Significance
Our findings demonstrate a function for Down syndrome cell adhesion molecule (DSCAM) in the embryonic development of the mouse visual system. We found that DSCAM promotes fasciculation and growth of axons in the developing mouse optic pathway, at least in part, through homotypic interactions. We also found that shed DSCAM can act independently of direct cell–cell contact to provide growth-promoting signals. Because the Dscam mutant phenotypes are mediated in a dose-dependent manner, these results are potentially relevant to human Down syndrome, in which Dscam is overexpressed as a result of trisomy of chromosome 21, and fragile-X syndrome, in which Dscam is overexpressed because of misregulation of Dscam mRNA.
Keywords: axon guidance, development, growth cone, optic chiasm, visual system
Abstract
Although many aspects of optic pathway development are beginning to be understood, the mechanisms promoting the growth of retinal ganglion cell (RGC) axons toward visual targets remain largely unknown. Down syndrome cell adhesion molecule (Dscam) is expressed by mouse RGCs shortly after they differentiate at embryonic day 12 and is essential for multiple aspects of postnatal visual system development. Here we show that Dscam is also required during embryonic development for the fasciculation and growth of RGC axons. Dscam is expressed along the developing optic pathway in a pattern consistent with a role in regulating RGC axon outgrowth. In mice carrying spontaneous mutations in Dscam (Dscamdel17; Dscam2J), RGC axons pathfind normally, but growth from the chiasm toward their targets is impaired, resulting in a delay in RGC axons reaching the dorsal thalamus compared with that seen in wild-type littermates. Conversely, Dscam gain of function results in exuberant growth into the dorsal thalamus. The growth of ipsilaterally projecting axons is particularly affected. Axon organization in the optic chiasm and tract and RGC growth cone morphologies are also altered in Dscam mutants. In vitro DSCAM promotes RGC axon growth and fasciculation, and can act independently of cell contact. In vitro and in situ DSCAM is required both in the RGC axons and in their environment for the promotion of axon outgrowth, consistent with a homotypic mode of action. These findings identify DSCAM as a permissive signal that promotes the growth and fasciculation of RGC axons, controlling the timing of when RGC axons reach their targets.
The developing vertebrate visual system has proven to be one of the most informative model systems for studying axon growth and guidance decision (1). In mice, retinal ganglion cell (RGC) axons grow into the brain from embryonic day (E) 12.5, although mapping within visual targets is not complete until postnatal stages (1). Molecules have been identified that are important for multiple aspects of RGC axon growth and pathfinding, including guidance out of the eye, constraining the general path followed by RGC axons and midline routing at the optic chiasm underlying the establishment of stereovision (1); however, the majority of these molecules induce inhibitory responses in RGC axons. Much less is known about the growth-promoting mechanisms that drive RGC axon extension.
Down syndrome cell adhesion molecule 1 (Dscam1) is a homophilic adhesion molecule essential for multiple aspects of neural circuit formation in Drosophila, including axon growth, fasciculation, and pathfinding; dendritic field organization; and synaptic specificity and targeting (2, 3). Although vertebrate Dscam lacks the extensive alternative splicing of fly Dscam1, it is required for many of the same developmental processes, including dendritic arborization and synaptic lamination and refinement (4).
Dscam is expressed by mouse RGCs from E12.5 (5) and, through regulation of cell death and homophilic antiadhesive interactions, controls the mosaic spacing and dendritic arborization of RGCs in the postnatal retina (5–7). However, the expression of Dscam in RGCs from early embryonic stages raises the possibility that DSCAM may facilitate additional aspects of RGC development, such as controlling axon outgrowth. In support of this idea, DSCAM has been implicated as a receptor for the guidance cue netrin-1 (8, 9), a key factor in controlling the growth of RGC axons out of the eye (10); however, in mice lacking Dscam, netrin-dependent guidance of spinal commissural axons occurs normally, arguing against a critical role for DSCAM in axon patterning in vivo (11).
Using two different Dscam mutant alleles and mice overexpressing Dscam (7), we have found that DSCAM is not essential for the netrin-dependent process of exiting the eye but is expressed along the developing optic pathway, promoting axon fasciculation and providing growth-promoting interactions that help drive RGC axon growth toward visual targets.
Results and Discussion
Dscam Is Expressed Along the Developing Optic Pathway.
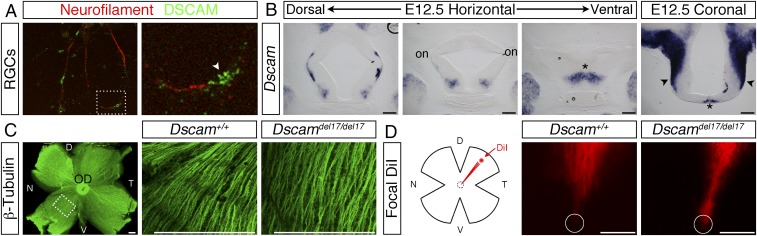
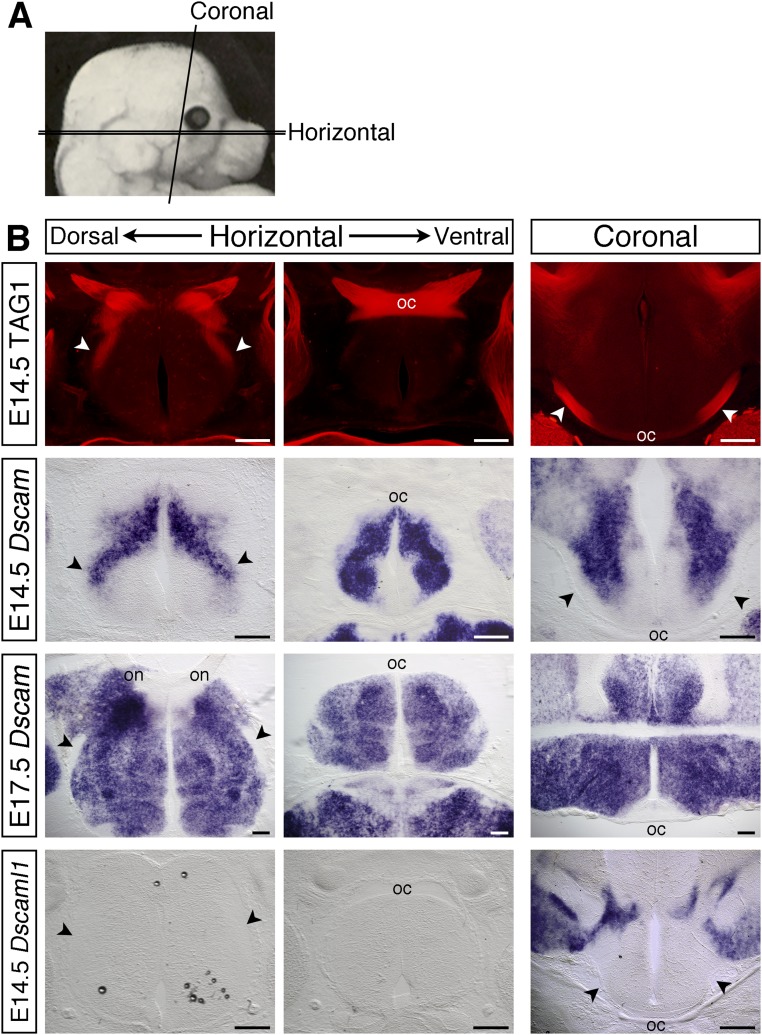
From E12.5 through to postnatal ages Dscam is expressed strongly by most, if not all, RGCs (5). Double immunofluorescent staining of cultured P0 retinal ganglion cells with antibodies validated previously against DSCAM (12–14) and neurofilament confirmed that DSCAM localized to retinal axons, with expression concentrated at the growth cone (Fig. 1A, white arrowhead). Dscam is also expressed along the developing optic pathway (Figs. 1B and Fig. S1). At E12.5, Dscam was expressed around the region where the optic nerves join with the brain, posterior to the developing optic chiasm and bordering the presumptive optic tracts (Fig. 1B). As development proceeded, the level of Dscam increased, and by E17.5, expression was present throughout the ventral diencephalon (Fig. S1). In contrast, expression of the related gene, Dscaml1 (15), was not detected in the embryonic retina (5) or along the developing optic pathway (Fig. S1).
Fig. 1.
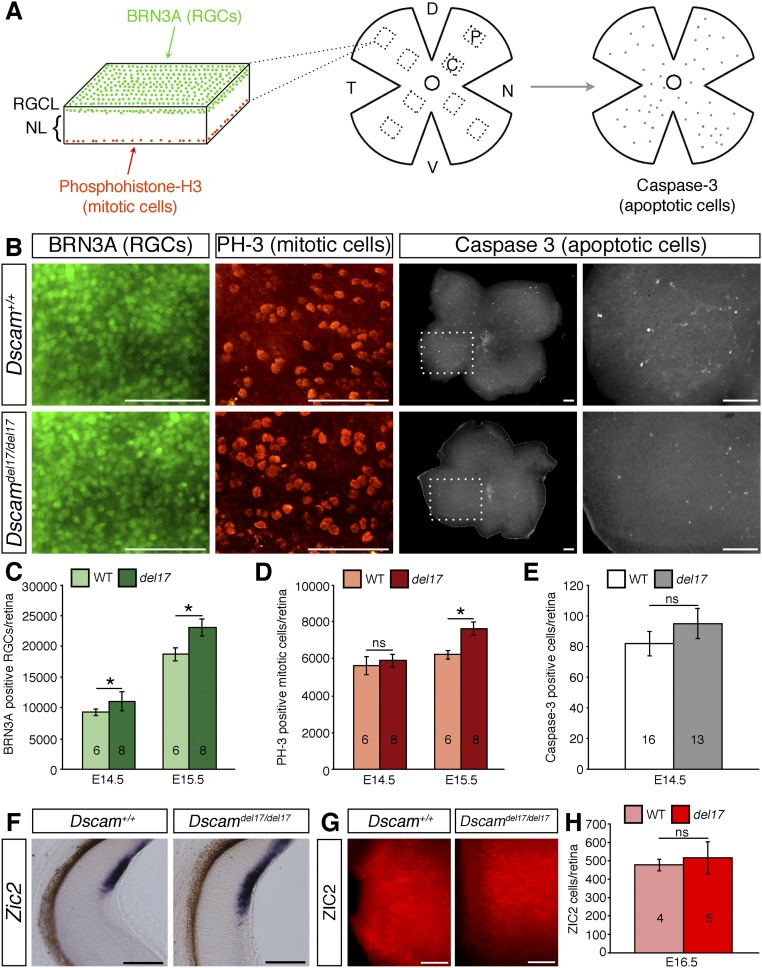
DSCAM is expressed by retinal axons and along the developing optic pathway, but is not essential for intraretinal axon pathfinding. (A) Double immunofluorescent staining of cultured P0 retinal neurons with antibodies against DSCAM (green) or neurofilaments (red). (Right) Boxed region shown at higher power. The white arrowhead indicates the growth cone. (B) In situ hybridization for Dscam on horizontal and coronal sections through the ventral diencephalon of E12.5 WT embryos. on, optic nerve; asterisk, presumptive chiasm; black arrowheads, presumptive optic tracts. (C) Flat-mounted E15.5 WT retina stained with antibodies against neuron-specific β-tubulin to label all retinal axons. The box indicates the region in which confocal images through the optic fiber layer were captured in E15.5 Dscam+/+ and Dscamdel17/del17 retinas (Right). (D) Schematic illustration of the method used to label small groups of RGC axons and images of labeled RGC axons in dorsal retina of E15.5 Dscamdel17/del17 WT and mutants. Circles indicate the position of the optic disk. D, dorsal; N, nasal; OD, optic disk; T, temporal; V, ventral. (Scale bars: 200 µm.)
