Abstract
With granulosa and theca cells, the ovaries are responsible for producing oocytes and secreting sex steroids such as estrogen and progesterone. Endoplasmic reticulum stress (ERS) plays an important role in follicle atresia and embryo implantation. In this study, goat granulosa cells were isolated from medium-sized (4–6 mm) healthy follicles. Primary granulosa cells were immortalized by transfection with human telomerase reverse transcriptase (hTERT) to establish a goat granulosa cell line (hTERT-GGCs). These hTERT-GGCs expressed hTERT and had relatively long telomeres at passage 50. Furthermore, hTERT-GGCs expressed the gonadotropin receptor genes CYP11A1, StAR, and CYP19A1, which are involved in steroidogenesis. Additionally, progesterone was detectable in hTERT-GGCs. Although the proliferation potential of hTERT-GGCs significantly improved, there was no evidence to suggest that the hTERT-GGCs are tumorigenic. In addition, thapsigargin (Tg) treatment led to a significant dose-dependent decrease in progesterone concentration and steroidogenic enzyme expression. In summary, we successfully generated a stable goat granulosa cell line. We found that Tg induced ERS in hTERT-GGCs, which reduced progesterone production and steroidogenic enzyme expression. Future studies may benefit from using this cell line as a model to explore the molecular mechanisms regulating steroidogenesis and apoptosis in goat granulosa cells.
Keywords: Endoplasmic reticulum stress (ERS), Granulosa cells, Goat, Human telomerase reverse transcriptase (hTERT), Immortalization
In female mammals, only 1% of follicles reach ovulation, causing the remaining follicles to undergo atresia [1]. Apoptosis of granulosa cells and degeneration of theca cells are considered to be crucial reasons for follicular atresia [2]. Granulosa cells also play a vital role in the secretion of sex steroids, such as estrogen and progesterone, in the follicle and are indispensable for oocyte maturation [2,3,4]. Previous in vitro studies using primary granulosa cells have elucidated changes in the molecular mechanisms of steroid synthesis, and the regulation of granulosa cell function [5]. The use of primary granulosa cells can be beneficial for maintaining intrinsic cell properties and for elucidating molecular mechanisms. However, primary cells lack the ability to be cultured for long term in vitro, which may limit some investigations. By transfecting the SV40 large T antigen in granulosa cells, others studies have successfully established immortalized granulosa cell lines [6]. Primary granulosa cells are known to be regulated by follicle stimulating hormone (FSH) or forskolin to secrete progesterone. However, the concentration of progesterone could not be detected in the presence of FSH or forskolin in an SV40-immortalized porcine granulosa cell line [7]. Furthermore, the KGN granulosa-like cell line, derived from human tumors, can be stimulated with FSH to regulate the secretion of progesterone, but the cells lacked the response to human chorionic gonadotropin (hCG) [8]. Consequently, the granulosa cell lines that have been established to date are not ideal models for the study of the function of granulosa cells in normal folliculogenesis. To better understand normal folliculogenesis, we aimed to immortalize a goat granulosa cell line that can secrete steroids without neoplastic transformation.
For most somatic cells, decrease in telomerase activity can lead to shortened telomeres after repeated cell division, and ultimately to irreversible replicating senescence [9, 10]. Recently, some groups have reported that senescence can be overcome by overexpressing certain oncogenes [11, 12]. Epstein Barr virus (EBV) and Simian virus 40 (SV40) T antigen have been demonstrated to induce immortalization and are highly expressed in cancer cells. Most cell lines that overexpress an oncogene for immortalization are unable to maintain their normal physiological functions because of the lack of some key transcription factors or the presence of oncogenic factors resulting from transformation [7, 13,14,15]. Importantly, exogenous human telomerase reverse transcriptase (hTERT) can be introduced into cells to achieve immortalization, and these immortalized cells maintain their normal functions and features [16,17,18]. Importantly, we have previously shown that transfection with the hTERT gene can initiate telomerase activation and immortalize the goat Leydig cell line [16].
The endoplasmic reticulum (ER) plays an important role in protein synthesis and cellular homeostasis [19]. Many conditions can cause damage to ER homeostasis, including the loss of calcium, hypoxia, and dysfunction of glycosylation [20, 21]. To cope with stress, the unfolded protein response (UPR) is activated [22], which induces a cytoprotective effect and helps to reestablish homeostasis [23]. For example, the binding immunoglobulin protein (BiP), also called 78-kDa glucose-regulated protein (GRP78), serves as an ER stress sensor and interacts with activating transcription factor 6 (ATF6), inositol-requiring enzyme1 (IRE1), and protein kinase-like ER kinase (PERK) in the presence of ER stress (ERS) [24, 25]. If ERS is not alleviated, CHOP (C/EBP homologous protein) is triggered by UPR to mediate cell death. Previous studies have shown that UPR is involved in steroidogenic enzyme expression in hCG-stimulated Leydig cells and the regulation of corpus luteum (CL) function in mice [26, 27]. However, it remains unclear whether ERS affects steroid synthesis in goat granulosa cells.
Here, we established a stable steroidogenic goat granulosa cell line by using hTERT. We then used this immortalized cell line to investigate whether ERS affects the function of steroid synthesis and steroidogenic enzymes in goat granulosa cells.
Materials and Methods
Isolation and routine culture of goat primary granulosa cells
Fresh goat ovaries, obtained from a local slaughterhouse, were transferred to prewarmed (37°C) phosphate-buffered saline (PBS) within 2 h of slaughter, under laboratory conditions. Briefly, granulosa cells were aspirated with a fine needle from medium-sized (4–6 mm) healthy follicles. Follicles were considered healthy if they were uniformly translucent and vascularized. Next, granulosa cells were isolated and adapted as described by Lin et al. [28]. Granulosa cells were cultured in M-199 medium (Gibco, Life Technologies, NY, USA) containing 10% fetal calf serum (Gibco, Life Technologies) and incubated at 37°C in a humidified incubator with 5% CO2. The culture medium was changed every three days.
Cell transfection and selection: When goat granulosa cells (GGCs) reached 50% confluence, they were immortalized by transduction with pCI-neo-hTERT using the turbofect transfection reagent (Thermo Scientific, USA) according to the manufacturer′s instructions. After 24 h, the complete culture medium, containing 400 μg/ml G418 (Sigma, St. Louis, MO, USA), was used to select transfected cells. After two weeks, drug-resistant monoclonal cells were selected. Three positive colonies were propagated and cultured for the subsequent studies and were named hTERT-goat granulosa cells (hTERT-GGCs).
