Abstract
A novel neutral iridium carbene complex Ir(κC,O-L1)(COD) (1) [where COD = cyclooctadiene and L1 = 3-(2-methylene-4-nitrophenolate)-1-(2,4,6-trimethylphenyl) imidazolylidene] with a pendant alkoxide ligand has been prepared and characterized. It contains a strong Ir-O bond and X-ray analysis reveals a distorted square planar structure. NMR spectroscopy reveals dynamic solution state behavior commensurate with rapid seven-membered ring flipping. In CD2Cl2 solution, under hydrogen at low temperature, this complex dominates although it exists in equilibrium with a reactive iridium dihydride cyclooctadiene complex. 1 reacts with pyridine and H2 to form neutral Ir(H)2(κC,O-L1)(py)2 which also exists in two conformers that differ according to the orientation of the seven-membered metallocycle and whilst its Ir-O bond remains intact, the complex undergoes both pyridine and H2 exchange. As a consequence, when placed under parahydrogen, efficient polarization transfer catalysis (PTC) is observed via the Signal Amplification By Reversible Exchange (SABRE) approach. Due to the neutral character of this catalyst, good hyperpolarization activity is shown in a wide range of solvents for a number of substrates. These observations reflect a dramatic improvement in solvent tolerance of SABRE over that reported for the best PTC precursor IrCl(IMes)(COD). For THF, the associated 1H NMR signal enhancement for the ortho proton signal of pyridine shows an increase of 600-fold at 298 K. The level of signal enhancement can be increased further through warming or varying the magnetic field experienced by the sample at the point of catalytic magnetization transfer.
Introduction
The underlying sensitivity problem in NMR has led to the development and utilization of hyperpolarization methods to address this issue.1–3 ParaHydrogen Induced Polarization4 (PHIP) is one such method, which produces non-Boltzmann spin populations by the incorporation of parahydrogen (p-H2), a nuclear singlet, into unsaturated molecules.5, 6 This chemical transformation results in increased signal intensity in the corresponding NMR spectra of reaction product nuclei originating from, or coupled to, those that were p-H2 derived nuclei.7, 8
Unlike classical PHIP methods, SABRE (Signal Amplification By Reversible Exchange)9 does not involve incorporation of p-H2 in to the analyte that is to be polarized. Polarization is instead transferred via a J-coupling that exists between hydride ligands that are derived from p-H2 and those of the spin-½ analyte nuclei in the polarization transfer catalyst.9 These catalysts most commonly take the form of cationic iridium complexes, which possess either phosphine10–13 or N-heterocyclic carbene (NHC) ligands.14, 15 One of the most successful catalysts to date is based upon [Ir(H)2(IMes)(analyte)3]Cl and it contains magnetically inequivalent but chemically equivalent hydride ligands.15 This complex has proved active for a wide range of analytes which include pyridine,15, 16 nicotinamide,17–19 isoniazid,20 pyrazinamide,20 pyrazole10 and acetonitrile.13 The magnetic field experienced by the catalyst at the point of polarization transfer is instrumental in determining the amplitude and identity of the created magnetic states.18 The use of NHC ligands in the catalyst dramatically increased the enhancement levels relative to those with PCy3.14, 15, 20, 21 These charged systems contain three analyte molecules and are efficient SABRE catalysts in polar solvents such as methanol with benzene being less effective. In this class of catalyst, SABRE transfer proceeds into the two analyte molecules that are trans to hydride ligands. It is ligand exchange, a secondary process, which results in visible hyperpolarization in the analyte molecule in solution.
An alternative catalyst system has been reported that is based on [Ir(H)2(IMes)(PCy3)(analyte)2]Cl.13 Now the exemplified analytes are pyridine and acetonitrile. The mixed analyte containing catalyst, [Ir(H)2(IMes)(PCy3)(pyridine)(CH3CN)]Cl, which is also charged, proved most effective for SABRE even though it contains a pair of chemically and magnetically inequivalent hydride ligands. These results confirmed that it is possible to block the two axial coordination sites in the SABRE catalyst with non-labile ligands without suppressing activity, whilst retaining a charged system. Species such as [Ir(H)2(IMes)(PCy3)(pyridine)2]Cl with magnetically inequivalent but chemically equivalent hydride ligands, whilst being directly related to [Ir(H)2(IMes)(analyte)3]Cl, proved less effective due to poor ligand exchange. Herein we present data dealing with the use of a neutral iridium catalyst containing a cis spanning bidentate NHC to mediate polarization transfer from p-H2 to pyridine and nicotinamide. The use of the chelate effect to promote and control catalysis is well exemplified by phosphines,22 bipyridyl,23 pincer24,25 and pincer carbene ligands.26–29 We seek to retain a carbene functionality whilst adding a hard anionic oxygen ligand and have selected phenolate because it has found wide use in many inorganic systems.30–32 Many iridium aliphatic alkoxide systems exist although these can readily undergo β-hydride elimination.33, 34 In comparison, phenolate ligands are more stable due to the π-interaction and lack of β-protons. A related system has been shown to act as a hydrogenation catalyst with no elimination of the phenol.35 Here we employ a phenolate substituted NHC that can coordinate to the same face of the metal to make Ir(κC,O-L1)(COD) (1) [where COD = cyclooctadiene and L1 = 3-(2-methylene-4-nitrophenolate)-1-(2,4,6-trimethylphenyl) imidazolylidene] in order to achieve this and demonstrate good activity in non-polar solvents whilst retaining optimal catalytic behavior.
Results and Discussion
Synthesis and Characterization
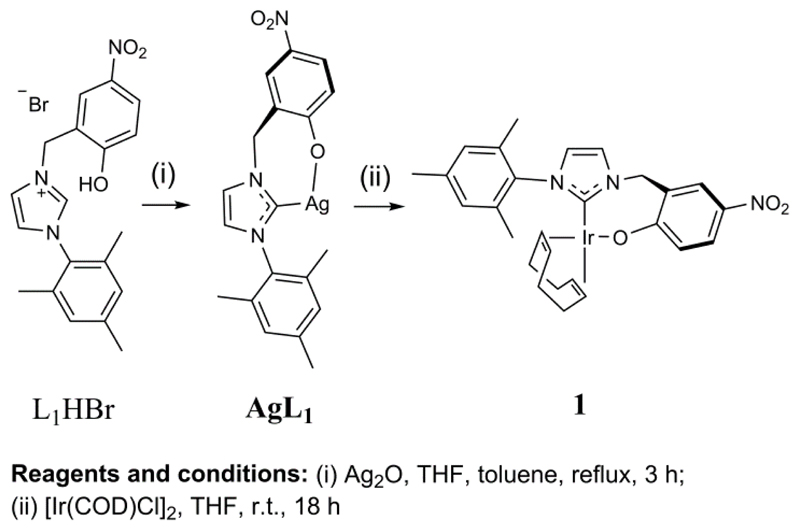
The synthesis of Ir(κC,O-L1)(COD) (1) via a transmetallation reaction was achieved according to Scheme 1. Occhipinti et al. have previously reported the synthesis of the silver carbene complex AgL136 and attached it to ruthenium. Here we use a similar route to make 1. Notably, the precursor, L1HBr in Scheme 1, shows a broad infrared (IR) signal at ~3000 cm−1 for a phenolic O-H stretch as well as signals at 1339 cm−1 and 1279 cm−1 for the O-H bend and C-OH stretching mode respectively.36 Complex 1 was prepared in 96% yield and its structure confirmed by NMR, X-ray and mass spectrometry. A peak is present in the IR spectrum of 1 at 1293 cm−1 due to a C-OIr stretch.
Scheme 1. Reaction scheme for the formation of Ir(κC,O-L1)(COD), 1 from AgL136 and [Ir(COD)Cl]237.
Reagents and conditions: (i) Ag2O, THF, toluene, reflux, 3 h; (ii) [lr(COD)CI]2, THF, r.t., 18 h
Comparison of the NMR data for 1 at 298 K in CD2Cl2, with that at 253 K revealed that its proton chemical shifts are strongly temperature dependent. At 298 K, the two aromatic proton resonances for the mesityl group appear as a broad singlet at δ ~7.03 but upon cooling its rotation is hindered and two singlets separate from it, which resonate at δ 7.06 and 6.96. Lineshape analysis38, 39 of this behavior enabled the activation enthalpy for mesityl group rotation, ΔH≠, to be estimated as 65.5 ± 3.1 kJ mol−1 with the corresponding value for ΔS≠ being +28.0 ± 10.9 J K−1 mol−1. These ΔH≠ and ΔS≠ values are commensurate with those expected for an intramolecular ring rotation40, 41 (see Supplementary Information (SI) for the raw data). We note that similar coalescence effects are also observed when 1 is present in a THF-d8 solution in accordance with a process where there is little charge separation.40
In a separate dynamic process, the two proton resonances of the CH2 linker are also broadened, in this case into the baseline at 298 K. At 253 K these protons provide two distinct doublets at δ 6.60 and 4.79 that share a common coupling of 13.97 Hz. This behavior is a consequence of the fact that the two CH2 protons are diastereotopic, due to their differing exo and endo orientations. They become equivalent by inversion of the conformation of the seven-membered ring through ring-flip and alkoxide lone pair inversion.42 The complexity of the associated NMR spectral changes, and the large chemical shift difference that exists between these resonances, precludes the quantitative analytical assessment of this process through lineshape analysis.
