Abstract
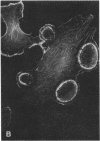
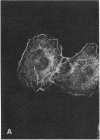
Porcine aorta endothelial cells are devoid of receptors for platelet-derived growth factor (PDGF). We have transfected such cells with cDNA for the PDGF B-type receptor, both the wild-type receptor and a mutant form of the receptor (K634A), in which the putative nucleotide-binding lysine of the protein-tyrosine domain has been changed to alanine. Immunoprecipitation studies of metabolically labeled cells showed that both types of receptors were synthesized and processed to the mature form of Mr 190,000. In cells expressing the wild-type receptor, PDGF-BB, the natural ligand for the B-type receptor, induced membrane ruffling and reorganization of actin. Such a response has previously been seen in cells expressing the natural PDGF B-type receptor in response to PDGF-BB. No such effect was induced in nontransfected cells or in cells expressing the K634A mutant receptor. PDGF was also shown to be chemotactic for cells expressing the wild-type receptor, whereas no chemotactic response was elicited in control cells or in cells expressing the K634A mutant receptor. Our study thus provides formal evidence that the PDGF B-type receptor mediates a motility response including actin reorganization and chemotaxis. Furthermore, the results establish a role for the receptor-associated protein-tyrosine kinase in the transduction of the chemotactic signal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Heaysman J. E., Pegrum S. M. The locomotion of fibroblasts in culture. II. "RRuffling". Exp Cell Res. 1970 Jun;60(3):437–444. doi: 10.1016/0014-4827(70)90537-9. [DOI] [PubMed] [Google Scholar]
- Albini A., Allavena G., Melchiori A., Giancotti F., Richter H., Comoglio P. M., Parodi S., Martin G. R., Tarone G. Chemotaxis of 3T3 and SV3T3 cells to fibronectin is mediated through the cell-attachment site in fibronectin and a fibronectin cell surface receptor. J Cell Biol. 1987 Oct;105(4):1867–1872. doi: 10.1083/jcb.105.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Bretcher M. S. Fibroblasts on the move. J Cell Biol. 1988 Feb;106(2):235–237. doi: 10.1083/jcb.106.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Morén A., Severinsson L., Ek B., Ostman A., Betsholtz C., Heldin C. H. cDNA cloning and expression of a human platelet-derived growth factor (PDGF) receptor specific for B-chain-containing PDGF molecules. Mol Cell Biol. 1988 Aug;8(8):3476–3486. doi: 10.1128/mcb.8.8.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Westermark B., Heldin C. H. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4917–4921. doi: 10.1073/pnas.86.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Hammacher A., Westermark B., Heldin C. H., Nistér M. Identification and structural analysis of the A type receptor for platelet-derived growth factor. Similarities with the B type receptor. J Biol Chem. 1989 Jan 25;264(3):1742–1747. [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Use of an antiserum against phosphotyrosine for the identification of phosphorylated components in human fibroblasts stimulated by platelet-derived growth factor. J Biol Chem. 1984 Sep 10;259(17):11145–11152. [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Barr P. J., Williams L. T. Role of tyrosine kinase and membrane-spanning domains in signal transduction by the platelet-derived growth factor receptor. Mol Cell Biol. 1988 Dec;8(12):5126–5131. doi: 10.1128/mcb.8.12.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. E., Selden L. A., Gershman L. C. Mechanism of action of phalloidin on the polymerization of muscle actin. Biochemistry. 1981 Feb 17;20(4):708–712. doi: 10.1021/bi00507a006. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., Kleinman H. K., Martin G. R., Schiffmann E. Role of attachment factors and attractants in fibroblast chemotaxis. J Lab Clin Med. 1980 Dec;96(6):1071–1080. [PubMed] [Google Scholar]