RNA extraction and RT-PCR
Total RNA was extracted from primary GGCs, hTERT-GGCs, and hTERT-GGCs treated with 0, 0.01, 0.1, or 1 μM of the ER stress inducer thapsigargin (Tg) using Trizol Reagent (TaKaRa Bio, Dalian, China) according to the manufacturer′s instructions. The RNA concentration and purity were measured as previously described [28]. cDNA was synthesized using the PrimeScriptTM RT reagent kit (TaKaRa Bio) following the manufacturer′s instructions. The primer sequences and PCR amplification product sizes are shown in Table 1. RT-PCR amplification was performed as previously reported by Zhou et al. [16]. PCR products were separated by gel electrophoresis on 2% agarose gels containing ethidium bromide (Sigma) and visualized under UV illumination [29].
Table 1. Primer pairs used for PCR and Real time PCR.
| Gene | Sequences (5ˊ→3ˊ) | Product length (bp) |
Annealing temperature (°C) |
References or GenBank accession number |
| TERT | Forward: tatgccgtggtccagaagg | 384 | 56 | [33] |
| Reverse: caagaaatcatccaccaaacg | ||||
| StAR 1 | Forward: tactaaaggagccgtggataaag | 576 | 56 | XM_005698829 |
| Reverse: ctacaagtggtaatggttgggtt | ||||
| LHCGR | Forward: ctctttctttgccatctcag | 270 | 58 | [39] |
| Reverse: cgggagggcttatttga | ||||
| CYP19A1 | Forward: ggcatcatatttaacaatccagca | 547 | 60 | [53] |
| Reverse: cagacatggtgtctggcgctgcgatca | ||||
| CYP11A1 1 | Forward: gcagagggcgacataagca | 233 | 57 | [30] |
| Reverse: ggtcacggagatagggtgga | ||||
| CYP17A1 | Forward: tttcaaggtgaagatcgaggtg | 146 | 58 | [30] |
| Reverse: cggggaggaagaaggaatg | ||||
| ESR1 | Forward: caagaaggtccaatccgagt | 846 | 55 | XM_013966607.1 |
| Reverse: aaagagcagtttccgtgaac | ||||
| ESR2 | Forward: tccattgccagtcgtcacttc | 634 | 60 | NM_001285688.1 |
| Reverse: gccagcttggtcaaggacatc | ||||
| FSHR | Forward: gggctgggtctttgcttttg | 494 | 60 | KJ817181.1 |
| Reverse: ctgacctgtaggtctgggct | ||||
| Telomere | Forward: ggtttttgagggtgagggtgagggtgagggtgagggt | 39 | 58 | [50] |
| Reverse: tcccgactatccctatccctatccctatccctatcccta | ||||
| GAPDH 1 | Forward: tctgctgatgcccccatgtt | 289 | 56 | [48] |
| Reverse: tgaccttgcccacagccttg | ||||
| GAPDH 2 | Forward: gatggtgaaggtcggagtgaac | 100 | 56 | [5] |
| Reverse: gtcattgatggcgacgatgt | ||||
| StAR2 | Forward: aggccatgggcgagtggaac | 145 | 60 | XM_005698829.1 |
| Reverse: gtacagcgcacgctcacaaa | ||||
| CYP11A1 2 | Forward: tgatggctccagaggcaataaa | 150 | 60 | NM_001287574.1 |
| Reverse: caaaggcaaagtgaaacaggtc | ||||
| 3β-HSD | Forward: tccacaccagcaccatagag | 143 | 60 | NM_001285716.1 |
| Reverse: ttccagcacagccttctcg |
The numbers (in bp) in the right margin indicate the sizes of the expected amplified products for each set of primers. GAPDH1 was used as an internal control in PCR, GAPDH2 was used as an internal control in Real time PCR.
Immunofluorescence
hTERT-GGCs and GGCs were seeded into six-well slide chambers, fixed with 4% paraformaldehyde (PFA) for 30 min and permeabilized in 1% Triton X-100 for 5 min. After blocking in PBS containing 1% bovine serum albumin for 1 h, the cells were incubated with primary antibodies against TERT (Boster Biotech, Wuhan, China) and against aromatase (Abcam, Cambridge, MA, USA) overnight at 4°C. After washing three times with PBS and incubating for 1 h at room temperature in a 1:500 dilution mixture of Alexa-labeled donkey anti-rabbit IgG (Invitrogen, Life Technologies) at 37°C for 2 h, the nuclei were counterstained by DAPI (4,6-diamidino-2-phenylindole) and the cells were observed by laser-scanning confocal microscopy (Nikon, Melville, NY, USA).
Estradiol and progesterone measurement
Primary GGCs and hTERT-GGCs were plated into 24-well plates (5 × 104 cells/well) and cultured in M-199 medium with 10% FBS. After 24 h, the cells were cultured in serum-free M-199 medium with 10 μM androstenedione (4-androstene-3, 17-dione; Dr. Ehenstorfer, GmbH). The cells were then stimulated with 0.5 and 5 IU/ml FSH (Sigma) for 24 h. The culture supernatants were collected, and the concentration of estradiol and progesterone were measured by ELISA kits according to manufacturer′s instructions.
Similarly, hTERT-GGCs were cultured in serum-free M-199 medium with 0, 0.01, 0.1, or 1 μM Tg for 12 h. Progesterone concentration was measured as described above. The coefficient of variation was less than 15% for each group. The minimum assay sensitivity of estradiol was 25 pg/ml, and the progesterone was 0.2 ng/ml.
Proliferation assays
hTERT-GGCs at passage 50 and GGCs at passage 7 were seeded in 96-well plates at 2,000 cells / well and allowed to adhere overnight. After growing for a week, proliferation was measured by adding the Cell Counting Kit-8 (Beyotime, Haimen, Jiangsu, China) to the cells (10 μl/well) and incubating for 2 h at 37°C. The number of viable cells was measured at 450 nm by a Microplate Reader (Bio-Rad 680). Independent experiments were performed in triplicate and repeated three times.
