Abstract
Introduction
Since 1947, Zika virus has been identified sporadically in humans in Africa and Asia; however, clinically consequential Zika virus disease had not been documented prior to the current outbreak in the Americas. Considering 6 decades have passed since the first identification of the virus, it is perhaps unexpected that Zika virus was recognised only recently as capable of causing disease epidemics. Substantial work on understanding the epidemiology of Zika virus has been conducted since the virus' first outbreak in 2007 in Micronesia; however, there has been little study of the earlier data on Zika virus.
Methods
A systematic literature search was conducted to identify evidence of Zika virus infection in humans from 1947 to 2007. Data extracted included seroprevalence of Zika virus infection, age distributions of positive test results and serologic test modalities used. Country-level and age-specific seroprevalence was calculated. Estimates of seroprevalence by different serologic test modalities were compared.
Results
12 026 citations were retrieved by the literature search, and 76 articles were included in this review. Evidence of Zika virus infection in humans was found in 29 countries in Africa, 8 countries in Asia and 1 country in Europe. Country-level seroprevalence of Zika virus infection ranged from 0.4% to 53.3%. Seroprevalence of Zika virus infection was found to increase across the lifespan; 15–40% of reproductive-age individuals may have been previously infected. No significant difference was found between estimates of seroprevalence by different serologic test modalities.
Discussion
Zika virus has likely been endemic for decades in certain regions of the world; however, the majority of reproductive-age individuals have likely not been infected. Historical evidence of Zika virus infection exists regardless of the serologic test modality used.
Key questions.
What is already known about this topic?
Existing historical reviews of Zika virus infection have documented the countries where Zika virus infection of humans has been reported, but there still exist gaps in completeness, pertinent detail and accuracy.
Some non-English language articles have not been included in these reviews, which results in the omission of evidence of Zika virus infection in some countries. These reviews have not synthesised or reported descriptive data on Zika virus infection; thus, little is known about the historical epidemiology of Zika virus.
Consequently, conclusions about Zika virus epidemiology have been drawn based on incomplete data.
Without context, these historical reports of Zika virus infection may not be helpful to current efforts to understand and address the current outbreak in the Americas.
What are the new findings?
This review provides the most comprehensive evidence of Zika virus in humans, from its first identification to its first documented outbreak.
To provide context for past reports of Zika virus, we synthesised the data on country-level seroprevalence, age distribution of seroprevalence and estimates of seroprevalence by serologic test modality.
We were then able to identify the countries with the highest seroprevalence of Zika virus, that may be investigated further to understand the natural history of Zika virus infection and disease.
We also determined the proportion of reproductive-aged individuals who displayed evidence of past infection with Zika virus (15–40%), and who may be protected against reinfection during pregnancy.
Finally, by comparing results from different serologic test modalities, we have supported the credibility of these historical reports of Zika virus infection in humans.
Recommendations for policy
The historical evidence of Zika virus infection in humans may direct future research towards the populations and settings where Zika virus may have been endemic for decades.
The synthesised data reported in this review may provide context for the current outbreak in the Americas, and motivate new directions in research and policy to address this outbreak.
Introduction
Since 1947, when Zika virus was first isolated in Uganda,1 it has been identified in humans sporadically throughout sub-Saharan Africa and parts of Asia;2 3 however, it was not until 2007 that the virus was recognised as a clinically important pathogen capable of causing disease epidemics.4
The 2007 outbreak, on Yap Island in Micronesia, was the world's first major outbreak of Zika virus disease in humans. No hospitalisations or deaths were reported during this outbreak, and the clinical features observed were similar to those described in earlier case reports: mild fever, malaise, headache, arthralgia and rash.5–8 In 2015, Zika virus infection on the American continents was reported for the first time. This outbreak has spread rapidly through much of Central and South America, and the Caribbean.2
The current outbreak in the Americas has featured a series of first occurrences for Zika virus, including a new geographic range.2 3 9 However, the most remarkable outcome of this outbreak has been the scientific consensus that Zika virus is a cause of congenital infection and associated birth defects, including microcephaly, and Guillain-Barré syndrome.3 10 11 There exist substantial concerns that this outbreak may portend consequential threats to human health around the world.2 12 13
The primary vector of Zika virus, mosquitoes of the Aedes genus, is present worldwide, including throughout Africa, Asia and the Americas.14 Considering that six decades have passed since the first identification of the virus, it is perhaps unexpected that clinically consequential Zika virus disease had not been documented prior to the current outbreak. Substantial work on understanding the epidemiology of Zika virus has been conducted since the 2007 outbreak on Yap; however, there has been little study of the extant data that were collected beforehand. Several recent publications have reviewed and mapped reports of Zika virus,2 15–20 but there still exist gaps in completeness, pertinent detail and accuracy. Under the threat of the current outbreak in the Americas, there has been a call for research to fill these gaps.3
In this review, we present the most comprehensive evidence of human infection with Zika virus, from its discovery in 1947 in Uganda to its first outbreak in 2007 on Yap. Our aim is to elucidate the historical epidemiology of Zika virus in countries with evidence of past infection.
Methods
Search strategy and selection criteria
A systematic literature review was conducted to identify microbiological evidence of human Zika virus infection, as well as its epidemiology and clinical documentation, from 1947 to 2007. Embase, PubMed, Scopus and CINAHL Plus were searched using the terms Zika OR ((flavivirus OR arbovirus) AND (seroprevalence OR serosurvey OR prevalence OR epidemiology)). A search of the authors' personal libraries was also conducted. Articles written in all languages and published until 31 December 2007 were included. We did not contact individuals to obtain additional data.
Article titles were screened for mention of Zika virus, mosquito-borne arbovirus surveillance, incidence, prevalence or epidemiology in any geographic location, serosurvey of arboviruses, especially flaviviruses, reports of an outbreak of a flavivirus-borne illness, especially yellow fever or dengue fever, and reports of fevers of unknown origin in a tropical region. Full-text articles were then excluded if they did not report quantitative data on Zika virus infection, seroprevalence or epidemiology in humans, if Zika virus seroprevalence was assessed but reported as zero, if sera collection occurred after 1 April 2007, the beginning of the outbreak on Yap, or if they reported data that were documented more completely in another article captured by the search.
Data extraction and aggregation
Seroprevalence of Zika virus infection was extracted, as were the age and sex distributions of positive test results. If seroprevalence was not reported, it was calculated by dividing the number of positive sera by the number of sera tested. In studies that reported testing individuals multiple times, only the results from the most recent test were extracted. Also extracted were the original purpose for each study, serologic test modalities used, titre at which a test result was considered positive, years and sites of sera collection and clinical features observed in individuals reported to be ill.