- Gronwald R. G., Grant F. J., Haldeman B. A., Hart C. E., O'Hara P. J., Hagen F. S., Ross R., Bowen-Pope D. F., Murray M. J. Cloning and expression of a cDNA coding for the human platelet-derived growth factor receptor: evidence for more than one receptor class. Proc Natl Acad Sci U S A. 1988 May;85(10):3435–3439. doi: 10.1073/pnas.85.10.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R., Chang T., Seppä H. E., Kleinman H. K., Martin G. R. Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol. 1982 Nov;113(2):261–266. doi: 10.1002/jcp.1041130213. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hammacher A., Hellman U., Johnsson A., Ostman A., Gunnarsson K., Westermark B., Wasteson A., Heldin C. H. A major part of platelet-derived growth factor purified from human platelets is a heterodimer of one A and one B chain. J Biol Chem. 1988 Nov 5;263(31):16493–16498. [PubMed] [Google Scholar]
- Hammacher A., Mellström K., Heldin C. H., Westermark B. Isoform-specific induction of actin reorganization by platelet-derived growth factor suggests that the functionally active receptor is a dimer. EMBO J. 1989 Sep;8(9):2489–2495. doi: 10.1002/j.1460-2075.1989.tb08385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Johnsson A., Wennergren S., Wernstedt C., Betsholtz C., Westermark B. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986 Feb 6;319(6053):511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: identification of constituent polypeptide chains. Biochem Biophys Res Commun. 1982 Jan 15;104(1):66–74. doi: 10.1016/0006-291x(82)91941-6. [DOI] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Becker K. M., Feltner D. E., Brown C. S., Ward P. A., Nairn R. Covalent affinity labeling, detergent solubilization, and fluid-phase characterization of the rabbit neutrophil formyl peptide chemotaxis receptor. Biochemistry. 1985 Apr 23;24(9):2227–2236. doi: 10.1021/bi00330a017. [DOI] [PubMed] [Google Scholar]
- Matsui T., Heidaran M., Miki T., Popescu N., La Rochelle W., Kraus M., Pierce J., Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989 Feb 10;243(4892):800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- Mellstroöm K., Höglund A. S., Nistér M., Heldin C. H., Westermark B., Lindberg U. The effect of platelet-derived growth factor on morphology and motility of human glial cells. J Muscle Res Cell Motil. 1983 Oct;4(5):589–609. doi: 10.1007/BF00712117. [DOI] [PubMed] [Google Scholar]
- Mellström K., Heldin C. H., Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp Cell Res. 1988 Aug;177(2):347–359. doi: 10.1016/0014-4827(88)90468-5. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Okabe T., Urabe A., Takaku F., Heldin C. H. Purification and properties of an endothelial cell growth factor from human platelets. J Biol Chem. 1987 Mar 25;262(9):4098–4103. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nistér M., Hammacher A., Mellström K., Siegbahn A., Rönnstrand L., Westermark B., Heldin C. H. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988 Mar 25;52(6):791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Ostman A., Bäckström G., Fong N., Betsholtz C., Wernstedt C., Hellman U., Westermark B., Valenzuela P., Heldin C. H. Expression of three recombinant homodimeric isoforms of PDGF in Saccharomyces cerevisiae: evidence for difference in receptor binding and functional activities. Growth Factors. 1989;1(3):271–281. doi: 10.3109/08977198908998003. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Seyer J. M., Kang A. H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci U S A. 1978 Feb;75(2):871–875. doi: 10.1073/pnas.75.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Molski T. F. Activation of the neutrophil. Prog Allergy. 1988;42:1–64. doi: 10.1159/000318681. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Smith C. D., Verghese M. W. Model for leukocyte regulation by chemoattractant receptors: roles of a guanine nucleotide regulatory protein and polyphosphoinositide metabolism. J Leukoc Biol. 1986 Dec;40(6):785–800. doi: 10.1002/jlb.40.6.785. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Waterfield M. D. Purification and properties of porcine platelet-derived growth factor. EMBO J. 1984 Dec 1;3(12):2963–2967. doi: 10.1002/j.1460-2075.1984.tb02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]