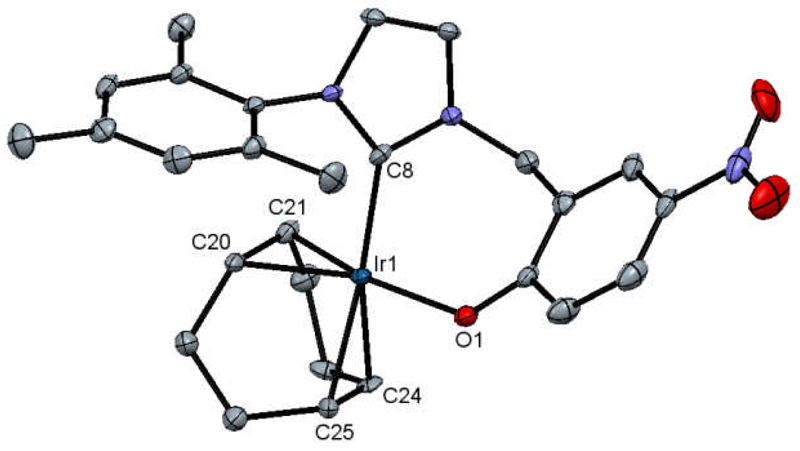
The structure of 1 was further confirmed by X-ray diffraction. Figure 1 shows the corresponding ORTEP plot, determined at 110 K. The crystals employed for this analysis were grown by the slow diffusion of hexane into a dichloromethane solution of 1. The crystal exhibited both pseudo-merohedral and multicrystal twinning, see SI for further details. The structure has a distorted square planar geometry around iridium where the corresponding bond angles are close to 90°. 1 can be compared to two related Ir(I) complexes, Ir(IMes)(COD)(OSiMe3) containing a siloxide ligand43 and [Ir(IMes)(COD)(py)]Cl containing a pyridine ligand.44 These complexes also proved to adopt distorted square planar arrangements. 1 has an Ir-O bond length of 2.067(3) Ǻ which compares well to the value of 2.020(4) Ǻ reported for Ir(IMes)(COD)(OSiMe3). For [Ir(IMes)(COD)(py)]Cl the Ir-N bond length is 2.0954(17) Ǻ. The C=C bond of COD which lies trans to the phenoxide in 1 is the longest of the series and hence the weakest (1.444(7) Ǻ, compared to 1.407(5) Å for Ir(IMes)(COD)(OSiMe3) and 1.399(3) Å for [Ir(IMes)(COD)(py)]Cl). Consequently, the phenoxide ligand in 1 must be the most electron donating ligand of this series and its iridium metal center must therefore be the most electron rich. Due to this increase in electron density at iridium the oxidative addition of H2 to 1 will be disfavored due to repulsion between it and the electron density provided by the ligands in the five-coordinate transition state.45
Figure 1.
ORTEP plot of 1. Hydrogens have been omitted for clarity. Selected bond lengths (Å) and angles (°): Ir1-O1, 2.067(3); Ir1-C8, 2.049(4); Ir1-C20, 2.111(5); Ir1-C21, 2.101(5); Ir1-C24, 2.185(4); Ir1-C25, 2.183(4); C20-C21, 1.444(7); C24-C25, 1.393(7); C8-Ir1-O1, 92.88(14).
The bond angle in 1 that exists between C(carbene)-Ir-O is 92.88(14)° and smaller than that of C(carbene)-Ir-N (94.45(7)°) in [Ir(IMes)(COD)(py)]Cl. This change is a consequence of the chelate effect which acts here to restrict the bond angle but when the freely orientating complex Ir(IMes)(COD)(OSiMe3) is compared, the C(carbene)-Ir-OSi angle is now compressed to 88.2(2)°. Therefore steric interactions must play a role in determining the shape of these complexes.
Reactivity of 1 towards H2 or pyridine
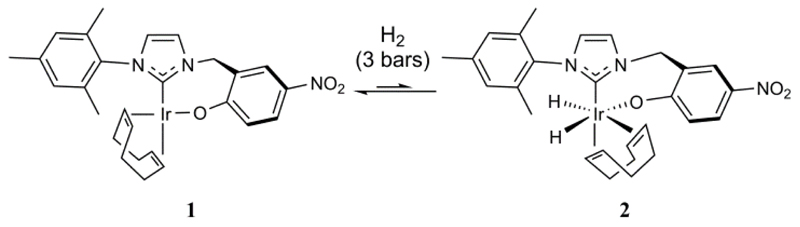
Upon the addition of p-H2 at 3 bars pressure to a CD2Cl2 solution of 1 at 253 K an immediate reaction is observed to take place and the formation of a new dihydride complex (2) is indicated (see Scheme 2). This species yields a pair of PHIP polarized hydride resonances at δ −10.84 and −21.51 in the corresponding 1H NMR spectrum where the chemical shift values are diagnostic of sites that lie trans to alkene and alkoxide respectively. We note that similar spectral changes have been reported when H2 adds to [Ir(COD)(NCCH3)(PMe3)]BF4 to form [Ir(H)2(COD)(NCCH3)(PMe3)]BF4.46 The product of the reaction with 1 is Ir(H)2(κC,O-L1)(COD) (2) and it exists in equilibrium with 1 where a steady-state of just 5% conversion is achieved under these conditions.
Scheme 2. Formation of dihydride 2 from 1.
The very broad hydride and hydrogen signals that are seen in the associated 1H NMR spectra confirm that the interconversion between 1 and 2 is rapid; upon heating this sample to 303 K the hydride resonances broaden into the baseline of the NMR spectrum. The H2 / Ir-H exchange process that is indicated by this observation provides a route to destroy the p-H2 that is present in solution because the relaxation times of metal hydride ligands are relatively short.47, 48 Therefore, despite PHIP being observed when a sample at 243 K is removed from the magnet, shaken and then returned to the magnet for measurement this effect does not last. Furthermore, at 298 K, this pair of hydride signals shows very small levels of PHIP and appears only transiently, thereby confirming that the p-H2 present in solution is consumed very rapidly. We note that significant signal averaging is necessary to observe the hydride resonances of 2 with normal, thermally equilibrated NMR polarization.
In the corresponding low temperature spectrum, a second pair of very weak hydride signals is observed at 243 K that also exhibit PHIP. These signals are only observed after a considerable number of scans under conditions where the magnetism of the molecules is thermally equilibrated. These new hydride ligand signals appear with just ca. 6% of the intensity of those for 2 and are hence too weak to allow the associated product to be characterized. They are likely, however, to arise from a minor isomer of 2 that differs from the main isomer according to the conformation of the seven-membered ring. These signals do not appear in the corresponding high temperature NMR spectrum, presumably due to dynamic averaging between the rapidly interconverting isomers.
Upon adding pyridine to a CD2Cl2 solution of 1 the slow displacement of COD is indicated in the resulting 1H NMR spectrum. However, no clearly identifiable reaction product is observed in this spectrum and decomposition occurs. After 6 days of reaction with pyridine, the subsequent addition of H2 to the resulting solution leads to the formation of a new dihydride species in 60% yield after a further 3 days of activation.
Formation of 3 from 1 upon addition of pyridine and H2
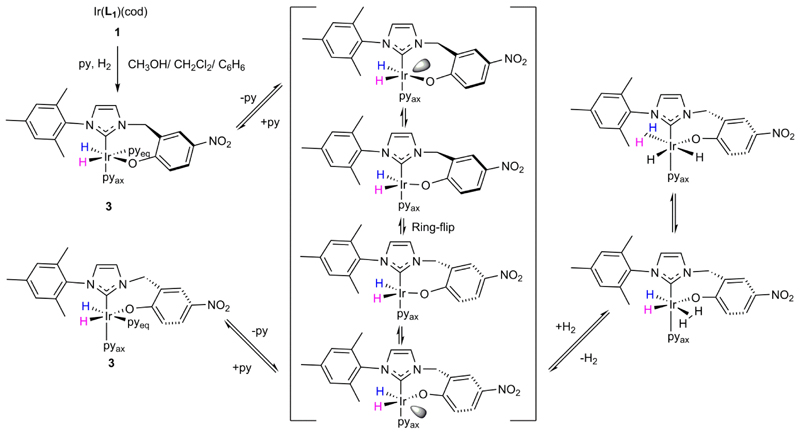
In contrast, 1 reacts very slowly and cleanly with H2 when it is located in a freshly prepared CD2Cl2 solution containing pyridine at 298 K to form the same reaction product. We have identified this product as neutral Ir(H)2(κC,O-L1)(py)2, 3 (see Scheme 3) through a series of observations that we now describe. We note that after 24 hours, 40% conversion of a 1.6 x 10−5 mol solution of 1 with an 8-fold excess of pyridine under 3 bars pressure of H2 to 3 is achieved; this reaction goes to completion on a longer timescale.
Scheme 3. Formation of Ir(H)2(κC,O-L1)(py)2, 3, from 1 with the vacant site represented through the shaded orbital and the purple and magenta hydride ligand labels detailing the hydride interchange processes.