Seroprevalence was aggregated to the country level from all articles reporting on a country. This was carried out by dividing the sum of all positive sera in a country by the sum of all sera tested in that country. In calculating aggregate country-level seroprevalence, results from the most conservative test modality were used from articles that reported results from multiple test modalities.21–23
Seroprevalence by age has been presented using the age intervals reported in the articles reviewed. Seroprevalence was aggregated within each age interval. To control for selection bias, sera from individuals reporting signs of illness were excluded from these calculations of seroprevalence by age.5 8 21 24–32
Sera were categorised as positive or negative according to the titre thresholds defined in the individual article. For articles that reported titres but not positive test thresholds, we used the following titre thresholds to align with the majority of other articles in this review: haemagglutination inhibition assay (HI) titre of 1:20,21 23 33–48 viral neutralisation antibody (NT) titre of 1:2049 and complement fixation (CF) titre of 1:8.50 51
Interpretation of serologic test results
In antibody detection test modalities, Zika virus is cross-reactive with other flaviviruses, such as yellow fever, dengue and West Nile.52–55 As such, seroprevalence of Zika virus infection may be overestimated by these test modalities.9 Titre threshold to determine a positive test result also factors into test specificity. To interpret appropriately the meaning of the seroprevalence data in the articles reviewed, results from different serologic test modalities were compared in two ways: first, in a between-test comparison of seroprevalence across studies, and second, in a between-test comparison of seroprevalence within studies that tested sera with multiple test modalities. For these two analyses, the individual study was the unit of analysis. To eschew aggregating data, articles reporting on more than one country were divided into separate studies; the same was performed for articles reporting on different time periods of sample collection within a country. For the analysis of seroprevalence across studies, data series were created for each serologic test modality, and for each positive test titre threshold used for HI. These series were compared using a non-parametric test of equality of populations (Kruskal-Wallis H-test).56 The significance level was set at α<0.05. For the analysis of seroprevalence within studies, the data were analysed by inspection. To control for selection bias, articles reporting signs of illness in their sample population were excluded from these analyses of seroprevalence by test modality.5 8 21 24–32
Results
Search
A flow diagram of the article selection process57 has been presented elsewhere (see online supplementary appendix A). The database search identified 12 026 articles. An additional 106 articles were identified through searches of the authors' personal libraries and relevant bibliographies. After removal of 11 591 articles through elimination of duplicates and title screening, 541 articles were selected for full-text assessment. Of these, 33 could not be located (see online supplementary appendix B). Four hundred and thirty-five articles did not fulfil inclusion criteria, including four that reported testing for Zika virus in humans with no positive results.58–61 An additional three articles were identified through a review of references of included articles. This search yielded 76 articles that were used for analysis.5 8 21–51 62–104 Articles included in this review were written in English (n=43), French (n=30), German (n=1), Portuguese (n=1) and Spanish (n=1).
bmjgh-2016-000087supp_appendixA.pdf (306.1KB, pdf)
bmjgh-2016-000087supp_appendixB.pdf (88.3KB, pdf)
Countries and years reported
Microbiological evidence of Zika virus infection was identified in 29 countries in Africa,5 21–38 40 43–47 50 62–72 74–78 80–88 90–101 104 eight countries in Asia8 42 48 49 51 73 79 89 102 103 and one country in Europe39 41 (table 1). No articles from the American continents were eligible for inclusion. Only one article reported testing for evidence of Zika virus anywhere in the Americas (Trinidad, 1953–1954),60 but it reported zero seroprevalence and thus was not included in this review. The number of articles reporting on each country ranged from 1 to 12. Multiple articles reported on more than one country.66 75 76 88 89 100 104 Current country names have been used.
Table 1.
Countries with evidence of Zika virus infection in humans prior to 1 April 2007
| Year(s) of sera collection | Source of sample population* |
Serologic test modalities* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of articles reporting on country | General population | Patients at a healthcare facility | Not reported | HI | IP | NT | CF | ELISA | ||
| Africa | ||||||||||
| West/Central Africa | ||||||||||
| Angola38 83 | 2 | 1960, 1971–1972 | X | X | X | X | ||||
| Benin66 | 1 | 1967 | X | X | ||||||
| Burkina Faso31 33 | 2 | 1963–1964, 1979 | X | X | X | |||||
| Cameroon45 46 63 90 | 4 | 1963–1966, 1971–1972, 1984 | X | X | X | |||||
| Central African Republic28 32 34–37 40 88 97 | 9 | 1954†, 1961–1967, 1975, 1979 | X | X | X | X | X | |||
| Chad88 | 1 | 1954† | X | X | ||||||
| Côte d'Ivoire21 24 44 92 | 4 | 1963–1965, 1969, 1977, 1999 | X | X | X | X | X | X | ||
| Gabon66 78 98 | 3 | 1967, 1975, 1979–1980 | X | X | X | |||||
| Guinea-Bissau43 | 1 | 1964–1965 | X | X | ||||||
| Liberia47 66 | 2 | 1967, 1981–1982 | X | X | ||||||
| Mali66 | 1 | 1964–1967 | X | X | ||||||
| Niger66 | 1 | 1965 | X | X | ||||||
| Nigeria5 26 27 29 50 62 66 68 69 84–86 | 12 | 1951–1953, 1955, 1964–1975, 1980 | X | X | X | X | X | X | ||
| Republic of the Congo88 | 1 | 1954† | X | X | ||||||
| Senegal22 25 65 87 91 | 5 | 1962, 1965–1966, 1970–1975, 1988–1990 | X | X | X | X | X | X | ||
| Sierra Leone93 | 1 | 1972 | X | X | ||||||
| Togo66 | 1 | 1964–1966 | X | X | ||||||
| Africa, other | ||||||||||
| Burundi94 | 1 | 1980–1982 | X | X | ||||||
| Djibouti30 | 1 | 1991–1992 | X | X | ||||||
| Egypt101 | 1 | 1954 | X | X | X | |||||
| Ethiopia74 95 99 104 | 4 | 1961–1964, 1966, 1971 | X | X | X | |||||
| Kenya64 72 76 104 | 4 | 1961–1962, 1966–1969 | X | X | X | X | ||||
| Madagascar70 71 | 2 | 1977, 1986 | X | X | X | |||||
| Mozambique82 | 1 | 1957 | X | X | ||||||
| Republic of Seychelles80 | 1 | 1970† | X | X | ||||||
| Somalia74 76 | 2 | 1966, 1969 | X | X | ||||||
| Sudan23 | 1 | 1976 | X | X | X | |||||
| Tanzania76 100 104 | 3 | 1945–1948, 1961–1962, 1967–1969 | X | X | X | X | ||||
| Uganda67 75–77 81 96 100 104 | 8 | 1945–1948, 1961–1962, 1967–1969, 1970, 1984 | X | X | X | X | ||||
| Asia | ||||||||||
| South Asia | ||||||||||
| India103 | 1 | 1952 | X | X | ||||||
| Pakistan51 | 1 | 1979–1980 | X | X | ||||||
| East Asia | ||||||||||
| Hong Kong79 | 1 | 1973–1977 | X | X | ||||||
| Southeast Asia | ||||||||||
| Indonesia8 42 48 | 3 | 1973, 1977–1978 | X | X | X | X | ||||
| Malaysia49 89 102 | 3 | 1953–1954, 1996–1997 | X | X | X | X | ||||
| Philippines73 | 1 | 1953 | X | X | ||||||
| Thailand89 | 1 | 1954 | X | X | ||||||
| Vietnam89 | 1 | 1954 | X | X | ||||||
| Europe | ||||||||||
| Spain39 41 | 2 | 1977†, 1980 | X | X | X | |||||
*Marked if applicable to one or more studies reporting on country.
†Year of article publication for articles not reporting year(s) of sera collection.
CF, complement fixation test; ELISA, enzyme-linked immunosorbent assay; HI, haemagglutination inhibition assay; IP, intracerebral mouse-protection test; NT, viral neutralisation antibody test (plaque reduction neutralisation test or microneutralisation).