Fig. S1.
Expression of Dscam along the developing optic pathway. (A) Schematic illustration of the plains of sections used. (B) Serial horizontal sections through the ventral diencephalon and coronal sections at the level of the optic tracts of E14.5 and E17.5 C57BL/6J embryos stained with antibodies against TAG-1 to label the RGC axons (Top), or by in situ hybridization with probes specific for Dscam or Dscaml1. Horizontal sections, anterior up; coronal sections, dorsal up. oc, optic chiasm; on, optic nerve; arrowheads, optic tracts. (Scale bars: 200 µm.)
DSCAM Is Not Essential for the Guidance of RGC Axons Out of the Eye.
Because DSCAM has been implicated as a receptor for netrin-1 (8, 9), a key factor controlling the growth of RGC axons out of the eye (10), we analyzed RGC axon patterning in retinas of E15.5 and E17.5 mice carrying a spontaneous mutation in Dscam (Dscamdel17) (16). Homozygous Dscamdel17 mice display a 70% reduction in Dscam mRNA levels (16) and express low amounts of a truncated protein that is unlikely to be functional (14). On the C57BL/6J background used in this study, Dscamdel71/del17 mutants appear relatively normal until birth, but die perinatally (16).
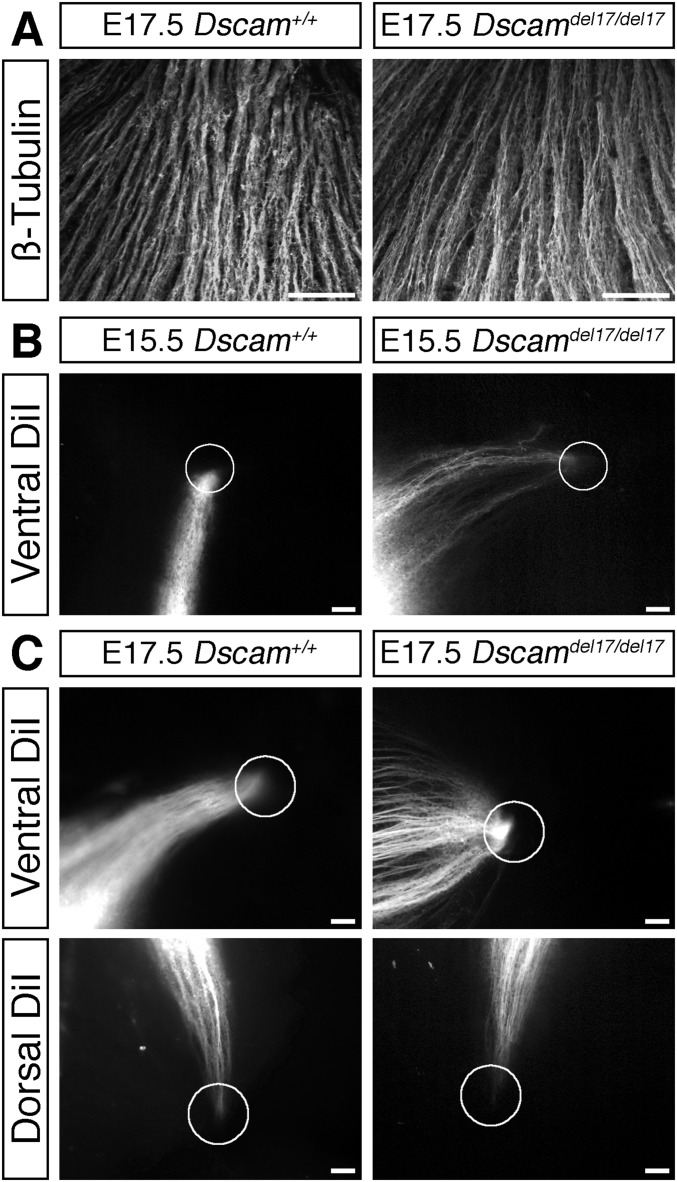
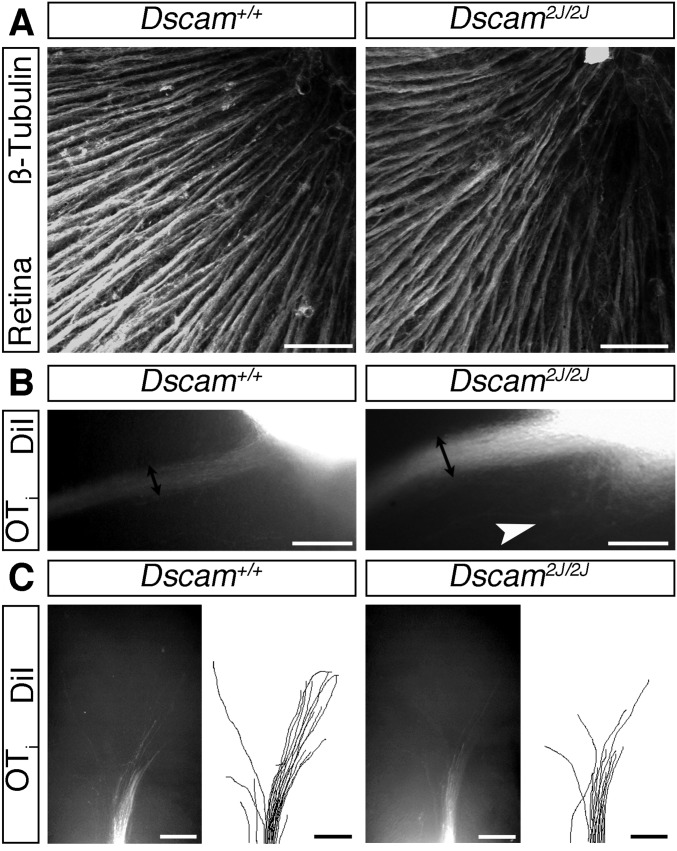
We found no defects in Dscamdel71/del17 mutants in the growth of RGC axons toward or out of the optic disk. Labeling of all RGC axons revealed no obvious defects in axon organization in the retina of Dscamdel17/del17 mutants (Figs. 1C and Fig. S2A). Moreover, labeling small groups of axons demonstrated normal targeting and exit of RGC axons at the optic disk (Figs. 1D and Fig. S2 B and C). Identical results were obtained using a second Dscam mutant allele (Dscam2J) that we have confirmed to be protein-null (14) (Fig. S3A). These findings demonstrate that DSCAM is not essential for the netrin-dependent process of exiting the eye, in agreement with previous analyses of mice carrying a targeted null mutation in Dscam (Dscam−/−) that failed to identify a critical requirement for DSCAM in the netrin-induced outgrowth and guidance of spinal commissural axons (11).
Fig. S2.
DSCAM is not required for intraretinal RGC axon guidance. (A) Confocal images of RGC axons labeled with antibodies against β-tubulin in the optic fiber layer of E17.5 Dscamdel17 WT and mutant retinas. The direction of the optic disk is up. (B and C) Small groups of DiI-labeled RGC axons originating in ventral or dorsal retina in E15.5 (B) and E175 (C) WT and Dscamdel17 mutant littermates. Circles indicate the positions of the optic disk. (Scale bars: 50 µm.)
Fig. S3.
Dscam2J mutants display similar RGC axon guidance phenotypes to Dscamdel17 mutants. (A) Confocal images of RGC axons in the optic fiber layer of E16.5 Dscam2J WT and mutant retinas stained with antibodies against β-tubulin. The direction of the optic disk is up. (B and C) Whole-mount views of anterograde DiI-labeled RGC axons in the ipsilateral optic tract adjacent to the chiasm (B) and dorsal thalamus (C) of E16.5 Dscam2J WT and mutant littermates. In C, the cortex has been removed, and the brains are viewed from the side. Double-headed black arrows indicate the width of the optic tract; white arrowhead, axons straying away from their normal pathway. (Scale bars: 50 µm in A; 250 µm in B and C.)
Evidence that DSCAM is a netrin-1 receptor has come from binding studies and temporal disruption of DSCAM function using siRNA-knockdown and dominant-negative constructs (8, 9). Studies of other putative netrin-signaling components also have found functional differences following acute pharmacologic- or siRNA-induced knockdown compared with genetic loss of function (17, 18). Thus, future studies may examine whether netrin-signaling is exquisitely sensitive to acute down-regulation of potentially redundant proteins or can compensate for the longer-term loss of signaling components that occurs in genetic null mutants. However, redundancy of DSCAM with DCC, a known netrin-1 receptor, has been excluded, at least for spinal commissural neurons (11).
More RGC Axons Are Present at the Optic Chiasm of Dscam Mutants, but Growth Toward Visual Targets Is Impaired.