The infrared spectrum of 3 contains a noteworthy C-O Ir stretch at 1274 cm−1 and no evidence for either an OH or OD stretch. The corresponding stretch in 1, an Ir(I) complex, is 1293 cm−1 which suggests that the Ir(III)-alkoxide bond in 3 is strengthened. Furthermore, starting material 1 possesses a strong yellow/orange color when in solution, and this is retained upon activation with pyridine and H2. UV-Vis analysis of 1 in CH2Cl2 reveals the complex yields an absorption band at 405.5 nm with a molar extinction coefficient, ε, of 21060 dm3 mol−1 cm−1. Similar analysis of the activated complex, 3, reveals an absorption band at the slightly higher energy of 398.6 nm with a reduced ε value of 10975 dm3 mol−1 cm−1. Interestingly, when the related complex [Ir(H)2(IMes)(pyridine)3]Cl is monitored in methanol, no equivalent transition is present, the most prominent feature being a broad blue shifted band at 341.0 nm with an ε of 3413 dm3 mol−1 cm−1. The large molar absorptivity values exhibited by 3 are consistent with its intense color and the detection of a charge transfer band.
As expected, COD is released during the formation of 3, and it proved to be hydrogenated on a slower timescale, first to form COE and then COA [where COE = cyclooctene and COA = cyclooctane] according to NMR analysis. The substitution of COD for pyridine in a related cyclometallated square planar palladium system has been reported49 as has the hydrogenation of COD in series of related systems.50–52
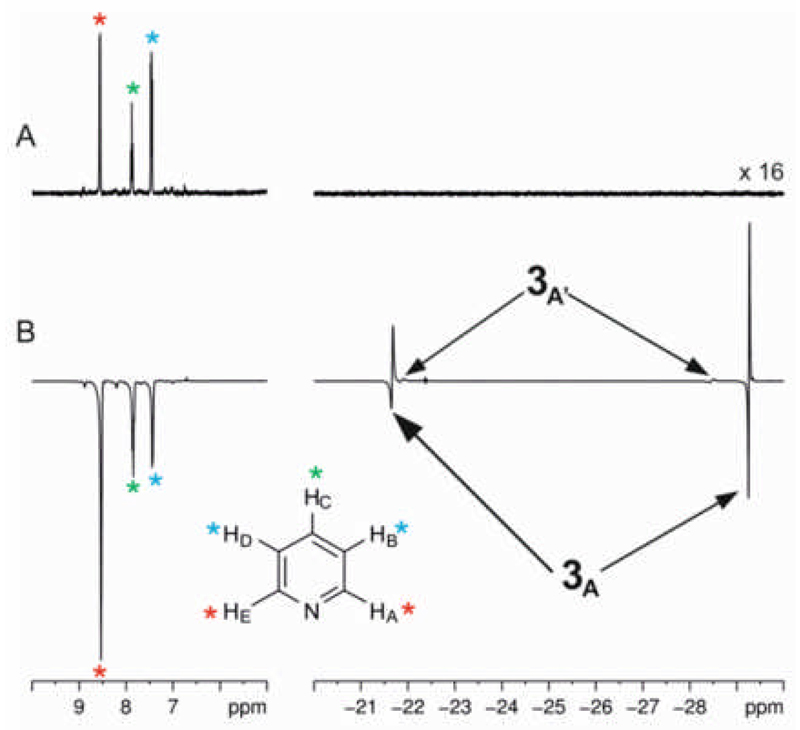
Neutral 3 forms according to Scheme 3 and the alternative zwitterionic Ir-O bond cleaved product [Ir(H)2(κC-L1-O−)(py)3]+ is not observed. At 298 K, the 1H NMR spectrum of 3 contains two distinct hydride ligands which yield a broad singlet at δ −21.80 for the trans pyridine site (HA) and a sharp doublet at δ −28.90 for the trans oxygen site (HB). Further analysis of these NMR spectra of 3 revealed that upon cooling to 223 K, each hydride signal pair separates into two sets of hydride resonances, at δ −21.40 (HA) and −29.80 (HB), and at δ −22.60 (HA’) and −29.00 (HB’) respectively with relative intensity 83:17. The two pairs of hydride resonances share common −8.9 and −7.3 Hz couplings respectively and are due to two conformers, 3A and 3A′, that result from differing orientations of the seven-membered metallocycle. A 15N-HMQC 2D NMR measurement completed at 233 K, located two signals at δ 258.4 and 240.7 for the equatorial and axial pyridine ligands of the major isomer 3A respectively. Given that the chemical shift of free pyridine is 303 ppm, and the difference between it and the bound signal has been suggested to be indicative of bond strength, the ligand trans to the carbene is more strongly bound.53 Full characterization data for 3A at 233 K in CD2Cl2 is presented in the SI.
Ligand exchange pathways in Ir(H)2(κC,O-L1)(py)2, 3.
When the hydride resonance for HA of 3 in CD2Cl2 is selectively irradiated in an EXSY54 experiment at 233 K magnetization transfer into only the HA’ signal of the second conformer is observed. The rate of this process is 4.00 ± 0.08 s−1 and the pair of exchanging hydride ligands remain trans to their original partner throughout. Probing the reverse step, by selective irradiation of HA’, enabled the rate of transfer of HA’ into HA to be quantified as 19.80 ± 0.33 s−1. This means that the equilibrium constant connecting 3A′ and 3A is 4.95 and we note that it matches that determined by integration.
Exchange of these two hydride ligands into free H2, or into a site that is trans to nitrogen is not observed at 233 K. The latter process, which corresponds to that of isomer interconversion, is however evident at 298 K alongside that of H2 loss as shown in Scheme 3. In fact, the H2 loss process proved to be inhibited by the addition of pyridine, while the process of hydride site interchange was unaffected.
When the corresponding 1H EXSY measurement procedure was used to monitor pyridine exchange in 3 only loss of the equatorial ligand was observed, in a process that followed saturation kinetics. These observations confirm that the loss of pyridine from 3 is therefore dissociative. Furthermore, the resulting 16-electron intermediate must have a trigonal bipyramidal shape, or undergo very rapid hydride site interchange, to equilibrate hydrides HA and HB. This is reflected in the fact that the hydride ligand exchange process is unaffected by added pyridine. It can also be concluded that H2 loss from 3 must therefore proceed after pyridine loss (see Table 1). These deductions are supported by the fact that the rate of H2 loss for a solution containing a 50-fold excess of pyridine was 0.042 ± 0.002 s−1 whilst the corresponding pyridine loss rate was 0.28 ± 0.03 s−1. In addition, a H2 exchange mechanism based on the transient formation of the related complex IrCl(H)2(η2-H2)(PPri3)255, 56 has been described previously. We note that these measurements were relatively straightforward to complete in CD2Cl2 as no H/D exchange is evident on the timescales of these measurements.
Table 1.
Summary of the specified ligand loss rates from 3 as a function of pyridine concentration in CD2Cl2. Data confirms pyridine loss is dissociative and that both hydride site interchange and H2 loss occurs after this step.
| No. of py eq. by NMR ratio | Rate of py loss / s −1 | Rate of H2 loss / s −1 |
|---|---|---|
| 15 | 0.28 ± 0.03 | 0.078 ± 0.003 |
| 25 | 0.28 ± 0.03 | 0.071 ± 0.003 |
| 30 | 0.31 ± 0.01 | 0.053 ± 0.002 |
| 50 | 0.28 ± 0.03 | 0.042 ± 0.002 |
When examined in MeOD-d4, 1 still reacts with H2 and pyridine to form 3 in an analogous way to that described in CD2Cl2. The hydride ligand resonances of 3 appear at δ −21.62 and −29.22 in this solvent and are hence very similar in value to those in CD2Cl2. In this case, 100% conversion of a 7.9 x 10−6 mmol solution of 1 is observed after just 24 hours and thus the activation of 1 is much more facile in this polar protic solvent when compared to CD2Cl2. At 243 K, H/D exchange between the hydrides and the solvent is not observed. However, at 270 K, deuterium exchange from the solvent into the hydride resonances of 3 proceeds with an experimentally determined rate constant of 4.24 x 10−5 ± 6 x 10−7 s−1 and both HD and MeOH-d3 are formed. At 298 K, the rate constant for H/D exchange increases to 8.19 x 10−4 ± 8 x 10−6 s−1.
In order to examine the pyridine and H2 exchange processes more reliably, the solvent MeOH-d3 was employed. At 298 K, a sample containing 15 eq. of pyridine enabled the observation that the hydride ligand trans to the oxygen donor proved to exchange into H2 with an experimentally determined rate constant of 0.54 ± 0.03 s−1, a ΔH≠ of 67.5 ± 7 kJ mol−1 and a ΔS≠ of −17.3 ± 25 J K−1 mol−1. In contrast, pyridine dissociation at 298 K occurs with a rate constant of 1.33 ±0.23 s−1, a ΔH≠ of 71.1 ± 3 kJ mol−1 and a ΔS≠ of 2.4 ± 12 J K−1 mol−1. In MeOH-d3, H2 loss therefore proceeds at a slower rate than pyridine loss and upon increasing the number of equivalents of pyridine, the rate of H2 loss is decreased, whilst the rate of pyridine loss remained constant. This is again consistent with the mechanism shown in Scheme 3 and shows that the exchange process remains dissociative in methanol. A mechanism for such an H/D exchange process in a closely related system has been proposed by Brookhart et al.57, 58
Reaction of 3 with p-H2
Upon addition of p-H2, to a CD2Cl2 solution of 1 at 298 K, a single pair of PHIP enhanced hydride signals are observed that are indicative of 3.59 The enhanced signal at δ −28.90 that is due to the HB hydride ligand of 3 and which lies trans to oxygen, has a line-width at half height of 14.6 Hz and signal integration confirms a signal enhancement of 346-fold relative to that seen in the normal trace. Its partner appears at δ −21.40 with a half-height width of 45.1 Hz. When the same measurement is made at 268 K or below, neither the hydride resonances of 3A or 3A′ show PHIP in accordance with the suppression of their exchange with the free H2 pool.