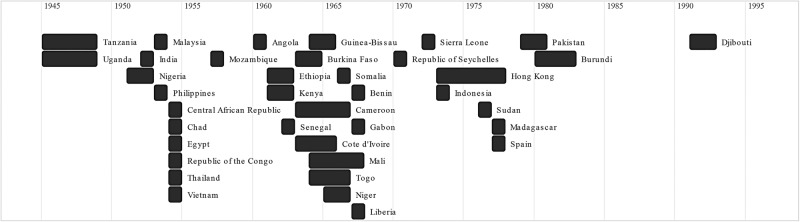
Years of sera collection ranged from 1945 to 1999 (table 1). For articles that did not document years of sera collection, the year of article publication has been reported.39 80 88 102
Original purpose of studies and populations sampled
These studies were originally conducted for a variety of purposes. Five of these studies were investigations of Zika virus specifically,5 8 27 67 68 and 55 were general serosurveys of mosquito-borne arboviruses.22 26 28 29 33–35 37–45 48 49 51 62–66 69–74 76–85 87 89–92 94–98 100–104 Ten studies were investigations of yellow fever,21 24 25 36 46 50 75 86 93 99 often as a response to an outbreak,21 24 25 46 50 86 and one study was an investigation into an outbreak of dengue fever.30 Of the remaining five studies, one investigated exanthematous fevers and fevers of unknown origin,32 one investigated a case of Spondweni virus infection,31 one was a survey of neurotropic viruses,88 one was a survey of schistosomiasis23 and one was a study on epilepsy.47
Studies sampling the general population were often conducted as a representative serosurvey of a country or subnational area. Of the 70 723 individuals tested across all studies, 32 433 were recruited from the general population.21–26 33–38 41 42 44–49 62–65 69 71–73 84 85 89 92 94 95 101 103 Sampling from healthcare facilities was performed either to investigate disease or as a matter of convenience. Eighteen thousand three hundred and forty-one individuals were recruited from healthcare facilities.5 8 21 24 26–32 39 50 51 76 77 79 81 85 89 96 98 101 The sources for the remaining 19 949 sampled individuals were not adequately described.40 43 66–68 70 74 75 78 80 82 83 86–88 90 91 93 97 99 100 102 104
Clinical features
Twelve articles reported signs or symptoms of illness in their entire sample population.5 8 21 24–32 Five articles described severe clinical features: two reported bleeding,21 24 one reported ‘meningoencephalomyelitis’,32 one reported ‘meningeal irritation’30 and one reported ‘liver damage’.5 All 12 of these articles mentioned the presence of fever. Other features described include exanthema, headache, bilious vomiting, arthralgia, muscle aches, stomach aches, jaundice and conjunctivitis. These findings should be considered alongside the original purposes of these studies, some of which were conducted to investigate endemic haemorrhagic fevers. There likely exist significant confounders of the association between clinical features and Zika virus infection reported in these articles. As a result, it is not possible to attribute these clinical features to Zika virus infection. No articles reported on microcephaly or other congenital birth defects, or Guillain-Barré Syndrome. In addition, no articles reported on sexual transmission of Zika virus.
Geographic correlates of seroprevalence
Of the 76 articles, 72 reported seroprevalence, or exact data that could be used to calculate seroprevalence in the population sampled. Three articles reported seroprevalence only in graphs34 78 89—these data were estimated and have been reported separately. One article reported only the number of positive sera without the number of sera tested; therefore, seroprevalence could not be determined.28
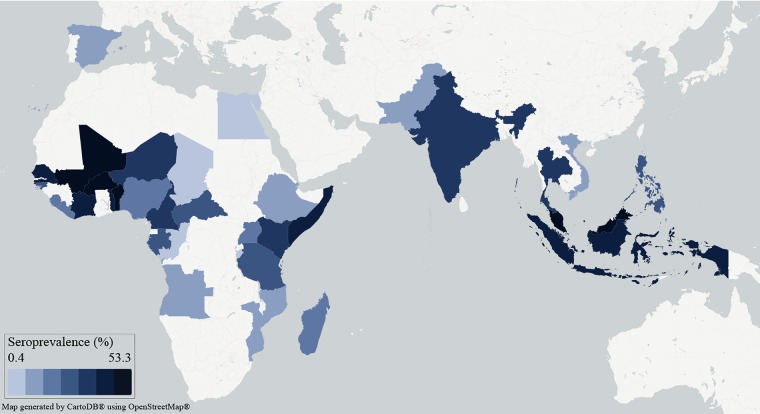
The mean seroprevalence across all countries included in this review was 15.7% (table 2). The aggregate country-level seroprevalence ranged from 0.4% in the Republic of the Congo, where 460 sera were tested, to 53.3% in Burkina Faso, where 1897 sera were tested (figure 1). The number of sera tested per country ranged from 43 in Pakistan, where the seroprevalence was reported as 2.3%, to 15 122 in Nigeria, where the seroprevalence was calculated to be 6.8%. The spatial distribution of seroprevalence was not specifically analysed, but West Africa and parts of Southeast Asia appear to have had higher seroprevalence than other regions. Subnational variability in seroprevalence was occasionally prominent, such as in Kenya between the Kitui District (dry plateau, seroprevalence=1.3%) and Malindi District (coastal region, seroprevalence=52.0%).72
Table 2.
Seroprevalence of Zika virus infection by country
| Number of sera tested | Seroprevalence (%) | |
|---|---|---|
| Africa | ||
| West/Central Africa | ||
| Angola | 5082 | 4.0 |
| Benin | 244 | 44.0 |
| Burkina Faso | 1897 | 53.3 |
| Cameroon | 5811 | 16.5 |
| Central African Republic* | 7657 | 10.8 |
| Central African Republic† | 908 | 27.1 |
| Chad | 140 | 1.4 |
| Côte d'Ivoire‡ | 3006 | 24.7 |
| Gabon | 970 | 8.4 |
| Gabon† | 1276 | 32.4 |
| Guinea-Bissau | 1154 | 10.6 |
| Liberia | 527 | 4.7 |
| Mali | 2369 | 52.0 |
| Niger | 308 | 18.0 |
| Nigeria§¶ | 15 122 | 6.8 |
| Republic of the Congo | 460 | 0.4 |
| Senegal** | 4734 | 39.3 |
| Sierra Leone | 899 | 6.9 |
| Togo | 1294 | 31.0 |
| Africa, other | ||
| Burundi | 623 | 1.4 |
| Djibouti | 91 | 2.2 |
| Egypt | 180 | 0.6 |
| Ethiopia | 1939 | 3.9 |
| Kenya | 3719 | 14.9 |
| Madagascar | 392 | 7.7 |
| Mozambique | 249 | 4.0 |
| Republic of Seychelles | 300 | 0.7 |
| Somalia | 477 | 19.7 |
| Sudan†† | 109 | 0.0 |
| Tanzania | 1063 | 12.7 |
| Uganda | 4236 | 6.3 |
| Asia | ||
| South Asia | ||
| India | 197 | 16.8 |
| Pakistan | 43 | 2.3 |
| East Asia | ||
| Hong Kong | 235 | 4.7 |
| Southeast Asia | ||
| Indonesia | 323 | 33.7 |
| Malaysia | 340 | 43.5 |
| Malaysia† | 358 | 49.2 |
| Philippines | 153 | 12.4 |
| Thailand | 50 | 16.0 |
| Vietnam | 50 | 4.0 |
| Europe | ||
| Spain | 1738 | 2.9 |
| World | ||
| Total across all countries | 70 723 | 15.7 |
*Excludes one article for which seroprevalence could not be calculated.28
‡Reports results of NT for sera that were tested with multiple test modalities.21
¶Reports results from the more recent test for individuals tested twice.69
**Reports results of IP for sera that were tested with multiple test modalities.22
††Reports results of NT for sera that were tested with multiple test modalities.23
Figure 1.
