Stroke is the leading cause of long-term adult disability and the fifth leading cause of death in the US, with approximately 795,000 stroke events in the US each year.1, 2 The aging of the population, coupled with the reduction in case fatality after stroke, is expected to increase the prevalence of stroke by 3.4 million people between 2012 and 2030.3, 4 While stroke mortality had decreased in the US over the past two decades, recent trends in mortality indicate that these decreases may have leveled off, and that stroke mortality may even be rising again. Reasons for this remain uncertain, but could reflect the consequences of the obesity epidemic, and associated diabetes. The morbidity associated with stroke remains high, with costs estimated at $34 billion per year for healthcare services, medications and missed days of work.3, 5 It is likely that estimates of morbidity and cost burden, moreover, based on studies of clinical stroke and using traditional measures such as physical disability and healthcare costs, underestimate the burden of cerebrovascular disease. It is increasingly appreciated, for example, that subclinical cerebrovascular disease—including so-called “silent infarction” identified on brain imaging in up to 28% of the population over age 656, and ischemic white matter disease—is associated with memory loss, dementia, gait impairment, and other functional disability. Furthermore, the global burden of stroke is high, with stroke remaining the fourth leading cause of death worldwide, with a particularly large impact in developing nations.7, 8
Stroke Risk Factors
Unlike myocardial infarction, which is almost always due to large vessel atherosclerotic disease affecting the coronary arteries, identification of risk factors for stroke is complicated by the fact that strokes come in many varieties. At the most basic level, stroke is divided into hemorrhagic and ischemic strokes. The majority (approximately 80%) of strokes are ischemic, although the relative burden of hemorrhagic versus ischemic stroke varies among different populations. Hemorrhagic strokes can be either primarily intraparenchymal or subarachnoid. Ischemic stroke can be further divided into what have been referred to as etiologic subtypes, or categories thought to represent the causes of the stroke: cardioembolic, atherosclerotic, lacunar, other specific causes (dissections, vasculitis, specific genetic disorders, others), and strokes of unknown cause.9 Risk factors for hemorrhagic and ischemic stroke are similar, but there are some notable differences; there are also differences in risk factors among the etiologic categories of ischemic stroke. Hypertension is a particularly important risk factor for hemorrhagic stroke, though it contributes to atherosclerotic disease that can lead to ischemic stroke as well. Hyperlipidemia, on the other hand, is a particularly important risk factor for strokes due to atherosclerosis of extracranial and intracranial blood vessels,10 just as it is a risk factor for coronary atherosclerosis. Atrial fibrillation is a risk factor for cardioembolic stroke.
There is evidence that a high proportion of hemorrhagic stroke, relative to ischemic stroke, can be found in developing countries, where the burden of hypertensive disorders is greater. As the recognition and treatment of hypertension has improved in those countries, often with an increase in Western style diets, the proportion of hemorrhagic strokes declines, and the proportion of ischemic strokes, as well as cardiovascular disease in general, increases. This pattern of the epidemiologic transition, from hypertensive hemorrhagic stroke to ischemic strokes, and their associated risk factors, has been particularly well-illustrated over a relatively short period of time in studies of stroke in Beijing, China, during that country’s rapid economic development over recent decades.11 From 1984 through 2004, for example, the incidence of hemorrhagic stroke declined by 1.7% annually, while the incidence of ischemic stroke increased by 8.7%. The proportion of deaths due to cerebrovascular disease declined, moreover, and the proportion of ischemic heart disease increased.
Reducing the burden of stroke in the population requires identification of modifiable risk factors and demonstration of the efficacy of risk reduction efforts. There are numerous risk factors for stroke, including both modifiable (e.g., diet, comorbid conditions) and non-modifiable risk factors (e.g., age, race). In addition, risk factors may also be thought of as short-term risks or triggers (e.g., infectious events, sepsis, stress), intermediate-term risk factors (e.g., hypertension, hyperlipidemia) and long-term risk factors for stroke (e.g., sex, race). Risk factors for stroke in the young also likely differ from those in older patients.
Estimating stroke risk based on an individual’s particular combination of risk factors, particularly for a first stroke event, is an important component of primary care. Patients indicate a preference for knowing their stroke risk.12 Investigators have therefore sought to create valid risk scoring systems to identify those patients at greatest risk for stroke, with the aim of both modifying these risk factors to reduce the risk of stroke and identifying thresholds of risk that would indicate a role for preventive therapies.13, 14 The Framingham Stroke Risk Profile (FSRP), a continuously-updated, well-known and widely used score, combines stroke predictors such as age, systolic blood pressure, anti-hypertensive therapy, diabetes, cigarette smoking, left ventricular hypertrophy by electrocardiogram, and the presence of cardiovascular disease (coronary heart disease, peripheral vascular disease, congestive heart failure), and can be used to estimate 10 year stroke risk stratified by sex (Table 1). 15-18 Other stroke risk scores have been developed from different sample populations, and while these additional scores include many of the same risk factors as the FSRP, they add additional metrics such as physical disabilities, depression, and marital status. 19-21 Risk prediction scores focused solely on stroke, however, are likely to have limited utility, since patients at risk of stroke are at risk of other cardiovascular events, as well, and so risk scores that include cardiac events and cardiovascular mortality are likely to be more useful.22 Recently, the ACC/AHA Pooled Cohort 10 year atherosclerotic cardiovascular disease (ASCVD) risk estimator was the first to include large amount of data from African Americans, allowing it to incorporate race as a predictive variable and making it particularly useful in black communities.23
Table 1.
The Framingham Stroke Risk Profile: 10 year Stroke Probability for Men and Women age 70 years with Systolic Blood Pressure of 160 mm Hg
| % Probability | |||||||
| Men | 8% | 15% | 18% | 30% | 40% | 60% | 85% |
| Women | 6% | 10% | 16% | 34% | 42% | 80% | 90% |
| Impact of Other Risk Factors | |||||||
| Hypertension medication | None | + | + | + | + | + | + |
| Diabetes | None | + | + | + | + | + | |
| Cigarette Use | None | + | + | + | + | ||
| Cardiovascular Disease | None | + | + | + | |||
| Atrial Fibrillation | None | + | + | ||||
| ECG_Left ventricular hypertrophy | None | + | |||||
From D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40-43
A recent international (22 nation) case-control study (INTERSTROKE) found that 10 modifiable risk factors explained 90% of the risk of stroke (Table 2).24 The investigators enrolled 3000 patients with stroke (n=2337 ischemic, n=663 hemorrhagic), and found that hypertension, current smoking, waist-to-hip ratio, diet risk score, regular physical activity, diabetes mellitus, binge alcohol consumption, psychosocial stress and depression, cardiac disease, and ratio of apolipoprotein B to A1 were all associated with ischemic stroke risk. Risk factors for intracerebral hemorrhage included hypertension, smoking, waist-to-hip ratio, diet, and heavy alcohol consumption. Considering the majority of strokes are first strokes within this study, these findings further illustrate the importance of primary prevention through the reduction of modifiable risk factors, particularly those that confer the greatest risk, to reduce the risk of a first stroke event.25 While much is known about long-term stroke risk factors, such as hypertension, diabetes, and atherosclerotic disease, much less is known about short-term risk factors, or triggers, for stroke.26
Table 2.
Major non-modifiable and modifiable stroke risk factors
| Non-Modifiable Risk Factors |
Modifiable Risk Factors | |
|---|---|---|
| Ischemic Stroke | Age | Hypertension |
| Sex | Current Smoking | |
| Race/Ethnicity | Waist-to-hip ratio | |
| diet | ||
| Physical Inactivity | ||
| Hyperlipidemia | ||
| Diabetes | ||
| Alcohol Consumption | ||
| Cardiac Causes | ||
| Apolipoprotein B to A1 | ||
| Genetics* | ||
| Hemorrhagic Stroke | Age | Hypertension |
| Sex | Current Smoking | |
| Race/Ethnicity | Waist-to-hip ratio | |
| Alcohol Consumption | ||
| Diet | ||
| Genetics* | ||
Genetics is placed in an overlapping location between modifiable and non-modifiable to represent the fact that genetic risk factors are increasingly recognized as potentially modifiable, either directly or through modification of gene-environment interactions.
Modifiable risk factors from O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet. 2010;376:112-123
Non-modifiable Stroke Risk Factors
Non-modifiable risk factors (also called risk markers) for stroke include age, sex, race-ethnicity and genetics. In general, stroke is a disease of aging. The incidence of stroke increases with age, with the incidence doubling for each decade after age 55.27 The mean age of incident ischemic stroke in 2005 was 69.2 years. Recent evidence suggests, however, that the incidence and prevalence of ischemic stroke has been increasing in the 20-54 year old age group, from 12.9% in 1993/1994 to 18.6% in 2005.28 In a retrospective analysis of the population-based Greater Cincinnati/Northern Kentucky cohort, the proportion of incident stroke occurring among those aged 20-54 years increased at each of three one-year time intervals, from 12.9% in 1993/1994, to 13.3% in 1999, to 18.6% in 2006. In an analysis of the US Nationwide Inpatient Sample, among adults 14-44 years ischemic stroke admissions increased annually from 1995-2008.29 In hemorrhagic stroke patients, the incidence increases after the age of 45.30 Some of the recent increases in incidence among younger persons may reflect changes in diagnostic testing as well, leading to greater sensitivity for the detection of stroke among those with minor symptoms.
The relationship of sex to stroke risk depends on age. At young ages, women have as high or higher risk of stroke as men, though at older ages, the relative risk is slightly higher for men.31 The higher stroke risk among women at younger ages likely reflects risks related to pregnancy and the post-partum state, as well as other hormonal factors, such as use of hormonal contraceptives. Overall, more strokes occur in women than men, due to the longer lifespan of women compared to men.27, 32 A study performed in 8 different European countries found that the risk of stroke increased by 9% per year in men, and 10% per year in women.33
There are well-documented racial disparities in stroke. 34 African Americans are at twice the risk of incident stroke when compared to their white counterparts, and have higher mortality associated with stroke.34-44 Hispanic/Latino Americans also have an increased risk of stroke in some cohorts. The disparity in stroke incidence is particularly prominent among younger black adults where the risk for subarachnoid hemorrhage and intracerebral hemorrhage is substantially higher than age-matched whites.38, 40 Furthermore, American Indians have an increased incidence of stroke compared to non-Hispanic whites.45 As illustrated recently by the REGARDS study, one reason for the racial disparities could be the higher prevalence of stroke risk factors, such as hypertension, obesity, and diabetes, among African Americans.46-52 However, these additional risk factors do not completely explain the increased risk seen in these racial-ethnic groups.53 Black race has been identified as a factor in the relationship between rurality and stroke risk,53, 54 but this could be attributed to issues with access to healthcare.34, 55, 56 Other factors that may influence racial-ethnic differences in stroke risk include other social determinants of disease, language, and nativity.57-60 The racial disparity in stroke mortality is being driven by the racial disparities in stroke incidence, highlighting the importance of stroke prevention interventions aimed at minority groups.61 Interestingly, the association seen between black race and stroke, while strong for incident stroke, does not remain for recurrent stroke.62 This could be due to stroke risk factors being addressed upon discharge from the primary stroke event.
Genetic factors are also known to be non-modifiable risk factors for stroke with parental history and family history increasing the risk of stroke.63-65 As is the case with other risk factors for stroke, the genetic risks of stroke vary by age, sex and race. Genetic risk factors and heritability will be more thoroughly discussed in the section on genetics below.
Modifiable Risk Factors
The modifiable risk factors are of utmost importance, as intervention strategies aimed at reducing these factors can subsequently reduce the risk of stroke. Early identification and modification of risk factors is imperative.27 Modifiable risk factors can be further divided into medical conditions and behavioral risk factors. The role of many “traditional” risk factors in causing stroke, such as hypertension, diabetes, hyperlipidemia, and smoking are well-established. The investigation of novel or “emerging” risk factors remains an area of active research.
Hypertension
Hypertension is the most important modifiable risk factor for stroke, with a strong, direct, linear, and continuous relationship between blood pressure and stroke risk.66, 67 In INTERSTROKE, hypertension was by far the most important stroke risk factor: using a definition of hypertension that included both a history of hypertension as well as a blood pressure measurement of 160/90 mm Hg, the population attributable risk, or proportion of strokes in the population attributable to hypertension, was 54%.24 Although this was a case-control study, and thus measurements of blood pressure were likely confounded by recent stroke, the results still imply a major effect of blood pressure on stroke risk, and are consistent with other studies. The effect of blood pressure was also greater for hemorrhagic than ischemic stroke.
Even among those who are not defined as hypertensive, the higher the blood pressure, the higher the risk of stroke. 55 Blood pressure, regardless of hypertension status, rises with increasing age, thereby increasing the lifetime risk of developing hypertension. 68, 69 Of people who are 65 and older, more than two-thirds are hypertensive.66 Hypertension control has improved due to heightened awareness and treatment options, with approximately 50% controlled in 2008, and the prevalence of hypertension in the US has remained at 29%.70, 71 In addition to medication for hypertension control, hypertensive patients are encouraged to engage in behavioral lifestyle changes, such as diet change and increase physical activity, to reduce the impact of hypertension. 66 Treatment of hypertension, whether through medication or lifestyle changes, remains one of the most effective strategies in reducing stroke risk, but hypertension remains undertreated in the community.
Recent studies have suggested that intraindividual variability in blood pressure measurements, or differences in blood pressure measures taken at different points in time within an individual, are associated with stroke risk beyond the risk due to elevated mean blood pressure alone. For example, British investigators, using data from 4 randomized controlled trials of patients with hypertension, prior stroke, or prior transient ischemic attack, found that variability in 2–10 blood pressure measures over approximately 2 years is a risk factor for stroke, independent of mean blood pressure.72 The measure of blood pressure variability may serve as an indication of the absence of cardiovascular homeostasis within the individual. These results suggest that blood pressure agents that reduce variability and not just mean blood pressure, such as calcium channel blockers, may have greater benefits. Other studies have not confirmed this association, however. In the Cardiovascular Health Study (CHS), for example, using a model that also accounted for intraindividual change in blood pressure over time, blood pressure variability was not associated with stroke risk, though it was associated with cardiac events and all-cause mortality.73
Diabetes
Diabetes is an independent risk factor for stroke with a 2-fold increased risk in stroke for diabetic patients, and stroke accounts for approximately 20% of deaths in diabetics. Pre-diabetics are also at increased risk of stroke.74, 75 Approximately 8% of Americans have diabetes, with nearly half of Americans over 65 pre-diabetic.76 The duration of diabetes is also associated with increased stroke risk; in the Northern Manhattan Study, duration of diabetes was associated with ischemic stroke (adjusted HR=1.03 per year with diabetes, 95% CI=1.02-1.04). Compared to non-diabetic participants, those with diabetes for 0-5 years (adjusted HR=1.7, 95% CI=1.1-2.7) and 5-10 years (adjusted HR=1.8, 95% CI=1.1-3.0) were at increased risk, and the risk for those with diabetes for ≥10 years increased markedly (adjusted HR=3.2, 95% CI=2.4-4.5).74 Diabetic patients who have a stroke tend to be younger, are more likely to be African American, and have a higher prevalence of other stroke risk factors. The increase in diabetes may explain some of the increase in the risk of stroke in younger populations.77 The use of combined behavioral modification and medical therapy in diabetics has been shown to reduce the risk of stroke. 78, 79 Interestingly, glycemic control alone in diabetics does not confer the reduced risk that intensive interventions with behavior modification plus medical intervention confer. 80, 81
Atrial fibrillation and atrial cardiopathy
Atrial fibrillation (AF) has long been recognized to be a major risk factor for stroke, and this has only increased with the aging of the US population. Incident stroke related to AF has nearly tripled in the past three decades.82 The association between AF and stroke has long been assumed to be due to stasis of blood in the fibrillating left atrium causing thrombus formation and embolization to the brain. Recent data, however, challenge this assumption. First, there is a poor temporal relationship between AF, which may come and go at irregular and infrequent intervals, and timing of a stroke; one-third of patients do not show evidence of AF until after a stroke despite months of preceding continuous heart-rhythm monitoring.83 Second, other paroxysmal supraventricular tachycardias (PSVT), without fibrillation, have also been associated with stroke risk; in an analysis of claims-based data, PSVT was associated with a doubling in stroke risk, even after adjusting for AF.84 Third, patients with genetic mutations associated with AF (such as in the gene for Natriuretic Peptide Precursor A) can have strokes even before the onset of AF.85 Furthermore, in some settings, the atrium may be in electromechanical disassociation, such that there is fibrillation of the atrium even when the electrocardiogram shows normal sinus rhythm; thus the EKG may not be a perfect indicator of the presence of normal atrial contractility. Finally, other studies have found associations between markers of atrial dysfunction and embolic stroke even in patients without diagnosed AF, suggesting that left atrial thromboembolism can occur in the absence of AF.86 Elevated N-terminal pro-brain natriuretic protein (NT-proBNP), is associated with a doubling of stroke risk in observational cohorts. Abnormalities of the electrocardiographic P wave on lead V1, which reflects left atrial contractility, is also associated with stroke risk independently of AF.87-89
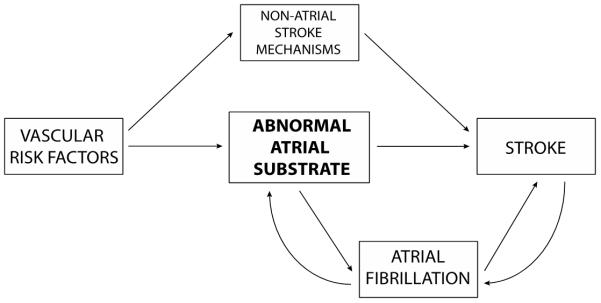
These findings suggest the need for an updated model that emphasizes the atrial substrate as well as rhythm. 90 Aging and vascular risk factors, according to this model, lead to an abnormal atrial tissue substrate, or “atrial cardiopathy,” that causes both AF and thromboembolism (Figure 1). AF, according to this model, may be just another marker of stroke risk associated with left atrial dysfunction.90 Once stroke occurs, the risk of subsequent atrial fibrillation may be further transiently increased by autonomic derangements and a post-stroke inflammatory state.90 There is even evidence from tertiary analyses of randomized trials that anticoagulation may be of benefit for ischemic stroke patients with evidence of elevations in NT-proBNP who are not known to have AF.91 Further trials are planned to test this concept of atrial cardiopathy.