Measurement of telomere length and quantitative real-time PCR
Telomere length was measured by quantitative real-time PCR. Briefly, genomic DNA was extracted from HeLa cells and GGCs at passage 7 and hTERT-GGCs at passage 50 using a TIANamp Genomic DNA Kit (TIANGEN Biotech, Beijing, China). Real-time quantitative PCR was carried out using SYBR Premix Ex Taq II Kit (TaKaRa Bio) in a Bio-Rad iQ5 instrument (iQ5, Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer′s protocol. The cycling conditions included a denaturation step at 95°C for 30 sec followed by 45 PCR cycles at 95°C for 15 sec and 58°C for 20 sec. The quantitative PCR protocol was adapted from our previously published study [16] and was performed in triplicate by laboratory technicians under identical conditions. Primer sequences, product lengths, and annealing temperatures are listed in Table 1. Telomere length and mRNA quantification were calculated using the 2-ΔΔCt method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control gene, and we ensured that the GAPDH expression was stable (Supplementary Fig. 1: online only) [16, 30, 31].
Karyotype analysis
Karyotype analysis of passage 50 hTERT-GGCs was performed using standard methods described by Wei et al. [17].
Soft agar assay
The lower soft agar layer was prepared using 2.0 ml of M-199 medium containing 10% FBS and 0.4% agar (Sigma) in 6-well plates. Subsequently, hTERT-GGCs at passage 50 were suspended in the top layer of M-199 medium containing 10% FBS and 0.4% agar. The cell suspension was then mixed rapidly and poured onto the lower layer. The agar culture was incubated at 37°C with 5% CO2 for 14 days. Colonies were observed and analyzed by microscopy. The mouse myeloma SP2/0 cell line (Sp2/0) was treated in the same way and used as a positive control [32].
Cell cycle analysis
hTERT-GGCs at passage 50 and GGCs at passage 7 were washed with ice-cold PBS and harvested with trypsin. Next, the cells were centrifuged at 2,000 rpm for 5 min and fixed in cold 70% ethanol overnight at 4°C. All samples were then stained using the Cell Cycle Detection Kit (KeyGEN Biotech, Nanjing, China) as we have previously reported [16]. Finally, the samples were analyzed by flow cytometry. Each test was performed in triplicate.
Western blot analysis
After treatment with 0, 0.01, 0.1, or 1 μM Tg hTERT-GGCs were lysed with RIPA buffer to extract the protein (Beijing Solarbio Science & Technology, Beijing, China). Total protein concentration was measured by the BCA assay (Nanjing Keygen Biotech). Thirty micrograms of total protein was loaded into each well of a 12% SDS-PAGE gel, and the proteins were separated by electrophoresis. The proteins were then transferred to PVDF membranes (Millipore; Bedford, MA). After blocking in Tris-buffered saline containing 0.5% Tween-100 (TBST) with 10% nonfat milk for 2 h, samples were incubated with phosphor-IRE1α (Abcam, diluted 1:1000), GRP78 (CST, diluted 1:1000), and CHOP (Santa Cruz, diluted 1:200) antibodies for 12 h at 4°C. Anti-β-actin mouse monoclonal antibody (Tianjin Sanjian Biotech, diluted 1:2000) served as a loading control. Subsequently, the membranes were incubated for 1 h with HRP-labeled secondary antibody at room temperature. Finally, the protein bands were visualized using the Gel Image System (Tannon Biotech, Shanghai, China) and measured with Quantity One software (Bio-Rad Laboratories).
Statistical analysis
All experiments were performed in triplicate and the data were analyzed using one-way ANOVA, followed by Tukey′s post hoc test and Fisher LSD. All data are expressed as mean ± SEM and analyzed using SPSS software (Version13.0; SPSS, Chicago, IL). Statistical differences were considered significant when the P value was less than 0.05.
Results
Establishment and characterization of immortalized GGCs
hTERT-GGCs at passages 30 and 50 were typically elongated and fibroblastic in morphology and had a high nuclei to cytoplasm ratio (Fig. 1A). To identify the purity of GGCs and hTERT-GGCs, we stained the cells with the granulosa cell marker, aromatase [33]. All the GGCs and hTERT-GGCs were specifically stained with aromatase (Supplementary Fig. 2: online only), demonstrating that GGCs and hTERT-GGCs are pure granulosa cells.
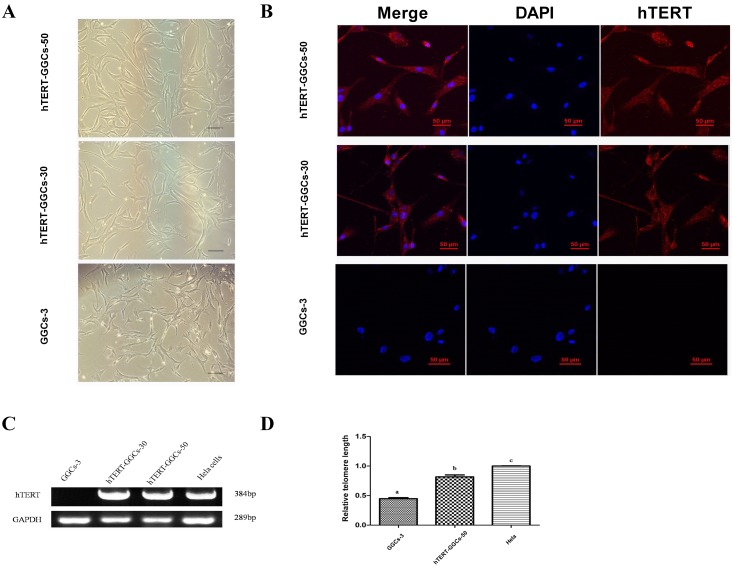
Fig. 1.
Cell morphology, hTERT mRNA detection, and telomere length in GGCs and hTERT-GGCs. (A) Morphology of GGCs at passage 3, and hTERT-GGCs at passage 30 and 50. Bar = 100 μm. (B) Immunofluorescence detection of the hTERT protein in GGCs and hTERT-GGCs. Immunofluorescence staining analysis of GGCs at passage 3, hTERT-GGCs at passage 30, and hTERT-GGCs at passage 50 were performed using hTERT antibodies. Bar = 50 μm. (C) RT-PCR analyses confirming the expression of hTERT mRNA. GGCs at passage 3 in lane 1, hTERT-GGC at passage 30 in lane 2, hTERT-GGCs at passage 50 in lane 3, and HeLa cells as positive controls in lane 4. (D) The relative telomere length detection by quantitative PCR. The telomere length is measured as the ratio of telomere copy number/ single copy gene copy number (T/S). For each sample, the values of T and S are measured by the standard curve method. The T/S ratio reflects the length of the telomeres. Data are presented as means ± SEM of three independent experiments. Bars with different letters are significantly different (P < 0.05).