In order to test for the formation of zwitterionic [Ir(H)2(κC-L1-O−)(py)3]+ we have also examined the formation of 3 when a 1:1 mixture of d5- and h5-pyridine is employed. Under these conditions no new PHIP enhanced hydride ligand signals are seen even though related studies on [Ir(H)2(IMes)(pyridine)3]Cl would suggest that the presence of the 2H-label is sufficient to break hydride ligand symmetry and thereby enable it to exhibit PHIP. For these reasons we conclude that the Ir-O bond in 3 is retained throughout these measurements in CD2Cl2. Analogous results are observed when the solvent is changed to methanol-d4 so again the Ir-O bond is retained.
Solvent effects on SABRE catalysis by 3
Evidence for the SABRE effect is also seen in many of the 1H NMR signals that are observed in these NMR spectra. The simplest experimental procedure to view the SABRE effect involves taking an NMR tube under an atmosphere of p-H2 and shaking it for a set time period in a known magnetic field before rapidly making an NMR observation. We now describe the results of this process in the solvents CD2Cl2, benzene-d6, THF-d8, methanol-d4 and ethanol-d6. We discuss the catalyst activation process and highlight the species that are formed. Table 2 details the performance of 1 under SABRE in the indicated solvents after 2 and 24 days respectively. The hydride ligand that is trans to oxygen in 3 always gives rise to a sharp doublet in these 1H NMR spectra while the resonance for the hydride ligand that lies trans to pyridine is considerably broader. This suggests that pyridine loss is possible in accordance with the observations we described earlier in the manuscript.
Table 2.
Pyridine 1H NMR signal enhancements produced for a series of 0.05 M pyridine and 15 mol% 1 solutions at 298 K using 3 bars of p-H2 after an activation period of 48 hours and 24 days.
| Deuterated solvent | Pyridine 1H NMR SABRE signal enhancements (fold) | |||||
|---|---|---|---|---|---|---|
| 48 hours | 24 days | |||||
| ortho | meta | para | ortho | meta | para | |
| Benzene | 121 | 93 | 56 | 561 | 469 | 253 |
| THF | 98 | 61 | 49 | 600 | 442 | 265 |
| DCM | 141 | 105 | 65 | 346 | 212 | 159 |
| Ethanol | 86 | 35 | 36 | 282 | 145 | 142 |
| Methanol | 185 | 130 | 82 | 29 | 24 | 18 |
When a sample containing 0.05 M pyridine and 15 mol% 1 in CD2Cl2 is examined in this way, after polarization transfer in a 6.5 x 10−3 T field, strong SABRE is visible in the NMR signals observed for free pyridine that is present in solution. This effect manifests itself clearly in the resulting single scan 1H NMR measurement as a 141-fold enhanced ortho pyridine proton resonance when compared to the corresponding signal without polarization transfer. We note that complex 3 is detected as the sole inorganic reaction product in these solutions for periods out to 24 days and that no deuterium exchange with the solvent is evident.
3 also forms cleanly in both benzene-d6 and THF-d8 solutions where it is again stable for extended periods of time. We observed, however, that the slow reaction means that significant time is necessary for full activation. When a benzene-d6 system is examined for SABRE, after 2 days of activation, a 121-fold signal enhancement is seen for the same ortho pyridine proton signal under similar conditions. In THF-d8 the analogous level of signal gain reduces to 98-fold for a similar sample under similar conditions. Despite this drop in SABRE efficiency in THF-d8, these observations in CD2Cl2, benzene-d6 and THF-d8, confirm our original hypothesis that neutral 3 is a good SABRE catalyst in a variety of low polarity solvents. When the same systems are examined after 24 days the signal enhancements for the ortho protons of pyridine in these samples have now increased to a maximum of 346-fold, 561-fold and 600-fold for CD2Cl2, benzene-d6 and THF-d8 respectively. This information confirms that while the catalyst takes longer to fully form in these less polar solvents it delivers good activity.
When the solvent is changed to polar protic methanol or ethanol different behavior is observed. In MeOD-d4 for a sample containing 0.1 M pyridine and 5 mol% 1, the initial levels of SABRE that were observed in the 1H NMR resonances of pyridine are small (~6 fold for the ortho 1H resonances) after shaking in a polarization transfer field (PTF) of 6.5 × 10−3 T at 40°C. However, when the same sample is tested 72 hours later, the signal enhancement levels are far greater (178, 96, 52 fold observed for ortho, meta and para 1H resonances of pyridine respectively). A typical trace is shown in Figure 2. These data confirm our earlier observation that 1 takes a significant time to fully activate. However, upon longer time scales, the level of delivered polarization falls as the pyridine substrate becomes 2H-labelled; 50% deuteration of the ortho proton signal for pyridine was observed after 24 days in methanol-d4. The analogous deuterium exchange process is seen in ethanol-d6, but it now proceeds more slowly in accordance with the slower formation of 3; no deuterium exchange is seen in the non-protic solvents.
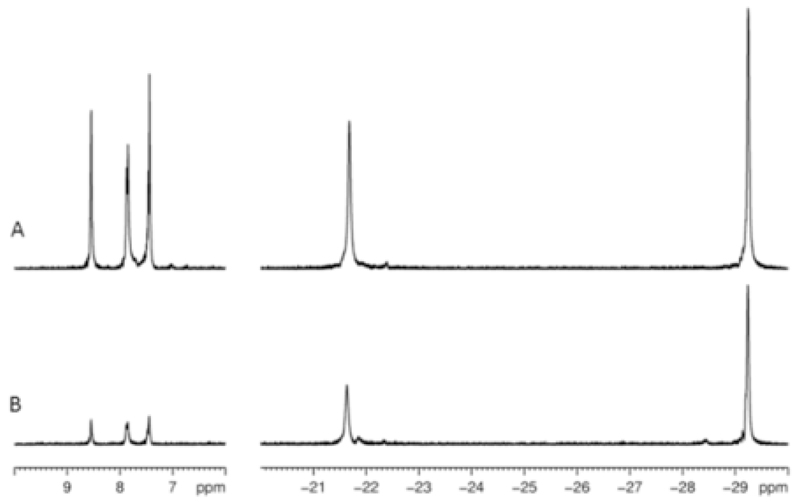
Figure 2.
1H NMR spectra of a sample containing 0.05 M pyridine and 15 mol% 1 in MeOD-d4 after; (A) thermal equilibrium at high field; and (B) polarization transfer from p-H2 at 6.5 × 10−3 T, using a 45° pulse. Pyridine and hydride species are labelled.
In order to demonstrate that the polarization transfer to pyridine observed in these systems arises from SABRE rather than one-proton PHIP, a d5-pyridine containing solution was also examined. These measurements were completed after shaking the sample in a PTF of 6.5 × 10−3 T and involved the application of a 45° excitation pulse. The 1H NMR signal for residual d4-pyr-idine was shown to polarize, delivering an in-phase emission peak at δ 8.56 which rapidly decayed to thermal magnetization over a time course of ~30 s. If this signal was to be enhanced through the one-proton PHIP effect it should remain enhanced over this period. For this reason we conclude that SABRE is the only polarization transfer pathway operating.60, 61
The magnetization transfer process was also probed by OPSY (Only Parahydrogen SpectroscopY62, 63). This pulse sequence enables the detection of signals that are either derived directly from p-H2 or nuclei that have received polarization from it. Two representative 1H NMR spectra that were recorded after shaking in a PTF of 2 x 10−4 T at 0°C or 60°C respectively are shown in Figure 3. It can be readily seen from these NMR spectra that greater polarization transfer is evident at 60°C and that polarization transfer via SABRE into free pyridine occurs. The two NMR spectra are presented in magnitude mode and the signals for pyridine and the hydride resonances of 3 dominate which confirms that they receive visible polarization from p-H2‥
Figure 3.
1H OPSY-dq NMR spectra of a sample containing 0.1 M pyridine and 5 mol% 1 in MeOD-d4 after polarization transfer from p-H2 at 2 x 10−4 T and 60°C (A) or 0°C (B).
SABRE catalysis optimization using 3
In order to optimize the efficiency of polarization transfer in methanol from p-H2 to substrates using 1 a number of conditions have been investigated. These include varying the temperature, the PTF and the catalyst. The most widely used catalyst to date is IrCl(IMes)(COD), 4.64,15 When activated with pyridine and H2, 4 has been reported to form [Ir(H)2(IMes)(py)3]Cl which then enables polarization transfer. The route to H2 addition to 4 is via the formation of [Ir(IMes)(COD)(py)]Cl which actually adds H2 faster than the precursor IrCl(IMes)(COD). In contrast, 1 takes a significant time to activate due to the analogous indirect reaction pathway being no longer available because of the strong Ir-O bond.