Figure 1.
Atrial Substrate Model of Thromboembolic Stroke.
The model emphasizes the importance of systemic and atrial substrate in explaining the relationship between atrial fibrillation (AF) and stroke. Systemic vascular risk factors produce an abnormal atrial tissue substrate that in turn causes both AF and thromboembolism. Once AF develops, the dysrhythmia causes atrial structural remodeling, worsening atrial tissue substrate and atrial dysfunction and thereby further increasing the risk of thromboembolism. Concurrently, the systemic risk factors driving this process separately increase stroke risk by driving specific mechanisms of stroke outside the atrium, such as large-artery atherosclerosis, ventricular systolic dysfunction with reduced ejection fraction, and in-situ cerebral small-vessel occlusion. Once stroke occurs, changes in autonomic tone and a post-stroke inflammatory state may transiently increase the risk of AF. (from Kamel H, Okin P, Elkind MSV, Iadecola C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke 2016;47(3):895-900.)
Dyslipidemia
The relationship between dyslipidemia and stroke risk is complex, with an increased risk for ischemic stroke with increased total cholesterol, and a decreased risk for ischemic stroke with elevated HDL cholesterol. 92-98 Evidence for the influence of triglycerides on stroke risk is conflicting. Risk appears to depend on stroke subtype, moreover, with a stronger association of cholesterol levels with large artery ischemic stroke than other ischemic stroke subtypes. 10 Total cholesterol, meanwhile, is inversely associated with hemorrhagic stroke, with hemorrhagic stroke risk increasing as total cholesterol decreases.99-101 The data on lipids and intracerebral hemorrhage are further complicated by the fact that some observational studies have found no increased risk of intracerebral hemorrhage with statin therapy, while some treatment trials have.102 While these studies show potentially inconsistent and opposite findings between dyslipidemia and risk of ischemic and hemorrhagic stroke, in the general patient population, the use of statins appears to reduce the risk of total and ischemic stroke with no definite increase in the risk of hemorrhagic stroke.103, 104 The relatively large reduction in risk of ischemic stroke and other ischemic events with statins, moreover, outweighs any small increased risk of hemorrhage in most patients. Among some patients with stroke, however, and particularly those with prior hemorrhage, small vessel disease, or cerebral amyloid angiopathy, statins may be associated with an increased risk of intracerebral hemorrhage.105, 106
Sedentary behavior, Diet/Nutrition, Obesity and Metabolic Syndrome
Physical inactivity is associated with many poor health effects, including stroke. People who are physically active have a lower risk of stroke and stroke mortality than those who are inactive. 107 The relationship between physical activity and stroke may be due to the associated decrease in blood pressure, reduction in diabetes, and reduction in excess body weight. 108
Diet influences the risk of stroke as well as the risk of other stroke risk factors such as diabetes, hypertension, and dyslipidemia. 109, 110 There are several limitations to diet studies including recall bias and measurement error, but some specific components of diet and nutrition are well-established risk factors for stroke. Salt intake, for example, is associated with an increased risk of hypertension and stroke, 111-115 whereas increased potassium intake is associated with a decreased stroke risk. 116-119 A Mediterranean diet, or a diet high in fruits and vegetables, reduces the risk of stroke. 120, 121
Body weight and obesity are risk factors for stroke, although the specific ways in which they increase stroke risk continue to be debated. Obesity is related to stroke risk factors such as hypertension and diabetes.122, 123 A recent large meta-analysis, including 1.8 million participants from 97 cohort studies, found that 76% of the effect of body mass index (BMI, a common measure of obesity) on stroke risk was mediated by blood pressure, cholesterol, and glucose levels. Blood pressure alone accounted for 65% of the risk due to weight. The importance of distinguishing between increased abdominal adiposity, as measured by the waist-hip ratio, as the major contributor to risk, rather than overall increased weight, as indicated by the BMI, is increasingly recognized.124 In INTERSTROKE, for example, waist-to-hip ratio was associated with stroke risk, though BMI was not. 24
The concept of Metabolic Syndrome incorporates obesity, dyslipidemia, pre-hypertension and pre-diabetes. There is evidence that a sedentary lifestyle contributes to the Metabolic Syndrome. Metabolic Syndrome is prevalent in the United States with approximately 34% of the population meeting the criteria, and while it is associated with a clear increase in risk of cardiovascular disease, the relationship between Metabolic Syndrome and stroke appears to be increased but not as well described.125 The risk of ischemic stroke from metabolic syndrome appears to be double, with the risk increasing as the number of components in the syndrome increase.126, 127 Considering the components of the metabolic syndrome are related to stroke individually, the combination of these risk factors should be related to increased stroke risk.127
Alcohol consumption, substance abuse and smoking
The relationship of alcohol consumption to stroke risk depends on stroke type. There is evidence of a J-shaped relationship between alcohol consumption and risk of ischemic stroke, with light to moderate alcohol consumption (up to 2 drinks per day in men and up to one drink per day in women) being protective against stroke, and heavy drinking associated with an increased risk of ischemic stroke. 128-133 Alcohol consumption has a more direct linear relationship with hemorrhagic stroke, such that consumption of even small amounts of alcohol appear to increase risk of hemorrhage. Heavy alcohol consumption is linked to hypertension, as well as poor blood pressure control in hypertensive patients who consume alcohol. 134-140
Abuse of illicit substances, including cocaine, heroin, amphetamines, and ecstasy, is associated with an increased risk of ischemic and hemorrhagic subtypes of strokes. 141-143 Cigarette smoking remains a major risk factor for stroke, nearly doubling the risk with a dose response relationship between pack-years and stroke risk. 15, 144 It is estimated that smoking contributes to nearly 15% of all stroke deaths per year. 145 Smoking cessation rapidly reduces the risk of stroke, with excess risk nearly disappearing 2-4 years after smoking cessation. 146-149 Second hand smoke has been identified as an independent risk factor for stroke in the REGARDS cohort, with the risk of stroke increasing 30% after accounting for other stroke risk factors, for those who have been exposed to second hand smoke versus those who have not been exposed.150
Inflammation and Infection
Levels of inflammatory biomarkers have been associated with increased risk of stroke, just as they have been associated with risk of other cardiovascular diseases and all-cause mortality. C-reactive protein, measured using a high-sensitivity assay (hsCRP), is one marker that has been particularly well-studied. HsCRP has become the inflammatory marker of choice in the clinical setting because of its consistent association with cardiovascular events, its long half-life, and its stability when stored frozen for prolonged periods of time. A meta-analysis of 54 prospective cohort studies, including a total of >160,309 individuals, found a modest association between hsCRP levels and ischemic stroke (relative risk per standard deviation increase in the log CRP concentration 1.27, 95% CI 1.15–1.40).151 Similar results were obtained in a meta-analysis of 12 observational studies of hsCRP and stroke risk152. Genetic studies, however, have not confirmed a causal association between hsCRP and ischemic stroke risk. In one study, single nucleotide polymorphisms in the CRP gene were associated with elevations in hsCRP levels, but these polymorphisms were not associated with an increase in stroke risk.153
The reasons for the association of inflammation with stroke risk remain uncertain. Because atherosclerosis is recognized to have a highly inflammatory character, with plaque containing high levels of activated macrophages and inflammatory mediators, it may be that elevated levels of inflammatory markers reflect a high burden of atherosclerosis, or perhaps a highly active form of atherosclerosis. Thus elevated inflammatory markers may simply serve as a marker of inflammatory burden from these plaques, making elevations in hsCRP a kind of epiphenomenon of vascular disease burden due to other conventional risk factors. Observational studies have generally tried to eliminate confounding by these other risk factors through the use of statistical adjustment, but the possibility of residual confounding, due to the inability to completely measure all such risk factors or their severity, remains. The genetic studies that have failed to confirm that CRP gene mutations cause an increase in risk would be consistent with possibility of residual confounding. There is, however, some evidence that CRP, an acute phase protein, may directly contribute to risk of stroke. Monomeric CRP, for example, interacts with other immune mediators to activate platelets and complement proteins.154 Functionally, each CRP monomeric subunit has a recognition face and an effector face. The recognition face can bind to a diverse set of structural groups, including phosphocholine residues in the C-polysaccharide fraction of Streptococcus pneumoniae and apoptotic cells, nuclear autoantigens, and lipoproteins.155 Binding of the recognition face induces a conformational change that allows the effector face to activate the complement pathway by binding to C1q and Fc receptors, some of which are found on endothelial cells.156 Through this and other mechanisms, CRP, cytokines, and other inflammatory mediators may directly contribute to stroke risk.157
One other way in which inflammation may contribute to stroke risk is through infection. Recent data suggest that chronic exposure to common infections is a potential risk factor for stroke, and that acute infections may also act as triggers for stroke. In an analysis from NOMAS, for example, a composite measure of chronic infection assessed by serologies against several common bacterial and viral infections (Chlamydia pneumoniae, Helicobacter pylori, herpes simplex virus 1 and 2, and cytomegalovirus), weighted for the effect of each individual infection on stroke risk, was associated with increased long-term stroke risk.158 While each individual infection was positively, though not significantly, associated with stroke risk after adjusting for other risk factors, the weighted infectious burden index was associated with an increased risk of all strokes (adjusted hazard ratio per standard deviation 1.39, 95% CI 1.02 – 1.90) after adjusting for demographics and risk factors. Results were similar after adjusting for inflammatory biomarkers. This same measure of infectious burden was also associated with carotid plaque thickness and ulceration, and with cognitive status and decline.159-161
Recent studies have also found that Human Immunodeficiency Virus (HIV) infection is associated with a modest increased risk of both ischemic and hemorrhagic stroke, even in the era of highly active anti-retroviral therapy.162, 163 Mechanisms for this increase in risk remain uncertain, but the risk appears to be higher among those with evidence of greater immunosuppression, such as lower (<200 cells/mm3) CD4+ T-cell counts and higher number of HIV-1 RNA copies. HIV may directly injure the arterial wall. There is evidence, for example, that outward arterial remodeling, or relative thinning of the arterial wall, occurs more commonly in patients with HIV who have protracted infections and greater viral load prior to death.164 Other studies do not suggest a direct effect of immunosuppression on vascular risk, though the risk may differ between cardiac and cerebrovascular events.165 Other explanations include a higher burden of cardiovascular risk factors among those with HIV infection and adverse metabolic effects of the anti-retroviral drugs themselves.166
Stroke triggers
A new area of investigation in stroke epidemiology involves the determination of stroke triggers. Addressing this issue reflects an increasing recognition that while we have a good understanding of the major stroke risk factors (the “why me?” question), our understanding of why strokes occur at a particular point in time (the “why now?” question) remains rudimentary.26 Several potential stroke triggers have been identified, but recent infection may be one that best lends itself to modification. Evidence suggests, for instance, that acute infections may serve as a short-term trigger for stroke in patients. In a case-crossover analysis from the Cardiovascular Health Study (n=5888), for example, a recent hospitalization for infection was associated with an increased risk of stroke.167 In this type of analysis, each individual serves as his own control, thereby limiting confounding factors and enabling the discovery of time-dependent associations. Among 669 participants who experienced a stroke, the risk of stroke was increased following hospitalization for infection within the previous 90 days (odds ratio (OR) 3.4, 95% CI 1.8-6.5). The risk increased as the time interval after hospitalization decreased: OR 7.3 (95% CI 1.9-40.9) for a time window of 30 days, and OR 8.0 (95% CI 1.7- 77.3) for a window of 14 days. In confirmatory survival analyses, hospitalization for an infection was associated with an increased risk of incident ischemic stroke in the following 30 days (adjusted HR=2.5, 95% CI 1.4-4.5). The finding that risk increased as shorter time windows were explored indicates that the triggering effects of the hospitalization with infection diminish over time. Other evidence suggests that more minor respiratory and urinary tract infections are associated with increased stroke risk, and that vaccinations may help prevent stroke. A Cochrane review of eight randomized controlled trials with a total of 12,029 participants provides evidence that influenza vaccination decreased cardiovascular outcomes, and a case series study that used a within-person method of comparison found that the risk of stroke was increased after respiratory tract infection but was reduced after vaccination against influenza, pneumococcal infection and tetanus.168,169 On the basis of this evidence, annual vaccination against influenza may affect stroke rates and could be given to individuals who are at moderate to high risk of stroke, as emphasized in recent guidelines.
Recent evidence also suggests that severe sepsis is associated with new-onset atrial fibrillation, thereby increasing risk of stroke.170 Research using administrative datasets has identified sepsis as a risk factor for stroke, though there may be a low absolute risk of stroke after sepsis, and those who are at risk appear to remain at risk for up to a year after their sepsis event. It is therefore plausible that acute infectious events lead to a prolonged pro-inflammatory state that can contribute to stroke risk. This phenomenon has been found for the risk of heart failure after pneumonia, for example, and it may also apply to stroke risk.171, 172 On the other hand, a population-based cohort study from Denmark showed that about 80% of cardiovascular events after exposure to bacteremia occurred during the index hospitalization, with the risk of stroke highest in the first 3 to 15 days post infection.167, 173, 174
The identification of a short-term state of elevated stroke risk after acute infection could have direct therapeutic implications, as well. For example, increased doses of antiplatelet agents or statins may be warranted during times of fever or infection. In addition, the period during and soon after hospitalization for infection could constitute a “treatable moment,” during which patients can be evaluated for cardiovascular risk and standard preventive strategies instituted.
Other potential triggers for stroke include air pollution. Air pollution, a largely ubiquitous environmental exposure, is quickly becoming a widespread public health hazard, particularly in urban areas. As of 2011, 124 million people in the United States were living in areas that did not meet the United States Environmental Protection Agency (EPA) National Ambient Air Quality Standards. Air pollution has been identified as a novel risk factor for stroke. Long-term exposure to pollution has been associated with increased risk of stroke.175-177 Evidence of the relationship between risk of ischemic stroke hospitalization and mortality and short-term peaks of pollutant levels, primarily particulate matter < 2.5 microns in diameter (PM2.5), have been seen primarily in case-crossover and time-series studies.178 A large case cross-over study of Medicare beneficiaries in 9 US cities also found that an interquartile range increase in PM10 was associated with a 1.03% increase in same-day stroke admissions, with similar results seen with CO, NO2, and SO2.179 Long-term exposure over time has generally been shown to increase the risk of a cerebrovascular event180-183, though several studies have also reported null associations.184 Meta-analyses have found a consistent positive association between air pollution and stroke; an analysis of 20 studies identified that exposure to a 5-ug/m3 increase in PM2.5 increases the risk of a stroke by 6% and stroke mortality by 12.5%. 185
Genetic Risk Factors for Stroke
Hereditary factors contribute to stroke risk, although teasing apart risk due to genetic mutations and due to shared familial exposures remains challenging. The task has been complicated by the heterogeneity of stroke, the multitude of conventional risk factors that cause stroke, and the variability among populations and studies. Genetic variability may, however, contribute to stroke risk through several potential mechanisms (Table 3). First, specific rare single gene disorders may contribute to individual familial syndromes for which stroke is the primary or unique manifestation (e.g., cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, or CADASIL). Second, single gene disorders may cause a multisystem disorder of which stroke is just one manifestation (e.g., sickle cell anemia). Third, some common variants of genetic polymorphisms have been associated with stroke risk, though the individual contribution of such polymorphisms is regarded as modest (e.g., variants in 9p21).186 Fourth, genetic causes of conventional stroke risk factors, such as atrial fibrillation, diabetes, and hypertension, are also, not surprisingly, associated with risk of stroke.187 Emerging evidence suggests that genetic studies could help to distinguish stroke subtypes and even contribute to patient management. For example, there is an association between gene variations that confer an increased risk of atrial fibrillation and ischemic stroke. This raises the possibility that genetic tests could help to make the diagnosis of strokes likely to be due to atrial fibrillation.