RT-PCR and immunofluorescence analysis showed that hTERT-GGCs at passages 30 and 50 expressed hTERT mRNA and protein. As expected, GGCs at passage 3 did not express hTERT mRNA and protein (Fig. 1B and Fig. 1C). Furthermore, the telomeres of hTERT-GGCs passage 50 chromosomes had longer telomeres than the chromosomes of GGCs at passage 7 (Fig. 1D). These results indicate that the telomeres of hTERT-GGCs were maintained at a sufficient length and functioned normally.
Proliferation and cell cycle of immortalized GGCs
Cell viability of hTERT-GGCs at passage 50 was significantly increased compared with that of the GGCs (Fig. 2A). Moreover, the cell cycles of GGCs and hTERT-GGCs, detected by flow cytometry, were significant different (Fig. 2B). The percentage of hTERT-GGCs in S-phase was markedly elevated compared to GGCs under the same conditions. A corresponding reduction in hTERT-GGCs in G1-phase was also observed (Supplementary Fig. 4: online only). These results demonstrate that hTERT-GGCs have greater viability than GGCs, and that hTERT expression accelerated cell cycle progression.
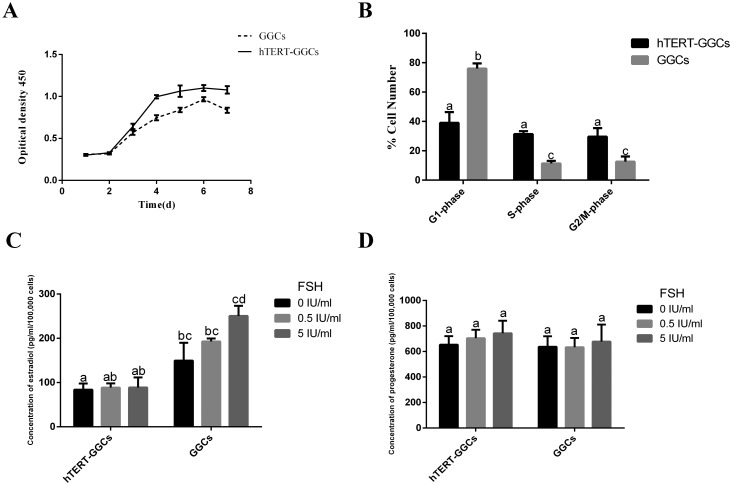
Fig. 2.
The detection of proliferation, cell cycle, and steroidogenic activity in hTERT-GGCs. (A) Growth curves of GGCs and hTERT-GGCs as determined by the CCK-8 method. Data presented are means ± SEM of three independent experiments. (B) Cell cycle distribution of GGCs at passage 7 and hTERT-GGCs at passage 50 as detected by flow cytometry. The percentage of hTERT-GGCs in S-phase is significantly higher than that of GGCs under the same culture conditions. (C and D) The 24 h secretion of estradiol and progesterone in GGCs and hTERT-GGCs was measured using an ELISA kit. Androstenedione was added to the culture medium and served as the substrate, followed by stimulation with 0, 0.5, or 5 IU/ml FSH. Data are presented as means ± SEM of three independent experiments. Bars with different letters are significantly different (P < 0.05).
Induction of steroidogenic activity in immortalized GGCs
To measure the steroidogenic activity of hTERT-GCCs, we stimulated GGCs and hTERT-GGCs with FSH and measured the estradiol and progesterone levels. After the addition of androstenedione, the estradiol level in hTERT-GGCs was lower than that in GGCs. Furthermore, estradiol levels in hTERT-GGCs were not appreciably increased by stimulation with FSH (Fig. 2C). Similarly, there was no significant difference between progesterone levels in hTERT-GGCs and GGCs (Fig. 2D).
Expression of the steroidogenesis-related genes in immortalized GGCs
It was important to determine whether hTERT-GGCs maintain the same phenotype as GGCs. Therefore, we evaluated the expression of a series of key genes using RT-PCR in GGCs at passage 5 and hTERT-GGCs at passages 30 and 50 (Fig. 3A). Expression of genes encoding enzymes involved in steroidogenesis including StAR, CYP11A1, CYP19A1, and CYP17A1 were detected in both GGCs and hTERT-GGCs by RT-PCR. Additionally, expression of genes encoding receptors such as LHCGR and ESR was observed in both GGCs and hTERT-GGCs. However, FSHR expression in hTERT-GGCs was relatively low compared to that in GGCs.
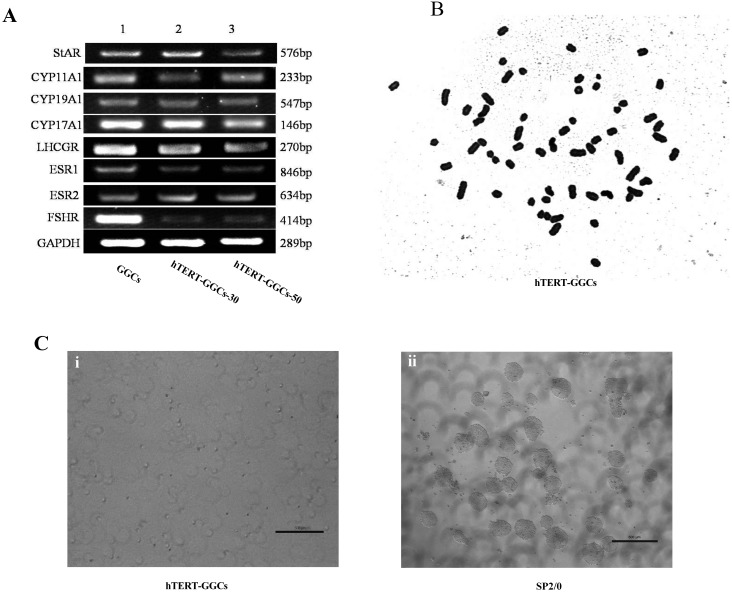
Fig. 3.