Table 3 displays a series of enhancement data that results from the SABRE of the substrates pyridine and nicotinamide in MeOD-d4 as a function of temperature and transfer field using both 4 and 1. It is clear from this table that 4 is the superior room temperature catalyst, delivering in some instances 30 times more signal intensity when compared to that produced by 1. For 1, the signal enhancement seen for bulk nicotinamide proton HA increases by a factor of ~5 on going from 2 x 10−4 T to 6.5 x 10−4 T at 30°C, and a factor of ~6 at 60°C. This compares with a drop in signal intensity when 4 is employed at the higher temperatures. Now, under transfer at 6.5 x 10−4 T, proton HA of nicotinamide shows better enhancement at 30°C rather than 60°C. These investigations on nicotinamide were conducted because of the recent publications which investigate the biological implications of hyperpolarizing nicotinamide in a move towards developing imaging applications that utilize the SABRE technique.17–19, 21
Table 3.
Signal enhancements obtained for each of the listed 1H resonances of pyridine and nicotinamide after polarization transfer in fields of either 2 x 10 −4 T or 6.5 x 10−3 T using catalyst 1 or 4 in MeOD-d4. For proton assignments see the SI.
| Polarization transfer at 2 x 10−4 T | Polarization transfer at 6.5 x 10−3 T | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 1 | 4 | |||||
| 1H resonance | 30°C | 60°C | 30°C | 60°C | 30°C | 60°C | 30°C | 60°C |
| Pyridine HA, HE | 44 | 72 | 616 | 269 | 116 | 254 | 677 | 474 |
| Pyridine HB, HD | 20 | 20 | 188 | 35 | 70 | 158 | 182 | 212 |
| Pyridine HC | 10 | 20 | 247 | 113 | 28 | 66 | 295 | 205 |
| Nicotinamide HA | 4 | 7 | 147 | 117 | 20 | 40 | 469 | 393 |
| Nicotinamide HB | 2 | 2 | 74 | 59 | 15 | 33 | 306 | 242 |
| Nicotinamide HC | 2 | 4 | 99 | 54 | 6 | 18 | 64 | 55 |
| Nicotinamide HD | 3 | 5 | 107 | 85 | 20 | 40 | 364 | 293 |
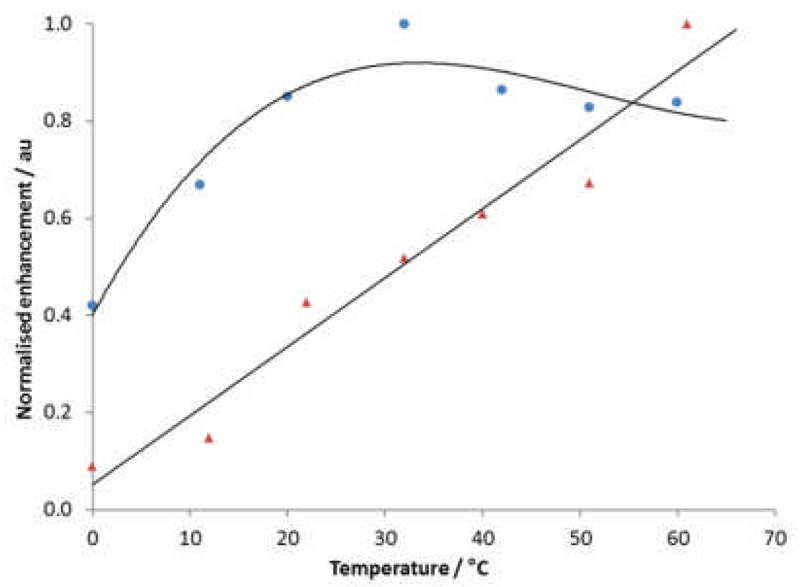
The effect of temperature is also clearly illustrated in Figure 4. The continued improvement in the activity of 1 with an increase in temperature is associated with an increase in ligand dissociation rate.15 In contrast, the rate of ligand exchange with 4 actually becomes too rapid for good catalysis upon exceeding 30°C. A full comparison of temperature dependent 1H NMR enhancements for pyridine and nicotinamide in MeOD-d4 as polarized by 1 and 4 is detailed in the SI.
Figure 4.
Normalized enhancement of the HA
1H NMR resonance of nicotinamide after polarization transfer at 6.5 x 10−3 T using either 1 ( ) or 4 (
) or 4 ( ).
).
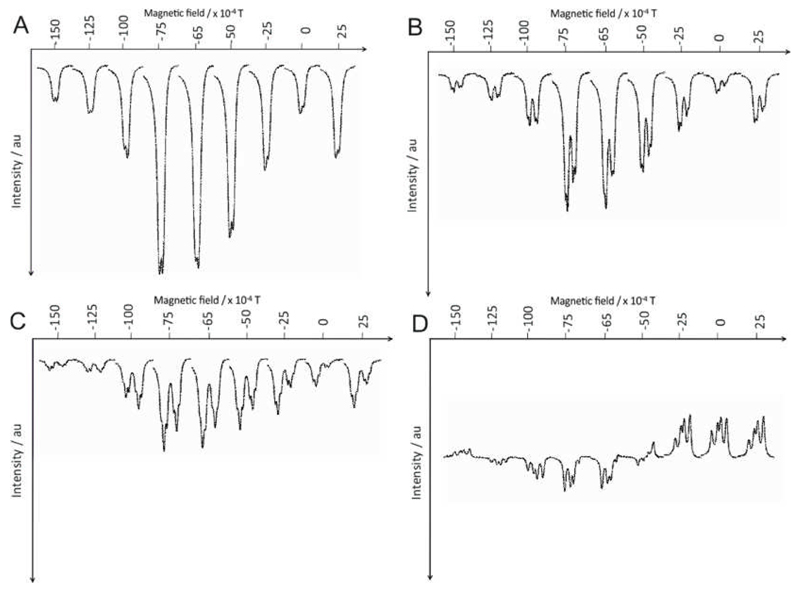
Further optimization was achieved by altering the PTF. This study was completed using a polarizer equipped with a vertically aligned coil that is capable of producing a well-defined vertical field between 0.5 x 10−4 T and ±1.5 x 10−2 T in steps of 0.5 x 10−4 T.15, 18 The four hyperpolarized 1H resonances of nicotinamide resulting from polarization in set fields between −1.5 x 10−2 T and +2.5 x 10−3 T are illustrated in Figure 5. The maximum signal enhancement proved to be delivered by 1 when the PTF lies between −6.5 x 10−3 T and −7.5 x 10−3 T for sites HA, HB and HC and their signals all appear in emission. The corresponding meta proton (HC) resonance undergoes two phase changes on moving from −1.5 x 10−2 T to −1.25 x 10−2 T and −6.5 x 10−3 T to −5.0 x 10−3 T respectively, and shows relatively weak signal amplification levels. Related observations have been reported for pyridine with 4 and the profiles are remarkably similar even though the two catalysts are very different in their nature.13
Figure 5.
1H NMR field plots showing the signal intensity of the four 1H (A-HA, B-HD, C-HB, D-HC) resonances of nicotinamide over the range of –1.5 x 10−2 T to +2.5 x 10−3 T.
Two other substrates that have been shown to polarize using 4 for SABRE in methanol include quinoline65 and nicotine.21 We have therefore examined these substrates using 1 in benzene as this is one of the most efficient solvent systems for this neutral catalyst. Both compounds show enhanced signals, with polarization transfer to nicotine being more efficient than quinolone. Nicotine exhibits a total proton enhancement of 360-fold across the four aromatic proton sites with quinolone showing a 54-fold increase across six of its proton sites. This shows that the use of specially designed catalysts in non-polar solvents could prove very important for identifying low concentration analytes in solution via the SABRE approach.
Conclusions
The iridium precatalyst Ir(κC,O-L1)(COD) (1) which contains a phenolate substituted NHC has been synthesized and shown to act as a SABRE catalyst precursor in the five solvents, dichloromethane, benzene, tetrahydrofuran, methanol and ethanol. This overcomes the current low polarity solvent limitations shown by existing SABRE catalysts. 1 contains strong Ir-O and Ir-NHC bonds which are preserved in all of these solvents and the chelate effect acts to create a seven-membered metallocycle that adopts two interconverting conformations in solution because of the non-linearity of the Ir-O-C bond. In addition, the square planar catalyst precursor 1, exhibits a strong charge transfer transition at 398 nm.
We have shown that 1 and H2 exist in equilibrium with dihydride 2 at low temperature in CD2Cl2 solution. The hydride resonances of this product exhibit PHIP in the associated 1H NMR spectra. 2 is an example of an unusual M(H)2(alkene) intermediate, for which other related complexes have been implicated in hydrogenation catalysis in many systems.66, 67 Upon warming 1 in the presence of pyridine and H2 in the five described solvents, slow conversion into neutral 3, Ir(H)2(κC,O-L1)(py)2 is observed. The stepwise hydrogenation of COD, first into COE and then COA is also evident upon studying this reaction at 298 K.
Neutral 3 has been shown to undergo the dissociative loss of pyridine, according to Scheme 3, prior to H2 exchange but no evidence for the reductive elimination of the cis IrO and IrH groups is observed even though the corresponding alcohol would be expected to be stable. The pyridine and H2 ligand loss processes enables the incorporation of p-H2 into 3 and thereby facilitates its action as a good SABRE catalyst.