Table 3.
Selected genetic causes of stroke
| Disease | Mode of inheritance |
Gene/protein | Mechanism of stroke |
Common clinical manifestations |
|---|---|---|---|---|
| Single gene disorders that primarily cause stroke | ||||
| Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) |
Autosomal dominant |
NOTCH3/NOTCH3 | Small vessel disease |
Ischemic stroke, leukoencephalopathy, migraine, psychiatric manifestations, dementia |
| Cerebral Autosomal Recessive Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CARASIL) |
Autosomal recessive |
HTRA1/ HtrA serine peptidase-1 |
Small vessel disease |
Ischemic stroke, leukoencephalopathy, premature baldness, spondylosis |
| Familial amyloid angiopathy |
Autosomal dominant |
APP/beta-amyloid precursor protein |
Rupture of small cortical vessels |
Lobar hemorrhage, microbleeds, leukoencephalopathy, dementia, “amyloid spells” |
| Collagen 4 (COL4A1) mutations |
Autosomal dominant |
Col4A1/α1 chain of collagen type IV |
Rupture of cortical and subcortical vessels |
Superficial and deep hemorrhages, intracranial aneurysms, hematuria, cystic kidney disease |
| Genetic Disorders that include stroke as manifestation | ||||
| Ehlers-Danlos Type 4 | Autosomal dominant |
Col3A1/type III procollagen |
Arterial dissection |
Aortic, renal, splenic, iliac dissection/rupture; aneurysms/pseudoaneurysms; visceral and muscular rupture; uterine rupture during preganncy |
| Fabry Disease | X-linked |
GAL/α- galactosidase A |
Large and small artery disease |
Ischemic stroke and vasculopathy, angiokeratomas, corneal opacities and cataracts, neuropathy (acroparesthesias, hypohidrosis), renal failure |
| Marfan syndrome | Autosomal dominant |
FBN1/fibrillin 1 | Arterial dissection, cardiac embolism |
Ischemic stroke, arterial dissection, scoliosis, pectus excavatum, aortic dilatation, valvular diysfunction/heart failure, ectopia lentis |
| Mitochondrial encephalopathy with lactic acid and stroke- like episodes (MELAS) |
Maternal | Mitochondrial DNA(MT-TL1)/ mitochondrially encoded tRNA leucine 1 (UUA/G); others |
Energy failure, “metabolic stroke” |
Ischemic stroke that does not observe vascular boundaries, short stature, developmental delay, seizures, vision loss, myopathy, diabetes |
| Sickle cell disease | Autosomal recessive |
HBB/beta-globin (hemoglobin subunit) |
Large and small vessel disease, moyamoya syndrome |
Ischemic stroke, painful crises, vascular crises, bacterial infections |
| Smooth Muscle Alpha- Actin (ACTA2) mutation associated disorders |
Autosomal dominant |
ACTA2/Smooth Muscle Alpha-Actin |
Moyamoya syndrome |
Ischemic stroke, coronary artery disease, thoracic aortic aneurysms, moyamoya syndrome |
| Common genetic variants | ||||
| TSPAN2 | Common variant |
TSPAN2/ tetraspanin-2 |
Vascular development; atherosclerosis |
Large vessel ischemic stroke; |
| FOXF2 | Common variant |
FOXF2/forkhead transcription factor |
Small vessel disease; abnormalities of smooth muscle cell and pericyte coverage of cerebral vessels |
All stroke, small vessel stroke, premature and extensive white matter disease |
| ABO | Common and rare variants |
ABO/blood group protein |
Thrombosis | Thrombosis, ischemic stroke |
| HDAC9 | Common and rare variants |
HDAC9/ histone deacetylase |
Atherosclerosis | Large vessel ischemic stroke |
| PITX2 | Common and rare variants |
PITX2 | Sinoatrial node development and regulation of ion channels; modulation of cardiac conduction; atrial fibrillation |
Cardioembolic ischemic stroke, atrial fibrillation |
| ZFHX3 | Common and rare variants |
ZFHX3 | Atrial fibrillation |
Cardioembolic ischemic stroke, atrial fibrillation |
Currently, heredity is generally considered a non-modifiable risk factor, although genetic therapies may change this in the future. Some genetic factors may even be modifiable, if not curable, already; for example, those with sickle cell anemia can be treated with exchange transfusion to reduce stroke risk. Genetic factors may also be modifiable since environmental factors may also interact with genetic mutations (i.e., gene-environment interactions); thus those with a predisposition to diabetes or hypertension could engage in dietary and other lifestyle modifications to reduce their risk of disease.
Heritability of stroke
Recent estimates of heritability using genome-wide single nucleotide polymorphism (SNP) data show similar heritability for cardioembolic (32.6%) and large vessel disease (40.3%), but lower for small vessel disease (16.1%).188 Family history of stroke increases stroke risk by 30%.189 Monozygotic twins are at 1.65-fold higher risk of stroke than dizygotic twins.189 Age, sex, and stroke subtype further affect stroke heritability 64, 65. Younger patients are more likely to have a first-degree relative with stroke,64 and women with stroke are more likely to have a parental history of stroke than men.65 MRI measures of small vessel ischemic disease, however, have concordance rates of 0.61 for monozygotic twins and 0.38 for dizygotic twins, suggesting a genetic susceptibility to small vessel ischemic stroke.190
Single gene disorders with stroke as a primary manifestation
CADASIL is a small vessel vasculopathy that affects skin and brain, though its clinical manifestations are restricted to the central nervous system. The pathology includes degeneration of the media of small vessels and a prominent and progressive leukoencephalopathy. Clinically, patients present with migraine-like headaches, psychiatric complaints including depression and psychosis, and recurrent strokes, often leading to pseudobulbar palsy and subcortical dementia. Characteristic imaging findings include white-matter lesions in the external capsule and anterior temporal poles; the imaging findings can usually be distinguished from those of more typical white matter changes associated with hypertension and aging.
CADASIL is associated with mutations of the Notch3 gene, located on chromosome 19q12.191 Most of these are missense mutations altering the number of cysteine residues expressed in an extracellular receptor domain.192 Though Notch3 is widely expressed in the body and plays an important role in development, CADASIL only affects the nervous system clinically, for unknown reasons. While the defective receptors usually do not interfere with phenotypic signaling, they have been shown to accumulate in the basal lamina of the small arteries.193 Skin biopsy revealing granular osmiophilic material can be pathognomonic for the diagnosis, sometimes detecting the disease in patients who have relatively minor findings on imaging studies and negative genetic test results for the most common mutations.194
Other rare single gene disorders that cause stroke include cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL),195 caused by mutations in the HtrA serine peptidase-1 gene ;196 arterial tortuosity syndrome, caused by mutations in the SLC2A10 gene encoding a glucose transporter, GLUT10;197, 198 and familial cerebral amyloid angiopathy, due to mutations affecting cystatin C.199
Single gene multisystem disorders with stroke as an important manifestation
Cerebrovascular complications are well-recognized in sickle cell anemia (SCA), and result from polymerized red blood cells at low oxygen tensions, leading to small vessel occlusion and sickle-related arterial disease (moyamoya syndrome). SCA is seen in approximately 6% of children with stroke, but 25% of individuals with SCA will experience a stroke by 45 years of age; the highest incidence for ischemic stroke is at 2-5 years.200 Untreated, the risk of a recurrent stroke is as high as 90%.201 Multiple silent infarcts may be more common than clinical infarcts and they can impair cognitive processing and school performance.202
Fabry disease is an X-linked deficiency in α-galactosidase A, a lysosomal enzyme, caused by both missense and nonsense mutations in the GLA gene. Loss of function of α-galactosidase A leads to accumulation of globotriaosylceramide in cells throughout the body, including the vascular endothelium. Fabry disease is the second most common lysosomal storage disorder. Cerebrovascular involvement typically occurs in both large and small vessels, especially affecting the posterior circulation, and can occur in young stroke patients.203 Mechanisms of stroke include cardioembolism from cardiac involvement, large artery thromboembolism from dolichoectasia and tortuosity of large vessels, and occlusive disease of small vessels due to glycolipid accumulation in the endothelium and vascular smooth muscle cells. In the Fabry Outcome Survey, the frequency of stroke among males aged 25–44 years was approximately 12 times the expected frequency in the general population.204Enzyme replacement therapy can modify the natural course of this disease.
The syndrome of mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is a mitochondrial disorder caused by mutations in mitochondrial DNA, leading to respiratory chain failure and reduced energy production. The most commonly reported genetic defect is an A3243G substitution within a tRNA gene, present in 80% of cases.205 The failure of energy production leads to dysfunction and injury of brain tissues from a metabolic cause rather than from an occlusive vascular process; thus, brain lesions seen in MELAS typically do not observe classic vascular territories. Patients present with attacks of encephalopathy and focal neurological dysfunction, and may also have migraine headaches, nausea, and vomiting. Episodes may be precipitated by fever, which increases metabolic demands. Multiple organ systems can be affected, leading to short stature, hearing loss, developmental delay, diabetes mellitus, and other problems. There is significant heterogeneity in the phenotype of family members, due to heteroplasmy, or the variable expression of mutated mitochondrial DNA within different tissues.
Genetic disorders of collagen can also affect multiple tissues, including the cerebral vasculature. The Ehlers-Danlos spectrum of disorders represents a group of inherited disorders characterized by connective tissue abnormalities and vascular fragility. The most worrisome is Ehlers-Danlos Type IV, which is associated with arterial dissections, cerebral aneurysms, and stroke. Patients may have complications of other organs as well, however, including uterine rupture during pregnancy. Type IV EDS is due to mutations of collagen type-III (COL3A1). Mutations in the gene encoding the alpha-1 chain of type IV collagen (COL4A1) can also result in impaired vessel-wall integrity. Cerebrovascular manifestations include small-vessel steno-occlusive disease, aneurysms, and dolichoectasia, with resulting ischemia, intracranial hemorrhage, leukoencephalopathy, and retinal arteriolar tortuosity.206 An increased tendency for cerebral hemorrhage can occur with head trauma, participation in sports, and anticoagulant use.207
Other hereditary connective tissue diseases, such as Marfan syndrome and ACTA2-associated vasculopathy, are similarly associated with vascular fragility and can lead to arterial dissections. Mutations in the ACTA2 gene, encoding actin alpha 2, an isoform of actin found in vascular smooth-muscle, leads to actin polymerization and smooth-muscle cell proliferation. Phenotypically, individuals have evidence of smooth muscle dysfunction throughout the body, including fixed dilated pupils, hypotonic bladder, gut malrotation and hypoperistalsis, and pulmonary hypertension.208There is a risk for aortic, cervical and intracranial arterial dissection.209They are also at risk for small vessel disease, moyamoya disease, and aneurysmal large-vessel disease.210
Genes associated with common ischemic stroke and stroke risk factors
There is emerging evidence that genetics contribute to the risk of common ischemic stroke. Several genetic variants have been identified, though the magnitude of effect of each variant is regarded as small. Genetic mutations in genes related to coagulation have been extensively investigated. MetaStroke is one of the largest genetic collaborations in ischemic stroke, and includes 15 European, North American, and Australian case-control stroke cohorts. Participants are subtyped by ischemic stroke etiology according to a modified TOAST classification system. After detection of potential disease-causing mutations in initial somewhat smaller European cohorts, MetaStroke confirmed an association of stroke with gene variants in the blood type gene ABO (rs505922), which is associated with levels of the coagulation proteins von Willebrand Factor and Factor 8; the associations were present for large-vessel and cardioembolic stroke subtypes, but not for small-vessel disease.211
Other subtype-specific genetic variants have been identified. Icelandic investigators first reported an association between genetic variants conferring an increased risk of atrial fibrillation and ischemic stroke, especially those thought to be cardioembolic. Interestingly, there was also a significant association in the events classified as noncardiogenic, perhaps due to underdiagnosis of atrial fibrillation. In a subsequent collaborative analysis among MetaStroke and several other cohorts, the PITX2 and ZFHX3 genes were also associated with cardioembolic stroke, and the HDAC9 gene and 9p21 locus were associated with large-vessel stroke.187 In a separate genome wide association study by the National Institute of Neurological Disorders and Stroke (NINDS)-Stroke Genetics Network (SiGN) project, a locus near the TSPAN2 gene on chromosome 1 was also associated with large-artery stroke.212 These findings provide insight into the pathophysiology of stroke: PITX2, for example, encodes a transcriptional activator that is involved in the development of the sinoatrial node and in regulation of ion channels that modulate cardiac conduction; ZFHX3 also encodes a transcription factor. The HDAC9 gene encodes histone deacetylase, though its mechanism for causing atherosclerotic stroke remains uncertain. TSPAN2 encodes tetraspanin-2, a member of a superfamily of transmembrane proteins that regulate signal transduction and play a role in cell development and growth; TSPAN2 is expressed in arterial tissue and blood cells, and TSPAN2 knock-out mice have activation of microglia and astrocytes.
A separate meta-analysis of genome-wide association studies, including 18 population-based cohorts with almost 85,000 participants (n=4348 strokes), identified a novel locus on chromosome 6p25 (rs12204590), near the forkhead transcription factor FOXF2 gene, that was associated with both the risk of all strokes and with the white matter hyperintensity burden seen on MRI scans, a marker of small vessel disease, in a subcohort (n=21,079 individuals).213 Deletion of FOXF2 in young patients was associated with extensive white matter disease, and mutations in other forkhead transcription factors (FOXC1) have been associated with white matter disease syndromes.214 Functional experiments in mice confirmed that deletion of Foxf2 is associated with cerebral infarction, reactive gliosis, and microhemorrhages. The zebrafish orthologs of FOXF2, moreover, are expressed in brain pericytes, and zebrafish without this gene have abnormalities of smooth muscle cell and pericyte coverage of cerebral vessels. These data suggest that FOXF2, a transcription factor that plays a role in cerebral vascular wall morphogenesis and function, may be a contributor to small vessel disease in humans, as well.
Much of the research thus far has focused on common genetic variants. More recent research, however, has confirmed that low-frequency genetic variants (i.e., allele frequency <5%) may contribute to risk of large vessel and small vessel stroke.215 GUCY1A3, for example, a gene that has been associated with early myocardial infarction, was associated with large vessel stroke, and had an allele frequency in the lead single nucleotide polymorphism (SNP) of 1.5%. This gene encodes the α1 subunit of soluble guanylyl cyclase, which plays a role both in nitric oxide induced vasodilation and platelet inhibition.216 Another gene, GCH1, also with an allele frequency of only 1.5%, was associated with small vessel stroke; GCH1 encodes GTP cyclohydrolase 1, which plays a role in endothelial nitric oxide synthase. Thus rare variants may account for some of the unexplained heritability in stroke risk.
Variation in chromosome 9p21 has been strongly associated with coronary artery disease and myocardial infarction in multiple cohorts.217 Fifteen SNPs in that region were evaluated within large samples from South Germany, the UK, Europe and North America, and six were associated with atherosclerotic or large-vessel stroke in adjusted analyses. A population attributable risk of 20% for large-vessel stroke due to SNPs at 9p21 was estimated from the analysis.218
Stroke Prevention
The aim of stroke prevention is to decrease stroke incidence through targeted modification of a single risk factor, or a cluster of multiple risk factors, employed on a population, community, or individual level. In some cases, however, as with the use of anti-platelets, the goal may be to use an intervention that is known to reduce ischemic stroke risk among those who are deemed to be at elevated risk, rather than as a treatment for a specific risk factor. There are three broad levels of stroke prevention: 1) primordial prevention is the most generalizable, and broadly deals with healthy living measures that, when applied on a group level, aim to decrease the population incidence of physiologic stroke risk factors; 2) primary prevention, which aims to improve the risk factor profile of individuals who do not have a history of stroke or TIA with the goal of preventing a first cerebrovascular event; and 3) secondary prevention, which is the most targeted and is only employed after an individual has suffered a stroke or TIA, with the goal of preventing stroke recurrence.219, 220 (Figure 2) Examples of primordial stroke prevention include efforts to encourage smoking cessation, a healthy diet, increased physical activity, and weight control. Primary and secondary stroke prevention target a person’s specific lifestyle-related and medical stroke risk factors, like hypertension and diabetes. We will focus this discussion on primordial and primary prevention of ischemic stroke.
Figure 2.