Map of Zika virus seroprevalence in countries with evidence of Zika virus infection in humans prior to 1 April 2007.
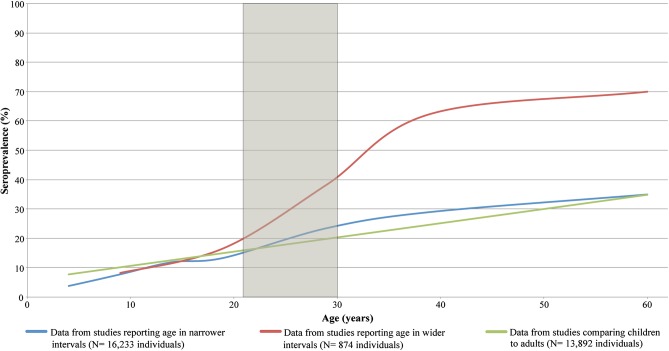
Demographic correlates of seroprevalence
Seroprevalence by age interval was documented for 30 989 individuals in 20 countries (figure 2).5 21 22 37 40 43 44 46 49 62 63 66 67 70 72 73 76 77 79 81–87 89 91–93 97 98 100 104 An additional 6139 individuals were reported only to be 20 years or younger at the time of sera collection. The aggregate seroprevalence for this group was calculated to be 3.0%.36 38 49 66 79 In comparison, seroprevalence among individuals over 20 years of age tended to be much greater.
Figure 2.
Years of first identification of Zika virus seropositivity by country.
Seroprevalence of Zika virus infection was found to increase with age, regardless of the age intervals or cut-points used. To correlate this finding with the epidemiology of the current outbreak in the Americas, the graph in figure 2 was overlaid with a shaded area representing the IQR of maternal age in Brazil, the origin of the current outbreak in the Americas. While some articles reported upwards of 70% seroprevalence among reproductive-aged individuals,62 89 97 the mean seroprevalence in this age group was evidently much lower, in the range of 15–40%.
One article reported testing sera from 28 mother–newborn pairs by HI.98 The newborns were tested using cord blood. One newborn tested positive for Zika virus after his mother tested positive. HI titres were not reported, and there were no signs of illness in either this mother or her newborn. No other mothers in this study tested positive.
To more completely assess the historical demographics of Zika virus infection, we would benefit from knowing the sex ratio among individuals who tested positive; however, this information was reported for only 362 individuals.5 31 42 48 66 79 89 97 98 These data were not analysed because the representativeness of the populations sampled could not be ascertained.
Variability in seroprevalence between serologic test modalities
Seroprevalence by serologic test modality was documented for 57 782 individuals not displaying clinical features of illness across 73 studies reported in 67 articles (figure 3).5 21–24 33–51 62–104 Sera were tested by HI in the majority of studies. Studies reported HI positive test titre thresholds of 1:10,62 63 70–72 78 85 86 90 92–96 or 1:20;21 23 33–48 however, many studies that used HI did not report a positive test titre threshold.22 64–66 74–77 79 81 83 91 97–99 104 Few studies used plaque reduction neutralisation (PRNT),21 23 49 a modality of NT and the most specific of the test modalities documented in this review.3 9 52 Among the three studies reporting results of PRNT, the median seroprevalence was 16.7%. IgM-capture ELISA (MAC-ELISA) is currently the most regularly used antibody detection test modality;3 52 however, in this review, it was used in only one study87 that reported a seroprevalence of 6.7%. IgG ELISA was used in another study,24 that reported a seroprevalence of 54.2%. A non-parametric equality-of-populations test (Kruskal-Wallis test) detected no significant difference in the scale of seroprevalence between test modalities (p=0.63). This finding implies that none of the test modalities was uniformly more prone to overestimate seroprevalence of Zika virus infection. Reverse transcription PCR (RT-PCR) was not used by any studies included in this review.
Figure 3.
Age distribution of seroprevalence of Zika virus infection. The shaded area represents the interquartile range for maternal age in Brazil, the origin of the current outbreak of Zika virus. Age intervals: narrower intervals report on individuals aged 0–4, 5–9, 10–14, 15–19, 20–29, 30–39 and ≥40 years; wider intervals report on individuals aged 0–9, 10–19, 20–29, 30–39 and ≥40 years; and dual age intervals with a cut-point at 1346 71 or 15 years,43 66 67 73 76 77 82 87 91 93 100 104 compare children to adults. Data were reported in the narrower intervals for 16 223 individuals tested in Cameroon, Central African Republic, Côte d'Ivoire, Kenya, Nigeria, Philippines, Senegal and Uganda,22 37 44 45 62 63 72 73 76 81 84 86 92 in the wider intervals for 874 individuals in Central African Republic,40 97 and in dual intervals for 13 892 individuals in all 20 countries where seroprevalence by age was reported. Methods: Bézier spline smoothed scatterplot with point data for age intervals placed at the upper bound of each interval. Point data from studies comparing children to adults were placed at 15 and 39 years, and the line was then projected under an assumption of constant slope. The IQR for maternal age in Brazil was estimated by indirect standardisation using 2010 age-specific fertility rates and female population by age in Brazil.105 106
Three studies tested a total of 1074 sera with more than one serologic test modality.21–23 In these three studies, seroprevalence depended on the test used. The highest seroprevalence reported by each of these studies was found by HI (range: 1.8–83.3%). IP, PRNT and CF found lower seroprevalence (range: 0.0–21.3%). Two of these studies found seroprevalence to be 0.0% with either CF21 or PRNT;23 however, one of these two studies had a small sample size (N=18), and the other reported a low seroprevalence by HI (1.8%), the least conservative of the test modalities. The third study had the largest sample size (N=947), and its most conservative seroprevalence was reported to be 20.8% by IP.22 This study's sample population consisted entirely of children. The reports of 0.0% seroprevalence may be evidence of inconsistency of results between test modalities. However, the entirety of this analysis indicates that estimates of seroprevalence are generally accordant.
Discussion
This review emphasises three important features of Zika virus epidemiology. First, that Zika virus has likely been endemic for decades in parts of sub-Saharan Africa and Asia, possibly with upwards of 50% seroprevalence at the country level. Second, that seroprevalence of Zika virus infection increases across the lifespan, with a possible seroprevalence of 15–40% among reproductive-age individuals. Third, that historical evidence of Zika virus infection in these regions exists regardless of the serologic test modality used.