Steroidogenesis-related gene expression, karyotype, and tumorigenicity in hTERT-GGCs. (A) Phenotypic features of hTERT-GGCs. The expression of some key genes related to steroidogenesis was measured by RT-PCR. GGCs at passage 5 in lane 1, hTERT-GGCs at passage 30 in lane 2, and hTERT-GGCs at passage 50 in lane 3. GAPDH was used as an internal control. (B) Karyotype analysis of hTERT-GGCs at passage 50. The chromosomal rearrangements of hTERT-GGCs showed the normal karyotype for goat. (C) Soft agar colony comparison between hTERT-GGCs at passage 50 (i) and Sp2/0 (ii). (i) No colonies were formed in hTERT-GGCs over 10 days of culture. (ii) Colony formation can be observed after 10 days of culture from Sp2/0 cells.
Karyotype and tumorigenicity analysis of immortalized GGCs
To ensure that no chromosomal abnormalities resulted from immortalization of the GGCs, we performed hTERT-GGC karyotype analysis. hTERT-GGCs showed the normal goat chromosome number (2n = 60), consistent with a normal chromosome profile (Fig. 3B).
Next, we investigated the oncogenicity of hTERT-GGCs at passage 50 using a soft agar assay. After 10 days, we observed the formation of colonies in soft agar from Sp2/0 cells, a cell line with known oncogenicity (Fig. 3Cii). We did not observe any hTERT-GGC colony formation (Fig. 3Ci), suggesting that hTERT-GGCs do not have oncogenic capacity.
ER stress affects progesterone production and steroidogenic enzyme expression
To explore whether progesterone production and steroidogenic enzyme expression is affected in hTERT-GGCs under ERS conditions, hTERT-GGCs were treated with 0, 0.01, 0.1, or 1 μM of Tg. Tg is a drug that inhibits Ca2+ pumping into the ER and increases the accumulation of misfolded proteins. The expression of phosphor-IRE1α, GRP78, and CHOP proteins was significantly increased by Tg in a dose-dependent manner in hTERT-GGCs (Fig. 4A–D).
Fig. 4.
Effect of Tg on the expression of ERS-related proteins in hTERT-GGCs. (A) hTERT-GGCs were treated with 0, 0.01, 0.1, or 1 μM Tg for 12 h. (B-D) The band intensity analysis of phosphor-IRE1α, GRP78, and CHOP. Data presented are means ± SEM of three independent experiments. Bars with different letters are significantly different (P < 0.05).
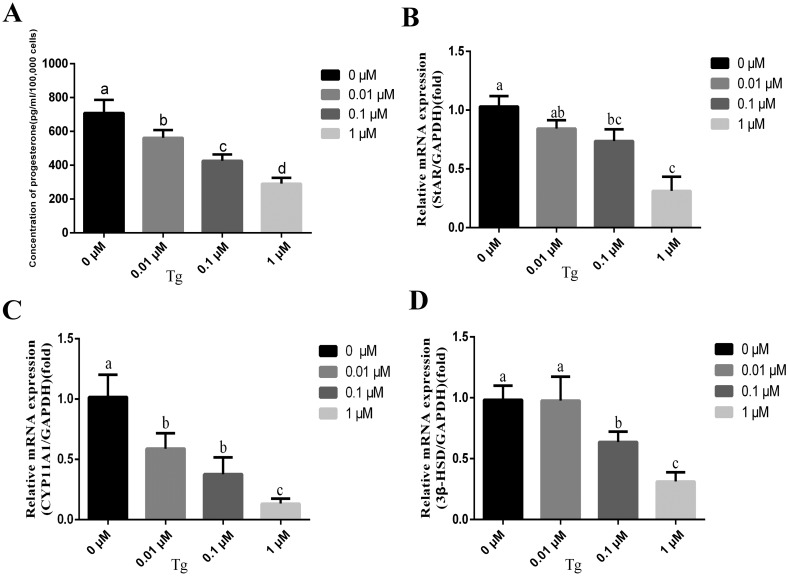
We then assessed whether the ERS inducer affected progesterone production in hTERT-GGCs. We measured the concentration of progesterone in the culture supernatants 12 h after Tg treatment. Tg treatment significantly decreased the concentration of progesterone in the culture supernatant and slightly inhibited cell viability (Fig. 5A and Supplementary Fig. 3: online only). In addition, Tg affected the expression levels of steroidogenic enzymes. Specifically, Tg significantly decreased the mRNA levels of StAR, CYP11A1, and 3β-HSD in a dose-dependent manner (Fig. 5B–D). Taken together these results showed that ERS suppressed progesterone production and steroidogenic enzyme expressions in hTERT-GGCs.
Fig. 5.
ER stress suppressed progesterone production and steroidogenic enzyme expression in hTERT-GGCs. (A) The secretion of progesterone in hTERT-GGCs treated with 0, 0.01, 0.1 and 1 μM Tg for 12 h was measured using an ELISA kit. (B) Key genes related to steroidogenesis (StAR, CYP11A1, and 3β-HSD) were measured by real-time-PCR. Data presented are means ± SEM of three independent experiments. Bars with different letters are significantly different (P < 0.05).
Discussion
Recent studies have shown that the immortalization of cells such as goat Leydig cells, goat luteal cells, myoblasts, human gingival epithelial cells, human germline stem cells, and human ovary surface epithelial cells can be achieved by induction of CDK4R24C, Cyclin D1, or hTERT and maintaining an intact genome [16, 34,35,36,37,38,39]. Here, we present the successful establishment of an immortalized steroidogenic goat granulosa cell line, hTERT-GGCs, derived from primary GGCs by transfection with the hTERT gene. hTERT-GGCs possess phenotypic characteristics of primary goat granulosa cells, as demonstrated by the detection of similar expression levels of a number of steroidogenesis-related enzymes, including StAR, CYP11A1, CYP19A1, and ESR. In addition, we observed that hTERT-GGCs maintained a similar morphological appearance as primary GGCs and secreted normal levels of progesterone. Our research indicated that this cell line could serve as a model with which to study molecular mechanisms regulating steroidogenesis and apoptosis.
Previous studies have confirmed that cellular senescence is related to telomere DNA loss. However, hTERT can reactivate telomerase and prevent telomeric DNA loss [40,41,42,43,44]. Moreover, the overexpression of hTERT can increase the proliferation potential of cells by allowing cells to obtain a remarkably higher growth rate and escape cellular senescence. Previous studies have demonstrated that cells transfected with hTERT preserve a similar characteristic and morphology of freshly isolated primary cells [45, 46].