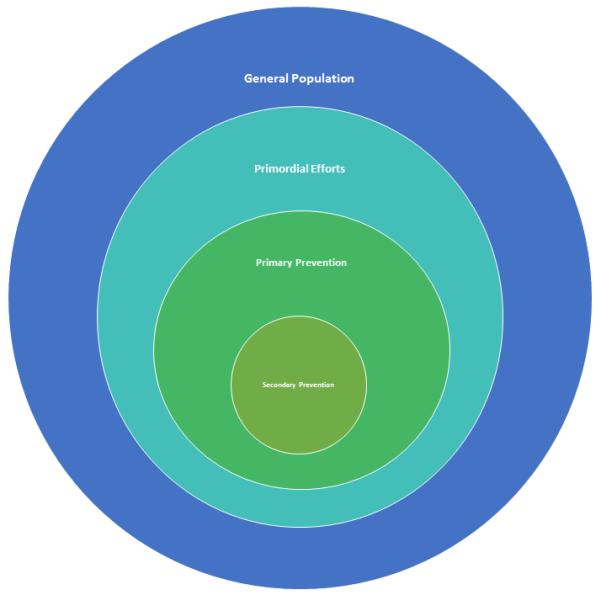
Levels of stroke prevention in a population
Mass Approaches
The cornerstone of lifetime CVD risk reduction is behavioral modification. In primordial prevention, healthy lifestyle behaviors such as abstaining from tobacco, a healthy diet and regular exercise should start in childhood and continue through one’s lifetime.219, 221 Prevention efforts targeting behavioral modification are especially well suited for mass interventions such as public health campaigns. Though the immediate result is steps removed from the outcome of stroke, such campaigns have the potential to affect multiple outcomes, including stroke, coronary artery disease, heart failure, diabetes, dementia and others.222, 223 Further, these primordial efforts are effective because of the small and widely dispersed magnitude of treatment-related adverse events.
Recently, a more specific form of population-based cardiovascular prevention has been proposed – the polypill. This is an example of a primary prevention effort employed on a mass scale. The best-studied is a pill that contains three anti-hypertensives, a hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor (statin), and an anti-platelet agent.224 In a phase 2, double–blind, randomized controlled clinical trial of healthy adults, the polypill was shown to favorably affect blood pressure, LDL, heart rate and urinary thromboxane B2 level (anti-platelet effect), when compared to single-drug regimens, without increased intolerability. Such a formulation could be particularly useful in places where the infrastructure for individualized care is underdeveloped or lacking.225 Because stroke incidence has a linear correlation with blood pressure no matter the baseline, such standardized methods can be justified.226 In an effort to simplify pre-therapy serum testing and medical follow up, limit side-effects, and ultimately decrease associated cost, the mini-polypill has recently received attention as a more refined standardized method of community cardiovascular disease prevention. The formulation that is being actively studied contains candesartan (angiotensin-receptor blocker), hydrochlorothiazide (thiazide diuretic), and a low dose of rosuvastatin. The strongest evidence for this approach to mass primary prevention came from The Heart Outcomes Prevention Evaluation (HOPE)–3 trial, a 2-by-2 factorial design, double-blind, placebo-controlled trial in which 12,705 participants with intermediate risk of cardiovascular disease, but without a history of CVD, were assigned to receive either rosuvastatin 10mg + placebo, rosuvastatin 10mg + candesartan and HCTZ (polypill), candesartan and HCTZ + placebo, or a double placebo. 227-230 The analysis that reported the combined therapy with rosuvastatin and the two antihypertensive agents administered versus placebo included 3,180 participants in the active arm versus 3,168 participants in the control arm. The treatment arm was associated with a 1.4% absolute risk reduction in combined cardiovascular outcomes (hazard ratio [HR], 71%, 95% CI 0.56-0.90), half of which was driven by a decrease in stroke incidence. Except for an increase in muscle weakness and dizziness, the polypill used in this study was well tolerated, had a similar compliance rate to placebo, and led to fewer hospitalizations for cardiovascular causes. This is the first study to validate the efficacy of a mass approach to primary prevention therapy through a standard combination of targeted drug therapies and minimal pre-treatment screening. Such an effort hinges on the notion that population-wide approaches are effective in reducing outcomes in intermediate- and high-risk individuals who may otherwise be naïve to individualized primary prevention efforts. The downside of this method is that those in the population who are low risk are also exposed to the side effects of the treatment, skewing the risk to benefit ratio. Despite this evidence that the polypill approach may be effective in preventing cardiovascular outcomes, it is unclear how and where to translate such efforts into clinical practice. This is especially true in the United States, where access to healthcare varies, but is relatively high compared to global standard.
Targeted Lifestyle Modification
Physical Activity
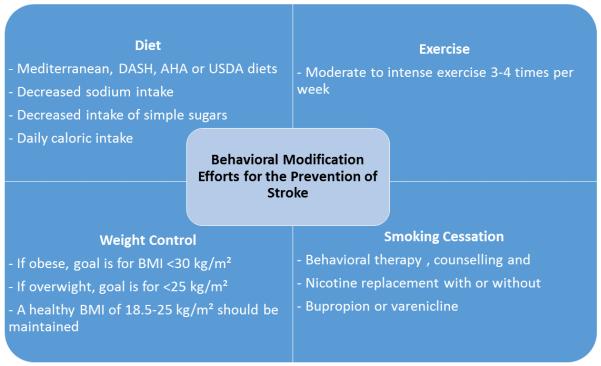
Classically, the lack of standard definitions of exercise intensity and variability in exercise routines, along with difficulty measuring the exposure and long time frame needed to see an effect, have all made studying the effects of exercise on the outcome of stroke difficult. It is now standard to report energy expenditure as metabolic equivalents (METs). Using this model, physical activity is classified as sedentary between 1-1.5 METs, light between 1.6 and 2.9 METs (e.g. playing an activity-promoting video game), moderate between 3–5.9 METs (e.g. ballet dancing), and vigorous when >6 MET (e.g. outdoor bicycling).231 Based on biological plausibility and evidence from pooled analysis from large prospective cohort and retrospective case-control studies, it is generally accepted that there is a lifetime-long inverse relationship between physical exercise and stroke.223, 232 Compared to physically inactive individuals (<600 MET minutes/week) those who are highly active (>8,000 MET minutes/week, or around 2 hours of daily vigorous activity) are estimated to have a 25-30% lower risk of stroke.223, 233 There is also evidence that the there is a gradient in protective effect depending on level of activity, although the optimal exercise regimen for specific subsets of the population have not been established.234 At this time, a generally accepted and useful recommendation for primary stroke prevention, which can be tailored to an individual’s lifestyle needs and preference, is the AHA/ACC CVD prevention guideline of at least 40 minutes per day of moderate to vigorous intensity exercise, 3 to 4 days per week.221, 235 (Figure 3)
Figure 3.
Healthy lifestyle related practices for improved cardiovascular health
DASH: Dietary Approaches to Stop Hypertension; AHA: American Heart Association; USDA: United States Department of Agriculture122, 123
Diet
A Cochrane review in 2013 suggested that adherence to a healthy diet can decrease lifetime risk of stroke by nearly 20%.236 The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), AHA, and US Department of Agriculture (USDA) food patterns diets are all alike in that they promote a combination of plant-derived micro and macronutrients, decreased caloric intake related to saturated and trans fats, increased intake of fruits and vegetables, and decreased salt intake.237 The Nurses’ Health Study, a prospective cohort of 71,768 participants, followed dietary patterns from 1984-1998. It was the first to show that a “Western diet”, high in saturated fats, processed grains, and simple sugars was associated with an increase in stroke incidence (RR 1.58, 95% CI 1.15-2.15; p < 0.001), while adherence to a “prudent diet”, high in fruits and vegetable, whole grains, legumes, and fish, was associated with a decreased stroke incidence when comparing extreme quintiles (RR 0.78, 95% CI 0.61-1.01).238 Perhaps the strongest evidence in favor of diet reducing cardiovascular events is the Mediterranean diet.239 It is defined by high intake of vegetable, fruits, legumes; olive oil as the principal source of fat; preferential consumption of fish and poultry over red meat; low dairy intake; and an option of low intake of red wine.240 The Primary Prevention of Cardiovascular Disease with a Mediterranean Diet (PREDIMED) study, a multicenter randomized trial that compared the effects of the Mediterranean diet on cardiovascular outcomes found an approximate 30% (95% CI 0.46-0.98, P<0.04) reduction of stroke incidence for a Mediterranean diet high in olive oil compared to a standard low fat diet, and a 50% (95% CI 0.35-0.84, P<0.006) reduction for a Mediterranean diet high in mixed nuts.241 Similar to the Mediterranean diet, a meta-analysis showed that the DASH diet was associated with a nearly 20% lowered risk of stroke (95% CI 0.72, 0.92).242 In general, any diet that revolves around the high intake of plant-based nutrients, low salt, and curbing of saturated fats and simple sugars, such as the Mediterranean, DASH, USDA food patterns or AHA diets, are recommended for the purpose of good cardiovascular health and primary stroke prevention. It may also be reasonable to supplement any of these diets with a high intake of mixed nuts, defined by 6 weekly servings of 30 g of mixed nuts. 241
Smoking cessation
Among smokers, cessation leads to a decrease in stroke risk to levels similar to non-smokers by 5 years.243 The U.S. Public Health Service Clinical Practice Guidelines Executive Summary recommends physician-led screening, counseling and referral to further behavior support, of those who smoke, as well as the routine use of nicotine replacement products, bupropion and varenicline, in those who are seeking to quit smoking - unless medically contra-indicated.244 Although the benefits are likely to far outweigh the risk of therapy, the FDA has advised careful monitoring of patients on varenicline, based on three systematic reviews suggesting an increased risk of cardiovascular events, including stroke, in patients treated with varenicline compared to placebo. For the purpose of cardiovascular disease prevention, it is reasonable to use a combination of behavior therapy, nicotine replacement, and/or bupropion and varenicline with close monitoring of high risk patients on varenicline.245
Targeted Risk Factor Modification
Hypertension
A meta-analysis of 147 trials, including 464,000 participants without a history of vascular disease or stroke, found that blood pressure reductions of 10 mm Hg systolic or 5 mm Hg diastolic were associated with a 40 percent reduction in stroke risk. 60 The effect is present even at levels below those thought to be normotensive, and down to 110 mm Hg systolic and 60 mm Hg diastolic. 226, 246 The disproportionately high prevalence of hypertension and diabetes among American blacks is likely a major driver of their higher rates of stroke. Further, blacks are more likely to suffer from increased blood pressure variability and are less likely to be adequately treated, all poor prognostic factors in the development of blood pressure-related morbidity.61 Beta blockers, thiazide diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers are the most widely studied agents, and while there are are some class differences, the majority of benefit is conferred by the level of blood pressure control rather than the class of medication used.247
AHA/ACC guidelines recommend regular blood pressure screening and promotion of lifestyle modification for patients found to have pre-hypertension, defined as systolic blood pressure of 120 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg.248 In hypertensive patients, blood pressure goals for the purpose of primary stroke prevention can generally be adapted from the recommendations from the Eighth Joint National Committee (JNC8) guidelines for blood pressure management. For individuals younger than 60 who are hypertensive, defined as a blood pressure >140 mm Hg systolic or >90 mm Hg diastolic, medical therapy is indicated. For those older than 60 who do not have a history of diabetes or kidney failure, the JNC8 guidelines call for a more liberal target of <150/90 mm Hg.249 The different recommendation based on age is countered, however, by a recent large randomized control trial that included 9,361 persons who were 50 years of age or older and with a systolic blood pressure of 130 mm Hg or higher. Intensive treatment targets of <120 mm Hg systolic compared to standard treatment of <140 mm Hg, reduced the risk of composite cardiovascular outcomes (HR 0.75, 95% CI 0.64-0.89, p<0.001) and showed a trend toward reduced stroke risk (HR 0.89, 95% CI 0.63-1.25). Adverse events were not significantly different between the two groups and the benefits extended out to those older than 75.250 For the purpose of primary stroke prevention, lifestyle modification and pharmacological therapy should be combined to achieve strict blood pressure goals less than 140/90 mm Hg. While the effects on stroke prevention are still unproven, it may be reasonable to target systolic blood pressure goals <120 mm Hg in individuals who are at low risk for complications from anti-hypertensive therapy. Self-measured blood pressure monitoring devices are also recommended to better gauge treatment effects, limit adverse effects and optimize blood pressure control.248
Diabetes
Aggressive management of hyperglycemia in diabetics has not been shown to decrease the incidence of stroke and may actually be harmful. The Action to Control Cardiovascular Risk in Diabetes Study (ACCORD) compared intensive glucose lowering to a goal glycated hemoglobin (HgbA1C) level below 6%, versus liberalized goals of 7 - 7.9%, and found no difference in stroke incidence, but a statistically significant increase in overall mortality in the intensive management group. The results suggest that it may be that the greatest effects of hyperglycemia on stroke risk accumulate early in the course of the disease and in the pre-diabetes stage, rather than late in the course when co-morbid cardiovascular risk factors are more likely to be present. This notion is supported by a recent randomized controlled trial of pioglitazone used after stroke or TIA in those with pre-diabetes. 251 It enrolled 3,876 individuals after a recent stroke or TIA who also had pre-diabetes. The combined outcome of recurrent stroke and myocardial infarction was seen in 9.0% of the pioglitazone group and 11.8% of the placebo group (HR 0.76, 95% CI 0.62-0.93), over a nearly 5 year period of follow up. Importantly, there was also corresponding 52% reduction in incidence of diabetes. Unfortunately, treatment with pioglitazone resulted in a greater frequency of weight gain, edema and bone fracture, limiting its practical utility. The 2016 recommendation by the American Diabetes Association calls for HgbA1C targets of less than 7% for the purpose of preventing cardiovascular complications of diabetes. It may be reasonable to liberalize the HgbA1C goal to 8% in the elderly or frail.252 At this time, the presence of prediabetes necessitates intensive lifestyle intervention, while targeted medical pharmacological therapy with metformin, for example, may be optimal for certain individuals.253
Hyperlipidemia
The role of cholesterol reduction, particularly with HMG-CoA reductase inhibitors, or statins, has been demonstrated in several observational studies and clinical trials. Large epidemiological studies, like the Multiple Risk Factor Intervention Trial (MRFIT), which included over 350,000 men, have shown a positive association between increased cholesterol levels and stroke mortality. In primary stroke prevention trials, several statins have been associated with reductions in risk of stroke ranging from 11-40%. The Heart Protection Study (HPS), a randomized multi-center, placebo-controlled trial of simvastatin therapy that included 20,536 individuals with either coronary artery disease, peripheral vascular disease, or diabetes, showed a 5-year stroke risk reduction of 25% in the simvastatin group compared to placebo (p<0.0001).254 The effect was driven by the decrease in ischemic strokes without an increase in risk of hemorrhagic strokes. Importantly, these benefits remained in those with LDL less than 100 mg/dL. More aggressive treatment was associated with a further reduction in risk. In the Treating to New Targets (TNT) study, compared to atorvastatin 10 mg daily, atorvastatin 80 mg daily was associated with a 25% reduction in stroke risk that correlated with reductions in LDL. Furthermore, meta-analyses of lipid therapy and stroke showed that with each 1 mmol/L reduction in LDL cholesterol, there was an approximate 20% relative risk reduction in ischemic stroke. 255, 256
The role for statin therapy in stroke prevention is also evident in secondary prevention studies. A post-hoc analysis of HPS showed that in patients with a history of cerebrovascular disease without coronary disease (n=3280), simvastatin was associated with a 5% reduction in the risk of major cardiovascular events or death compared to placebo.257 The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, however, provides the most direct evidence regarding the role of statins in stroke patients. Patients (n=4731) with stroke or TIA and baseline LDL 100-190 mg/dL were randomized to atorvastatin 80 mg versus placebo beginning 1-6 months after their event. Atorvastatin was associated with an approximate 2% absolute reduction in recurrent stroke risk (13.1% vs. 11.2%) over a median follow up of 5 years, with a relative risk reduction of 16%.258 The benefits of statins on risk reduction were similar across subtypes of the index stroke subtype as well, implying that all ischemic stroke patients, regardless of subtype, should receive statin therapy.259 While there was a 0.9% absolute increase in risk of hemorrhagic stroke among patients on atorvastatin (2.3% at 5 years) compared to placebo (1.4%; hazard ratio 1.66, 95% CI 1.08 to 2.55), this detrimental effect was more than outweighed by the effect on ischemic stroke, which occurred much more commonly, such that the benefits overall favored atorvastatin for total stroke prevention (11.2% on atorvastatin versus 13.1% on placebo).
While SPARCL was the first clinical trial to prove the benefit of high dose statin therapy in secondary stroke prevention, the effect was modest with a number needed to treat of 50 over 5 years. There were several limitations, moreover. For instance, patients were eligible for randomization beginning one month after stroke, a period where the risk of stroke recurrence falls, especially in patients with large artery atherosclerosis in whom statins may provide the most stroke prevention benefit. Ongoing trials are addressing the role of statin therapy given immediately after stroke, not only to reduce lipid levels and prevent recurrent stroke, but also to ameliorate cerebral injury related to the stroke itself. For example, the Neuroprotection with Statin Therapy for Acute Recovery (NeuSTART) trial is a Phase II trial randomizing patients with acute ischemic stroke to lovastatin 640 mg daily for three days versus placebo to determine the safety and efficacy of lovastatin in reducing infarct size and promoting stroke recovery, which may be a function of the pleiotropic effects of statins.260 In addition, SPARCL did not establish a target LDL.