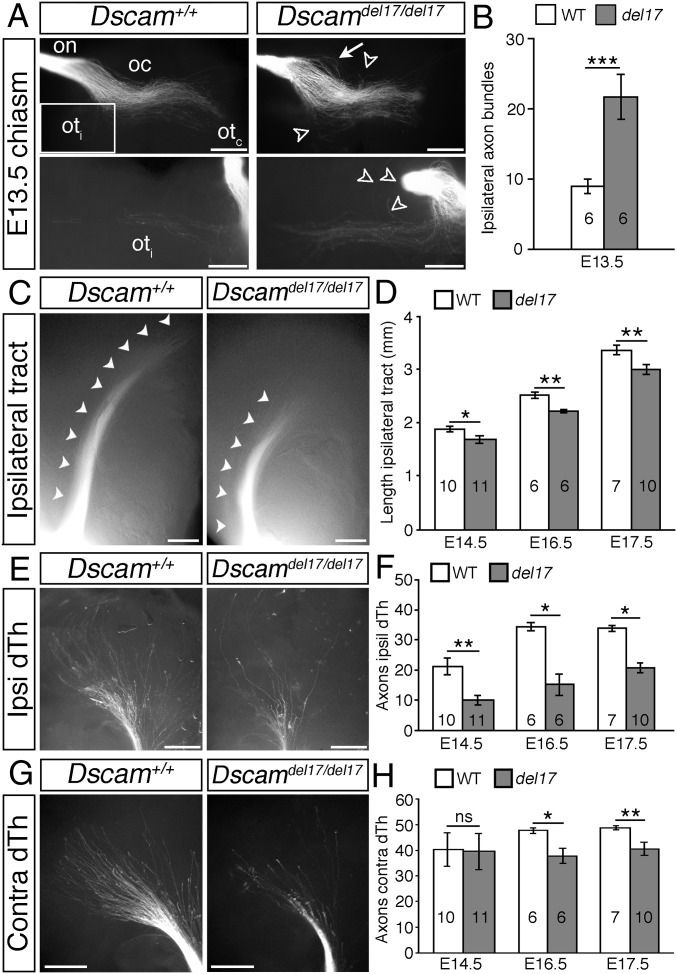
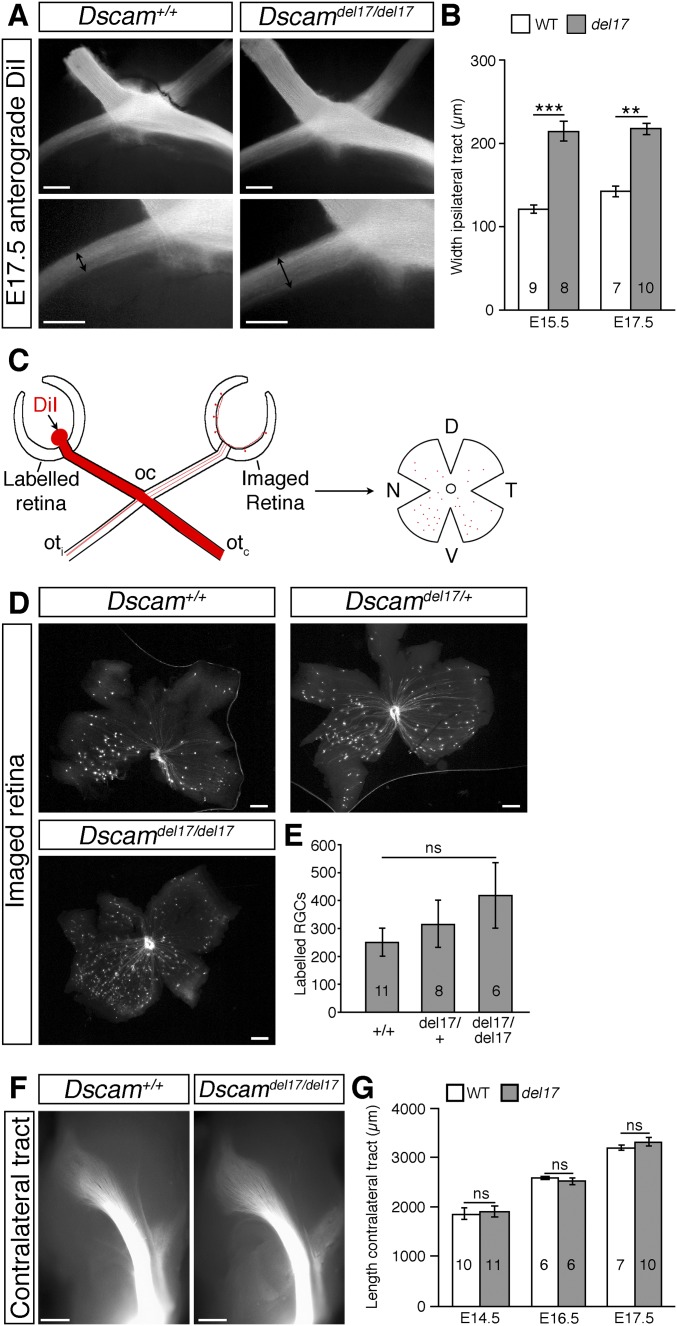
Because DSCAM has been well characterized as a homophilic binding molecule (19, 20) and is expressed both by RGCs and bordering the developing optic pathway, we next asked whether DSCAM regulates RGC growth from the eye toward visual brain targets. The number of RGCs is significantly increased in postnatal Dscamdel17/del17 retinas (5). Quantitation revealed a significant increase in RGCs also at embryonic stages (Fig. S4 A–C), likely driven by increased cell production (Fig. S4 A, B, and D) rather than reduced cell death (Fig. S4 A, B, and E). Associated with this increase in RGC number, was a significant increase in the number of RGC axon bundles at the optic chiasm of Dscamdel17 mutants. Following labeling with DiI of all axons from one eye of E13.5 embryos, more than twice as many RGC axon bundles were labeled in the ipsilateral optic tract of E13.5 Dscamdel17/del17 mutants compared with stage-matched littermates (Fig. 2 A and B). At later stages, the width of the ipsilateral optic tract was increased significantly, consistent with a sustained increase in the number of RGC axons (Fig. S5 A and B). Similar changes in the size of the ipsilateral optic tract were found in Dscam2J mutants (Fig. S3B). There was also a trend toward an increase in the number of RGC axons projecting to the contralateral eye in Dscamdel17/+ and Dscamdel17/del17 embryos compared with WT littermates (Fig. S5 C–E).
Fig. S4.
RGC genesis is increased in Dscamdel17 mutants, but specification of ipsilaterally projecting RGCs occurs normally. (A) Schematic illustration of the method used to quantify RGC, mitotic, and apoptotic cell numbers. Retinas were stained by double immunofluorescence with antibodies against BRN3A to label RGCs, with phosphohistone H to label mitotic cells, or with cleaved caspase-3 to label apoptotic cells. Confocal images were captured through the RGC, and neuroblastic layers from four peripheral (p) and four central (c) regions in each retina of BRN3A- and phosphohistone H3-positive cells, and the number of labeled cells in each image was quantified and used to calculate the total number of labeled cells in each retina. Caspase-3–positive cells were quantified by counting all labeled cells. D, dorsal; N, nasal; NL, neuroblastic layer; RGCL, RGC layer; T, temporal; V, ventral. (B) Confocal images of BRN3A-positive RGCs and phosphohistone H3-positive mitotic cells in the RGC layer and neuroblastic layer respectively, and caspase-3–positive cells in E14.5 Dscamdel17 WT and mutant retinas. (C–E) Mean ± SEM number of BRN3A-positive RGCs (C), phosphohistone-H3–positive mitotic cells (D), and caspase-3–positive apoptotic cells (E) in retinas from E14.5 and E15.5 Dscamdel17 WT and mutant (del17) retinas. (F) Sections of E15.5 Dscamdel17 WT and mutant retinas stained by in situ hybridization with a probe specific for Zic2, a marker of ipsilaterally specified RGCs. (G) Peripheral ventrotemporal retina from E16.5 Dscamdel17 WT and mutant littermates stained with an antibody against ZIC-2. (H) Mean ± SEM number of ZIC2-positive cells in retinas from E16.5 Dscamdel17 mutant (del17) and WT littermates. *P < 0.05; ns, not significant. The numbers analyzed at each age and genotype are indicated on the bars. (Scale bars: 100 µm.)
Fig. 2.
RGC axon growth through the optic tracts is impaired in Dscam mutants. (A, C, E, and G) Whole-mount views of anterograde DiI-labeled RGC axons in the optic nerve (on), optic chiasm (oc), and proximal contralateral (otc) and ipsilateral (oti) optic tracts of E13.5 Dscamdel17 WT and mutant littermates (A) and in the ipsilateral optic tracts (C) and ipsilateral and contralateral dorsal thalamus (E and G) of E16.5 Dscamdel17 WT and mutant littermates. The boxed region in A indicates the region of the ipsilateral optic tract shown at higher power in the lower panels. White arrows indicate defasciculated axons in the optic nerve; open arrowheads, straying axons. (A) Ventral views. (C, E, and G) Side views following removal of the cortex. (B, D, F, and H) Mean ± SEM number of axon bundles in the ipsilateral optic tract of E13.5 Dscamdel17 WT and mutant (del17) littermates (B), and ipsilateral optic tract length (D) and number of axon bundles in the ipsilateral (F) and contralateral (H) dorsal thalamus (dTh) of E14.5–E17.5 Dscamdel17 WT and mutant littermates. Analyses were performed blinded to genotype. Numbers on bars indicate the numbers analyzed. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant. (Scale bars: 250 µm.)
Fig. S5.
RGC axon projections in Dscamdel17 mutant and WT littermates. (A) Whole-mount views of anterograde DiI-labeled RGC axons at the optic chiasm (Top) and proximal ipsilateral optic tract (Bottom) of E17.5 Dscamdel17 WT and mutant littermates. Double-headed black arrows indicate the width of the ipsilateral optic tract. (B) Mean ± SEM width of the ipsilateral optic tract in E15.5 and E17.5 Dscamdel17 WT and mutant (del17) littermates. (C) Schematic diagram illustrating the method used to quantify RGC axons projecting between the two eyes. RGC axons from one eye were labeled with DiI, and after allowing time for the dye to diffuse, the contralateral retina was removed and flat-mounted, and the number of labeled cells was quantified. (D) Labeled cells in the contralateral retina of E16.5 Dscamdel17 WT, heterozygous, and homozygous mutant embryos. (E) Mean ± SEM number of labeled cells in the contralateral retina of E16.5 Dscamdel17 WT, heterozygous, and homozygous mutant embryos. (F) Whole-mount views of anterograde DiI-labeled RGC axons in the contralateral optic tracts of E16.5 Dscamdel17 WT and mutant littermates. Here the cortex has been removed, and the diencephalon is viewed from the side in each image. (G) Mean ± SEM contralateral optic tract length in E14.5–E17.5 Dscamdel17 WT and mutant littermates. Analyses were performed blinded to genotype. Numbers on the bars indicate the numbers analyzed at each age and genotype. **P < 0.01; ***P < 0.001; ns, not significant. (Scale bars: 250 µm in A and F; 200 µm in D.)