Though the timeline of Zika virus identification over the past three decades is sparse, evidence of Zika virus infection was found in data from 37 countries in Africa and Asia, and one country in Europe. Since the current outbreak in the Americas is widely believed to be a result of the first introduction of Zika virus to this region,2 3 9 105 it is unsurprising that we found no historical evidence of Zika virus infection in the Americas. However, it was unexpected to find evidence in two articles of Zika virus infection in Spain.39 41 These findings could represent false positives, or infection acquired during travel; however, these possibilities were not discussed in the articles. Locally acquired Zika virus infection, though unlikely, could also be considered. Mosquitoes of the Aedes aegypti species, the primary vector of Zika virus, are known to have existed in Spain in the decades prior to the publication of these data from Spain.106 107
Pertinent to the current outbreak is the age distribution of Zika virus seroprevalence. The presumed de novo introduction of Zika virus to the Americas has caused significant morbidity due to congenital infection, including microcephaly. This relationship had not been documented prior to the current outbreak,3 but has now been determined to be causal.10 The lack of prior documentation may be explained by a hypothesis about the protective effect of Zika virus infection early in life. Infection in women prior to child bearing age may confer immunity that might later protect against congenital infection and gestational morbidity due to Zika virus. However, this review did not find evidence to strongly support this hypothesis. In countries where Zika virus infection has been reported, most reproductive-aged women did not display evidence of prior exposure to Zika virus (figure 2). The implications of this finding should be explored further.
Owing to the cross-reactivity in antibody detection between Zika virus and other flaviviruses, it is difficult to obtain an accurate estimate of the prevalence of past infection with Zika virus.9 52 55 Despite its status as the test of reference, PRNT is also prone to cross-reactivity among the flaviviruses.9 55 To overcome this problem in the current outbreak, RT-PCR is used to test for viral nucleic acid in acute cases;3 52 however, none of the studies in this review used RT-PCR. To identify evidence of presumptive Zika virus infection, MAC-ELISA may be run in parallel for Zika virus and dengue virus; a positive result for Zika virus with a negative result for dengue virus may indicate recent Zika virus infection.3 52 PRNT may then be used to help verify MAC-ELISA results. Without this comparative data from the studies under review, Zika virus seroprevalence likely overestimates the true prevalence of past infection with Zika virus.
Zika virus MAC-ELISAs and IgG-ELISAs were only developed in recent decades, and their use was therefore restricted to the later studies.24 30 87 These assays, along with other Zika virus serology assays, have only been available in reference laboratories. On 26 February 2016, to assist with expanding the use of the CDC Zika virus IgM serology assay, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the CDC Zika virus MAC-ELISA.108 Although several vendors have developed commercial assays that detect Zika virus IgM and IgG antibodies, none of these have been FDA approved for clinical testing, and are currently undergoing clinical evaluation.
Our study had several other limitations. We were not able to locate 33 articles that were listed for full-text assessment; references for these articles have been listed in the online supplementary appendix. The reference lists from many of the older articles reviewed did not include titles, and thus they were not included in our search. Data extraction was performed by only one investigator, but the integrity of the data was repeatedly verified as it was manipulated and analysed. We extracted data on infection and prevalence only in humans, and we did not consider vectors or animal infections, though few articles reviewed discussed these issues in detail.42 49 51 66 85 87 We aggregated data from each country without respect to time, and as such we were not able to analyse trends over time, either in seroprevalence or in test accuracy. The serologic test modalities discussed have dissimilar sensitivities and specificities; thus, their results are not directly comparable. To account for this, we set the test results in context and compared modalities across studies.
There exist several plausible hypotheses to explain the current outbreak in the Americas.2 3 18 105 They are all founded on the belief that Zika virus circulated in Africa and Asia for decades, and was only recently introduced to the Americas. This review provides the most comprehensive evidence to support this foundational belief. It is not clear why the morbidity and rapidity of spread of the current outbreak were not previously seen in Africa or Asia; however, under-recognition and misattribution of observations are possible drivers. It is also possible that genetic alterations have resulted in increased virulence and different virus–host dynamics, but none of the articles included in this review addressed this issue. Future research on the epidemiology of Zika virus may be targeted to the populations and settings that have been identified by this review. Future seroprevalence studies in regions where Zika virus was recently introduced may also provide valuable information regarding Zika virus spread and history of exposure.
Acknowledgments
The authors thank Marcelo Cerullo for assisting with statistical analyses and language translation, Dr Lisa Pell for reviewing the manuscript and providing valuable input, and Jenna Craig for assisting with retrieval of papers.
Footnotes
Handling editor: Seye Abimbola
Contributors: This review was conceptualised by SKM, who also provided overall supervision for the project. The literature search, data extraction and writing of the manuscript were performed by HJP. All authors reviewed the extracted data and contributed equally to its interpretation and analysis for this review.
Funding: JBG has received research grants from GlaxoSmithKline and Hoffman-La Roche to study antiviral resistance in influenza, and from Pfizer to conduct microbiological surveillance of Streptococcus pneumoniae.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dick GW, Kitchen SF, Haddow AJ. Zika virus: (I) isolations and serological specificity. Trans R Soc Trop Med Hyg 1952;46:509–20. 10.1016/0035-9203(52)90042-4 [DOI] [PubMed] [Google Scholar]
- 2.Kindhauser MK, Allen T, Frank V et al. . Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ 2016. Published Online 9 Feb 2016;171082 10.2471/BLT.16.171082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen LR, Jamieson DJ, Powers AM et al. . Zika virus. N Engl J Med 2016;374:1552–63. 10.1056/NEJMra1602113 [DOI] [PubMed] [Google Scholar]
- 4.Duffy MR, Chen TH, Hancock WT et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009;360:2536–43. 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 5.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954;48:139–45. 10.1016/0035-9203(54)90006-1 [DOI] [PubMed] [Google Scholar]
- 6.Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956;50:442–8. 10.1016/0035-9203(56)90091-8 [DOI] [PubMed] [Google Scholar]
- 7.Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg 1964;58:335–8. 10.1016/0035-9203(64)90200-7 [DOI] [PubMed] [Google Scholar]
- 8.Olson JG, Ksiazek TG, Suhandiman et al. . Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981;75:389–93. 10.1016/0035-9203(81)90100-0 [DOI] [PubMed] [Google Scholar]
- 9.Chang C, Ortiz K, Ansari A et al. . The Zika outbreak of the 21st century. J Autoimmun 2016;68:1–13. 10.1016/j.jaut.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Zika virus, microcephaly, and Guillain-Barré syndrome: situation report, 31 March 2016. http://www.who.int/emergencies/zika-virus/situation-report/31-march-2016/en/ (accessed 21 July 2016).
- 11.World Health Organization. Zika virus, microcephaly, and Guillain-Barré syndrome: situation report, 7 April 2016. pp 117–34. http://www.who.int/emergencies/zika-virus/situation-report/7-april-2016/en/ (accessed 21 July 2016).
- 12.Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 2016;374:601–4. 10.1056/NEJMp1600297 [DOI] [PubMed] [Google Scholar]
- 13.Heymann DL, Hodgson A, Sall AA et al. . Zika virus and microcephaly: why is this situation a PHEIC? Lancet 2016;387:719–21. 10.1016/S0140-6736(16)00320-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraemer MU, Sinka ME, Duda KA et al. . The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. ELife 2015;4:e08347 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009;15:1347–50. 10.3201/eid1509.090442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ioos S, Mallet HP, Leparc Goffart I et al. . Current Zika virus epidemiology and recent epidemics. Med Maladies Infect 2014;44:302–7. 10.1016/j.medmal.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev 2016;29:487–524. 10.1128/CMR.00072-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paixao ES, Barreto F, Teixeira Mda G et al. . History, epidemiology, and clinical manifestations of Zika: a systematic review. Am J Public Health 2016;106:606–12. 10.2105/AJPH.2016.303112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plourde AR, Bloch EM. A literature review of Zika virus. Emerg Infect Dis 2016;22:1185–92. 10.3201/eid2207.151990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Map displaying infected countries from 1947-2016. http://www.who.int/emergencies/zika-virus/zika-historical-distribution.pdf?ua=1 (accessed 31 Mar 2016).