Beyond the primary effect of statins on lowering levels of LDL, there is evidence that they may also improve endothelial function and attenuate inflammation. In one large trial in which healthy individuals with normal LDL levels and mildly elevated hsCRP levels received high-dose rosuvastatin or a placebo, after a 2 year follow up period, the rate of stroke was 48% lower in the treatment group than in the placebo group (absolute risk reduction of 0.16%)261. The additional reduction in risk of other cardiovascular outcomes could provide a rationale for the use of rosuvastatin for primary prevention in patients with elevated hsCRP levels but normal lipid levels. Furthermore, the absolute reduction in risk was greatest among those with the highest levels of hsCRP, reflecting the fact that these patients were at higher risk of cardiovascular events overall.
Antiplatelet therapies
Unlike in secondary stroke prevention, where a large body of evidence supports the role for anti-platelet therapy in preventing recurrent stroke, there is little evidence to recommend the use of anti-platelet therapy for sole purpose of primary stroke prevention. A meta-analysis of vascular events in six primary prevention trials that included 95,000 individuals contributing 660,000 person-years, done by the Antithrombotic Trialists’ Collaboration in 2009, showed no overall effect of aspirin on stroke incidence (0.20% vs 0.21% per year, p=0.4).262 The 2015 guidelines from the US Preventive Services Task Force on aspirin use for primary CVD prevention can be applied to primary prevention of stroke. They recommend aspirin use for primary CVD prevention only in high risk individuals aged 50-59. 263 There is no convincing evidence for the use of dual antiplatelet therapies, such as aspirin and clopidogrel combined, for primary stroke prevention.264 In the Clopidogrel and Aspirin versus Aspirin Alone for the Prevention of Atherothrombotic Events (CHARISMA) trial, for example, 15,603 patients with cardiovascular disease or multiple risk factors were randomized to aspirin 75-162 mg daily alone or in combination with clopidogrel 75 mg daily, and followed for 28 months. The primary end-point was a composite myocardial infarction, stroke, or vascular death. There was no statistical difference in outcome between those in the dual antiplatelet group (6.8%) versus those taking aspirin alone (7.3%; p=0.22). The risk of bleeding was higher on dual antiplatelets, however.
Atrial fibrillation and heart failure
Warfarin, a vitamin K antagonist (VKA), has been for many years the standard therapy for both primary and secondary stroke prevention in patients with non-valvular AF. In primary prevention, the AFASAK trial was the first to compare warfarin to aspirin or placebo in patients with chronic AF, and showed a significant reduction in stroke risk with warfarin.265 The Stroke Prevention in Atrial Fibrillation Study (SPAF), a double-blind placebo-controlled trial comparing warfarin, aspirin and placebo in patients without a history of stroke, also showed significant benefit with warfarin and a relative risk reduction of 76% (absolute risk reduction of 5.1%) when compared to placebo.266 There was also a reduced risk of stroke incidence when warfarin was compared to aspirin, albeit less than when compared to placebo.
Since those trials, there have been advances in thromboembolism prevention with the addition of a number novel anticoagulants from two separate classes. Dabigatran (a direct thrombin inhibitor) and edoxaban, rivaroxaban and apixaban (all factor Xa inhibitors) were shown to be non-inferior in efficacy to warfarin in reducing strokes in patients with AF.267-270 The rates of major hemorrhage have also been compared between the new agents and warfarin in a meta-analysis that included 102,607 patients. The novel agents as a group were associated with a lower risk of major hemorrhage (RR 0.72, p< 0.01), including ICH (RR 0.53, p < 0.01), without any difference in risk of gastrointestinal bleeding. The incidence of ICH was 0.51% in the novel agents group and 1.08% in the warfarin group.271
To assist with clinical decision making, validated AF scoring systems such as CHADS2 or the newer CHA2DS2-VASc score, can be used to approximate ischemic stroke event rates from underlying AF.272, 273 Depending on the scoring system used, a number of co-morbid conditions act as additional predictors of stroke risk. (Tables 4 and 5) For both scoring systems, anticoagulation is generally recommended for those with scores of 2 or higher.
Table 4.
Commonly used risk stratification schemes for Atrial Fbrillation
| CHADS2 items | Points | CHA2DS2-VASc items | Points |
|---|---|---|---|
| C=Congestive heart failure | 1 | C=Congestive heart failure | 1 |
| H=Hypertension | 1 | H=Hypertension | 1 |
| A=Age ≥75 years | 1 | A2=Age ≥75 years (double value) | 2 |
| D=Diabetes mellitus | 1 | D=Diabetes mellitus | 1 |
| S2=history of stroke, TIA, or thromboembolism (double value) |
2 | S2=history of stroke, TIA, or thromboembolism (double value) |
2 |
| V=Vascular Disease (prior MI, peripheral arterial disease, aortic plaque) |
1 | ||
| A=Age 65-74 years | 1 | ||
| Sc=sex category (female sex) | 1 | ||
| Range | 0-6 | 0-9 | |
| Maximum | 6 | 9 |
Table 5.
Commonly used risk prediction schemes, annual stroke risk, and antithrombotic recommendations based on guidelines for patients with atrial fibrillation
| CHADS2 score | Stroke risk (%/year) |
CHA2DS2-VASc score |
Stroke risk (%/year) |
Recommended antithrombotic treatment based on CHA2DS2-VASc (Class of recommendation, level of evidence) |
|---|---|---|---|---|
| 0 | 1.9 | 0 | 0 | None (IIb, B) |
| 1 | 2.8 | 1 | 1.3 | None, aspirin, or anticoagulant (IIb, C) |
| 2 | 4.0 | 2 | 2.2 | Anticoagulant (I, A [warfarin], B [dabigatran, rivaroxaban, apixaban] |
| 3 | 5.9 | 3 | 3.2 | |
| 4 | 8.5 | 4 | 4.0 | |
| 5 | 12.5 | 5 | 6.7 | |
| 6 | 18.2 | 6 | 9.8 | |
| 7 | 9.6 | |||
| 8 | 6.7 | |||
| 9 | 15.2 |
A new alternative to anticoagulation for patients with AF is left atrial appendage closure. Because an estimated 90% of thromboembolic material originates in the atrial appendage in patients with non-rheumatic AF,274 closing the atrial appendage was theorized as a way to prevent AF-related thromboembolism. The two ways to achieve atrial appendage closure and make it functionally obsolete are through surgical ligation and through a trans-aortic catheter approach using the Watchman or Amplatzer trans-aortic devices.275, 276 Patients are generally expected to be on therapeutic doses of warfarin for at least 45 days after a closure procedure, followed by 6 months of dual anti-platelet therapy and indefinite aspirin use. There is a small subset of patients in whom there has been inadequate seeding of the device, necessitating a longer course of anticoagulation. Two randomized controlled trials compared the Watchman device to warfarin in patients with non-valvular AF: the PROTECT-AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) and the more recent PREVAIL (Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy) trials. In both trials, the Watchman device was non-inferior in efficacy for the prevention of ischemic stroke.277, 278 Pericardial effusion, peri-procedural stroke, and device embolization accounted for the major complications. Based on these two studies, the Watchman device was approved by the FDA for use in patients who: a) have non-valvular AF, b) carry an increased risk of stroke, c) are indicated for and may take warfarin, and d) have a reason to seek a non-drug alternative to anticoagulation.279
The Warfarin and Aspirin in Patients with Heart Failure and Sinus Rhythm (WARCEF) study evaluated the role of anti-coagulation with warfarin, versus aspirin, for the prevention of ischemic stroke, intracerebral hemorrhage or death in patients with congestive heart failure and an ejection fraction (EF) <35%.280 It included 2,305 participants with a mean EF of 27%, followed for up to 6 years. There was no difference in the composite outcome (26.4% vs. 27.5%, HR 0.93, 95% CI 0.79-1.10, p=0.4), but there was a reduction in ischemic stroke (2.5% vs. 4.7%, HR 0.52, 95% CI 0.33-0.82, p=0.005) with no increase in ICH. Further accounting for time on treatment, there was a marginal benefit of warfarin over aspirin by the 4th year of follow up but there was also higher rate of major systemic hemorrhage, mostly gastrointestinal hemorrhage, with warfarin. The study concluded that a reduced risk of ischemic stroke with anti-coagulation was offset by an increased risk of major hemorrhage. A secondary analysis showed that increasing the time that patients are in the therapeutic range with warfarin was significantly associated with better outcomes and improved net clinical benefit.281 Based on a meta-analysis that included 3,663 patients, there is no clear evidence at this time to recommend the use of anti-coagulation in this subgroup of patients.282 With the availability of newer oral anticoagulants with a more favorable risk profile, however, it is possible that future trials could demonstrate a benefit.
Extracranial and intracranial atherosclerotic disease
Another major source of preventable cerebral infarction is large vessel atherosclerotic disease, and specifically internal carotid artery stenosis. In asymptomatic (i.e., those without a history of recent stroke or TIA on the side of the stenosis) men with greater than 60% carotid stenosis and low perioperative risk, carotid endarterectomy (CEA) was shown beneficial; approximately 20 procedures must be performed to prevent one stroke over a five year period. There is no clear benefit in women or those with a peri-operative risk of >3%.283, 284 There is no benefit of CEA in asymptomatic patients with less than 50% stenosis.285,286
For patients with symptomatic extracranial carotid stenosis of at least 70% (i.e., those with stroke or TIA and a stenosis on the symptomatic side), there is evidence that CEA reduces the risk of stroke by 50% in relative terms, with an absolute risk reduction of about 13% over two years. 287 The studies that demonstrated a benefit of surgical revascularization were conducted 10-25 years ago, however. Since the advent of more aggressive medical approaches to atherosclerotic disease, particularly statins, there have been temporal changes in the rates of stroke among medically-treated patients with atherosclerotic carotid artery disease. Stroke rates among medically-treated patients were significantly lower in studies that completed recruitment after 2000 (1.13% per year) than in those that completed recruitment earlier (2.38% per year; P < 0.001).288 More recent studies (e.g., the Carotid Revascularization Endarterectomy Versus Stenting Trial 2) are ongoing.289
For patients with asymptomatic intracranial stenosis, there is no evidence of benefit to stenting or angioplasty. Even for those with symptomatic intracranial stenosis, recent trials do not provide any evidence of a benefit to stenting intracranial arteries of 50% stenosis or greater. The Comparison of Warfarin and Aspirin for Symptomatic Intracranial Stenosis (WASID) trial studied aspirin versus warfarin in patients with symptomatic intracranial stenosis of 50-99%. It was stopped early because of a high rate of adverse events in the warfarin group, leading to a conclusion that warfarin does not provide a benefit over aspirin, and may even be harmful in this subset of patients. The subsequent Stenting Versus Aggressive Medical Management of Intracranial Atherosclerotic Stenosis (SAMMPRIS) trial enrolled patients with symptomatic high-grade intracranial stenosis (>70%), comparing percutaneous stenting to best medical management with aggressive risk factor control and dual antiplatelet therapy for three months.290 During a median follow up of 32 months the primary end-point of recurrent stroke or death was seen in 23% of the stenting group and 15% in the medical management group. 291 Interestingly, the medical group had a lower-than-expected recurrence of stroke, compared to that which was predicted from previous studies. The results suggested that aggressive medical therapy is superior to intervention for high grade intracranial stenosis with currently available technology, particularly in the context of current medical approaches, as was seen with extracranial disease, as noted above, as well.
Conclusion
Recent years have witnessed tremendous strides in in our understanding of risk factors and prevention of stroke. Research into stroke risk factors has increasingly addressed the heterogeneous subtypes of strokes, including not only hemorrhagic versus ischemic stroke, but also the several etiologic subtypes of stroke. Thus studies have confirmed that lipids are a risk factor for atherosclerotic stroke, while atrial fibrillation and related atrial cardiopathies are associated with cardioembolic strokes. Genetic analyses have benefited in particular from the emphasis on specific categories of stroke. Mutations in particular genes have now been associated with, and replicated in, large vessel, cardioembolic, and small vessel stroke subtypes. Genetic studies have also suggested new avenues to pursue in teasing out stroke pathogenesis. These studies suggest that in the future precision medicine will enable clinicians to better focus treatments in cerebrovascular disease. Meanwhile, several large clinical trials have provided evidence of the benefits of several medical and behavioral treatments in reducing stroke risk. Blood pressure reduction, statin therapy, antiplatelets, anticoagulants, carotid revascularization, and dietary changes have all been proven to reduce stroke risk in various patient populations. Just as importantly, some treatments, such as stenting of stenosed intracranial vessels, have not yet been proven of benefit. Observational studies provide strong evidence for other behavioral approaches, including smoking cessation and regular physical activity. Considerations of alternative approaches to prevention, in general, such as the mass approach using therapies like the polypill, utilizing risk estimation in primary care, and focusing on high-risk intervals of time after stroke triggers, also have the potential to dramatically change the future of stroke prevention.
Supplementary Material
Acknowledgments
Sources of funding: Dr. Esenwa was supported during the writing of the manuscript by NINDS NIH/NINDS T32-NS07153 (PI: Elkind). The other authors report no specific funding for this manuscript.