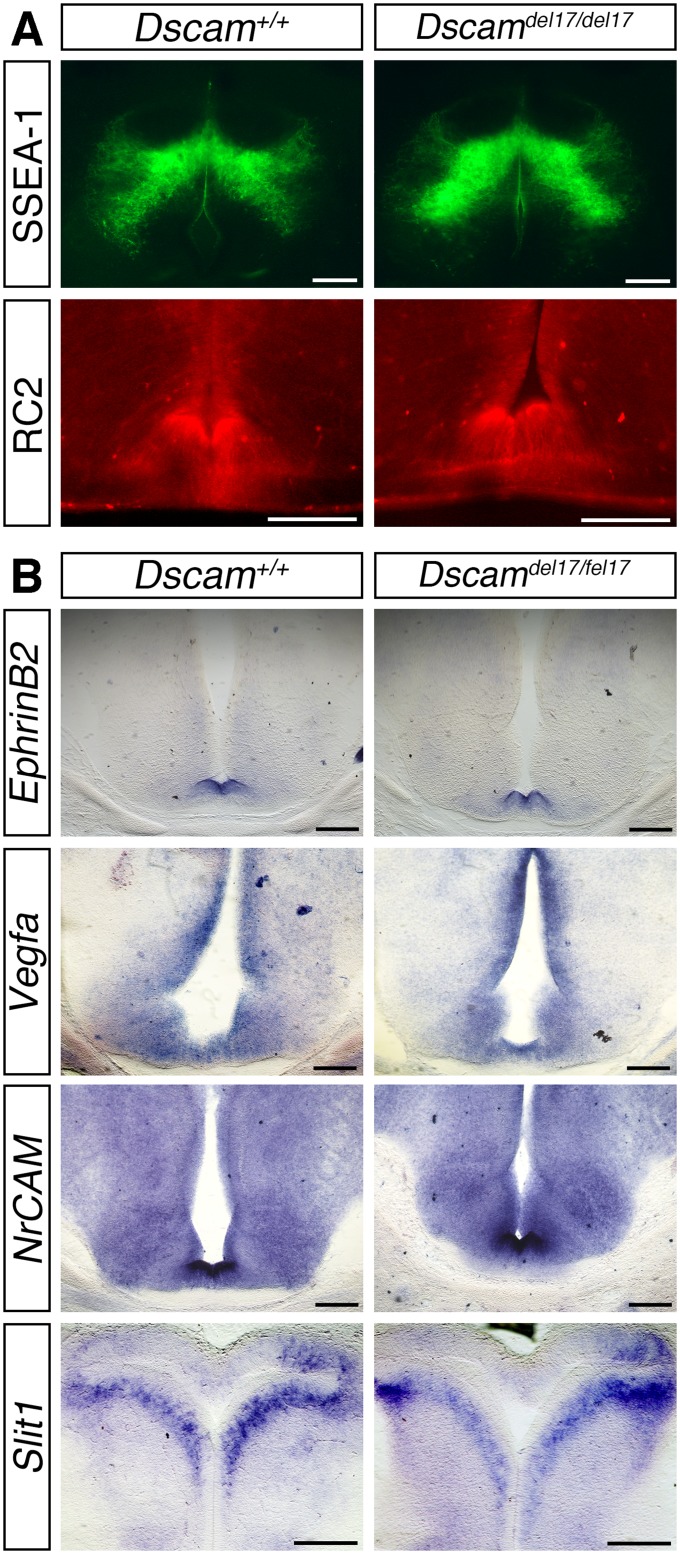
Although we found that more RGC axons reached the optic chiasm of Dscamdel17/del17 mutants, extension of these axons into the dorsal thalamus was severely impaired. In the same DiI-labeled embryos used for the chiasm studies, the ipsilateral optic tracts were significantly shorter in Dscamdel17/del17 mutants compared with stage-matched WT littermates at all ages examined (Fig. 2 C and D). The number of RGC axon bundles in the ipsilateral dorsal thalamus was also decreased significantly (Fig. 2 E and F). The length of the contralateral optic tract of Dscamdel17/del17 mutants was not significantly different compared with WT (Fig. S5 F and G), but from E16.5, the number of RGC axon bundles in the contralateral dorsal thalamus was decreased significantly (Fig. 2 G and H). A reduced number of RGC axons in the dorsal thalamus was also found in E16.5 Dscam2J mutants (Fig. S3C). Retrograde labeling of RGCs by placing small crystals of DiI into a consistent site in the dorsal thalamus on one side confirmed that fewer RGC axons reached the dorsal thalamus of Dscamdel17/del17 embryos compared with WT littermates (Fig. S6). Because the same embryos were used for the analyses of the optic chiasm and tract and more RGC axons were present at the optic chiasm (Fig. 2 A and B), we consider it highly unlikely that a labeling problem or delay in RGC axonogenesis underlies the stunted appearance of the ipsilateral optic tract in Dscamdel17 mutant embryos. The similar lengths of the contralateral tracts in Dscamdel17/del17 mutants and WT littermates (Fig. S5 F and G) also was inconsistent with a developmental delay or significant reduction in brain size in the mutants. The morphology and patterning of the ventral diencephalon of the mutants also appeared relatively normal. The organization of the SSEA-1–positive neurons located posterior to the chiasm and RC2-positive radial glia (21) was similar in Dscamdel17/del17 mutants and WT littermates (Fig. S7A). The expression of ephrinB2, Vegfa, and Nrcam, important for the divergent growth of RGC axons at the chiasm midline (22–24), and Slit1, which helps keep RGC axons from straying from their normal pathway (25), also appeared similar in WT and mutants (Fig. S7B). Taken together, these findings are consistent with a role for DSCAM in promoting the growth or guidance of RGC axons through the optic tracts.
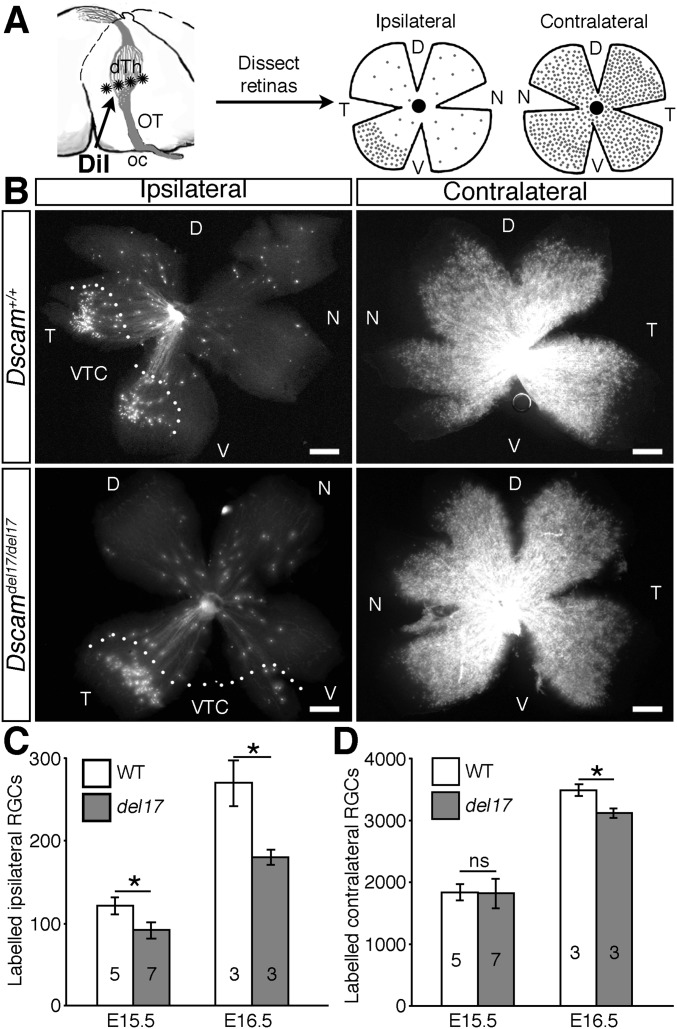
Fig. S6.
The number of RGC axons reaching the dorsal thalamus is lower in Dscamdel17 mutant embryos compared with WT littermates. (A) Schematic illustration of the method used to retrogradely label RGCs from the dorsal thalamus (dTh). Crystals of DiI (asterisks) were placed in the dorsal thalamus on one side of the brain to label all RGC axons that had extended into that region. After allowing time for diffusion back to the eyes, the ipsilateral and contralateral retinas were flat-mounted, and the number of labeled cells was quantified. (B) Flat-mounted ipsilateral and contralateral retinas from E16.5 Dscamdel17 WT and mutant littermates after retrograde labeling from the dorsal thalamus. (C and D) Mean ± SEM numbers of retrogradely labeled RGCs in the ipsilateral (C) and contralateral (D) retinas of E15.5 and E16.5 Dscamdel17 WT and mutant (del17) littermates. D, dorsal; dTh, dorsal thalamus; N, nasal; oc, optic chiasm; OT, optic tract; T, temporal; V, ventral; ns, not significant. Numbers on the bars indicate the numbers analyzed at each age and genotype. *P < 0.05. (Scale bars: 250 µm.)
Fig. S7.
Cellular organization and guidance cue expression are similar in the ventral diencephalon of Dscamdel17 WT and mutant littermates. (A) Immunostaining of the SSEA-1–positive neurons located posterior to the developing optic chiasm and RC2-positive radial glia in horizontal (SSEA-1) and coronal (RC2) sections through the optic chiasm of E14.5 Dscamdel17 WT and mutant embryos. (B) In situ hybridization for ephrinB2, Vegfa, NrCAM, and Slit1 in coronal (ephrinB2, Vegfa, and NrCAM) and horizontal (Slit1) sections through the optic chiasm of E14.5 Dscamdel17 WT and mutant littermates. (Scale bars: 200 µm.)
DSCAM Is Required for Growth and Fasciculation, but Not Guidance, of RGC Axons.
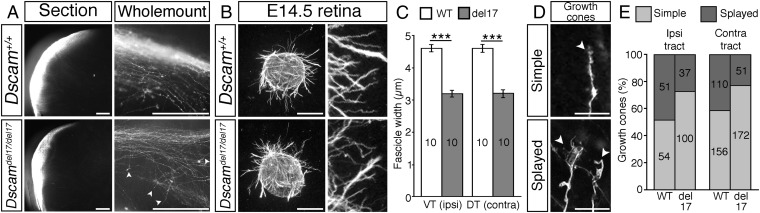
Imaging of the optic tracts in whole mounts or sections revealed no significant defects in the path followed by RGC axons as they extended through the optic tracts (Fig. 3A). A small number of RGC axons in the optic tracts of Dscamdel17/del17 mutants projected orthogonally to the normal direction of growth (Fig. 3A). At the optic chiasm, we also found small numbers of RGC axons that had strayed from their normal path in the optic nerve, anterior to the optic chiasm and the proximal optic tract (Fig. 2A). However, the small number of these aberrant axons does not likely account for the substantial reduction in RGC axons reaching the dorsal thalamus.
Fig. 3.
DSCAM modulates RGC axon fasciculation and growth cone morphology. (A) Coronal sections at the level of the optic tracts and whole-mount views of the ipsilateral optic tract of anterograde DiI-labeled Dscamdel17 WT and mutant littermates. Sections, E17.5 embryos; whole mounts, E14.5 embryos. White arrowheads indicate straying axons. (B) E14.5 retinal explants from Dscamdel17 mutant and WT littermates cultured in collagen gels for 24 h and stained with antibodies against neuron-specific β-tubulin. (Right) Higher-magnification images of the axon bundles. (C) Mean ± SEM axon bundle width of Dscam+/+ and Dscamdel17/del17 retinal axons. Results are the mean from four independent experiments analyzed blinded to genotype. (D) DiI-labeled simple and splayed growth cones (white arrowheads) in the ipsilateral optic tract of E14.5 WT embryos. (E) Percentage of simple and splayed growth cones in the ipsilateral and contralateral optic tracts of E14.5 Dscamdel17 mutants and WT littermates. Analyses were performed blinded to genotype. Numbers on the bars indicate the numbers analyzed. ***P < 0.001. (Scale bars: 250 µm in A and B; 25 µm in D.)