- 21.Chippaux A, Chippaux-Hyppolyte C, Monteny-Vandervorst N et al. . Diagnostic de plusieurs cas de fièvre jaune en zone d’émergence endémique en Côte-d'Ivoire. Med Trop 1981;41:53–61. [PubMed] [Google Scholar]
- 22.Le Gonidec G, Dhiver F. Le virus de la fièvre jaune et autres arbovirus dans le Sénégal oriental: étude des serums humains. Bull Soc Pathol Exot 1973;66:603–15. [Google Scholar]
- 23.Omer AH, McLaren ML, Johnson BK et al. . A seroepidemiological survey in the Gezira, Sudan, with special reference to arboviruses. J Trop Med Hyg 1981;84:63–6. [PubMed] [Google Scholar]
- 24.Akoua-Koffi C, Diarrassouba S, Benie VB et al. . Investigation autour d'un cas mortel de fièvre jaune en Côte d'Ivoire en 1999. Bull Soc Pathol Exot 2001;94:227–30. [PubMed] [Google Scholar]
- 25.Chambon L, Wone I, Brès P et al. . Une épidémie de fièvre jaune au Sénégal en 1965: l’épidémie humaine. Bull World Health Organ 1967;36:113–50. [PMC free article] [PubMed] [Google Scholar]
- 26.Fagbami A. Human arthropod-borne virus infections in Nigeria: serological and virological investigations at Shaki, Oyo State. J Hyg Epidemiol Microbiol Immunol 1978;22:184–9. [PubMed] [Google Scholar]
- 27.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979;83:213–19. 10.1017/S0022172400025997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georges AJ, Saluzzo JF, Gonzalez JP et al. . Arboviroses en Centrafrique: incidence et aspects diagnostiques chez l'homme. Med Trop 1980;40:561–8. [PubMed] [Google Scholar]
- 29.Moore DL, Causey OR, Carey DE et al. . Arthropod-borne viral infections of man in Nigeria, 1964-1970. Ann Trop Med Parasitol 1975;69:49–64. 10.1080/00034983.1975.11686983 [DOI] [PubMed] [Google Scholar]
- 30.Rodier GR, Gubler DJ, Cope SE et al. . Epidemic dengue 2 in the city of Djibouti 1991-1992. Trans R Soc Trop Med Hyg 1996;90:237–40. 10.1016/S0035-9203(96)90228-X [DOI] [PubMed] [Google Scholar]
- 31.Wolfe MS, Calisher CH, Mcguire K. Spondweni virus infection in a foreign resident of Upper Volta. Lancet 1982;320:1306–8. 10.1016/S0140-6736(82)91511-2 [DOI] [PubMed] [Google Scholar]
- 32.Chippaux A, Chippaux-Hyppolite C, Guerin J et al. . Syndromes exanthématiques et “fièvres inexpliquées” en Centrafrique. Bull Soc Pathol Exot 1965;58:34–47. [PubMed] [Google Scholar]
- 33.Brès P, Carrié J, Desbois A et al. . Les arbovirus en Haute Volta: enquête sérologique. Ann Inst Pasteur 1965;108:341–52. [Google Scholar]
- 34.Chippaux A, Chippaux-Hyppolite C. Immunologie des arbovirus chez des pygmées-babinga de Centrafrique. Bull Soc Pathol Exot 1965;58:820–33. [PubMed] [Google Scholar]
- 35.Chippaux-Hyppolite C. Enquête immunologique sur l'incidence des arbovirus chez l'homme en République centrafricaine: note préliminaire. Bull Soc Pathol Exot 1965;58:812–20. [PubMed] [Google Scholar]
- 36.Chippaux-Hyppolite C, Chippaux A. Les anticorps antiamarils chez les enfants en République centrafricaine. Bull World Health Organ 1966;34:105–11. [PMC free article] [PubMed] [Google Scholar]
- 37.Digoutte JP, Luong PNT. Contribution à l’étude des arboviroses en Afrique Centrale: (I) enquête immunologique chez l'homme dans le centre et l'ouest de la République centrafricaine. Bull Soc Pathol Exot 1968;6:803–17. [PubMed] [Google Scholar]
- 38.Filipe AR, De Carvalho RG, Relvas A et al. . Arbovirus studies in Angola: serological survey for antibodies to arboviruses. Am J Trop Med Hyg 1975;24:516–20. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez MT, Filipe AR. Antibodies to arboviruses in Northwestern Spain. Am J Trop Med Hyg 1977;26:792–7. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez JP, Saluzzo JF, Herve JP et al. . Enquête sérologique sur la prévalence des arbovirus chez l'homme en milieu forestier et périforestier de la région de la Lobaye, République centrafricaine. Bull Soc Pathol Exot 1979;72:416–23. [PubMed] [Google Scholar]
- 41.Lozano A, Filipe AR. Anticuerpos frente a virus West Nile y otros virus transmitidos por artropodos en la poblacion del Delta del Ebro. Rev Esp Salud Pública 1998;72:245–50. 10.1590/S1135-57271998000300009 [DOI] [PubMed] [Google Scholar]
- 42.Olson JG, Ksiazek TG, Gubler DJ et al. . A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Ann Trop Med Parasitol 1983;77:131–7. [DOI] [PubMed] [Google Scholar]
- 43.Pinto MR. Survey for antibodies to arboviruses in the sera of children in Portuguese Guinea. Bull World Health Organ 1967;37:101–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Robin Y, Brès P, Lartigue JJ et al. . Les arbovirus en Côte-d'Ivoire: enqête sérologique dans la population humaine. Bull Soc Pathol Exot 1968;61:833–45. [PubMed] [Google Scholar]
- 45.Salaun JJ, Brottes H. Les arbovirus au Cameroun: enquête sérologique. Bull World Health Organ 1967;37:343–61. [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai TF, Lazuick JS, Ngah RW et al. . Investigation of a possible yellow fever epidemic and serosurvey for flavivirus infections in northern Cameroon, 1984. Bull World Health Organ 1987;65:855–60. [PMC free article] [PubMed] [Google Scholar]
- 47.Van der Waals FW, Asher DM, Goudsmit J et al. . Post-encephalitic epilepsy and arbovirus infections in an isolated rainforest area of central Liberia. Trop Geogr Med 1986;38:203–8. [PubMed] [Google Scholar]
- 48.Van Peenen PFD, Joseph SW, Casals J et al. . Arbovirus antibodies in Indonesians at Malili, South Sulawesi (Celebes) and Balikpapan, E. Kalimantan (Borneo). Ann Trop Med Parasitol 1975;69:475–82. 10.1080/00034983.1975.11687035 [DOI] [Google Scholar]
- 49.Wolfe ND, Kilbourn AM, Karesh WB et al. . Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg 2001;64:310–16. [DOI] [PubMed] [Google Scholar]
- 50.Carey DE, Kemp GE, Troup JM et al. . Epidemiological aspects of the 1969 yellow fever epidemic in Nigeria. Bull World Health Organ 1972;46:645–51. [PMC free article] [PubMed] [Google Scholar]
- 51.Darwish MA, Hoogstraal H, Roberts TJ et al. . A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg 1983;77:442–5. 10.1016/0035-9203(83)90106-2 [DOI] [PubMed] [Google Scholar]
- 52.Revised diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. http://www.cdc.gov/zika/pdfs/denvchikvzikv-testing-algorithm.pdf (accessed 31 Mar 2016).