Footnotes
Disclosures: Dr. Elkind receives compensation for providing consultative services for Biotelemetry/Cardionet, BMS-Pfizer Partnership, Boehringer-Ingelheim, and Sanofi-Regeneron Partnership; receives compensation for serving as an expert witness in litigation for BMS-Sanofi (Plavix), Merck/Organon (Nuvaring), and Hi-Tech Pharmaceuticals (dimethylamylamine); serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association; and receives royalties from UpToDate for chapters related to stroke. Drs. Esenwa and Boehme report no disclosures.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics—2011 update. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Stroke Association Stroke 101 fact sheet. 2011 [Google Scholar]
- 3.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the future of stroke in the united states: A policy statement from the american heart association and american stroke association. Stroke; a journal of cerebral circulation. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, et al. American heart association guide for improving cardiovascular health at the community level, 2013 update: A scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation. 2013;127:1730–1753. doi: 10.1161/CIR.0b013e31828f8a94. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 6.Vermeer SE, Longstreth WT, Jr., Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 7.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 8.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: Estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8:345–354. doi: 10.1016/S1474-4422(09)70023-7. [DOI] [PubMed] [Google Scholar]
- 9.Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke; a journal of cerebral circulation. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Jr., Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 11.Zhao D, Liu J, Wang W, Zeng Z, Cheng J, Liu J, et al. Epidemiological transition of stroke in china: Twenty-one-year observational study from the sino-monica-beijing project. Stroke; a journal of cerebral circulation. 2008;39:1668–1674. doi: 10.1161/STROKEAHA.107.502807. [DOI] [PubMed] [Google Scholar]
- 12.Powers BJ, Danus S, Grubber JM, Olsen MK, Oddone EZ, Bosworth HB. The effectiveness of personalized coronary heart disease and stroke risk communication. Am Heart J. 2011;161:673–680. doi: 10.1016/j.ahj.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Pasternak R, Greenland P, Smith S, Jr., Fuster V. Aha/acc scientific statement: Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: A statement for healthcare professionals from the american heart association and the american college of cardiology. J Am Coll Cardiol. 1999;34:1348–1359. doi: 10.1016/s0735-1097(99)00387-3. [DOI] [PubMed] [Google Scholar]
- 14.Pocock SJ, McCormack V, Gueyffier F, Boutitie F, Fagard RH, Boissel JP. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ. 2001;323:75–81. doi: 10.1136/bmj.323.7304.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 16.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 17.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication. The framingham study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D'Agostino RB, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: The framingham heart study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 19.Lumley T, Kronmal RA, Cushman M, Manolio TA, Goldstein S. A stroke prediction score in the elderly: Validation and web-based application. J Clin Epidemiol. 2002;55:129–136. doi: 10.1016/s0895-4356(01)00434-6. [DOI] [PubMed] [Google Scholar]
- 20.Simons LA, McCallum J, Friedlander Y, Simons J. Risk factors for ischemic stroke: Dubbo study of the elderly. Stroke. 1998;29:1341–1346. doi: 10.1161/01.str.29.7.1341. [DOI] [PubMed] [Google Scholar]
- 21.Chien KL, Su TC, Hsu HC, Chang WT, Chen PC, Sung FC, et al. Constructing the prediction model for the risk of stroke in a chinese population: Report from a cohort study in taiwan. Stroke. 2010;41:1858–1864. doi: 10.1161/STROKEAHA.110.586222. [DOI] [PubMed] [Google Scholar]
- 22.Lackland DT, Elkind MS, D'Agostino R, Sr., Dhamoon MS, Goff DC, Jr., Higashida RT, et al. Inclusion of stroke in cardiovascular risk prediction instruments: A statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2012;43:1998–2027. doi: 10.1161/STR.0b013e31825bcdac. [DOI] [PubMed] [Google Scholar]
- 23.Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–147. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): A case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 25.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkind MS. Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51–57. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]
- 27.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 28.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Age at stroke: Temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995-2008. Ann Neurol. 2011;70:713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 30.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 31.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, et al. Sex differences in stroke care and outcomes: Results from the registry of the canadian stroke network. Stroke; a journal of cerebral circulation. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 32.Reeves MJ, Fonarow GC, Zhao X, Smith EE, Schwamm LH, Get With The Guidelines-Stroke Steering C et al. Quality of care in women with ischemic stroke in the gwtg program. Stroke; a journal of cerebral circulation. 2009;40:1127–1133. doi: 10.1161/STROKEAHA.108.543157. [DOI] [PubMed] [Google Scholar]
- 33.Asplund K, Karvanen J, Giampaoli S, Jousilahti P, Niemela M, Broda G, et al. Relative risks for stroke by age, sex, and population based on follow-up of 18 european populations in the morgam project. Stroke. 2009;40:2319–2326. doi: 10.1161/STROKEAHA.109.547869. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, et al. Racial-ethnic disparities in stroke care: The american experience: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 35.Gillum RF. Stroke mortality in blacks. Disturbing trends. Stroke; a journal of cerebral circulation. 1999;30:1711–1715. doi: 10.1161/01.str.30.8.1711. [DOI] [PubMed] [Google Scholar]
- 36.Howard G, Howard VJ, Geographic REf. Racial Differences in Stroke I. Ethnic disparities in stroke: The scope of the problem. Ethnicity & disease. 2001;11:761–768. [PubMed] [Google Scholar]
- 37.Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, et al. Racial-ethnic disparities in stroke care: The american experience: A statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 38.Kleindorfer D, Broderick J, Khoury J, Flaherty M, Woo D, Alwell K, et al. The unchanging incidence and case-fatality of stroke in the 1990s: A population-based study. Stroke; a journal of cerebral circulation. 2006;37:2473–2478. doi: 10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- 39.Cooper ES. Cardiovascular diseases and stroke in african americans: A call for action. Journal of the National Medical Association. 1993;85:97–100. [PMC free article] [PubMed] [Google Scholar]
- 40.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: The excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 41.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, et al. Stroke incidence among white, black, and hispanic residents of an urban community: The northern manhattan stroke study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 42.Howard G, Anderson R, Sorlie P, Andrews V, Backlund E, Burke GL. Ethnic differences in stroke mortality between non-hispanic whites, hispanic whites, and blacks. The national longitudinal mortality study. Stroke; a journal of cerebral circulation. 1994;25:2120–2125. doi: 10.1161/01.str.25.11.2120. [DOI] [PubMed] [Google Scholar]
- 43.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, et al. Excess stroke in mexican americans compared with non-hispanic whites: The brain attack surveillance in corpus christi project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Eden SV, et al. Differences in intracerebral hemorrhage between mexican americans and non-hispanic whites. Neurology. 2006;66:30–34. doi: 10.1212/01.wnl.0000191402.41914.d2. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, et al. Incidence and risk factors for stroke in american indians: The strong heart study. Circulation. 2008;118:1577–1584. doi: 10.1161/CIRCULATIONAHA.108.772285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black-white differences in the risk of cerebral infarction. The national health and nutrition examination survey epidemiologic follow-up study. Arch Intern Med. 1995;155:1319–1324. [PubMed] [Google Scholar]
- 47.Gillum RF. Risk factors for stroke in blacks: A critical review. Am J Epidemiol. 1999;150:1266–1274. doi: 10.1093/oxfordjournals.aje.a009957. [DOI] [PubMed] [Google Scholar]
- 48.Kittner SJ, White LR, Losonczy KG, Wolf PA, Hebel JR. Black-white differences in stroke incidence in a national sample. The contribution of hypertension and diabetes mellitus. JAMA. 1990;264:1267–1270. [PubMed] [Google Scholar]
- 49.Liao Y, Greenlund KJ, Croft JB, Keenan NL, Giles WH. Factors explaining excess stroke prevalence in the us stroke belt. Stroke. 2009;40:3336–3341. doi: 10.1161/STROKEAHA.109.561688. [DOI] [PubMed] [Google Scholar]
- 50.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: The atherosclerosis risk in communities study, the cardiovascular health study, and the multi-ethnic study of atherosclerosis. Circulation. 2012;126:50–59. doi: 10.1161/CIRCULATIONAHA.111.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glasser SP, Judd S, Basile J, Lackland D, Halanych J, Cushman M, et al. Prehypertension, racial prevalence and its association with risk factors: Analysis of the reasons for geographic and racial differences in stroke (regards) study. American journal of hypertension. 2011;24:194–199. doi: 10.1038/ajh.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howard G, Safford MM, Moy CS, Howard VJ, Kleindorfer DO, Unverzagt FW, et al. Racial differences in the incidence of cardiovascular risk factors in older black and white adults. J Am Geriatr Soc. 2016 doi: 10.1111/jgs.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Annals of neurology. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joubert J, Prentice LF, Moulin T, Liaw ST, Joubert LB, Preux PM, et al. Stroke in rural areas and small communities. Stroke; a journal of cerebral circulation. 2008;39:1920–1928. doi: 10.1161/STROKEAHA.107.501643. [DOI] [PubMed] [Google Scholar]
- 55.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: Epidemiology, acute care, and postacute outcomes. Stroke; a journal of cerebral circulation. 2005;36:374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 56.Kimball MM, Neal D, Waters MF, Hoh BL. Race and income disparity in ischemic stroke care: Nationwide inpatient sample database, 2002 to 2008. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;23:17–24. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Jablecki J, Kaczmarzyk L, Domanasiewicz A, Paruzel M. [management of soft tissue defects in the region of finger pip joints by means of pedicular skin flaps taken from abdomen] Chirurgia narzadow ruchu i ortopedia polska. 2006;71:205–209. [PubMed] [Google Scholar]
- 58.Greer S, Casper M, Kramer M, Schwartz G, Hallisey E, Holt J, et al. Racial residential segregation and stroke mortality in atlanta. Ethn Dis. 2011;21:437–443. [PubMed] [Google Scholar]
- 59.Kleindorfer D, Lindsell C, Alwell KA, Moomaw CJ, Woo D, Flaherty ML, et al. Patients living in impoverished areas have more severe ischemic strokes. Stroke. 2012;43:2055–2059. doi: 10.1161/STROKEAHA.111.649608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moon JR, Capistrant BD, Kawachi I, Avendano M, Subramanian SV, Bates LM, et al. Stroke incidence in older us hispanics: Is foreign birth protective? Stroke. 2012;43:1224–1229. doi: 10.1161/STROKEAHA.111.643700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howard G, Moy CS, Howard VJ, McClure LA, Kleindorfer DO, Kissela BM, et al. Where to focus efforts to reduce the black-white disparity in stroke mortality: Incidence versus case fatality? Stroke; a journal of cerebral circulation. 2016;47:1893–1898. doi: 10.1161/STROKEAHA.115.012631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howard G, Kissela BM, Kleindorfer DO, McClure LA, Soliman EZ, Judd SE, et al. Differences in the role of black race and stroke risk factors for first vs. Recurrent stroke. Neurology. 2016;86:637–642. doi: 10.1212/WNL.0000000000002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seshadri S, Beiser A, Pikula A, Himali JJ, Kelly-Hayes M, Debette S, et al. Parental occurrence of stroke and risk of stroke in their children: The framingham study. Circulation. 2010;121:1304–1312. doi: 10.1161/CIRCULATIONAHA.109.854240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulz UG, Flossmann E, Rothwell PM. Heritability of ischemic stroke in relation to age, vascular risk factors, and subtypes of incident stroke in population-based studies. Stroke. 2004;35:819–824. doi: 10.1161/01.STR.0000121646.23955.0f. [DOI] [PubMed] [Google Scholar]
- 65.Touze E, Rothwell PM. Sex differences in heritability of ischemic stroke: A systematic review and meta-analysis. Stroke. 2008;39:16–23. doi: 10.1161/STROKEAHA.107.484618. [DOI] [PubMed] [Google Scholar]
- 66.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 67.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 68.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the us adult population. Results from the third national health and nutrition examination survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 69.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D'Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The framingham heart study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 70.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the united states 1999 to 2000: A rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 71.Egan BM, Zhao Y, Axon RN. Us trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 72.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 73.Suchy-Dicey AM, Wallace ER, Mitchell SV, Aguilar M, Gottesman RF, Rice K, et al. Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens. 2013;26:1210–1217. doi: 10.1093/ajh/hpt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Banerjee C, Moon YP, Paik MC, Rundek T, Mora-McLaughlin C, Vieira JR, et al. Duration of diabetes and risk of ischemic stroke: The northern manhattan study. Stroke; a journal of cerebral circulation. 2012;43:1212–1217. doi: 10.1161/STROKEAHA.111.641381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sui X, Lavie CJ, Hooker SP, Lee DC, Colabianchi N, Lee CD, et al. A prospective study of fasting plasma glucose and risk of stroke in asymptomatic men. Mayo Clin Proc. 2011;86:1042–1049. doi: 10.4065/mcp.2011.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Utsumi H, Elkind MM. Potentially lethal damage, deficient repair in x-ray-sensitive caffeine-responsive chinese hamster cells. Radiation research. 1986;107:95–106. [PubMed] [Google Scholar]
- 77.Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, et al. Epidemiology of ischemic stroke in patients with diabetes: The greater cincinnati/northern kentucky stroke study. Diabetes care. 2005;28:355–359. doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- 78.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 79.Anselmino M, Malmberg K, Ohrvik J, Ryden L. Evidence-based medication and revascularization: Powerful tools in the management of patients with diabetes and coronary artery disease: A report from the euro heart survey on diabetes and the heart. Eur J Cardiovasc Prev Rehabil. 2008;15:216–223. doi: 10.1097/HJR.0b013e3282f335d0. [DOI] [PubMed] [Google Scholar]
- 80.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 81.Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: The uk glucose insulin in stroke trial (gist-uk) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 82.Yiin GS, Howard DP, Paul NL, Li L, Luengo-Fernandez R, Bull LM, et al. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation-related embolic vascular events: A population-based study. Circulation. 2014;130:1236–1244. doi: 10.1161/CIRCULATIONAHA.114.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 84.Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Disertori M, Quintarelli S, Grasso M, Pilotto A, Narula N, Favalli V, et al. Autosomal recessive atrial dilated cardiomyopathy with standstill evolution associated with mutation of natriuretic peptide precursor a. Circulation. Cardiovascular genetics. 2013;6:27–36. doi: 10.1161/CIRCGENETICS.112.963520. [DOI] [PubMed] [Google Scholar]
- 86.O'Neal WT, Kamel H, Kleindorfer D, Judd SE, Howard G, Howard VJ, et al. Premature atrial contractions on the screening electrocardiogram and risk of ischemic stroke: The reasons for geographic and racial differences in stroke study. Neuroepidemiology. 2016;47:53–58. doi: 10.1159/000448619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamel H, Hunter M, Moon YP, Yaghi S, Cheung K, Di Tullio MR, et al. Electrocardiographic left atrial abnormality and risk of stroke: Northern manhattan study. Stroke; a journal of cerebral circulation. 2015;46:3208–3212. doi: 10.1161/STROKEAHA.115.009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamel H, O'Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the atherosclerosis risk in communities study. Ann Neurol. 2015;78:670–678. doi: 10.1002/ana.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okin PM, Kamel H, Kjeldsen SE, Devereux RB. Electrocardiographic left atrial abnormalities and risk of incident stroke in hypertensive patients with electrocardiographic left ventricular hypertrophy. J Hypertens. 2016;34:1831–1837. doi: 10.1097/HJH.0000000000000989. [DOI] [PubMed] [Google Scholar]
- 90.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: Time for a new model. Stroke; a journal of cerebral circulation. 2016;47:895–900. doi: 10.1161/STROKEAHA.115.012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Longstreth WT, Jr., Kronmal RA, Thompson JL, Christenson RH, Levine SR, Gross R, et al. Amino terminal pro-b-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44:714–719. doi: 10.1161/STROKEAHA.112.675942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Horenstein RB, Smith DE, Mosca L. Cholesterol predicts stroke mortality in the women's pooling project. Stroke. 2002;33:1863–1868. doi: 10.1161/01.str.0000020093.67593.0b. [DOI] [PubMed] [Google Scholar]
- 93.Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68:556–562. doi: 10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindenstrom E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The copenhagen city heart study. BMJ. 1994;309:11–15. doi: 10.1136/bmj.309.6946.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soyama Y, Miura K, Morikawa Y, Nishijo M, Nakanishi Y, Naruse Y, et al. High-density lipoprotein cholesterol and risk of stroke in japanese men and women: The oyabe study. Stroke. 2003;34:863–868. doi: 10.1161/01.STR.0000060869.34009.38. [DOI] [PubMed] [Google Scholar]
- 96.Tanne D, Yaari S, Goldbourt U. High-density lipoprotein cholesterol and risk of ischemic stroke mortality. A 21-year follow-up of 8586 men from the israeli ischemic heart disease study. Stroke. 1997;28:83–87. doi: 10.1161/01.str.28.1.83. [DOI] [PubMed] [Google Scholar]
- 97.Wannamethee SG, Shaper AG, Ebrahim S. Hdl-cholesterol, total cholesterol, and the risk of stroke in middle-aged british men. Stroke. 2000;31:1882–1888. doi: 10.1161/01.str.31.8.1882. [DOI] [PubMed] [Google Scholar]
- 98.Sacco RL, Benson RT, Kargman DE, Boden-Albala B, Tuck C, Lin IF, et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly: The northern manhattan stroke study. JAMA. 2001;285:2729–2735. doi: 10.1001/jama.285.21.2729. [DOI] [PubMed] [Google Scholar]