In methanol, the formation of 3 proceeds cleanly with no evidence being observed for zwitterionic [Ir(H)2(κC-LI-O−)(py)2(CD3OD)]+ or [Ir(H)2(κC-L1-O−)(py)3]+ in solution. The ligand exchange rates for substrate dissociation and H2 loss have been determined in MeOH-d3, and both increase with temperature. The H2 loss process has been suggested to proceed via Ir(H)2(H2)(κC,O-L1)(py) as shown in Scheme 3. The associated activation parameters for the H2 loss process, ΔH≠ and ΔS≠ are 67.5 ± 7 kJ mol−1 and −17.3 ± 25 J K−1 mol−1 respectively. In contrast, pyridine dissociation which precedes H2 loss occurs with a ΔH≠ of 71.1 ± 3 kJ mol−1 and a ΔS≠ of 2.4 ± 12 J K−1 mol−1. The entropy term for this latter reaction is smaller than expected for a dissociative exchange pathway and may reflect an early transition state or a reduction in the conformational freedom of the seven-membered ring.
Nevertheless, when 3 is monitored for activity as a polarization transfer catalyst good levels of hyperpolarization are seen in a range of substrates when utilizing the low polarity solvents, CD2Cl2, benzene-d6 and THF-d8 that find widespread use in NMR spectroscopy. For example, in THF, the ortho proton of the substrate pyridine shows an impressive 600-fold enhancement which equates to 2% polarization level. Furthermore, the level of PTC was found to be PTF dependent, and in methanol, the best single spin polarization resulted from transfer at a field strength of between −6.5 x 10−3 T and −7.5 x 10−3 T. When PTC by 3 in methanol is compared to that achieved by [Ir(H)2(IMes)(py)3]Cl, whilst lower catalyst efficiency is observed, the enhancement levels are still commensurate with significant time savings in both NMR and MRI measurements. Superior catalysis is, however, found in benzene using 1 and similar signal enhancements are seen in dichloromethane albeit with a different catalyst species present to that described previously for 4.
The range of substrates exemplified includes pyridine, quinoline and nicotine in order to demonstrate the wider utility of this catalyst in non-polar solvents. We note that catalyst activation takes on average 5 days in these relatively non-polar solvents but over 500-fold ortho proton enhancement values for pyridine result. These enhancement levels can be improved further still by warming and using an optimized PTF. We therefore expect derivatives of this neutral catalyst to dramatically widen the applicability of SABRE for NMR measurements in the future, and we are now seeking to prepare a precatalyst such as Ir(κC,O-L1)(COE)2 which may activate rapidly whilst retaining both the air stability and efficiency of 1.
Experimetnal Proceedures
Methods and equipment
All experimental procedures were performed under an atmosphere of either dinitrogen or argon, using standard Schlenk line techniques or an MBraun Unilab glovebox, unless otherwise stated. General solvents for synthetic chemistry were dried using an Innovative Technology anhydrous solvent engineering system or were distilled from an appropriate drying agent under N2 as necessary. Deuterated solvents (methanol-d4, methanol-d3, CDCl3, CD2Cl2, benzene-d6, THF-d8) were obtained from Sigma-Aldrich and Cambridge Isotope Laboratories (ethanol-d6) and used as supplied. NMR measurements were made on a Bruker Avance III series 400 MHz NMR spectrometer (1H at 400.13 MHz, 13C at 100 MHz) and a 500 MHz NMR spectrometer (1H at 500.13 MHz, 13C at 125.77 MHz). NMR samples were made up in Young’s tap equipped 5 mm NMR tubes. Parahydrogen (p-H2) was prepared by cooling hydrogen gas over charcoal in a copper block at 30 K. COSY, HMQC and EXSY54 pulse sequences were used as previously described. Polarization transfer experiments are described in the supplementary.
X-ray
Single crystals of C27H30Ir1.04N3O3 (1) were grown from slow diffusion of hexane into a DCM solution of 1. A suitable crystal was selected and diffraction data were collected at 110 K on an Oxford Diffraction SuperNova diffractometer with Cu-Kα radiation (λ = 1.54184 Ǻ) using an EOS CCD camera. The crystal was cooled with an Oxford Instruments Cryojet. Diffractometer control, data collection, initial unit cell determination, frame integration and unit-cell refinement was carried out with “Crysalis” (CrysAlisPro, Oxford Diffraction Ltd. Version 1.171.34.40). Face-indexed absorption corrections were applied using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm (Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm within CrysAlisPro software, Oxford Diffraction Ltd. Version 1.171.34.40). OLEX268 was used for overall structure solution, refinement and preparation of computer graphics and publication data. Within OLEX2, the algorithm used for structure solution was direct methods,69 within SHELXS. Refinement by full-matrix least-squares used the SHELXL algorithm within OLEX2. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed using a “riding model” and included in the refinement at calculated positions.
The crystal exhibited both merohedral and multi-crystal twinning. Only one form of twinning can be modelled. Modelling the merohedral twinning gave a statistically better result than the multi-crystal twin model. For the merohedral twinning, a twin matrix of -1 0 0 0 -1 0 0 0 1 was used with a BASF of 0.0863(5). This left large residual density of 8 e Ǻ−3 presumably due to the alternative site of iridium from the multi-crystal twinning. This was accounted for by adding a partially occupied iridium with a refined occupancy of 0.0418(12), all other atoms from this minor form were too weak to be modelled. Inclusion of the partial iridium gave a significant improvement in the structure quality (R1 [I>=2σ (I)] from 4.22 to 3.45%, wR2 [All data] from 10.06 to 7.01%, residual density from 8.08 to 1.81 e Å−3).
Synthesis of 1-(2,4,6-trimethylphenyl)-1H-imidazole70
Glacial acetic acid (10 ml), aqueous formaldehyde (3 ml, 37 wt %, 40 mmol) and aqueous glyoxal (4.6 ml, 40 wt %, 40 mmol) were added to a round bottom flask and heated at 70°C. A solution of glacial acetic acid (10 ml), ammonium acetate (3.08 g, 40 mmol, in 2 ml water) and mesitylamine (5.6 ml, 40 mmol) was added dropwise to the flask over a period of 30 min. The resulting solution was continuously stirred and heated for 18 h. The reaction mixture was cooled to room temperature and was added dropwise to a stirred solution of sodium hydrogen carbonate (29.4 g in 300 ml water). The product formed a precipitate which was filtered off and washed with water before being allowed to air dry. The brown solid was dissolved in the minimal amount of ethyl acetate and placed in a freezer o/n whereupon the required product crystallised and was isolated via filtration (4.36 g, yield 59%); 1H NMR [CDCl3, 400 MHz] δ 7.44 (s, 1H, CHimidazole), 7.24 (s, 1H, CHimidazole), 6.98 (s, 2H, 2×CHmesityl), 6.90 (s, 1H, CHimidazole), 2.35 (s, 3H, CH3), 2.00 (s, 6H, 2×CH3); 13C{1H} NMR [CDCl3] δ 138.8, 137.5, 135.4, 133.4, 129.5, 129.0, 120.1, 21.0 (CH3), 17.3 (2×CH3); MS [ESI] m/z 187.0 (M+H)+
Synthesis of 3-(2-methylene-4-nitrophenol)-1-(2,4,6-trimethylphenyl) imidazolium bromide, L1HBr36