Straying of axons in the optic nerve, chiasm, and tract of mice lacking Secreted frizzled-related proteins (Sfrp1−/− and Sfrp2−/−) has been attributed, at least in part, to changes in axon fasciculation (26). To test whether DSCAM modulates RGC axon fasciculation, we determined the mean axon bundle width from cultured E14.5 Dscamdel17/del17 mutant or WT retinal explants (Fig. 3 B and C). A similar approach has been used to investigate the role of Sfrps (26) and Slit/Robo signaling (27) in modulating axon fasciculation. Axon bundles from Dscamdel17/del17 retinal explants were significantly thinner than axon bundles from WT explants, and this was true for both presumptive ipsilaterally and contralaterally projecting axons (Fig. 3 B and C). These findings are consistent with a role for DSCAM in promoting RGC axon fasciculation, and suggest that disrupted axon fasciculation contributes to the straying of RGC axons in Dscamdel17/del17 mutants.
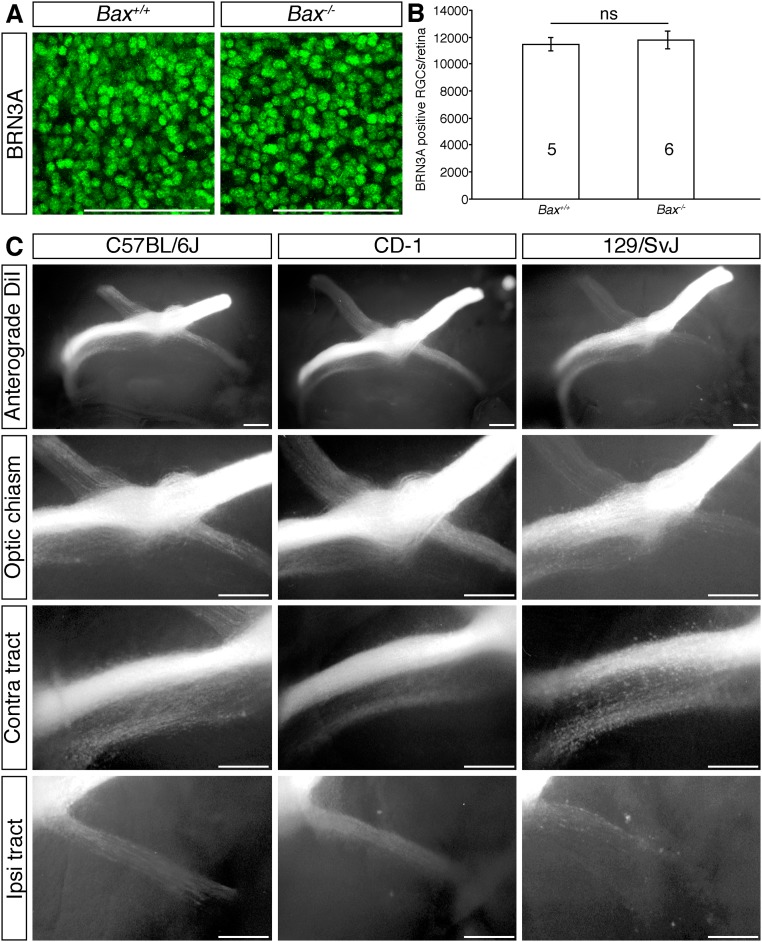
Although we cannot exclude the possibility that the significant increase in RGC number contributes to the disorganized appearance of the optic pathway in Dscamdel17/del17 mutants, we consider this unlikely. Mice with defective naturally occurring cell death do not exhibit an increase in RGC number embryonically (Fig. S8 A and B) (28); however, there is a natural, bimodal variation in RGC number in different strains of mice (29) driven by differences in RGC production (30). No significant differences in chiasm organization in mice on different genetic backgrounds have been reported, however (22, 23, 25, 31, 32) (Fig. S8C). Moreover, fasciculation defects of RGC dendrites in Dscam mutants can occur independently of increased cell number (6).
Fig. S8.
Quantitation of RGCs in E17.5 Bax-deficient mice and RGC axon organization in mice on different genetic backgrounds. (A) Confocal images of BRN3A-positive RGCs in flat-mounted E17.5 WT and Bax−/− retinas. (B) Mean ± SEM number of BRN3A-positive RGCs in E17.5 WT and Bax−/− retinas. The numbers on the bars indicate the number of each genotype analyzed. ns, not significant. (C) Whole-mount views following anterograde DiI labeling of all axons from one eye of E15.5 C57BL/6J, CD-1, and 129/SvJ mice. Higher-magnification views of the optic chiasm, contralateral (contra), and ipsilateral (ipsi) optic tract are shown. (Scale bars: 100 µm in A; 250 µm in C.)
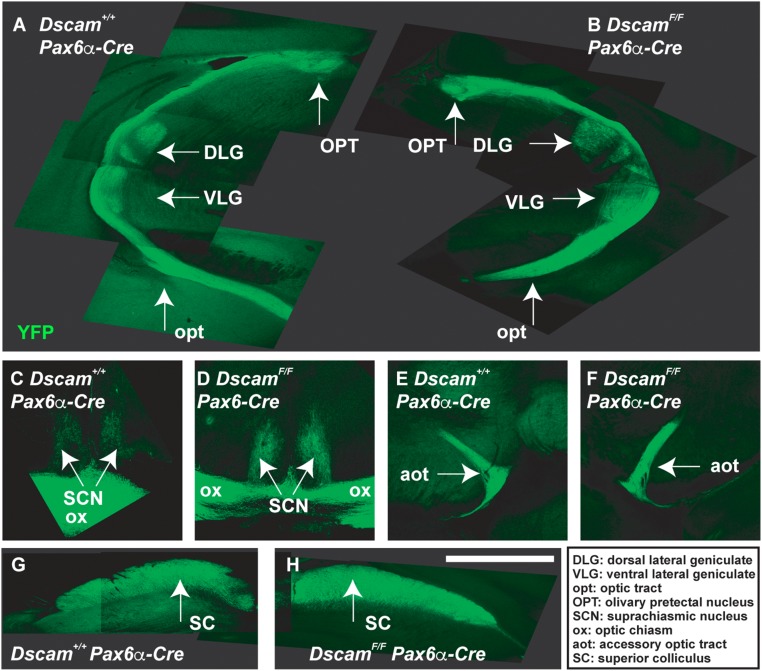
We considered the possibility that RGC axons fail to reach the dorsal thalamus because they stall along the optic tracts; however, we found no evidence for accumulation of growth cones within the optic tracts (Figs. 2 E and G and 3A). Moreover, comparison of the length of the ipsilateral optic tract and number of axon bundles within the ipsilateral dorsal thalamus at different ages demonstrated that RGC axons continued to grow in Dscam mutants (Fig. 2 D and F) and ultimately reached their targets (Fig. S9) (33). These findings are consistent with DSCAM providing permissive, but not guidance, signals that promote extension of RGC axons from, but not toward, the optic chiasm.
Fig. S9.
Dscam mutant RGCs target brain loci normally. A floxed allele of Dscam (DscamFF) was targeted with Pax6a-Cre, which induces recombination before RGC differentiation and activates a YFP reporter under control of the Thy1 promoter. No differences were observed when comparing targeting of WT and Dscam−/− RGC axons (n = 4 mice). Targeting of brain loci by WT (A, C, E, and G) and Dscam mutant (B, D, F, and H) RGCs. A, G, and H are composite pictures generated by overlying in Adobe Illustrator images captured at overlapping positions of the optic tract (A) or superior colliculus (G and H). Abbreviations in Inset: DLG, dorsal lateral geniculate; VLG, ventral lateral geniculate; opt, optic tract; OPT, olivary prectectal nucleus; SCN, suprachiasmatic nucleus; ox, optic chiasm; aot, accessory optic tract; SC, superior colliculus. (The scale bar in G is equivalent to 1.92 mm in A and B, 514 mm in C and D, 1.41 mm in E and F, and 880 mm in G and H.)
A similar, spatially restricted requirement for DSCAM in promoting axon extension has been reported for Drosophila mechanosensory neurons. In the absence of Dscam1, mechanosensory neurons grow normally into the CNS, but then extension is severely impaired (34). The differential requirement for DSCAM in growth of RGC axons toward and away from the optic chiasm may reflect redundancy with other signals that promote the growth of RGC axons toward the optic chiasm, or a switch in growth cone sensitivity. Further experiments are needed to distinguish between these possibilities.
Morphology of RGC Axon Growth Cones Is Altered in Dscamdel17/del17 Mutants.
In Drosophila Dscam1 mutants, the impaired extension of mechanosensory axons is associated with changes in growth cone morphology. Growth cones of mutant axons are less complex and have abnormally dense and short filopodia compared with WT axons (35). Analysis of growth cone morphology in the optic tracts of E14.5 Dscamdel17/del17 mutants and WT littermates revealed a similar loss of growth cone complexity in the mutants (Fig. 3 D and E). Growth cones were characterized as having either a simple, torpedo-like morphology (longer than wide, with few filopodia) or splayed morphology (wider than long with several filopodia; Fig. 3D). In both the ipsilateral and contralateral optic tract of Dscamdel17/del17 mutants, a greater percentage of growth cones had simple morphologies compared with WT littermates (Fig. 3E). Both in vitro and in vivo reductions in the number of growth cone filopodia decrease the rate of axon outgrowth (36, 37); thus, the reductions in growth cone complexity in Dscam mutants may contribute directly to the reduced axon extension in the murine optic tract.
DSCAM Promotes RGC Axon Outgrowth in Vitro.