In our study, we used hTERT to successfully establish a goat granulosa cell line. The hTERT-GGCs expressed hTERT at passages 30 and 50 as measured by RT-PCR and immunofluorescence. In addition, we confirmed that hTERT-GGCs had longer telomeres than GGCs, by RT-PCR. However, using a soft agar assay, a common method to identify oncogenicity, and proliferation assays we found no evidence of any malignant phenotype associated with the higher growth rate of hTERT-GGCs.
We also compared the mRNA expression levels of some genes related to steroidogenesis and steroidogenic enzymes in GGCs and hTERT-GGCs. We observed consistent steroidogenic phenotypes in GGCs and hTERT-GGCs, as measured by similar mRNA expression levels of StAR, CYP11A1, and CYP19A1. These results support the assertion that hTERT-GGCs maintain a normal phenotype, even though the expression of StAR in hTERT-GGCs at passage 50 decreased, compared with its expression at passage 30. Furthermore, our data suggest that the estrogen receptors ESR1 and ESR2 function properly in hTERT-GGCs. LHCGR, which is involved in steroidogenesis, is normally expressed in hTERT-GGCs [47]. Additionally, CYP17A1, the standard marker of thecal contamination of granulosa cell preparations and plays an important role in androstenedione synthesis, was expressed in hTERT-GGCs [48]. While some scholars agree that CYP17A1 is only expressed in theca cells and that GCs do not express CYP17A1 [49], it has been suggested that the expression of CYP17A1 can be detected in GCs [5]. Others studies have documented the relationship between FSHR expression and luteinization in humans and bovine. For example, downregulated FSHR expression indicates the initiation of GC luteinization [32, 49, 50]. In our studies, FSHR expression in hTERT-GGCs was low. Interestingly, a previous study reported that granulosa cells undergo functional luteinization when cultured in medium with serum [16]. The lack of FSHR expression in luteinized GCs might be consistent with this observation. This phenomenon underscores the fact that the hTERT-GGCs can be used as a model of early luteinizing granulosa cells.
The secretion of estradiol did not dramatically increase in hTERT-GGCs after stimulation with FSH. Therefore, the concentration of estradiol measured in GGCs was higher than that measured in hTERT-GGCs. A previous study showed that FSH treatment stimulates CYP19A1 activity and subsequent estradiol production [51]. StAR is considered to be the rate-limiting factor in steroid hormone synthesis [52]. We assumed that the relatively low level of FSHR and StAR affects estradiol production in hTERT-GGCs, although we did not observe downregulation of CYP19A1 at the mRNA level. In contrast, hTERT-GGCs secreted high levels of progesterone. Therefore, hTERT-GGCs appear to have functional luteinization, characterized by decreased estradiol levels and a sustained increase in progesterone throughout the culture period. It is important to point out that this phenomenon may be induced by serum [16]. For example, the culture conditions for granulosa cells supplemented with serum can induce granulosa cell differentiation. The serum itself already contains unknown ingredients including a variety of hormones, nutrients, and undefined growth factors that mainly cause granulosa cells to proliferate and luteinize [53]. Therefore, GGC culture conditions without serum need to be formally explored in future studies.
In animal cells the ER is an important organelle involved in protein folding and synthesis [54]. Cell stress or environmental change can destabilize intracellular homeostasis, leading to the accumulation of unfolded proteins and induce ERS [55]. Consequently, UPR is initiated to decrease ERS and restore homeostasis. GRP78 is a marker for ERS that interacts with three UPR signal transduction pathways [56]. IRE1, a dual-activity enzyme, dissociates from GRP78 and undergoes autophosphorylation to activate endoribonuclease activity and cleavage of downstream proteins [57]. CHOP is involved in the ERS induced apoptosis pathway, and functions to promote apoptosis under severe ERS [58]. Previous studies have reported that the three UPR-related signaling pathways are involved in the lifespan of the corpus luteum (CL) during the bovine estrous cycle [59]. Furthermore, heat-induced ERS affected testosterone production and steroidogenic enzyme expression in the Leydig cells of mice [60]. These results suggest that ERS can affect hormone secretion and the expression of steroidogenic enzymes in the secretory cells. Since granulosa cells are a type of endocrine secretory cell, we believed that some stimuli that produce ERS might affect the biosynthesis of estradiol and progesterone.
In this study, Tg treatment increased the protein levels of phospho-IRE1α, GRP78, and CHOP, suggesting that Tg can induce ERS in hTERT-GGCs in a dose-dependent manner. Moreover, with increasing doses of Tg, the concentration of progesterone was reduced in hTERT-GGCs, and steroidogenic enzyme expression was significantly decreased. Taken together, our results provide evidence that ERS inhibits the secretion of steroid hormones and the expression of steroidogenic enzymes in granulosa cells. However, further experiments need to be conducted to explore the mechanism by which UPR signal transduction pathways suppress the secretion of steroid hormones.
In conclusion, we established a stable immortalized goat granulosa cell line using hTERT. This cell line retained the same morphology, phenotype, and similar steroidogenic properties as goat primary granulosa cells. We believe that this cell line could be useful for studying the molecular mechanisms regulating steroidogenesis and apoptosis. In addition, treatment with Tg reduced progesterone production and decreased expression of steroidogenic enzymes in hTERT-GGCs, suggesting that Tg induced ERS.
Supplementary
Acknowledgments
We thank Dr Yuan Gao (Lanzhou Veterinary Research Institute) and Dr. Xiaochun Wu (College of Veterinary Medicine, Northwest A&F University) for their help with language editing. This research was funded by the National Natural Science Foundation of China (Grant No. 31372499, to YJ; Grant No. 31201966, to PL; Grant No. 31402077, to KT; Grant No. 31602125, to HC), the Scientific Research Foundation for Talents of Northwest A&F University (No. Z111021601, to HC), and the Specialized Natural Science Basic Research Plan in the Shaanxi Province of China (2014JQ3096).