- 53.American Committee on Arthropod Borne Viruses Subcommittee on Information Exchange, Center for Disease Control. Zika. In: Berge TO, ed. International catalogue of arboviruses: including certain other viruses of vertebrates. Bethesda: (MD: ) Atlanta: (GA: ): Public Health Service, 1975:782–3. [Google Scholar]
- 54.De Madrid AT, Porterfield JS. The flaviviruses (group B arboviruses): a cross-neutralization study. J Gen Virol 1974;23:91–6. 10.1099/0022-1317-23-1-91 [DOI] [PubMed] [Google Scholar]
- 55.Waggoner JJ, Pinsky BA. Zika virus: diagnostics for an emerging pandemic threat. J Clin Microbiol 2016;54:860–7. 10.1128/JCM.00279-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corder G, Foreman D. Comparing more than two unrelated samples: the Kruskal-Wallis H-test. In: Corder G, Foreman D, eds. Nonparametric statistics: a step-by-step approach. Hoboken: (NJ: ): John Wiley & Sons, 2014. [Google Scholar]
- 57.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 58.Botros BAM, Watts DM, Soliman AK et al. . Serological evidence of dengue fever among refugees, Hargeysa, Somalia. J Med Virol 1989;29:79–81. 10.1002/jmv.1890290202 [DOI] [PubMed] [Google Scholar]
- 59.Raoult D, Roussellier P, Rodhain F et al. . Enquête séroépidémiologique en Camargue sur les virus transmis par les arthropodes, 1983. Med Maladies Infect 1985;15:636–8. 10.1016/S0399-077X(85)80231-6 [DOI] [Google Scholar]
- 60.Spence L, Jonkers AH, Grant LS. Arboviruses in the Caribbean Islands. Prog Med Virol 1968;10:415–86. [PubMed] [Google Scholar]
- 61.Sureau P, Jaeger G, Pinerd G et al. . Enquête séro-épidémiologique sur les arbovirus chez les pygmées Bi-Aka de la Lobaye, Empire centrafricain. Bull Soc Pathol Exot 1977;70:131–7. [PubMed] [Google Scholar]
- 62.Adekolu-John EO, Fagbami AH. Arthropod-borne virus antibodies in sera of residents of Kainji Lake Basin, Nigeria 1980. Trans R Soc Trop Med Hyg 1983;77:149–51. 10.1016/0035-9203(83)90053-6 [DOI] [PubMed] [Google Scholar]
- 63.Boche R, Jan C, Le Noc P et al. . Enquête immunologique sur l'incidence des arbovirus dans la population pygmée de l'est du Cameroun (Région de Djoum). Bull Soc Pathol Exot 1974;67:126–40. [PubMed] [Google Scholar]
- 64.Bowen ETW, Simpson DIH, Platt GS et al. . Large scale irrigation and arbovirus epidemiology, Kano Plain, Kenya: (II) preliminary serological survey. Trans R Soc Trop Med Hyg 1973;67:702–9. 10.1016/0035-9203(73)90041-2 [DOI] [PubMed] [Google Scholar]
- 65.Brès P, Lacan A, Diop I et al. . Les arbovirus au Sénégal: enquête sérologique. Bull Soc Pathol Exot 1963;56:384–402. [PubMed] [Google Scholar]
- 66.Brès P. Données récentes apportées par les enquêtes sérologiques sur la prévalence des arbovirus en Afrique, avec référence spéciale à la fièvre jaune. Bull World Health Organ 1970;43:223–67. [PMC free article] [PubMed] [Google Scholar]
- 67.Dick GW. Zika virus: (II) pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952;46:521–34. 10.1016/0035-9203(52)90043-6 [DOI] [PubMed] [Google Scholar]
- 68.Dick GW. Epidemiological notes on some viruses isolated in Uganda (Yellow fever, Rift Valley fever, Bwamba fever, West Nile, Mengo, Semliki forest, Bunyamwera, Ntaya, Uganda S and Zika viruses). Trans R Soc Trop Med Hyg 1953;47:13–48. 10.1016/0035-9203(53)90021-2 [DOI] [PubMed] [Google Scholar]
- 69.Fagbami A. Epidemiological investigations on arbovirus infections at Igbo-Ora, Nigeria. Trop Geogr Med 1977;29:187–91. [PubMed] [Google Scholar]
- 70.Fontenille D, Mathiot C, Rodhain F et al. . Les arboviroses dans la région de Tsiroanomandidy à Madagascar: études entomologiques, virologiques et sérologiques. Ann Soc Belge Med Trop 1988;68:43–52. [PubMed] [Google Scholar]
- 71.Fontenille D, Mathiot C, Rodhain F et al. . Les arboviroses dans la région de Nosy-Bé, Madagascar: données sérologiques et entomologiques. Bull Soc Pathol Exot 1988;81:58–70. [PubMed] [Google Scholar]
- 72.Geser A, Henderson BE, Christensen S. A multipurpose serological survey in Kenya: (2) results of arbovirus serological tests. Bull World Health Organ 1970;43:539–52. [PMC free article] [PubMed] [Google Scholar]
- 73.Hammon WM, Schrack WD Jr, Sather GE. Serological survey for arthropod-borne virus infections in the Philippines. Am J Trop Med Hyg 1958;7:323–8. [DOI] [PubMed] [Google Scholar]
- 74.Henderson BE, Tukei PM, Sekyalo E et al. . Arbovirus serological survey. Entebbe, Report, vol. 17 East African Virus Research Institute, 1968;29–32. [Google Scholar]
- 75.Henderson BE, Metselaar D, Cahill K et al. . Yellow fever immunity surveys in northern Uganda and Kenya and eastern Somalia, 1966-67. Bull World Health Organ 1968;38:229–37. [PMC free article] [PubMed] [Google Scholar]
- 76.Henderson BE, Kirya GB, Hewitt LE et al. . Serological surveys in East Africa and Somalia. Entebbe, Report, vol. 18 East African Virus Research Institute, 1969;35–47. [Google Scholar]
- 77.Henderson BE, Kirya GB, Hewitt LE. Serological survey for arboviruses in Uganda, 1967-69. Bull World Health Organ 1970;42:797–805. [PMC free article] [PubMed] [Google Scholar]
- 78.Jan C, Languillat G, Renaudet J et al. . Enquête sérologique pour les arbovirus au Gabon. Bull Soc Pathol Exot 1978;71:140–6. [PubMed] [Google Scholar]
- 79.Johnson BK, Chanas AC, Gardner P et al. . Arbovirus antibodies in the human population of Hong Kong. Trans R Soc Trop Med Hyg 1979;73:594–6. 10.1016/0035-9203(79)90063-4 [DOI] [PubMed] [Google Scholar]
- 80.Kirya BG, Hewitt LE, Sekyalo E et al. . Arbovirus serology. Entebbe, Report, vol. 19 East African Virus Research Institute, 1970;30–1. [Google Scholar]
- 81.Kirya BG, Hewitt LE, Lule M et al. . Arbovirus serology. Entebbe, Report, vol. 20 East African Virus Research Institute, 1971;32–6. [Google Scholar]