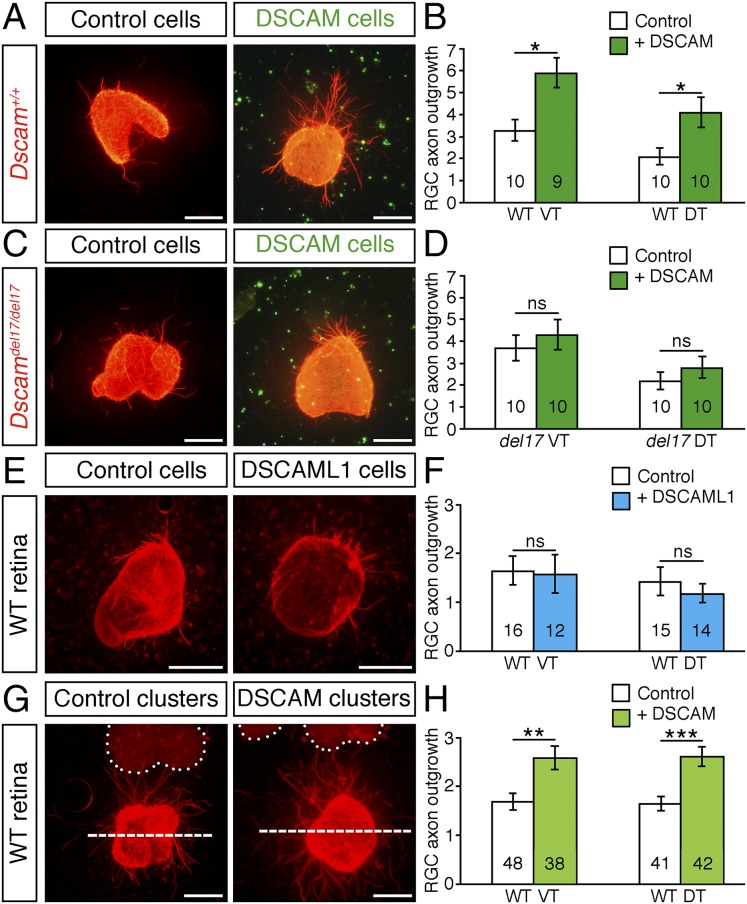
Because axon–axon interactions play an important role in controlling the rate of growth along the developing optic pathway (38), defasciculation could underlie the reduced growth of RGC axons in the absence of DSCAM. Alternatively, DSCAM expressed along the optic pathway may act directly on the RGC growth cones to increase the rate of axon extension. These possible mechanisms are not mutually exclusive and could act together to determine the overall rate of axon outgrowth. To test whether DSCAM provides growth-promoting signals to RGC axons, we cultured E14.5 retinal explants in collagen gels seeded with control or DSCAM-producing cells. In this assay, RGC axons grow among the cells that are dispersed throughout the collagen. Culturing of Dscam+/+ explants with DSCAM-producing cells induced a significant increase in the extent of axon outgrowth of both presumptive ipsilateral (ventrotemporal) and contralateral (dorsotemporal) RGCs compared with cultures containing control cells (Fig. 4 A and B).
Fig. 4.
DSCAM promotes RGC axon outgrowth in vitro. (A, C, E, and G) Retinal explants from E14.5 WT (A, E, and G) and Dscamdel17 mutants (C) cultured for 24 h in collagen gels seeded with control, Dscam-expressing cells (A and C), Dscaml1-expressing cells (E), or 100–300 µm from clusters of control or Dscam-expressing cells (G). Explants were fixed and stained with antibodies against β-tubulin (red) or DSCAM (green). Dotted lines in G indicate the outlines of the cell clusters. Outgrowth was quantified in the area above the dashed line. (B, D, F, and H) Mean ± SEM axon outgrowth of presumptive ipsilateral (VT) and contralateral (DT) RGCs from WT (B, F, and H) and Dscamdel17/del17 (D) retinal explants in the presence of control, DSCAM-producing, or DSCAML1-producing cells. The number of explants analyzed is indicated on the bars. Data are the mean of at least four independent experiments analyzed blinded to genotype and condition. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. (Scale bars: 250 µm.)
We next asked whether DSCAM is required in RGC axons, as well as the environment, for promotion of axon outgrowth. In the presence of control cells, the extent of axon outgrowth from WT and Dscamdel17/del17 mutant retinal explants was similar (Fig. 4 A–D); however, in cultures containing Dscamdel17/del17 retinal explants, the growth-promoting effect of the DSCAM-producing cells was abrogated completely (Fig. 4 C and D). We conclude that DSCAM is a potent promoter of RGC axon outgrowth and is required in both RGC axons and the environment for enhanced axon growth, consistent with a homotypic mode of action. The growth-promoting effect of DSCAM occurs not simply from the addition of large adhesion molecules to the cultures; culturing E14.5 WT retinal explants in collagen gels seeded with cells producing the highly related molecule DSCAML1 (15) had no effect on RGC axon outgrowth (Fig. 4 E and F).
DSCAM is constitutively cleaved from transfected cells and the N-terminal portion shed into the medium (14). This raises the possibility that DSCAM may promote RGC axon outgrowth independently of direct contact with expressing cells. To test this idea, we cocultured WT retinal explants at a short distance (100–300 µm) from clusters of mock- or Dscam-transfected cells. We found that the outgrowth of both presumptive ipsilateral and contralateral RGC axons was increased significantly from explants cultured a short distance from DSCAM-producing cell clusters compared with explants cultured at a distance from mock-transfected cells (Fig. 4 G and H). This finding demonstrates that shed DSCAM can act independently of direct cell–cell contact to modulate cell behavior.
In vitro, we found that DSCAM is a potent growth promoter of both presumptive ipsilateral and contralateral RGC axons (Fig. 4 A, B, G, and H); however, in vivo, extension of ipsilateral axons was more severely impaired in Dscam mutants (Fig. 2C–H and Fig. S5 F and G). We considered the possibility that a loss of ipsilaterally specified RGCs may contribute to the more severe reduction of ipsilateral projections in Dscam mutants, but found no evidence to support this idea. Specification of Zic2-positive ipsilaterally projecting RGCs occurred normally in Dscamdel17/del17 mutants (Fig. S4F), with a similar number of ZIC2-positive cells present in E16.5 WT and mutant retinas (Fig. S4 G and H). Contralateral RGC axons may be less sensitive to loss of DSCAM, owing to redundancy with other growth-promoting signals, such as VEGF-A, that act selectively on these axons (22). Alternatively, because ipsilateral axons are selectively sensitive to repellents such as ephrin B2 (24), loss of DSCAM may induce a greater shift toward inhibition in ipsilateral axons. Differential interaction of DSCAM with other potential binding partners, such as Netrin-1 and Slits (8, 9, 39), in ipsilateral vs. contralateral axons also could contribute to the greater dependency of ipsilateral axons on DSCAM for outgrowth in vivo.
DSCAM Is Required in Both RGC Exons and Their Environment for Optimal Axon Outgrowth in Situ.
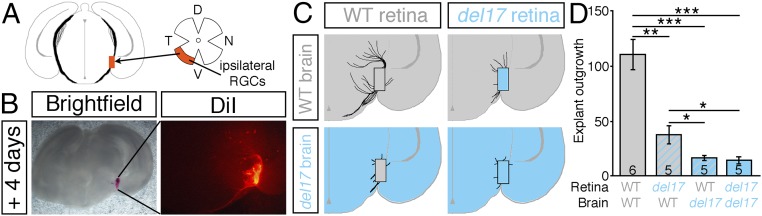
To determine whether in situ DSCAM localized to RGC axons, their environment, or both promotes RGC axon outgrowth, we used a chimeric culture approach adapted from a system used to study corpus callosum development (40). Retinal explants from E16.5 Dscamdel17 WT and mutant littermates were cultured on coronal slices at the level of the optic tract of embryos from the same litters, with explants and slices combined in different genetic combinations (Fig. 5 A–C). After 4 d, the cultures were fixed, and DiI was used to label outgrowth from the retinal explants. In cultures of WT retina on WT brain slices, RGC axons extended for some distance from the cultured explants, both toward and away from the optic chiasm (Fig. 5 B–D); however, loss of DSCAM from the RGC axons and/or their environment induced a significant decrease in the extent of outgrowth from the cultured explants (Fig. 5 C and D). Thus, in cultures containing Dscamdel17/del17 retinal explants on Dscam+/+ brain slices, Dscam+/+ retinal explants on Dscamdel17/del17 brain slices, or Dscamdel17/del17 retinal explants on Dscamdel17/del17 brain slices, the extent of axon outgrowth from the retinal explants was decreased significantly compared with cultures using only WT tissue (Fig. 5 C and D). The extent of axon outgrowth from the retinal explants was significantly less in cultures using Dscamdel17/del17 brain slices than in cultures using only mutant retinal tissue (Fig. 5D). This could reflect a generally less favorable environment of the mutant brain tissue owing to a reduced number of endogenous RGC axons in the optic tracts (Fig. 2 C–F) or to interaction of DSCAM with heterophilic partners in the retina (39).
Fig. 5.
DSCAM promotes RGC axon outgrowth in situ. (A) Schematic diagram of the culture approach. Ventrotemporal retinal explants, containing predominately ipsilaterally projecting RGCs, and brain slices at the level of the optic tract were prepared from E16.5 Dscamdel17 mutant and WT littermates. Retinal explants were cultured on top of the brain slices in different genetic combinations. (B) Bright-field and fluorescent (DiI) images of an E16.5 WT retinal explant cultured on a WT brain slice. (C) Manual tracing of RGC axons from Dscamdel17 WT (gray) or mutant (blue) retinal explants cultured on WT (gray) or mutant (blue) brain slices. (D) Mean ± SEM RGC axon outgrowth from E16.5 Dscamdel17 WT and mutant retinal explants cultured on brain slices from WT and mutant embryos. Numbers on the bars indicate the numbers analyzed. Results are from five independent experiments analyzed blinded to genotype. *P < 0.05; **P < 0.01; ***P < 0.001.
Dscam Gain of Function Promotes RGC Axon Growth in Vivo.
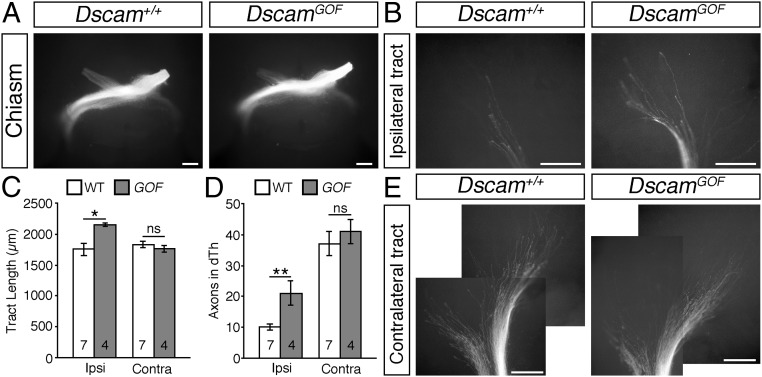
We used a gain-of-function Dscam allele (7) to investigate whether the overexpression of DSCAM affects RGC axon outgrowth in vivo. Anterograde DiI labeling of all axons from one eye of E14.5 DscamGOF mice and WT littermates demonstrated no significant defects in the growth of RGC axons toward and through the optic chiasm in the transgenic mice (Fig. 6A); however, the ipsilateral optic tracts were significantly longer than in WT littermates, with significantly more RGC axon bundles within the dorsal thalamus (Fig. 6 B–D). In contrast, the length of the contralateral optic tract and the number of axons bundles in the contralateral dorsal thalamus were not significantly different in DscamGOF embryos compared with WT littermates (Fig. 6 C–E). Thus, gain-of-function experiments induced an opposite phenotype (exuberant growth; Fig. 6 B–D) in the ipsilateral optic tract compared with reductions in Dscam levels (stunted growth; Fig. 2 C–F).
