed] [Google Scholar]
- 65.Marín-Briggiler CI, Lapyckyj L, González Echeverría MF, Rawe VY, Alvarez Sedó C, Vazquez-Levin MH. Neural cadherin is expressed in human gametes and participates in sperm-oocyte interaction events. Int J Androl 2010; 33: e228–e239. [DOI] [PubMed] [Google Scholar]
- 66.Correa LM, Cho C, Myles DG, Primakoff P. A role for a TIMP-3-sensitive, Zn(2+)-dependent metalloprotease in mammalian gamete membrane fusion. Dev Biol 2000; 225: 124–134. [DOI] [PubMed] [Google Scholar]
- 67.Khazaie Y, Nasr Esfahani MH. MicroRNA and Male Infertility: A Potential for Diagnosis. Int J Fertil Steril 2014; 8: 113–118. [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Q, Song R, Ortogero N, Zheng H, Evanoff R, Small CL, Griswold MD, Namekawa SH, Royo H, Turner JM, Yan W. The RNase III enzyme DROSHA is essential for microRNA production and spermatogenesis. J Biol Chem 2012; 287: 25173–25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Comazzetto S, Di Giacomo M, Rasmussen KD, Much C, Azzi C, Perlas E, Morgan M, O'Carroll D. Oligoasthenoteratozoospermia and infertility in mice deficient for miR-34b/c and miR-449 loci. PLoS Genet 2014; 10: e1004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu J, Bao J, Kim M, Yuan S, Tang C, Zheng H, Mastick GS, Xu C, Yan W. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc Natl Acad Sci USA 2014; 111: E2851–E2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou JH, Zhou QZ, Lyu XM, Zhu T, Chen ZJ, Chen MK, Xia H, Wang CY, Qi T, Li X, Liu CD. The expression of cysteine-rich secretory protein 2 (CRISP2) and its specific regulator miR-27b in the spermatozoa of patients with asthenozoospermia. Biol Reprod 2015; 92: 28. [DOI] [PubMed] [Google Scholar]
- 72.Brukman NG, Miyata H, Torres P, Lombardo D, Caramelo JJ, Ikawa M, Da Ros VG, Cuasnicú PS. Fertilization defects in sperm from Cysteine-rich secretory protein 2 (Crisp2) knockout mice: implications for fertility disorders. Mol Hum Reprod 2016; 22: 240–251. [DOI] [PubMed] [Google Scholar]
- 73.Cuasnicú PS, Da Ros VG, Weigel Muñoz M, Cohen DJ. Acrosome Reaction as a Preparation for Gamete Fusion. Adv Anat Embryol Cell Biol 2016; 220: 159–172. [DOI] [PubMed] [Google Scholar]
- 74.Margalit M, Yogev L, Yavetz H, Lehavi O, Hauser R, Botchan A, Barda S, Levitin F, Weiss M, Pastan I, Wreschner DH, Paz G, Kleiman SE. Involvement of the prostate and testis expression (PATE)-like proteins in sperm-oocyte interaction. Hum Reprod 2012; 27: 1238–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ying X, Liu Y, Guo Q, Qu F, Guo W, Zhu Y, Ding Z. Endoplasmic reticulum protein 29 (ERp29), a protein related to sperm maturation is involved in sperm-oocyte fusion in mouse. Reprod Biol Endocrinol 2010; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herrero MB, Mandal A, Digilio LC, Coonrod SA, Maier B, Herr JC. Mouse SLLP1, a sperm lysozyme-like protein involved in sperm-egg binding and fertilization. Dev Biol 2005; 284: 126–142. [DOI] [PubMed] [Google Scholar]
- 77.Anifandis G, Messini C, Dafopoulos K, Sotiriou S, Messinis I. Molecular and cellular mechanisms of sperm-oocyte interactions opinions relative to in vitro fertilization (IVF). Int J Mol Sci 2014; 15: 12972–12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venditti JJ, Swann JM, Bean BS. Hamster sperm-associated alpha-L-fucosidase functions during fertilization. Biol Reprod 2010; 82: 572–579. [DOI] [PubMed] [Google Scholar]
- 79.Phopin K, Nimlamool W, Lowe-Krentz LJ, Douglass EW, Taroni JN, Bean BS. Roles of mouse sperm-associated alpha-L-fucosidases in fertilization. Mol Reprod Dev 2013; 80: 273–285. [DOI] [PubMed] [Google Scholar]
- 80.Rojas FJ, Moretti-Rojas I. Involvement of the calcium-specific protease, calpain, in the fertilizing capacity of human spermatozoa. Int J Androl 2000; 23: 163–168. [DOI] [PubMed] [Google Scholar]
- 81.Lorenzetti D, Poirier C, Zhao M, Overbeek PA, Harrison W, Bishop CE. A transgenic insertion on mouse chromosome 17 inactivates a novel immunoglobulin superfamily gene potentially involved in sperm-egg fusion. Mamm Genome 2014; 25: 141–148. [DOI] [PubMed] [Google Scholar]