1-(2,4,6-trimethylphenyl)-1H-imidazole (0.61 g, 3.3 mmol) and 2-hydroxy-5-nitrobenzyl bromide (0.76 g, 3.3 mmol) were dissolved in toluene (8 ml). The resulting mixture was refluxed for 18 h and then the solution was cooled to room temperature. The precipitate was collected via vacuum filtration and was washed with diethyl ether (1.25 g, yield 91%); 1H NMR [DMSO, 400 MHz] δ 11.81 (s, 1H, OH), 9.55 (s, 1H, CHimidazole), 8.39 (d, 1H, 4J(HH) = 2.55 Hz, CHaromatic), 8.22 (dd, 1H, 4J(HH) = 2.55 Hz, 3J(HH) = 9.04 Hz, CHaromatic), 8.05 (s, 1H, CHimidazole), 7.94 (s, 1H, CHimidazole), 7.15 (s, 2H, 2×CHmesityl), 7.09 (d, 1H, 3J(HH) = 9.04 Hz, CHaromatic), 5.53 (s, 2H, CH2), 2.33 (s, 3H, CH3), 2.01 (s, 6H, 2×CH3); 13C{1H} NMR [DMSO, 500 MHz] δ 163.3 (Caromatic), 141.2 (Caromatic), 140.3 (Caromatic), 139.1 (CHimidazole), 135.2 (Caromatic), 132.0 (Caromatic), 130.1 (2×CHmesityl), 128.1 (CHaromatic), 127.9 (CHaromatic), 125.0 (CHimidazole), 124.1 (CHimidazole), 122.2 (Caromatic), 116.7 (CHaromatic), 49.2 (CH2), 21.5 (CH3), 17.7 (2×CH3); MS [ESI] m/z 338.1 (M-Br)+
Synthesis of silver(II) 3-(2-methylene-4-nitrophenolate)-1-(2,4,6-trimethylphenyl)imidazolylidene, AgL136
L1 (500 mg, 1.2 mmol), silver (I) oxide (569.4 mg, 2.5 mmol) and 4Å molecular sieves (1.12 g) were added to a Schlenk flask under a nitrogen atmosphere. Dried THF (8.5 ml) and toluene (8.5 ml) were added to the flask and the reaction mixture was stirred at reflux for 3 h. Once cooled to room temperature, the mixture was diluted with dichloromethane (80 ml), filtered through a pad of celite and washed through with dichloromethane (2 × 5 ml). The solvent was removed under pressure to produce a yellow solid (0.45 g, yield 85%); 1H NMR [CDCl3, 400 MHz] δ 8.16 (s br, 1H, CHaromatic), 7.78 (s br, 1H, CHaromtic), 7.38 (s br, 1H, CHaromatic), 7.01 (s br, 2H, 2xCHmesityl), 6.99 (s br, 1H, CHaromatic), 5.84 (s br, 1H, CHaromatic), 5.27 (s br, 2H, CH2), 2.42 (s, 3H, CH3), 1.96 (s br, 6H, 2×CH3); 13C{1H} NMR [CDCl3] δ 174.7, 139.6, 137.2, 135.2, 134.5, 129.2 (2×CHmesityl), 127.2 (CHaromatic), 126.8 (CHaromatic), 124.4, 122.2, 121.9 (CHaromatic), 121.5 (CHaromatic), 50.3 (CH2), 21.4 (CH3), 17.9 (2×CH3); MS [ESI] m/z 783 (Ag dimer, protonated phenol groups)
Synthesis of iridium(I) (3-(2-methylene-4-nitropheno-late)-1-(2,4,6-trimethylphenyl)imidazolylidene) (cyclooctadiene), 137
AgL1 (170 mg, 0.38 mmol) and [Ir(COD)Cl]2 (129 mg, 0.19 mmol) were added to a Schlenk flask, which was evacuated and filled with nitrogen. Dry THF (12 ml) was added to the flask and the reaction mixture was stirred overnight at room temperature. The mixture was diluted with DCM, filtered through a pad of celite then purified by column chromatography (silica, DCM:acetone 80:20). It was evaporated to dryness to produce an orange/brown powder (0.23 g, yield 96%); 1H NMR [CD2Cl2, 500 MHz, 298 K] δ 8.17 (d, 1H, 4J(HH) = 3.05 Hz, CHaromatic), 7.96 (dd, 1H, 4J(HH) = 3.05 Hz, 3J(HH) = 9.28 Hz, CHaromatic), 7.14 (d, 1H, 3J(HH) = 1.94 Hz, CHimidazole), 7.03 (s, br, 2H, 2×CHmesityl), 6.72 (d, 1H, 3J(HH) = 1.94 Hz, CHimidazole), 6.60 (s v. br, 1H, CH2 linker), 6.57 (d, 1H, 3J(HH) = 9.28 Hz, CHaromatic), 4.79 (s v. br, 1H, CH2 linker), 4.40 (s, v. br, 2H, 2×CHCOD), 3.30 (s v. br, 1H, CHCOD), 2.45 (s v. br, 1H, CHCOD), 2.39 (s, 3H, CH3), 2.09 (s br, 4H, CH2 COD), 2.03 (s br, 6H, 2×CH3), 1.58 (s br, 4H, CH2 COD); 1H NMR [CD2Cl2, 500 MHz, 253 K] δ 8.18 (d, 1H, 4J(HH) = 3.01 Hz, CHaromatic), 7.96 (dd, 1H, 4J(HH) = 3.01 Hz, 3J(HH) = 9.24 Hz), 7.14 (d, 1H, 3J(HH) = 1.87 Hz, CHimidazole), 7.06 (s, 1H, CHmesityl), 6.96 (s, 1H, CHmesityl), 6.72 (d, 1H, 3J(HH) = 1.87 Hz, CHimidazole), 6.60 (d, 1H, 2J(HH) = 14.02 Hz, CH2 linker), 6.55 (d, 1H, 3J(HH) = 9.24 Hz, CHaromatic), 4.79 (d, 1H, 2J(HH) = 14.02 Hz, CH2 linker), 4.42 (m, 1H, CHCOD), 4.26 (m, 1H, CHCOD), 3.27 (m, 1H, CHCOD), 2.41 (m, 1H, CHCOD), 2.36 (s, 3H, CH3), 2.20 − 2.00 (m, 4H, 2×CH2 COD), 2.07 (s, 3H, CH3), 1.96 (s, 3H, CH3), 1.68 − 1.53 (m, 4H, 2×CH2 COD); 13C{1H} NMR [CD2Cl2, 500 MHz, 253 K] δ 175.7 (C-OIr), 175.1 (C-Ir), 139.0 (Caromatic), 136.1 (Caromatic), 135.5 (Caromatic), 134.4 (Caromatic), 134.0 (Caromatic), 129.1 (CHaromatic), 128.4 (CHaromatic), 127.0 (CHaromatic), 126.3 (CHaromatic), 123.4 (CHimidazole), 123.2 (Caromatic), 121.5 (CHaromatic), 119.6 (CHimidazole), 84.7 (CH), 84.6 (CH), 51.2 (CH2), 49.7 (CH), 48.3 (CH), 34.5 (CH2), 33.8 (CH2), 28.9 (CH2), 28.4 (CH2), 21.0 (CH3), 18.7 (CH3), 17.6 (CH3); MS [ESI] m/z 636.19. Anal. Calcd for C27H30N3O3lr (Mr = 636.77): C, 50.93; H, 4.75; N, 6.60. Found: C, 50.64; H, 4.52; N, 6.14.
Supporting Information
Experimental details, exchange rate data, activation parameters, crystal structure data, UV-vis and IR analysis, SABRE analysis of pyridine in different solvents, 1H and 13C hyperpolarized spectra of nicotinamide and pyridine and enhancement vs temperature graphs. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We acknowledge the Nuffield Foundation for an undergraduate bursary for AJR (URB/39689), Bruker Biospin, the University of York, the EPSRC (grant no. EP/G009546/1) and the Wellcome Trust and Wolfson Foundation (092506 and 098335) for their generous funding.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. The authors declare no competing financial interests.
References
- (1).Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Nikolaou P, Coffey AM, Walkup LL, Gust BM, Whiting N, Newton H, Barcus S, Muradyan I, Dabaghyan M, Moroz GD, Rosen MS, et al. Proc Natl Acad Sci U S A. 2013;110:14150–14155. doi: 10.1073/pnas.1306586110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Acosta RH, Blumler P, Munnemann K, Spiess HW. Prog Nucl Magn Reson Spectrosc. 2012;66:40–69. doi: 10.1016/j.pnmrs.2012.03.003. [DOI] [PubMed] [Google Scholar]
- (4).Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL. J Am Chem Soc. 1987;109:8089–8091. [Google Scholar]
- (5).Bowers CR, Weitekamp DP. J Am Chem Soc. 1987;109:5541–5542. [Google Scholar]
- (6).Pravica MG, Weitekamp DP. Chem Phys Lett. 1988;145:255–258. [Google Scholar]
- (7).Natterer J, Bargon J. Prog Nucl Magn Reson Spectrosc. 1997;31:293–315. [Google Scholar]
- (8).Green RA, Adams RW, Duckett SB, Mewis RE, Williamson DC, Green GGR. Prog Nucl Magn Reson Spectrosc. 2012;67:1–48. doi: 10.1016/j.pnmrs.2012.03.001. [DOI] [PubMed] [Google Scholar]
- (9).Adams RW, Duckett SB, Green RA, Williamson DC, Green GGR. J Chem Phys. 2009;131 doi: 10.1063/1.3254386. [DOI] [PubMed] [Google Scholar]
- (10).Dücker EB, Kuhn LT, Münnemann K, Griesinger C. J Magn Reson. 2012;214:159–165. doi: 10.1016/j.jmr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- (11).Atkinson KD, Cowley MJ, Duckett SB, Elliott PIP, Green GGR, Lopez-Serrano J, Khazal IG, Whitwood AC. Inorg Chem. 2009;48:663–670. doi: 10.1021/ic8020029. [DOI] [PubMed] [Google Scholar]
- (12).Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Lopez-Serrano J, Whitwood AC. J Am Chem Soc. 2009;131:13362–13368. doi: 10.1021/ja903601p. [DOI] [PubMed] [Google Scholar]
- (13).Fekete M, Bayfield O, Duckett SB, Hart S, Mewis RE, Pridmore N, Rayner PJ, Whitwood A. Inorg Chem. 2013;52:13453–13461. doi: 10.1021/ic401783c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).van Weerdenburg BJA, Gloggler S, Eshuis N, Engwerda AHJ, Smits JMM, de Gelder R, Appelt S, Wymenga SS, Tessari M, Feiters MC, Blumich B, et al. Chem Commun. 2013;49:7388–7390. doi: 10.1039/c3cc43423k. [DOI] [PubMed] [Google Scholar]