Fig. 6.
DSCAM gain of function promotes RGC axon outgrowth in vivo. (A, B, and E) Whole-mount views of anterograde DiI-labeled RGC axons at the optic chiasm (A) and in the ipsilateral (B) and contralateral (E) optic tracts of E14.5 DscamGOF and WT littermates. Panels in E are composite pictures generated by overlying in Adobe Photoshop images captured at overlapping positions along the optic tract. (C and D) Mean ± SEM optic tract length (C) and number of axon bundles in the ipsilateral (ipsi) and contralateral (contra) dorsal thalamus (D) of E14.5 DscamGOF (GOF) and WT littermates. Analyses were performed blinded to genotype. Numbers on the bars indicate the numbers analyzed for each genotype. ns, not significant; *P < 0.05; **P < 0.01. (Scale bars: 250 µm.)
The timing at which RGC axons arrive in the brain correlates with the fidelity of target selection. Early-arriving axons initially innervate multiple targets, followed by pruning of connections to most of these regions. In contrast, later-arriving axons innervate a limited number of targets from the outset (41). By modulating the timing at which RGC axons reach brain targets, DSCAM may contribute to the specificity of brain wiring patterns. Consistent with this idea, Dscam gain- and loss-of-function mutants display defects in the eye-specific segregation or RGC axons in the lateral geniculate nucleus (33). Axon overgrowth defects associated with Dscam overexpression also have been demonstrated in fragile-X syndrome (42).
Methods
The methodology used in this study is described in detail in SI Methods. In brief, gene expression patterns and analyses of axon growth in vivo and in vitro were performed as described previously (22, 25, 43–45). Coculture of retina explants on brain slices was adapted from a method used to study corpus callosum development (40). Animal experiments were performed in accordance with UK Home Office Guidelines and approved by the University of Aberdeen Ethical Review Committee.
SI Methods
Mice.
All animal procedures were performed in accordance with institutional and UK Home Office guidelines. Experiments were performed using C57BL/6J WT, Dscamdel17 (B6.Cby-Dscamdel17.RwbJ) (16), Dscam2J (C3H/HeDiSn-Dscam2J/GrsrJ) (46), DscamGOF (gain-of-function mice overexpressing Dscam) (7), DscamF (6), Pax6α-Cre (47), Thy1-YFP (48), and Bax−/− (49) mice. The morning of vaginal plug formation was considered E0.5. Pregnant mothers were killed by cervical dislocation, and embryos were fixed overnight at 4 °C with 4% (wt/vol) PFA in PBS or used immediately in culture experiments. Genotyping protocols are available on request.
In Situ Hybridization.
In situ hybridization with digoxigenin-labeled riboprobes specific for Dscam, Dscaml1, Zic2, ephrinB2, Vegfa, NrCAM, and Slit1 (5, 22, 43) was performed on 100-µm vibratome-cut sections as described previously (44). In brief, sections were dehydrated and rehydrated in 25–100% (vol/vol) methanol in PBT (PBS plus 0.1% Tween-20), bleached with 6% (vol/vol) H202 in PBT, treated with 5 µg/mL proteinase K, refixed with 4% PFA in PBT, and incubated in hybridization buffer [50% (vol/vol) formamide, 5× SSC, 50 µg/mL tRNA, 1% SDS, and 50 µg/mL heparin] for 1 h at 65 °C. Probes were diluted by adding 15 µL to 1 mL of hybridization buffer. Then 100 µL was added to each slide under a plastic coverslip, and the slides were incubated in a humidified chamber overnight at 65 °C. Sections were washed three times with 50% (vol/vol) formamide, 5× SSC, and 1% SDS at 65 °C, followed by 50% formamide and 2× SSC at 60 °C. Sections were then washed with TBST (Tris- buffered saline with 1% Tween-20), blocked with 10% (vol/vol) sheep serum in TBST, and incubated in anti-digoxigenin alkaline phosphatase (AP)-Fab fragments (Roche Diagnostics) diluted 1:2,000 in 1% sheep serum/TBST overnight at 4 °C. Slides were washed extensively with TBST and incubated in color reaction (100 mM NaCl, 100 mM Tris⋅HCl pH 9.5, 50 mM MgCl2, 1% Tween-20, 337.5 µg/mL NBT, and 175 µg/mL BCIP). Slides were mounted in 90% glycerol/10% (vol/vol) PBS and photographed using a Nikon SMZ1500 microscope and DXM1200 camera with ACT-1 software.
DiI Labeling.
Focal labeling of small groups of RGC axons extending toward and out of the optic disk was performed as described previously (44). In brief, a small crystal of DiI [DiIC18 (3); D282; Thermo Fisher Scientific] was placed into the peripheral dorsal or ventral region of E14.5–E17.5 retinas. The tissue was incubated at 37 °C for 5–8 h in PBS plus 0.01% sodium azide, flat-mounted with the RGC layer facing upward using Vectashield (Vector Laboratories), and photographed using a Zeiss Axiophot microscope and DXM1200 digital camera or a Zeiss LSM510 confocal microscope.
Whole-tract anterograde DiI labeling was performed essentially as described previously (25, 45). The retinas were removed, a crystal of DiI placed onto the optic nerve on one side to label all of the axons, and the embryos were incubated at 37 °C in PBS plus 0.01% sodium azide for 3–10 d. The brain was dissected free of surrounding tissues and photographed using a Nikon SMZ1500 microscope and DXM1200 camera either ventral side up to view the optic chiasm, or laterally, after removing the cortex, to visualize the optic tracts. Dissected brains were also embedded in 3% (wt/vol) agarose in water and sectioned horizontally or coronally at 100 µm using a vibratome (Intracel Ltd). For labeling of projections between the eyes, DiI crystals were placed into one retina, the embryos kept at room temperature for 10–12 wk, the contralateral retina flat-mounted, and the number of labeled cells quantified (44).
Retrograde labeling was performed as described previously (22). The cortex was removed from one side of the brain of formaldehyde-fixed embryos, DiI crystals placed in a row over the dorsal thalamus, and the embryos were incubated in PBS containing 0.01% sodium azide at room temperature for 10–16 wk. Retinas were flat-mounted using Vectashield with the RGC layer facing upward, images were captured using a Nikon SMZ1500 or Zeiss Axiophot microscope with a DXM1200 digital camera, and the number of labeled cells was quantified. For contralateral retinas, the labeled cells within a 50× magnification image from four central and four peripheral regions of each retinal quadrant were counted, and the average cell density was determined. Together with the total retinal area, this value was used to calculate the average number of labeled cells in each retina. For ipsilateral retinas, all labeled cells in each retina were counted directly.
Immunofluorescence.
Retinal cells were cultured by dissociating entire P0 retinas in TrypLE (Thermo Fisher Scientific) for 30 min and plating the cells on coverslips coated with poly-d-lysine and laminin in neurobasal medium supplemented with B27 (Thermo Fisher Scientific). Cultures were grown for 7 d and then fixed in warm 4% PFA in PBS for 10 min. Cells were double immunostained with antibodies against neurofilaments (2H3, 1:100; Developmental Studies Hybridoma Bank) and DSCAM (goat anti-DSCAM, 1:250; R&D Systems) for 2 h at room temperature, followed by secondary antibodies (donkey anti-goat Cy3 and donkey anti-mouse Alexa Fluor 488, 1:1,000; Jackson Immunoresearch), and imaged with an Olympus confocal microscope.
Immunostaining of whole retinas and sections was performed as described previously (44). The following primary antibodies were used: mouse monoclonal anti–neuron- specific β-tubulin (TUJ1; MMS-435P, 1:1,000; Cambridge Biosciences), anti–TAG-1 (4D7, 1:40; Developmental Studies Hybridoma Bank), anti–SSEA-1 (MC-480; 1:9; Developmental Studies Hybridoma Bank), RC2 (1:50; Developmental Studies Hybridoma Bank), anti-BRN3A (MAB1585, 1:300; Millipore,), anti-phosphohistone H3 (06–570, 1:50; Millipore), anti–cleaved caspase-3 (9664, 1:100; New England Biolabs), and anti-ZiC2 (a gift from Eloisa Herrera), followed by the appropriate secondary antibody: Alexa Fluor 488 goat anti-mouse IgG (1:200; Thermo Fisher Scientific), Cy3 goat anti-rabbit IgG, or Cy3 goat anti-mouse IgG (1:1,000; Jackson ImmunoResearch). For quantification of BRN3A-positive and phosphohistone-H3–positive retinal cells, confocal Z-stacks through the entire RGC layer or from the outer surface of the retina adjacent to the retinal pigmented epithelium were captured from the central and peripheral regions of each retinal quadrant (Fig. S4 A and B), and the number and density of the labeled cells in each area were determined. Total retinal area was measured and, together with the average density of the labeled cells, used to calculate the total number of labeled cells in each retina.
Retinal Explant Cultures.
293T or COS-7 cells were transfected using Lipofectamine Plus or Lipofectamine 3000 (Thermo Fisher Scientific) with an expression vector encoding full-length mouse DSCAM with a myc-His tag (14), full-length mouse DSCAML1 (Addgene plasmid 18738), or vector alone. Explants were dissected from the periphery of E14.5 retinas and cultured in a 1:1 mixture of bovine dermis collagen and rat tail collagen (BD Biosciences) seeded with 8 × 104 293T cells, 100–300 µm from clusters of ∼500 transfected cells (43), or in collagen alone. The culture medium was composed of DMEM:F12 (Thermo Fisher Scientific) containing 1% BSA and insulin, transferrin, and sodium selenite media supplement (I1884; Sigma-Aldrich). After 24–30 h, the cultures were fixed and stained with antibodies specific for β-tubulin (T8660, 1:500; Sigma-Aldrich) or rabbit polyclonal TUJ1 (MRB-435P, 1:1,000; Cambridge Biosciences), MYC (9E10, 1:9; Developmental Studies Hybridoma Bank), or DSCAML1 (AF3315, 1:200; R&D Systems), followed by the appropriate secondary antibody: Cy3-conjugated goat anti-mouse IgG (1:2,000), Cy3-conjugated goat anti-rabbit IgG (1:2,000), or Alexa Fluor 488 goat anti-mouse IgG (1:500). The area covered by the RGC axons was quantified using Image J (https://imagej.nih.gov/ij/) as described previously (22). For measurements of fascicle widths, a line was drawn 50 µm from the explant body, and the width of all fascicles crossing the line was quantified. Analyses were performed blinded to genotype and condition. Data are the mean ± SEM from a minimum of three independent experiments. Statistical comparisons were made using ANOVA or the Mann–Whitney U test.
Retina-Brain Slice Cocultures.