References
- 1.Girard A, Dufort I, Douville G, Sirard MA. Global gene expression in granulosa cells of growing, plateau and atretic dominant follicles in cattle. Reprod Biol Endocrinol 2015; 13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen F, Wang N, Yang D, Wen X, Mahmoud TN, Zhou D, Tang K, Lin P, Wang A, Jin Y. Herp depletion arrests the S phase of the cell cycle and increases estradiol synthesis in mouse granulosa cells. J Reprod Dev 2016; 62: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehraiki A, Racine C, Krust A, Habert R, Levacher C. Phthalates impair germ cell number in the mouse fetal testis by an androgen- and estrogen-independent mechanism. Toxicol Sci 2009; 111: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong F, Hu L, Zhang Y, Xiao X, Xiao J. miR-22 inhibits mouse ovarian granulosa cell apoptosis by targeting SIRT1. Biol Open 2016; 5: 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu M, Quan F, Han C, Wu B, Liu J, Yang Z, Su F, Zhang Y. Effects of granulosa cells on steroidogenesis, proliferation and apoptosis of stromal cells and theca cells derived from the goat ovary. J Steroid Biochem Mol Biol 2013; 138: 325–333. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam A, Zauberman A, Meir G, Pinhasi-Kimhi O, Suh BS, Oren M. Cotransfection of granulosa cells with simian virus 40 and Ha-RAS oncogene generates stable lines capable of induced steroidogenesis. Proc Natl Acad Sci USA 1988; 85: 7582–7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin MT. Establishment of an immortalized porcine granulosa cell line (PGV) and the study on the potential mechanisms of PGV cell proliferation. Keio J Med 2005; 54: 29–38. [DOI] [PubMed] [Google Scholar]
- 8.Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata H. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001; 142: 437–445. [DOI] [PubMed] [Google Scholar]
- 9.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345: 458–460. [DOI] [PubMed] [Google Scholar]
- 10.Vaziri H, Schächter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley CB. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet 1993; 52: 661–667. [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeifer AM, Cole KE, Smoot DT, Weston A, Groopman JD, Shields PG, Vignaud JM, Juillerat M, Lipsky MM, Trump BF, et al. Simian virus 40 large tumor antigen-immortalized normal human liver epithelial cells express hepatocyte characteristics and metabolize chemical carcinogens. Proc Natl Acad Sci USA 1993; 90: 5123–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amicone L, Spagnoli FM, Späth G, Giordano S, Tommasini C, Bernardini S, De Luca V, Della Rocca C, Weiss MC, Comoglio PM, Tripodi M. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO J 1997; 16: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briers TW, van de Voorde A, Vanderstichele H. Characterization of immortalized mouse granulosa cell lines. In Vitro Cell Dev Biol Anim 1993; 29A: 847–854. [DOI] [PubMed] [Google Scholar]
- 14.Toouli CD, Huschtscha LI, Neumann AA, Noble JR, Colgin LM, Hukku B, Reddel RR. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene 2002; 21: 128–139. [DOI] [PubMed] [Google Scholar]
- 15.Inoshima Y, Ishiguro N. Establishment of vascular endothelial cell lines from the aortas of wild Japanese serows (Capricornis crispus). Cell Biol Int 2009; 33: 617–620. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Dai R, Lei L, Lin P, Lu X, Wang X, Tang K, Wang A, Jin Y. Establishment and evaluation of a stable steroidogenic goat Leydig cell line. Anim Sci J 2016; 87: 492–502. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Xu X, Huang Y, Li Z, Yu G, Wang Z, Ding L, Tong D. Establishment and evaluation of a stable steroidogenic caprine luteal cell line. Theriogenology 2012; 78: 263–272. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz-Gutiérrez JF, Schneider DA, Baszler TV, Greenlee JJ, Nicholson EM, Stanton JB. hTERT-immortalized ovine microglia propagate natural scrapie isolates. Virus Res 2015; 198: 35–43. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Lin P, Yin Y, Zhou J, Lei L, Zhou X, Jin Y, Wang A. Brucella suis vaccine strain S2-infected immortalized caprine endometrial epithelial cell lines induce non-apoptotic ER-stress. Cell Stress Chaperones 2015; 20: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 1999; 13: 1211–1233. [DOI] [PubMed] [Google Scholar]
- 21.Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med 2006; 6: 55–69. [DOI] [PubMed] [Google Scholar]
- 22.Sanderson TH, Gallaway M, Kumar R. Unfolding the unfolded protein response: unique insights into brain ischemia. Int J Mol Sci 2015; 16: 7133–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res 2005; 569: 29–63. [DOI] [PubMed] [Google Scholar]
- 24.Park SW, Ozcan U. Potential for therapeutic manipulation of the UPR in disease. Semin Immunopathol 2013; 35: 351–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol Chem 2014; 289: 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SJ, Kim TS, Park CK, Lee SH, Kim JM, Lee KS, Lee IK, Park JW, Lawson MA, Lee DS. hCG-induced endoplasmic reticulum stress triggers apoptosis and reduces steroidogenic enzyme expression through activating transcription factor 6 in Leydig cells of the testis. J Mol Endocrinol 2013; 50: 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HJ, Park SJ, Koo DB, Lee SR, Kong IK, Ryoo JW, Park YI, Chang KT, Lee DS. Progesterone production is affected by unfolded protein response (UPR) signaling during the luteal phase in mice. Life Sci 2014; 113: 60–67. [DOI] [PubMed] [Google Scholar]
- 28.Lin P, Yang Y, Li X, Chen F, Cui C, Hu L, Li Q, Liu W, Jin Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol Reprod Dev 2012; 79: 423–432. [DOI] [PubMed] [Google Scholar]
- 29.Yuan JH, Wang JZ, Lan GC, Sui HS, Yu JN, Tan JH. Expression of steroidogenic enzymes and synthesis of steroid hormones during development of ovarian follicles in prepubertal goats. Domest Anim Endocrinol 2008; 34: 451–460. [DOI] [PubMed] [Google Scholar]