- (15).Cowley MJ, Adams RW, Atkinson KD, Cockett MCR, Duckett SB, Green GGR, Lohman JAB, Kerssebaum R, Kilgour D, Mewis RE. J Am Chem Soc. 2011;133:6134–6137. doi: 10.1021/ja200299u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Barskiy DA, Kovtunov KV, Koptyug IV, He P, Groome KA, Best QA, Shi F, Goodson BM, Shchepin RV, Truong ML, Coffey AM, et al. ChemPhysChem. 2014;15:4100–4107. doi: 10.1002/cphc.201402607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hoevener J-B, Schwaderlapp N, Borowiak R, Lickert T, Duckett SB, Mewis RE, Adams RW, Burns MJ, Highton LAR, Green GGR, Olaru A, et al. Anal Chem. 2014;86:1767–1774. doi: 10.1021/ac403653q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Mewis RE, Atkinson KD, Cowley MJ, Duckett SB, Green GGR, Green RA, Highton LAR, Kilgour D, Lloyd LS, Lohman JAB, Williamson DC. Magn Reson Chem. 2014;52:358–69. doi: 10.1002/mrc.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Truong ML, Shi F, He P, Yuan B, Plunkett KN, Coffey AM, Shchepin RV, Barskiy DA, Kovtunov KV, Koptyug IV, Waddell KW, et al. J Phys Chem B. 2014;118:13882–13889. doi: 10.1021/jp510825b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zeng H, Xu J, Gillen J, McMahon MT, Artemov D, Tyburn J-M, Lohman JAB, Mewis RE, Atkinson KD, Green GGR, Duckett SB, et al. J Magn Reson. 2013;237:73–78. doi: 10.1016/j.jmr.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano J, Williamson DC. Science. 2009;323:1708–1711. doi: 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- (22).Chikkali SH, van der Vlugt JI, Reek JNH. Coord Chem Rev. 2014;262:1–15. [Google Scholar]
- (23).Espinet P, Soulantica K. Coord Chem Rev. 1999;193(5):499–556. [Google Scholar]
- (24).Zhang W-H, Chien SW, Hor TSA. Coord Chem Rev. 2011;255:1991–2024. [Google Scholar]
- (25).Selander N, Szabo KJ. Chem Rev. 2011;111:2048–2076. doi: 10.1021/cr1002112. [DOI] [PubMed] [Google Scholar]
- (26).Pugh D, Danopoulos AA. Coord Chem Rev. 2007;251:610–641. [Google Scholar]
- (27).Lin IJB, Vasam CS. Coord Chem Rev. 2007;251:642–670. [Google Scholar]
- (28).Douthwaite RE. Coord Chem Rev. 2007;251:702–717. [Google Scholar]
- (29).Diez-Gonzalez S, Marion N, Nolan SP. Chem Rev. 2009;109:3612–3676. doi: 10.1021/cr900074m. [DOI] [PubMed] [Google Scholar]
- (30).Fu R, Bercaw JE, Labinger JA. Organometallics. 2011;30:6751–6765. doi: 10.1021/om200364w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Noshiranzadeh N, Emami M, Bikas R, Slepokura K, Lis T. Polyhedron. 2014;72:56–65. [Google Scholar]
- (32).Kozlowska A, Dranka M, Zachara J, Pump E, Slugovc C, Skowerski K, Grela K. Chem-Eur J. 2014;20:14120–14125. doi: 10.1002/chem.201403580. [DOI] [PubMed] [Google Scholar]
- (33).Blum O, Milstein D. J Organomet Chem. 2000;593:479–484. [Google Scholar]
- (34).Zhao J, Hesslink H, Hartwig JF. J Am Chem Soc. 2001;123:7220–7227. doi: 10.1021/ja010417k. [DOI] [PubMed] [Google Scholar]
- (35).Weinberg DR, Hazari N, Labinger JA, Bercaw JE. Organometallics. 2010;29:89–100. [Google Scholar]
- (36).Occhipinti G, Jensen VR, Tornroos KW, Froystein NA, Bjorsvik HR. Tetrahedron. 2009;65:7186–7194. [Google Scholar]
- (37).Herde JL, Lambert JC, Senoff CV, Cushing MA. Inorg Syn. John Wiley & Sons, Inc; 2007. Cyclooctene and 1,5-Cyclooctadiene Complexes of Iridium(I) pp. 18–20. [Google Scholar]
- (38).Bell HM. J Chem Educ. 1976;53:665. [Google Scholar]
- (39).Harris DC. J Chem Educ. 1998;75:119–121. [Google Scholar]
- (40).Kappert W, Sander W, Landgrafe C. Liebigs Ann-Recl. 1997:2519–2524. [Google Scholar]
- (41).Coluccini C, Grilli S, Lunazzi L, Mazzanti A. J Org Chem. 2003;68:7266–7273. doi: 10.1021/jo034737t. [DOI] [PubMed] [Google Scholar]
- (42).Peng HM, Webster RD, Li X. Organometallics. 2008;27:4484–4493. [Google Scholar]
- (43).Kownacki I, Kubicki M, Szubert K, Marciniec B. J Organomet Chem. 2008;693:321–328. [Google Scholar]
- (44).Hillier AC, Lee HM, Stevens ED, Nolan SP. Organometallics. 2001;20:4246–4252. [Google Scholar]
- (45).Sargent AL, Hall MB. Inorg Chem. 1992;31:317–321. [Google Scholar]
- (46).Martin M, Sola E, Torres O, Plou P, Oro LA. Organometallics. 2003;22:5406–5417. [Google Scholar]
- (47).Castellanos A, Ayllon JA, Sabo-Etienne S, Donnadieu B, Chaudret B, Yao WB, Kavallieratos K, Crabtree RH. Comptes Rendus De L Academie Des Sciences Serie Ii Fascicule C-Chimie. 1999;2:359–368. [Google Scholar]
- (48).Oldham WJ, Hinkle AS, Heinekey DM. J Am Chem Soc. 1997;119:11028–11036. [Google Scholar]
- (49).Campora J, Lopez JA, Palma P, del Rio D, Carmona E, Valerga P, Graiff C, Tiripicchio A. Inorg Chem. 2001;40:4116–4126. doi: 10.1021/ic010114r. [DOI] [PubMed] [Google Scholar]
- (50).Crabtree R. Accounts Chem Res. 1979;12:331–337. [Google Scholar]
- (51).Schmidt A, Schomäcker R. Ind Eng Chem Res. 2007;46:1677–1681. [Google Scholar]
- (52).Moura FCC, dos Santos EN, Lago RM, Vargas MD, Araujo MH. J Mol Catal A-Chem. 2005;226:243–251. [Google Scholar]
- (53).Pazderski L. Magn Reson Chem. 2008;46:S3–S15. doi: 10.1002/mrc.2301. [DOI] [PubMed] [Google Scholar]
- (54).Stott K, Keeler J, Van QN, Shaka AJ. J Magn Reson. 1997;125:302–324. [Google Scholar]
- (55).Eckert J, Jensen CM, Koetzle TF, Lehusebo T, Nicol J, Wu P. J Am Chem Soc. 1995;117:7271–7272. [Google Scholar]
- (56).Li SH, Hall MB, Eckert J, Jensen CM, Albinati A. J Am Chem Soc. 2000;122:2903–2910. [Google Scholar]
- (57).Lenges CP, Brookhart M, Grant BE. J Organomet Chem. 1997;528:199–203. [Google Scholar]
- (58).Lenges CP, White PS, Brookhart M. J Am Chem Soc. 1999;121:4385–4396. [Google Scholar]
- (59).Duckett SB, Mewis RE. Accounts Chem Res. 2012;45:1247–1257. doi: 10.1021/ar2003094. [DOI] [PubMed] [Google Scholar]
- (60).Barskiy DA, Kovtunov KV, Koptyug IV, He P, Groome KA, Best QA, Shi F, Goodson BM, Shchepin RV, Coffey AM, Waddell KW, et al. J Am Chem Soc. 2014;136:3322–3325. doi: 10.1021/ja501052p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Lloyd LS, Asghar A, Burns MJ, Charlton A, Coombes S, Cowley MJ, Dear GJ, Duckett SB, Genov GR, Green GGR, Highton LAR, et al. Catal Sci Technol. 2014;4:3544–3554. [Google Scholar]
- (62).Aguilar JA, Adams RW, Duckett SB, Green GGR, Kandiah R. J Magn Reson. 2011;208:49–57. doi: 10.1016/j.jmr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- (63).Aguilar JA, Elliott PIP, Lopez-Serrano J, Adams RW, Duckett SB. Chem Commun. 2007:1183–1185. doi: 10.1039/b616307f. [DOI] [PubMed] [Google Scholar]
- (64).Torres O, Martin M, Sola E. Organometallics. 2009;28:863–870. [Google Scholar]
- (65).Lloyd LS, Adams RW, Bernstein M, Coombes S, Duckett SB, Green GGR, Lewis RJ, Mewis RE, Sleigh CJ. J Am Chem Soc. 2012;134:12904–12907. doi: 10.1021/ja3051052. [DOI] [PubMed] [Google Scholar]
- (66).Gruber S, Pfaltz A. Angew Chem-Int Edit. 2014;53:1896–1900. doi: 10.1002/anie.201309515. [DOI] [PubMed] [Google Scholar]
- (67).Cheng C, Kim BG, Guironnet D, Brookhart M, Guan C, Wang DY, Krogh-Jespersen K, Goldman AS. J Am Chem Soc. 2014;136:6672–6683. doi: 10.1021/ja501572g. [DOI] [PubMed] [Google Scholar]
- (68).Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. J Appl Crystallogr. 2009;42:339–341. doi: 10.1107/S0021889811041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Sheldrick GM. Acta Crystallogr A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- (70).Arduengo AJ. III US Patent 5077414. 1991
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.