The retina-brain slice coculture approach was adapted from a method used to study corpus callosum development (40). Rapid genotyping was performed using the KAPA mouse genotyping kit (Kapa Biosystems). Peripheral ventrotemporal retinal explants containing predominately ipsilaterally projecting RGCs were dissected from E16.5 Dscamdel17 WT and mutant littermates, and the brains from the same embryos were embedded in 4% (wt/vol) low-melting-point agarose in PBS and sectioned coronally at 300 µm on a vibratome. Sections were transferred onto polycarbonate membranes (TETP01300; EMD Millipore) in organ tissue culture dishes (Falcon) containing 1 mL of MEM with 10% (vol/vol) FCS, 0.5% glucose, and penicillin-streptomycin (Thermo Fisher Scientific), and retinal explants placed on top of the slice in the region of the optic tract. Cultures were incubated for 1 h at 37 °C, after which the medium was replaced with 1 mL of Neurobasal medium containing B27 supplement, 0.5% glucose, and penicillin-streptomycin (Thermo Fisher Scientific), followed by incubation for 4 d. After fixation with 4% PFA in PBS, DiI crystals were placed onto the retinal explant, and cultures were kept at room temperature in the dark for 1 wk. Cultures were photographed using a Zeiss Axiophot or LSM510 confocal microscope. The outgrowth from each explant was traced manually and quantified, blinded to genotype, using ImageJ to measure the total area covered by the axons. Data are from five independent experiments using tissue from separate litters. Statistical comparisons were made using the Mann–Whitney U test.
Axon Targeting.
Whole brains were fixed in 4% PFA overnight at 4 °C and rinsed in PBS. Then 100-μm sections of brain were cut using a vibratome. Brain sections were mounted in 80% (vol/vol) glycerol in PBS and imaged for YFP.
Acknowledgments
We thank Drs. Robert Burgess, Carol Mason, and Eloisa Herrera for helpful discussions; Dr. Thomas Theil for his invaluable advice on the slice culture methods; Francesca Lamb and Emma Smith for technical assistance; and the Institute of Medical Sciences Microscopy and Imaging Facility for assistance with confocal microscopy. This work was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) doctoral training award studentship and a BBSRC project grant (BB/J00815X/1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.S. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618606114/-/DCSupplemental.
References
- 1.Erskine L, Herrera E. Connecting the retina to the brain. ASN Neuro. 2014;6(6):1759091414562107. doi: 10.1177/1759091414562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrovic M, Schmucker D. Axonal wiring in neural development: Target-independent mechanisms help to establish precision and complexity. BioEssays. 2015;37(9):996–1004. doi: 10.1002/bies.201400222. [DOI] [PubMed] [Google Scholar]
- 4.Schmucker D, Chen B. Dscam and DSCAM: Complex genes in simple animals, complex animals yet simple genes. Genes Dev. 2009;23(2):147–156. doi: 10.1101/gad.1752909. [DOI] [PubMed] [Google Scholar]
- 5.Fuerst PG, et al. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64(4):484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuerst PG, Bruce F, Rounds RP, Erskine L, Burgess RW. Cell autonomy of DSCAM function in retinal development. Dev Biol. 2012;361(2):326–337. doi: 10.1016/j.ydbio.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, et al. DSCAM promotes refinement in the mouse retina through cell death and restriction of exploring dendrites. J Neurosci. 2015;35(14):5640–5654. doi: 10.1523/JNEUROSCI.2202-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, et al. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci USA. 2009;106(8):2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ly A, et al. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133(7):1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deiner MS, et al. Netrin-1 and DCC mediate axon guidance locally at the optic disc: Loss of function leads to optic nerve hypoplasia. Neuron. 1997;19(3):575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 11.Palmesino E, Haddick PC, Tessier-Lavigne M, Kania A. Genetic analysis of DSCAM’s role as a Netrin-1 receptor in vertebrates. J Neurosci. 2012;32(2):411–416. doi: 10.1523/JNEUROSCI.3563-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Andrade GB, Kunzelman L, Merrill MM, Fuerst PG. Developmentally dynamic colocalization patterns of DSCAM with adhesion and synaptic proteins in the mouse retina. Mol Vis. 2014;20:1422–1433. [PMC free article] [PubMed] [Google Scholar]
- 13.de Andrade GB, Long SS, Fleming H, Li W, Fuerst PG. DSCAM localization and function at the mouse cone synapse. J Comp Neurol. 2014;522(11):2609–2633. doi: 10.1002/cne.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schramm RD, et al. A novel mouse Dscam mutation inhibits localization and shedding of DSCAM. PLoS One. 2012;7(12):e52652. doi: 10.1371/journal.pone.0052652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwala KL, et al. Cloning and functional characterization of DSCAML1, a novel DSCAM-like cell adhesion molecule that mediates homophilic intercellular adhesion. Biochem Biophys Res Commun. 2001;285(3):760–772. doi: 10.1006/bbrc.2001.5214. [DOI] [PubMed] [Google Scholar]
- 16.Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451(7177):470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore SW, et al. Soluble adenylyl cyclase is not required for axon guidance to netrin-1. J Neurosci. 2008;28(15):3920–3924. doi: 10.1523/JNEUROSCI.0547-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu KY, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci. 2006;9(10):1257–1264. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118(5):619–633. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwala KL, Nakamura S, Tsutsumi Y, Yamakawa K. Down syndrome cell adhesion molecule DSCAM mediates homophilic intercellular adhesion. Brain Res Mol Brain Res. 2000;79(1-2):118–126. doi: 10.1016/s0169-328x(00)00108-x. [DOI] [PubMed] [Google Scholar]
- 21.Marcus RC, Blazeski R, Godement P, Mason CA. Retinal axon divergence in the optic chiasm: Uncrossed axons diverge from crossed axons within a midline glial specialization. J Neurosci. 1995;15(5 Pt 2):3716–3729. doi: 10.1523/JNEUROSCI.15-05-03716.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erskine L, et al. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70(5):951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwajima T, et al. Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron. 2012;74(4):676–690. doi: 10.1016/j.neuron.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams SE, et al. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39(6):919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Plump AS, et al. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33(2):219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 26.Marcos S, et al. Secreted frizzled related proteins modulate pathfinding and fasciculation of mouse retina ganglion cell axons by direct and indirect mechanisms. J Neurosci. 2015;35(11):4729–4740. doi: 10.1523/JNEUROSCI.3304-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaworski A, Tessier-Lavigne M. Autocrine/juxtaparacrine regulation of axon fasciculation by Slit-Robo signaling. Nat Neurosci. 2012;15(3):367–369. doi: 10.1038/nn.3037. [DOI] [PubMed] [Google Scholar]
- 28.Burne JF, Staple JK, Raff MC. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J Neurosci. 1996;16(6):2064–2073. doi: 10.1523/JNEUROSCI.16-06-02064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams RW, Strom RC, Rice DS, Goldowitz D. Genetic and environmental control of variation in retinal ganglion cell number in mice. J Neurosci. 1996;16(22):7193–7205. doi: 10.1523/JNEUROSCI.16-22-07193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strom RC, Williams RW. Cell production and cell death in the generation of variation in neuron number. J Neurosci. 1998;18(23):9948–9953. doi: 10.1523/JNEUROSCI.18-23-09948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez-Arrones L, et al. Shh/Boc signaling is required for sustained generation of ipsilateral projecting ganglion cells in the mouse retina. J Neurosci. 2013;33(20):8596–8607. doi: 10.1523/JNEUROSCI.2083-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams SE, et al. A role for Nr-CAM in the patterning of binocular visual pathways. Neuron. 2006;50(4):535–547. doi: 10.1016/j.neuron.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Blank M, et al. The Down syndrome critical region regulates retinogeniculate refinement. J Neurosci. 2011;31(15):5764–5776. doi: 10.1523/JNEUROSCI.6015-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen BE, et al. The molecular diversity of Dscam is functionally required for neuronal wiring specificity in Drosophila. Cell. 2006;125(3):607–620. doi: 10.1016/j.cell.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 35.He H, et al. Cell-intrinsic requirement of Dscam1 isoform diversity for axon collateral formation. Science. 2014;344(6188):1182–1186. doi: 10.1126/science.1251852. [DOI] [PubMed] [Google Scholar]
- 36.Chien CB, Rosenthal DE, Harris WA, Holt CE. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 1993;11(2):237–251. doi: 10.1016/0896-6273(93)90181-p. [DOI] [PubMed] [Google Scholar]
- 37.McCaig CD. Nerve growth in the absence of growth cone filopodia and the effects of a small applied electric field. J Cell Sci. 1989;93(Pt 4):715–721. doi: 10.1242/jcs.93.4.715. [DOI] [PubMed] [Google Scholar]
- 38.Bak M, Fraser SE. Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development. 2003;130(20):4999–5008. doi: 10.1242/dev.00713. [DOI] [PubMed] [Google Scholar]
- 39.Alavi M, et al. Dscam1 forms a complex with Robo1 and the N-terminal fragment of slit to promote the growth of longitudinal axons. PLoS Biol. 2016;14(9):e1002560. doi: 10.1371/journal.pbio.1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magnani D, et al. Gli3 controls corpus callosum formation by positioning midline guideposts during telencephalic patterning. Cereb Cortex. 2014;24(1):186–198. doi: 10.1093/cercor/bhs303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osterhout JA, El-Danaf RN, Nguyen PL, Huberman AD. Birthdate and outgrowth timing predict cellular mechanisms of axon target matching in the developing visual pathway. Cell Reports. 2014;8(4):1006–1017. doi: 10.1016/j.celrep.2014.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterne GR, Kim JH, Ye B. Dysregulated Dscam levels act through Abelson tyrosine kinase to enlarge presynaptic arbors. eLife. 2015;4:4. doi: 10.7554/eLife.05196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erskine L, et al. Retinal ganglion cell axon guidance in the mouse optic chiasm: Expression and function of robos and slits. J Neurosci. 2000;20(13):4975–4982. doi: 10.1523/JNEUROSCI.20-13-04975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson H, Andrews W, Parnavelas JG, Erskine L. Robo2 is required for Slit-mediated intraretinal axon guidance. Dev Biol. 2009;335(2):418–426. doi: 10.1016/j.ydbio.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson H, Barker D, Camand O, Erskine L. Slits contribute to the guidance of retinal ganglion cell axons in the mammalian optic tract. Dev Biol. 2006;296(2):476–484. doi: 10.1016/j.ydbio.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Fuerst PG, Harris BS, Johnson KR, Burgess RW. A novel null allele of mouse DSCAM survives to adulthood on an inbred C3H background with reduced phenotypic variability. Genesis. 2010;48(10):578–584. doi: 10.1002/dvg.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stacy RC, Wong RO. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J Comp Neurol. 2003;456(2):154–166. doi: 10.1002/cne.10509. [DOI] [PubMed] [Google Scholar]
- 48.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424(6947):430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 49.White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18(4):1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]