- 30.Lin P, Lan X, Chen F, Yang Y, Jin Y, Wang A. Reference gene selection for real-time quantitative PCR analysis of the mouse uterus in the peri-implantation period. PLoS ONE 2013; 8: e62462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3: research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab 2012; 97: E1524–E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatzirodos N, Hummitzsch K, Irving-Rodgers HF, Rodgers RJ. Transcriptome comparisons identify new cell markers for theca interna and granulosa cells from small and large antral ovarian follicles. PLoS ONE 2015; 10: e0119800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki R, Narisawa-Saito M, Yugawa T, Fujita M, Tashiro H, Katabuchi H, Kiyono T. Oncogenic transformation of human ovarian surface epithelial cells with defined cellular oncogenes. Carcinogenesis 2009; 30: 423–431. [DOI] [PubMed] [Google Scholar]
- 35.Shiomi K, Kiyono T, Okamura K, Uezumi M, Goto Y, Yasumoto S, Shimizu S, Hashimoto N. CDK4 and cyclin D1 allow human myogenic cells to recapture growth property without compromising differentiation potential. Gene Ther 2011; 18: 857–866. [DOI] [PubMed] [Google Scholar]
- 36.Zushi Y, Narisawa-Saito M, Noguchi K, Yoshimatsu Y, Yugawa T, Egawa N, Fujita M, Urade M, Kiyono T. An in vitro multistep carcinogenesis model for both HPV-positive and -negative human oral squamous cell carcinomas. Am J Cancer Res 2011; 1: 869–881. [PMC free article] [PubMed] [Google Scholar]
- 37.Evans JR, Schreiber NB, Williams JA, Spicer LJ. Effects of fibroblast growth factor 9 on steroidogenesis and control of FGFR2IIIc mRNA in porcine granulosa cells. J Anim Sci 2014; 92: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou J, Niu M, Liu L, Zhu Z, Wang X, Sun M, Yuan Q, Yang S, Zeng W, Liu Y, Li Z, He Z. Establishment and characterization of human germline stem cell line with unlimited proliferation potentials and no tumor formation. Sci Rep 2015; 5: 16922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moffatt-Jauregui CE, Robinson B, de Moya AV, Brockman RD, Roman AV, Cash MN, Culp DJ, Lamont RJ. Establishment and characterization of a telomerase immortalized human gingival epithelial cell line. J Periodontal Res 2013; 48: 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su F, Liu X, Liu G, Yu Y, Wang Y, Jin Y, Hu G, Hua S, Zhang Y. Establishment and evaluation of a stable cattle type II alveolar epithelial cell line. PLoS ONE 2013; 8: e76036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279: 349–352. [DOI] [PubMed] [Google Scholar]
- 42.Fiedler W, Reinicke D, Aurich H, Christ B, Fleig WE, Ballhausen WG. In vitro immortalisation of porcine hepatocytes by transfection with the gene for the human catalytic subunit of telomerase reverse transcriptase (hTERT). J Hepatol 2004; 40 (Suppl): 102. 14672620 [Google Scholar]
- 43.Meyerson M. Telomerase enzyme activation and human cell immortalization. Toxicol Lett 1998; 102−103: 41–45. [DOI] [PubMed] [Google Scholar]
- 44.Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology 2004; 45: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong HX, Zhang YM, Xu H, Su ZY, Sun P. Immortalization of swine umbilical vein endothelial cells with human telomerase reverse transcriptase. Mol Cells 2007; 24: 358–363. [PubMed] [Google Scholar]
- 46.He YL, Wu YH, He XN, Liu FJ, He XY, Zhang Y. An immortalized goat mammary epithelial cell line induced with human telomerase reverse transcriptase (hTERT) gene transfer. Theriogenology 2009; 71: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 47.Martinat N, Crépieux P, Reiter E, Guillou F. Extracellular signal-regulated kinases (ERK) 1, 2 are required for luteinizing hormone (LH)-induced steroidogenesis in primary Leydig cells and control steroidogenic acute regulatory (StAR) expression. Reprod Nutr Dev 2005; 45: 101–108. [DOI] [PubMed] [Google Scholar]
- 48.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nimz M, Spitschak M, Schneider F, Fürbass R, Vanselow J. Down-regulation of genes encoding steroidogenic enzymes and hormone receptors in late preovulatory follicles of the cow coincides with an accumulation of intrafollicular steroids. Domest Anim Endocrinol 2009; 37: 45–54. [DOI] [PubMed] [Google Scholar]
- 50.Wu YG, Barad DH, Kushnir VA, Lazzaroni E, Wang Q, Albertini DF, Gleicher N. Aging-related premature luteinization of granulosa cells is avoided by early oocyte retrieval. J Endocrinol 2015; 226: 167–180. [DOI] [PubMed] [Google Scholar]
- 51.Stapp AD, Gómez BI, Gifford CA, Hallford DM, Hernandez Gifford JA. Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells. PLoS ONE 2014; 9: e86432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q, Wang J, Zhu J, Liu J, Zhang J, Zhao M. Assessment of the endocrine-disrupting effects of short-chain chlorinated paraffins in in vitro models. Environ Int 2016; 94: 43–50. [DOI] [PubMed] [Google Scholar]
- 53.Piccinato CA, Montrezor LH, Collares CA, Vireque AA, Rosa e Silva AA. Norepinephrine stimulates progesterone production in highly estrogenic bovine granulosa cells cultured under serum-free, chemically defined conditions. Reprod Biol Endocrinol 2012; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sreedhar R, Giridharan VV, Arumugam S, Karuppagounder V, Palaniyandi SS, Krishnamurthy P, Quevedo J, Watanabe K, Konishi T, Thandavarayan RA. Role of MAPK-mediated endoplasmic reticulum stress signaling in the heart during aging in senescence-accelerated prone mice. Biofactors 2016; 42: 368–375. [DOI] [PubMed] [Google Scholar]
- 55.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 2013; 15: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaira K, Toyoda M, Shimizu A, Shino M, Sakakura K, Takayasu Y, Takahashi K, Asao T, Chikamatsu K. Expression of ER stress markers (GRP78/BiP and PERK) in adenoid cystic carcinoma. Acta Otolaryngol 2016; 136: 1–7. [DOI] [PubMed] [Google Scholar]
- 57.Martins AS, Alves I, Helguero L, Domingues MR, Neves BM. The unfolded protein response in homeostasis and modulation of mammalian immune cells. Int Rev Immunol 2016; 35: 457–476. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y, Pei X, Jin Y, Wang Y, Zhang C. The roles of endoplasmic reticulum stress response in female mammalian reproduction. Cell Tissue Res 2016; 363: 589–597. [DOI] [PubMed] [Google Scholar]
- 59.Park HJ, Park SJ, Koo DB, Kong IK, Kim MK, Kim JM, Choi MS, Park YH, Kim SU, Chang KT, Park CK, Chae JI, Lee DS. Unfolding protein response signaling is involved in development, maintenance, and regression of the corpus luteum during the bovine estrous cycle. Biochem Biophys Res Commun 2013; 441: 344–350. [DOI] [PubMed] [Google Scholar]
- 60.Kim JH, Park SJ, Kim TS, Kim JM, Lee DS. Testosterone production by a Leydig tumor cell line is suppressed by hyperthermia-induced endoplasmic reticulum stress in mice. Life Sci 2016; 146: 184–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.