- 99.Iso H, Jacobs DR, Jr., Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med. 1989;320:904–910. doi: 10.1056/NEJM198904063201405. [DOI] [PubMed] [Google Scholar]
- 100.Zhang X, Patel A, Horibe H, Wu Z, Barzi F, Rodgers A, et al. Cholesterol, coronary heart disease, and stroke in the asia pacific region. Int J Epidemiol. 2003;32:563–572. doi: 10.1093/ije/dyg106. [DOI] [PubMed] [Google Scholar]
- 101.Iribarren C, Jacobs DR, Sadler M, Claxton AJ, Sidney S. Low total serum cholesterol and intracerebral hemorrhagic stroke: Is the association confined to elderly men? The kaiser permanente medical care program. Stroke. 1996;27:1993–1998. doi: 10.1161/01.str.27.11.1993. [DOI] [PubMed] [Google Scholar]
- 102.Hackam DG, Austin PC, Huang A, Juurlink DN, Mamdani MM, Paterson JM, et al. Statins and intracerebral hemorrhage: A retrospective cohort study. Arch Neurol. 2012;69:39–45. doi: 10.1001/archneurol.2011.228. [DOI] [PubMed] [Google Scholar]
- 103.Amarenco P, Labreuche J, Lavallee P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: Systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902–2909. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 104.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 105.Lauer A, Greenberg SM, Gurol ME. Statins in intracerebral hemorrhage. Curr Atheroscler Rep. 2015;17:46. doi: 10.1007/s11883-015-0526-5. [DOI] [PubMed] [Google Scholar]
- 106.Goldstein LB, Amarenco P, Szarek M, Callahan A, 3rd, Hennerici M, Sillesen H, et al. Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology. 2008;70:2364–2370. doi: 10.1212/01.wnl.0000296277.63350.77. [DOI] [PubMed] [Google Scholar]
- 107.Zhou ML, Zhu L, Wang J, Hang CH, Shi JX. The inflammation in the gut after experimental subarachnoid hemorrhage. The Journal of surgical research. 2007;137:103–108. doi: 10.1016/j.jss.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 108.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Krolewski AS, Rosner B, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991;151:1141–1147. [PubMed] [Google Scholar]
- 109.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: A scientific statement from the american heart association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 110.Appel LJ, Angell SY, Cobb LK, Limper HM, Nelson DE, Samet JM, et al. Population-wide sodium reduction: The bumpy road from evidence to policy. Ann Epidemiol. 2012;22:417–425. doi: 10.1016/j.annepidem.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perry IJ, Beevers DG. Salt intake and stroke: A possible direct effect. J Hum Hypertens. 1992;6:23–25. [PubMed] [Google Scholar]
- 112.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027–2034. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 113.Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in japanese men and women. Stroke. 2004;35:1543–1547. doi: 10.1161/01.STR.0000130425.50441.b0. [DOI] [PubMed] [Google Scholar]
- 114.Li XY, Cai XL, Bian PD, Hu LR. High salt intake and stroke: Meta-analysis of the epidemiologic evidence. CNS Neurosci Ther. 2012;18:691–701. doi: 10.1111/j.1755-5949.2012.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ascherio A, Rimm EB, Hernan MA, Giovannucci EL, Kawachi I, Stampfer MJ, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among us men. Circulation. 1998;98:1198–1204. doi: 10.1161/01.cir.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 117.Khaw KT, Barrett-Connor E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N Engl J Med. 1987;316:235–240. doi: 10.1056/NEJM198701293160502. [DOI] [PubMed] [Google Scholar]
- 118.D'Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57:1210–1219. doi: 10.1016/j.jacc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 119.Larsson SC, Orsini N, Wolk A. Dietary potassium intake and risk of stroke: A dose-response meta-analysis of prospective studies. Stroke. 2011;42:2746–2750. doi: 10.1161/STROKEAHA.111.622142. [DOI] [PubMed] [Google Scholar]
- 120.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 121.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet. 2006;367:320–326. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 122.Suk SH, Sacco RL, Boden-Albala B, Cheun JF, Pittman JG, Elkind MS, et al. Abdominal obesity and risk of ischemic stroke: The northern manhattan stroke study. Stroke; a journal of cerebral circulation. 2003;34:1586–1592. doi: 10.1161/01.STR.0000075294.98582.2F. [DOI] [PubMed] [Google Scholar]
- 123.Walker SP, Rimm EB, Ascherio A, Kawachi I, Stampfer MJ, Willett WC. Body size and fat distribution as predictors of stroke among us men. Am J Epidemiol. 1996;144:1143–1150. doi: 10.1093/oxfordjournals.aje.a008892. [DOI] [PubMed] [Google Scholar]
- 124.Global Burden of Metabolic Risk Factors for Chronic Diseases C. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arenillas JF, Moro MA, Davalos A. The metabolic syndrome and stroke: Potential treatment approaches. Stroke; a journal of cerebral circulation. 2007;38:2196–2203. doi: 10.1161/STROKEAHA.106.480004. [DOI] [PubMed] [Google Scholar]
- 126.Koren-Morag N, Goldbourt U, Tanne D. Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: A prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke; a journal of cerebral circulation. 2005;36:1366–1371. doi: 10.1161/01.STR.0000169945.75911.33. [DOI] [PubMed] [Google Scholar]
- 127.Najarian RM, Sullivan LM, Kannel WB, Wilson PW, D'Agostino RB, Wolf PA. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke: The framingham offspring study. Arch Intern Med. 2006;166:106–111. doi: 10.1001/archinte.166.1.106. [DOI] [PubMed] [Google Scholar]
- 128.Kuo SH, Lee YT, Li CR, Tseng CJ, Chao WN, Wang PH, et al. Mortality in emergency department sepsis score as a prognostic indicator in patients with pyogenic liver abscess. The American journal of emergency medicine. 2013;31:916–921. doi: 10.1016/j.ajem.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 129.Gill JS, Zezulka AV, Shipley MJ, Gill SK, Beevers DG. Stroke and alcohol consumption. N Engl J Med. 1986;315:1041–1046. doi: 10.1056/NEJM198610233151701. [DOI] [PubMed] [Google Scholar]
- 130.Hillbom M, Numminen H, Juvela S. Recent heavy drinking of alcohol and embolic stroke. Stroke. 1999;30:2307–2312. doi: 10.1161/01.str.30.11.2307. [DOI] [PubMed] [Google Scholar]
- 131.Klatsky AL, Armstrong MA, Friedman GD, Sidney S. Alcohol drinking and risk of hospitalization for ischemic stroke. Am J Cardiol. 2001;88:703–706. doi: 10.1016/s0002-9149(01)01824-0. [DOI] [PubMed] [Google Scholar]
- 132.Mazzaglia G, Britton AR, Altmann DR, Chenet L. Exploring the relationship between alcohol consumption and non-fatal or fatal stroke: A systematic review. Addiction. 2001;96:1743–1756. doi: 10.1046/j.1360-0443.2001.961217434.x. [DOI] [PubMed] [Google Scholar]
- 133.Wannamethee SG, Shaper AG. Patterns of alcohol intake and risk of stroke in middle-aged british men. Stroke. 1996;27:1033–1039. doi: 10.1161/01.str.27.6.1033. [DOI] [PubMed] [Google Scholar]
- 134.Rantakomi SH, Laukkanen JA, Sivenius J, Kauhanen J, Kurl S. Alcohol consumption and the risk of stroke among hypertensive and overweight men. J Neurol. 2013;260:534–539. doi: 10.1007/s00415-012-6672-6. [DOI] [PubMed] [Google Scholar]
- 135.Hillbom M, Saloheimo P, Juvela S. Alcohol consumption, blood pressure, and the risk of stroke. Curr Hypertens Rep. 2011;13:208–213. doi: 10.1007/s11906-011-0194-y. [DOI] [PubMed] [Google Scholar]
- 136.Judd SE, McClure LA, Howard VJ, Lackland DT, Halanych JH, Kabagambe EK. Heavy drinking is associated with poor blood pressure control in the reasons for geographic and racial differences in stroke (regards) study. International journal of environmental research and public health. 2011;8:1601–1612. doi: 10.3390/ijerph8051601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wakabayashi I, Araki Y. Influences of gender and age on relationships between alcohol drinking and atherosclerotic risk factors. Alcohol Clin Exp Res. 2010;34(Suppl 1):S54–60. doi: 10.1111/j.1530-0277.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- 138.Foerster M, Marques-Vidal P, Gmel G, Daeppen JB, Cornuz J, Hayoz D, et al. Alcohol drinking and cardiovascular risk in a population with high mean alcohol consumption. Am J Cardiol. 2009;103:361–368. doi: 10.1016/j.amjcard.2008.09.089. [DOI] [PubMed] [Google Scholar]
- 139.Ohira T, Tanigawa T, Tabata M, Imano H, Kitamura A, Kiyama M, et al. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among japanese men. Hypertension. 2009;53:13–19. doi: 10.1161/HYPERTENSIONAHA.108.114835. [DOI] [PubMed] [Google Scholar]
- 140.Taylor B, Irving HM, Baliunas D, Roerecke M, Patra J, Mohapatra S, et al. Alcohol and hypertension: Gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction. 2009;104:1981–1990. doi: 10.1111/j.1360-0443.2009.02694.x. [DOI] [PubMed] [Google Scholar]
- 141.Brust JC. Neurological aspects of substance abuse. Butterworth-Heinemann; Philadelphia, Pa: 2004. [Google Scholar]
- 142.Esse K, Fossati-Bellani M, Traylor A, Martin-Schild S. Epidemic of illicit drug use, mechanisms of action/addiction and stroke as a health hazard. Brain Behav. 2011;1:44–54. doi: 10.1002/brb3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ho EL, Josephson SA, Lee HS, Smith WS. Cerebrovascular complications of methamphetamine abuse. Neurocrit Care. 2009;10:295–305. doi: 10.1007/s12028-008-9177-5. [DOI] [PubMed] [Google Scholar]
- 144.Bhat VM, Cole JW, Sorkin JD, Wozniak MA, Malarcher AM, Giles WH, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439–2443. doi: 10.1161/STROKEAHA.107.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: Confounding in the courtroom. JAMA. 2000;284:706–712. doi: 10.1001/jama.284.6.706. [DOI] [PubMed] [Google Scholar]
- 146.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46:11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 147.Fagerstrom K. The epidemiology of smoking: Health consequences and benefits of cessation. Drugs. 2002;62(Suppl 2):1–9. doi: 10.2165/00003495-200262002-00001. [DOI] [PubMed] [Google Scholar]
- 148.Robbins AS, Manson JE, Lee IM, Satterfield S, Hennekens CH. Cigarette smoking and stroke in a cohort of u.S. Male physicians. Ann Intern Med. 1994;120:458–462. doi: 10.7326/0003-4819-120-6-199403150-00002. [DOI] [PubMed] [Google Scholar]
- 149.Song YM, Cho HJ. Risk of stroke and myocardial infarction after reduction or cessation of cigarette smoking: A cohort study in korean men. Stroke. 2008;39:2432–2438. doi: 10.1161/STROKEAHA.107.512632. [DOI] [PubMed] [Google Scholar]
- 150.Malek AM, Cushman M, Lackland DT, Howard G, McClure LA. Secondhand smoke exposure and stroke: The reasons for geographic and racial differences in stroke (regards) study. Am J Prev Med. 2015;49:e89–97. doi: 10.1016/j.amepre.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Emerging Risk Factors C. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou Y, Han W, Gong D, Man C, Fan Y. Hs-crp in stroke: A meta-analysis. Clinica chimica acta; international journal of clinical chemistry. 2016;453:21–27. doi: 10.1016/j.cca.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 153.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated c-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 154.Eisenhardt SU, Habersberger J, Murphy A, Chen YC, Woollard KJ, Bassler N, et al. Dissociation of pentameric to monomeric c-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ Res. 2009;105:128–137. doi: 10.1161/CIRCRESAHA.108.190611. [DOI] [PubMed] [Google Scholar]
- 155.Thompson D, Pepys MB, Wood SP. The physiological structure of human c-reactive protein and its complex with phosphocholine. Structure. 1999;7:169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 156.Fujita Y, Yamaguchi S, Kakino A, Iwamoto S, Yoshimoto R, Sawamura T. Lectin-like oxidized ldl receptor 1 is involved in crp-mediated complement activation. Clin Chem. 2011;57:1398–1405. doi: 10.1373/clinchem.2011.168625. [DOI] [PubMed] [Google Scholar]
- 157.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Elkind MS, Ramakrishnan P, Moon YP, Boden-Albala B, Liu KM, Spitalnik SL, et al. Infectious burden and risk of stroke: The northern manhattan study. Archives of neurology. 2010;67:33–38. doi: 10.1001/archneurol.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Elkind MS, Luna JM, Moon YP, Boden-Albala B, Liu KM, Spitalnik S, et al. Infectious burden and carotid plaque thickness: The northern manhattan study. Stroke; a journal of cerebral circulation. 2010;41:e117–122. doi: 10.1161/STROKEAHA.109.571299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wright CB, Gardener H, Dong C, Yoshita M, DeCarli C, Sacco RL, et al. Infectious burden and cognitive decline in the northern manhattan study. J Am Geriatr Soc. 2015;63:1540–1545. doi: 10.1111/jgs.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Katan M, Moon YP, Paik MC, Sacco RL, Wright CB, Elkind MS. Infectious burden and cognitive function: The northern manhattan study. Neurology. 2013;80:1209–1215. doi: 10.1212/WNL.0b013e3182896e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with hiv infection. Neurology. 2011;76:444–450. doi: 10.1212/WNL.0b013e31820a0cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chow FC, He W, Bacchetti P, Regan S, Feske SK, Meigs JB, et al. Elevated rates of intracerebral hemorrhage in individuals from a us clinical care hiv cohort. Neurology. 2014;83:1705–1711. doi: 10.1212/WNL.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with hiv. Neurology. 2015;85:1139–1145. doi: 10.1212/WNL.0000000000001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sabin CA, Ryom L, De Wit S, Mocroft A, Phillips AN, Worm SW, et al. Associations between immune depression and cardiovascular events in hiv infection. AIDS. 2013;27:2735–2748. doi: 10.1097/01.aids.0000432457.91228.f3. [DOI] [PubMed] [Google Scholar]
- 166.Gutierrez J, Elkind MS, Marshall RS. Cardiovascular profile and events of us adults 20-49 years with hiv: Results from the nhanes 1999-2008. AIDS Care. 2013;25:1385–1391. doi: 10.1080/09540121.2013.769493. [DOI] [PubMed] [Google Scholar]
- 167.Elkind MS, Carty CL, O'Meara ES, Lumley T, Lefkowitz D, Kronmal RA, et al. Hospitalization for infection and risk of acute ischemic stroke: The cardiovascular health study. Stroke; a journal of cerebral circulation. 2011;42:1851–1856. doi: 10.1161/STROKEAHA.110.608588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Clar C, Oseni Z, Flowers N, Keshtkar-Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. The Cochrane database of systematic reviews. 2015;5 doi: 10.1002/14651858.CD005050.pub3. CD005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. The New England journal of medicine. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 170.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Corrales-Medina VF, Taljaard M, Yende S, Kronmal R, Dwivedi G, Newman AB, et al. Intermediate and long-term risk of new-onset heart failure after hospitalization for pneumonia in elderly adults. Am Heart J. 2015;170:306–312. doi: 10.1016/j.ahj.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dalager-Pedersen M, Sogaard M, Schonheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: A 20-year population-based cohort study. Circulation. 2014;129:1387–1396. doi: 10.1161/CIRCULATIONAHA.113.006699. [DOI] [PubMed] [Google Scholar]
- 174.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: Case-control study through a general practice database. Eur Heart J. 2008;29:96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 175.Tonne C, Halonen JI, Beevers SD, Dajnak D, Gulliver J, Kelly FJ, et al. Long-term traffic air and noise pollution in relation to mortality and hospital readmission among myocardial infarction survivors. Int J Hyg Environ Health. 2016;219:72–78. doi: 10.1016/j.ijheh.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 176.Mostofsky E, Schwartz J, Coull BA, Koutrakis P, Wellenius GA, Suh HH, et al. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol. 2012;176:317–326. doi: 10.1093/aje/kws018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O'Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA, Investigators of the Registry of Canadian Stroke N Fine particulate air pollution (pm2.5) and the risk of acute ischemic stroke. Epidemiology. 2011;22:422–431. doi: 10.1097/EDE.0b013e3182126580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Suissa L, Fortier M, Lachaud S, Staccini P, Mahagne MH. Ozone air pollution and ischaemic stroke occurrence: A case-crossover study in nice, france. BMJ Open. 2013;3:e004060. doi: 10.1136/bmjopen-2013-004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke; a journal of cerebral circulation. 2005;36:2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- 180.Johnson JY, Rowe BH, Villeneuve PJ. Ecological analysis of long-term exposure to ambient air pollution and the incidence of stroke in edmonton, alberta, canada. Stroke; a journal of cerebral circulation. 2010;41:1319–1325. doi: 10.1161/STROKEAHA.110.580571. [DOI] [PubMed] [Google Scholar]
- 181.Chen R, Zhang Y, Yang C, Zhao Z, Xu X, Kan H. Acute effect of ambient air pollution on stroke mortality in the china air pollution and health effects study. Stroke; a journal of cerebral circulation. 2013;44:954–960. doi: 10.1161/STROKEAHA.111.673442. [DOI] [PubMed] [Google Scholar]
- 182.Sorensen M, Luhdorf P, Ketzel M, Andersen ZJ, Tjonneland A, Overvad K, et al. Combined effects of road traffic noise and ambient air pollution in relation to risk for stroke? Environ Res. 2014;133:49–55. doi: 10.1016/j.envres.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 183.Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. Outdoor air pollution and incidence of ischemic and hemorrhagic stroke: A small-area level ecological study. Stroke; a journal of cerebral circulation. 2012;43:22–27. doi: 10.1161/STROKEAHA.110.610238. [DOI] [PubMed] [Google Scholar]
- 184.Wing JJ, Adar SD, Sanchez BN, Morgenstern LB, Smith MA, Lisabeth LD. Ethnic differences in ambient air pollution and risk of acute ischemic stroke. Environ Res. 2015;143:62–67. doi: 10.1016/j.envres.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. Long-term exposure to particulate matter air pollution is a risk factor for stroke: Meta-analytical evidence. Stroke; a journal of cerebral circulation. 2015;46:3058–3066. doi: 10.1161/STROKEAHA.115.009913. [DOI] [PubMed] [Google Scholar]
- 186.Matarin M, Brown WM, Singleton A, Hardy JA, Meschia JF, investigators I Whole genome analyses suggest ischemic stroke and heart disease share an association with polymorphisms on chromosome 9p21. Stroke. 2008;39:1586–1589. doi: 10.1161/STROKEAHA.107.502963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gretarsdottir S, Thorleifsson G, Manolescu A, Styrkarsdottir U, Helgadottir A, Gschwendtner A, et al. Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Annals of neurology. 2008;64:402–409. doi: 10.1002/ana.21480. [DOI] [PubMed] [Google Scholar]
- 188.Bevan S, Traylor M, Adib-Samii P, Malik R, Paul NL, Jackson C, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 189.Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004;35:212–227. doi: 10.1161/01.STR.0000107187.84390.AA. [DOI] [PubMed] [Google Scholar]
- 190.Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, et al. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke. 1998;29:1177–1181. doi: 10.1161/01.str.29.6.1177. [DOI] [PubMed] [Google Scholar]
- 191.Tournier-Lasserve E, Iba-Zizen MT, Romero N, Bousser MG. Autosomal dominant syndrome with strokelike episodes and leukoencephalopathy. Stroke. 1991;22:1297–1302. doi: 10.1161/01.str.22.10.1297. [DOI] [PubMed] [Google Scholar]
- 192.Peters N, Opherk C, Bergmann T, Castro M, Herzog J, Dichgans M. Spectrum of mutations in biopsy-proven cadasil: Implications for diagnostic strategies. Archives of neurology. 2005;62:1091–1094. doi: 10.1001/archneur.62.7.1091. [DOI] [PubMed] [Google Scholar]