- 82.Kokernot R, Smithburn K, Gandara A et al. . Provas de neutralização com soros de individuos residentes em Moçambique contra determinados virus isolados em Africa transmitidos por artrópodes. Anais Inst Med Trop 1960;17:201–30. [PubMed] [Google Scholar]
- 83.Kokernot RH, Casaca VMR, Weinbren MP et al. . Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of Angola. Trans R Soc Trop Med Hyg 1965;59:563–70. 10.1016/0035-9203(65)90159-8 [DOI] [PubMed] [Google Scholar]
- 84.Macnamara FN, Horn DW, Porterfield J. Yellow fever and other arthropod-borne viruses: a consideration of two serological surveys made in South Western Nigeria. Trans R Soc Trop Med Hyg 1959;53:202–12. 10.1016/0035-9203(59)90072-0 [DOI] [PubMed] [Google Scholar]
- 85.Monath TP, Lee VH, Wilson DC et al. . Arbovirus studies in Nupeko Forest, a possible natural focus of yellow fever virus in Nigeria: (I) description of the area and serological survey of humans and other vertebrate hosts. Trans R Soc Trop Med Hyg 1974;68:30–8. 10.1016/0035-9203(74)90248-X [DOI] [PubMed] [Google Scholar]
- 86.Monath TP, Wilson DC, Casals J. The 1970 yellow fever epidemic in Okwoga District, Benue Plateau State, Nigeria: (3) serological responses in persons with and without pre-existing heterologous group B immunity. Bull World Health Organ 1973;49:235–44. [PMC free article] [PubMed] [Google Scholar]
- 87.Monlun E, Zeller H, Le Guenno B et al. . Surveillance de la circulation des arbovirus d'intérêt médical dans la région du Sénégal oriental, 1988-1991. Bull Soc Pathol Exot 1993;86:21–8. [PubMed] [Google Scholar]
- 88.Pellissier A. Enquête sérologique sur l'incidence des virus neurotropes en Afrique Equatoriale Française. Bull Soc Pathol Exot 1954;47:223–7. [PubMed] [Google Scholar]
- 89.Pond WL. Arthropod-borne virus antibodies in sera from residents of South-East Asia. Trans R Soc Trop Med Hyg 1963;57:364–71. 10.1016/0035-9203(63)90100-7 [DOI] [PubMed] [Google Scholar]
- 90.Radda A. Antikörper gegen arboviren in der bevölkerung West-Kameruns. Zbl Bakt Hyg I Abt Orig A 1973;225:375–80. [PubMed] [Google Scholar]
- 91.Renaudet J, Jan C, Ridet J et al. . Enquête sérologique pour les arbovirus dans la population humaine du Sénégal. Bull Soc Pathol Exot 1978;71:131–40. [PubMed] [Google Scholar]
- 92.Robin Y, Gidel R, Le Gonidec G et al. . Les arbovirus en Côte d'Ivoire: enquête sérologique dans la population humaine de la région d'Abengourou. Bull Soc Pathol Exot 1971;64:434–46. [PubMed] [Google Scholar]
- 93.Robin Y, Mouchet J. Enquête sérologique et entomologique sur la fièvre jaune en Sierra Leone. Bull Soc Pathol Exot 1975;68:249–58. [PubMed] [Google Scholar]
- 94.Rodhain F, Carteron B, Laroche R et al. . Arboviroses humaines au Burundi: résultats d'une enquête séroépidémiologique, 1980-1982. Bull Soc Pathol Exot 1987;80:155–61. [PubMed] [Google Scholar]
- 95.Rodhain F, Hannoun C, Metselaar D. Enquête épidémiologique et sérologique sur les arboviroses dans la basse vallée de l'Omo, Ethiopie méridionale. Bull World Health Organ 1972;47:295–304. [PMC free article] [PubMed] [Google Scholar]
- 96.Rodhain F, Gonzalez JP, Mercier E et al. . Arbovirus infections and viral haemorrhagic fevers in Uganda: a serological survey in Karamoja district, 1984. Trans R Soc Trop Med Hyg 1989;83:851–4. 10.1016/0035-9203(89)90352-0 [DOI] [PubMed] [Google Scholar]
- 97.Saluzzo JF, Gonzalez JP, Herve JP et al. . Enquête sérologique sur la prévalence de certains arbovirus dans la population humaine du sud-est de la République centrafricaine en 1979. Bull Soc Pathol Exot 1981;74:490–9. [PubMed] [Google Scholar]
- 98.Saluzzo JF, Ivanoff B, Languillat G et al. . Enquête sérologique sur l'incidence des arbovirus parmi les populations humaines et simiennes du sud-est de la République gabonaise. Bull Soc Pathol Exot 1982;75:262–6. [PubMed] [Google Scholar]
- 99.Sérié C, Casals J, Panthier R et al. . Etudes sur la fièvre jaune en Ethiopie: (2) enquête sérologique sur la population humaine. Bull World Health Organ 1968;38:843–54. [PMC free article] [PubMed] [Google Scholar]
- 100.Smithburn KC. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J Immunol 1952;69:223–34. [PubMed] [Google Scholar]
- 101.Smithburn KC, Taylor RM, Rizk F et al. . Immunity to certain arthropod-borne viruses among indigenous residents of Egypt. Am J Trop Med Hyg 1954;3:9–18. [DOI] [PubMed] [Google Scholar]
- 102.Smithburn KC. Neutralizing antibodies against arthropod-borne viruses in the sera of long-time residents of Malaya and Borneo. Am J Hyg 1954;59:157–63. [DOI] [PubMed] [Google Scholar]
- 103.Smithburn KC, Kerr JA, Gatne PB. Neutralizing antibodies against certain viruses in the sera of residents of India. J Immunol 1954;72:248–57. [PubMed] [Google Scholar]
- 104.Woodall JP, Williams MC, Revell AH et al. . Serology. Entebbe, Report, vol. 12 East African Virus Research Institute, 1962;25–8. [Google Scholar]
- 105.Faria NR, Azevedo Rdo S, Kraemer MU et al. . Zika virus in the Americas: early epidemiological and genetic findings. Science 2016;352:345–9. 10.1126/science.aaf5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christophers SR. Aedes aegypti (L.) the yellow fever mosquito: its life history, bionomics and structure. Cambridge: Cambridge University Press, 2009. [Google Scholar]
- 107.Reiter P. Yellow fever and dengue: a threat to Europe? Euro Surveill 2010;15:19509. [PubMed] [Google Scholar]
- 108.New CDC laboratory test for Zika virus authorized for emergency use by FDA. http://www.cdc.gov/media/releases/2016/s0226-laboratory-test-for-zika-virus.html (accessed 22 Jun 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2016-000087supp_appendixA.pdf (306.1KB, pdf)
bmjgh-2016-000087supp_appendixB.pdf (88.3KB, pdf)