- 193.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the notch3 receptor accumulates within the cerebrovasculature of cadasil patients. The Journal of clinical investigation. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ebke M, Dichgans M, Bergmann M, Voelter HU, Rieger P, Gasser T, et al. Cadasil: Skin biopsy allows diagnosis in early stages. Acta neurologica Scandinavica. 1997;95:351–357. doi: 10.1111/j.1600-0404.1997.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 195.Menezes Cordeiro I, Nzwalo H, Sa F, Ferreira RB, Alonso I, Afonso L, et al. Shifting the carasil paradigm: Report of a non-asian family and literature review. Stroke. 2015;46:1110–1112. doi: 10.1161/STROKEAHA.114.006735. [DOI] [PubMed] [Google Scholar]
- 196.Hara K, Shiga A, Fukutake T, Nozaki H, Miyashita A, Yokoseki A, et al. Association of htra1 mutations and familial ischemic cerebral small-vessel disease. The New England journal of medicine. 2009;360:1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]
- 197.Annes JP, Munger JS, Rifkin DB. Making sense of latent tgfbeta activation. Journal of cell science. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 198.Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, et al. Mutations in the facilitative glucose transporter glut10 alter angiogenesis and cause arterial tortuosity syndrome. Nature genetics. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- 199.Levy E, Jaskolski M, Grubb A. The role of cystatin c in cerebral amyloid angiopathy and stroke: Cell biology and animal models. Brain pathology. 2006;16:60–70. doi: 10.1111/j.1750-3639.2006.tb00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 201.Russell MO, Goldberg HI, Hodson A, Kim HC, Halus J, Reivich M, et al. Effect of transfusion therapy on arteriographic abnormalities and on recurrence of stroke in sickle cell disease. Blood. 1984;63:162–169. [PubMed] [Google Scholar]
- 202.Schatz J, Brown RT, Pascual JM, Hsu L, DeBaun MR. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56:1109–1111. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- 203.Dichgans M. Genetics of ischaemic stroke. The Lancet. Neurology. 2007;6:149–161. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 204.Mehta A, Ginsberg L, Investigators FOS Natural history of the cerebrovascular complications of fabry disease. Acta paediatrica. 2005;94:24–27. doi: 10.1111/j.1651-2227.2005.tb02106.x. discussion 29-10. [DOI] [PubMed] [Google Scholar]
- 205.Martinez-Fernandez E, Gil-Peralta A, Garcia-Lozano R, Chinchon I, Aguilera I, Fernandez-Lopez O, et al. Mitochondrial disease and stroke. Stroke. 2001;32:2507–2510. doi: 10.1161/hs1101.098328. [DOI] [PubMed] [Google Scholar]
- 206.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of col4a1 in small-vessel disease and hemorrhagic stroke. The New England journal of medicine. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 207.Lanfranconi S, Markus HS. Col4a1 mutations as a monogenic cause of cerebral small vessel disease: A systematic review. Stroke. 2010;41:e513–518. doi: 10.1161/STROKEAHA.110.581918. [DOI] [PubMed] [Google Scholar]
- 208.Milewicz DM, Ostergaard JR, Ala-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, et al. De novo acta2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. American journal of medical genetics. Part A. 2010;152A:2437–2443. doi: 10.1002/ajmg.a.33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Disabella E, Grasso M, Gambarin FI, Narula N, Dore R, Favalli V, et al. Risk of dissection in thoracic aneurysms associated with mutations of smooth muscle alpha-actin 2 (acta2) Heart. 2011;97:321–326. doi: 10.1136/hrt.2010.204388. [DOI] [PubMed] [Google Scholar]
- 210.Munot P, Saunders DE, Milewicz DM, Regalado ES, Ostergaard JR, Braun KP, et al. A novel distinctive cerebrovascular phenotype is associated with heterozygous arg179 acta2 mutations. Brain : a journal of neurology. 2012;135:2506–2514. doi: 10.1093/brain/aws172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Williams FM, Carter AM, Hysi PG, Surdulescu G, Hodgkiss D, Soranzo N, et al. Ischemic stroke is associated with the abo locus: The euroclot study. Annals of neurology. 2013;73:16–31. doi: 10.1002/ana.23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Network NSG, International Stroke Genetics C Loci associated with ischaemic stroke and its subtypes (sign): A genome-wide association study. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)00338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Neurology Working Group of the Cohorts for H. Aging Research in Genomic Epidemiology C. Stroke Genetics N. International Stroke Genetics C Identification of additional risk loci for stroke and small vessel disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2016;15:695–707. doi: 10.1016/S1474-4422(16)00102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.French CR, Seshadri S, Destefano AL, Fornage M, Arnold CR, Gage PJ, et al. Mutation of foxc1 and pitx2 induces cerebral small-vessel disease. J Clin Invest. 2014;124:4877–4881. doi: 10.1172/JCI75109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Malik R, Traylor M, Pulit SL, Bevan S, Hopewell JC, Holliday EG, et al. Low-frequency and common genetic variation in ischemic stroke: The metastroke collaboration. Neurology. 2016;86:1217–1226. doi: 10.1212/WNL.0000000000002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, de Wit C, et al. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature. 2013;504:432–436. doi: 10.1038/nature12722. [DOI] [PubMed] [Google Scholar]
- 217.Schunkert H, Gotz A, Braund P, McGinnis R, Tregouet DA, Mangino M, et al. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation. 2008;117:1675–1684. doi: 10.1161/CIRCULATIONAHA.107.730614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gschwendtner A, Bevan S, Cole JW, Plourde A, Matarin M, Ross-Adams H, et al. Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Annals of neurology. 2009;65:531–539. doi: 10.1002/ana.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC, Jr., Hayman LL, et al. Value of primordial and primary prevention for cardiovascular disease: A policy statement from the american heart association. Circulation. 2011;124:967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 220.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 221.Howard VJ, McDonnell MN. Physical activity in primary stroke prevention: Just do it! Stroke; a journal of cerebral circulation. 2015;46:1735–1739. doi: 10.1161/STROKEAHA.115.006317. [DOI] [PubMed] [Google Scholar]
- 222.Marcus BH, Williams DM, Dubbert PM, Sallis JF, King AC, Yancey AK, et al. Physical activity intervention studies: What we know and what we need to know: A scientific statement from the american heart association council on nutrition, physical activity, and metabolism (subcommittee on physical activity); council on cardiovascular disease in the young; and the interdisciplinary working group on quality of care and outcomes research. Circulation. 2006;114:2739–2752. doi: 10.1161/CIRCULATIONAHA.106.179683. [DOI] [PubMed] [Google Scholar]
- 223.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the global burden of disease study 2013. Bmj. 2016;354:i3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Indian Polycap S. Yusuf S, Pais P, Afzal R, Xavier D, Teo K, et al. Effects of a polypill (polycap) on risk factors in middle-aged individuals without cardiovascular disease (tips): A phase ii, double-blind, randomised trial. Lancet. 2009;373:1341–1351. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
- 225.Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the pure study): A prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 226.Law MR, Wald NJ. Risk factor thresholds: Their existence under scrutiny. Bmj. 2002;324:1570–1576. doi: 10.1136/bmj.324.7353.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. The New England journal of medicine. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 228.Cushman WC, Goff DC., Jr. More hope for prevention with statins. The New England journal of medicine. 2016;374:2085–2087. doi: 10.1056/NEJMe1603504. [DOI] [PubMed] [Google Scholar]
- 229.Yusuf S, Lonn E, Pais P, Bosch J, Lopez-Jaramillo P, Zhu J, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. The New England journal of medicine. 2016;374:2032–2043. doi: 10.1056/NEJMoa1600177. [DOI] [PubMed] [Google Scholar]
- 230.Lonn EM, Bosch J, Lopez-Jaramillo P, Zhu J, Liu L, Pais P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. The New England journal of medicine. 2016;374:2009–2020. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 231.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr., Tudor-Locke C, et al. 2011 compendium of physical activities: A second update of codes and met values. Medicine and science in sports and exercise. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 232.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: A meta-analysis. Stroke; a journal of cerebral circulation. 2003;34:2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 233.Physical activity guidelines advisory committee report, 2008. To the secretary of health and human services. Part a: Executive summary. Nutrition reviews. 2009;67:114–120. doi: 10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- 234.Willey JZ, Moon YP, Paik MC, Boden-Albala B, Sacco RL, Elkind MS. Physical activity and risk of ischemic stroke in the northern manhattan study. Neurology. 2009;73:1774–1779. doi: 10.1212/WNL.0b013e3181c34b58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 aha/acc guideline on lifestyle management to reduce cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 236.Rees K, Dyakova M, Wilson N, Ward K, Thorogood M, Brunner E. Dietary advice for reducing cardiovascular risk. The Cochrane database of systematic reviews. 2013 doi: 10.1002/14651858.CD002128.pub5. CD002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Boden-Albala B, Southwick L, Carman H. Dietary interventions to lower the risk of stroke. Current neurology and neuroscience reports. 2015;15:15. doi: 10.1007/s11910-015-0538-0. [DOI] [PubMed] [Google Scholar]
- 238.Fung TT, Stampfer MJ, Manson JE, Rexrode KM, Willett WC, Hu FB. Prospective study of major dietary patterns and stroke risk in women. Stroke; a journal of cerebral circulation. 2004;35:2014–2019. doi: 10.1161/01.STR.0000135762.89154.92. [DOI] [PubMed] [Google Scholar]
- 239.Lakkur S, Judd SE. Diet and stroke: Recent evidence supporting a mediterranean-style diet and food in the primary prevention of stroke. Stroke; a journal of cerebral circulation. 2015;46:2007–2011. doi: 10.1161/STROKEAHA.114.006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: A cultural model for healthy eating. The American journal of clinical nutrition. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 241.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a mediterranean diet. The New England journal of medicine. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 242.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of dietary approaches to stop hypertension (dash)-style diet on fatal or nonfatal cardiovascular diseases--incidence: A systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–618. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 243.Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke. The framingham study. JAMA : the journal of the American Medical Association. 1988;259:1025–1029. [PubMed] [Google Scholar]
- 244.Phs Guideline Update Panel L, Staff Treating tobacco use and dependence: 2008 update u.S. Public health service clinical practice guideline executive summary. Respiratory care. 2008;53:1217–1222. [PubMed] [Google Scholar]
- 245.Chelladurai Y, Singh S. Varenicline and cardiovascular adverse events: A perspective review. Therapeutic advances in drug safety. 2014;5:167–172. doi: 10.1177/2042098614530421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Bmj. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen GJ, Yang MS. The effects of calcium channel blockers in the prevention of stroke in adults with hypertension: A meta-analysis of data from 273,543 participants in 31 randomized controlled trials. PloS one. 2013;8:e57854. doi: 10.1371/journal.pone.0057854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA : the journal of the American Medical Association. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 250.Group SR. Wright JT, Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. The New England journal of medicine. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. The New England journal of medicine. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Professional practice committee for the standards of medical care in diabetes-2016. Diabetes care. 2016;39(Suppl 1):S107–108. doi: 10.2337/dc16-S018. [DOI] [PubMed] [Google Scholar]
- 253.Hostalek U, Gwilt M, Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs. 2015;75:1071–1094. doi: 10.1007/s40265-015-0416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Heart Protection Study Collaborative G Mrc/bhf heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 255.Cholesterol Treatment Trialists C. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of ldl cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Amarenco P, Labreuche J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet neurology. 2009;8:453–463. doi: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- 257.Mrc/bhf heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 258.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. The New England journal of medicine. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 259.Amarenco P, Benavente O, Goldstein LB, Callahan A, 3rd, Sillesen H, Hennerici MG, et al. Results of the stroke prevention by aggressive reduction in cholesterol levels (sparcl) trial by stroke subtypes. Stroke; a journal of cerebral circulation. 2009;40:1405–1409. doi: 10.1161/STROKEAHA.108.534107. [DOI] [PubMed] [Google Scholar]
- 260.Elkind MS, Sacco RL, MacArthur RB, Fink DJ, Peerschke E, Andrews H, et al. The neuroprotection with statin therapy for acute recovery trial (neustart): An adaptive design phase i dose-escalation study of high-dose lovastatin in acute ischemic stroke. International journal of stroke : official journal of the International Stroke Society. 2008;3:210–218. doi: 10.1111/j.1747-4949.2008.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. The New England journal of medicine. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 262.Antithrombotic Trialists C. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Guirguis-Blake JM, Evans CV, Senger CA, Rowland MG, O'Connor EA, Whitlock EP. Aspirin for the primary prevention of cardiovascular events: A systematic evidence review for the u.S. Preventive services task force. Rockville (MD): 2015. [PubMed] [Google Scholar]
- 264.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. The New England journal of medicine. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 265.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The copenhagen afasak study. Lancet. 1989;1:175–179. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 266.Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84:527–539. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- 267.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 268.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. The New England journal of medicine. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 269.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 270.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 271.Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: A systematic review and meta-analysis. Blood. 2014;124:2450–2458. doi: 10.1182/blood-2014-07-590323. [DOI] [PubMed] [Google Scholar]
- 272.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. JAMA : the journal of the American Medical Association. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 273.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 274.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. The Annals of thoracic surgery. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 275.Bartus K, Han FT, Bednarek J, Myc J, Kapelak B, Sadowski J, et al. Percutaneous left atrial appendage suture ligation using the lariat device in patients with atrial fibrillation: Initial clinical experience. Journal of the American College of Cardiology. 2013;62:108–118. doi: 10.1016/j.jacc.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 276.Kebernik J, Jose J, Abdel-Wahab M, Stocker B, Geist V, Richardt G. Safety and efficacy of left atrial appendage closure with the amplatzer cardiac plug in very high stroke and bleeding risk patients with non-valvular atrial fibrillation. Cardiology and therapy. 2015;4:167–177. doi: 10.1007/s40119-015-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the protect af (watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 278.Holmes DR, Jr., Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The prevail trial. Journal of the American College of Cardiology. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 279.Waksman R, Pendyala LK. Overview of the food and drug administration circulatory system devices panel meetings on watchman left atrial appendage closure therapy. The American journal of cardiology. 2015;115:378–384. doi: 10.1016/j.amjcard.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 280.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. The New England journal of medicine. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Homma S, Thompson JL, Qian M, Ye S, Di Tullio MR, Lip GY, et al. Quality of anticoagulation control in preventing adverse events in patients with heart failure in sinus rhythm: Warfarin versus aspirin in reduced cardiac ejection fraction trial substudy. Circulation. Heart failure. 2015;8:504–509. doi: 10.1161/CIRCHEARTFAILURE.114.001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lee M, Saver JL, Hong KS, Wu HC, Ovbiagele B. Risk-benefit profile of warfarin versus aspirin in patients with heart failure and sinus rhythm: A meta-analysis. Circulation. Heart failure. 2013;6:287–292. doi: 10.1161/CIRCHEARTFAILURE.112.971697. [DOI] [PubMed] [Google Scholar]
- 283.Risk of stroke in the distribution of an asymptomatic carotid artery. The european carotid surgery trialists collaborative group. Lancet. 1995;345:209–212. [PubMed] [Google Scholar]
- 284.Mohammed N, Anand SS. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomized controlled trial. Mrc asymptomatic carotid surgery trial (acst) collaborative group. Lancet. 2004;363:1491–502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]; Vascular medicine. 2005;10:77–78. doi: 10.1191/1358863x05vm588xx. [DOI] [PubMed] [Google Scholar]
- 285.Rothwell PM. Endarterectomy for symptomatic and asymptomatic carotid stenosis. Neurologic clinics. 2008;26:1079–1097. x. doi: 10.1016/j.ncl.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 286.Benavente O, Moher D, Pham B. Carotid endarterectomy for asymptomatic carotid stenosis: A meta-analysis. Bmj. 1998;317:1477–1480. doi: 10.1136/bmj.317.7171.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.North American Symptomatic Carotid Endarterectomy Trial C Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. The New England journal of medicine. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 288.Raman G, Moorthy D, Hadar N, Dahabreh IJ, O'Donnell TF, Thaler DE, et al. Management strategies for asymptomatic carotid stenosis: A systematic review and meta-analysis. Ann Intern Med. 2013;158:676–685. doi: 10.7326/0003-4819-158-9-201305070-00007. [DOI] [PubMed] [Google Scholar]
- 289.Moore WS. Issues to be addressed and hopefully resolved in the carotid revascularization endarterectomy versus stenting trial 2. Angiology. 2016;67:408–410. doi: 10.1177/0003319715611281. [DOI] [PubMed] [Google Scholar]
- 290.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. The New England journal of medicine. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (sammpris): The final results of a randomised trial. Lancet. 2014;383:333–341. doi: 10.1016/S0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.