Abstract
Objective/Purpose
Laryngotracheal stenosis (LTS) is a chronic fibrotic disease characterized by fibroblast proliferation, collagen deposition, and matrix remodeling in the lamina propria of the larynx and/or trachea. Current medical therapies are limited by a poor understanding of the effector cell’s (fibroblasts) cellular biology and metabolism. The purpose of this study is to compare cellular proliferation, function, and metabolism between normal and LTS-derived fibroblasts in vitro.
Methods
Human biopsies of normal and iatrogenic LTS tissue (n=7) were obtained and fibroblasts were isolated and cultured in vitro. Cellular proliferation, cellular histology, gene expression and metabolic analyses were performed. Statistical analyses comparing normal and scar-derived fibroblasts were performed.
Results
LTS fibroblast proliferation rate, cellular surface area, and collagen-1 expression were increased compared to normal fibroblasts. Cellular metabolic analysis of LTS-derived fibroblasts demonstrated reduced oxidative phosphorylation and increased glycolysis/oxidative phosphorylation ratio compared with normal fibroblasts.
Conclusion
Human iatrogenic LTS-derived fibroblasts demonstrated aberrant behavior when compared with normal fibroblasts. A Warburg-like effect was revealed suggesting human iatrogenic LTS fibroblasts drive their proliferation with aerobic glycolysis. The distinct metabolism suggests metabolic inhibitors could reduce fibroblast hyperplasia and hypertrophy in LTS and fibrosis in general.
Keywords: fibrosis, laryngotracheal stenosis, larynx, Warburg effect, fibroblasts, cellular metabolism, mTOR
Introduction
Laryngotracheal stenosis (LTS) is the clinical entity of fibrosis within the larynx and trachea. It is a chronic disease characterized by fibroblast proliferation, collagen deposition, and matrix remodeling in the lamina propria of the posterior glottis, sub-glottis, and/or trachea. This leads to airway narrowing and can result in communication handicap, dyspnea, or airway collapse.1 LTS can be caused by prolonged intubation, local trauma, autoimmune disease, radiation exposure, or idiopathic reasons.2–4 However, despite similar clinical presentation different etiologies of LTS display variable histologic characteristics. Idiopathic LTS is primarily limited to the lamina propria while iatrogenic LTS often involves the lamina propria and underlying cartilage superstructure, suggesting a difference in pathophysiology. Treatment strategies for LTS include both medical and surgical therapies. Cricotracheal or tracheal resection can be a very effective surgical therapy but can also cause significant morbidity and at times mortality. Furthermore, many LTS patients are not candidates for surgical resection due to medical co-morbidities and/or significant laryngeal involvement. Medical therapies for LTS are of minimal benefit, a reflection of our limited understanding of LTS pathogenesis. Improved understanding of LTS pathophysiology as it pertains to each etiology would allow for the investigation and development of disease specific therapies.
Fibroblasts are the main effector cells in LTS. However, the underlying molecular and metabolic mechanism of fibroblasts involved in the pathogenesis of LTS is poorly understood. In physiologic wound repair, fibroblasts play a major role in extracellular matrix deposition and remodeling. However, when fibroblasts lose their normal cellular function they proliferate faster and deposit extracellular matrix at uncontrollable rates, leading to abnormal tissue remodeling.5 Fibrosis in other organs provides a model for what may be happening to fibroblasts in the larynx and trachea. In the lower airway, idiopathic pulmonary fibrosis (IPF) is a well characterized fibro-proliferative disease, with hyperfunctional pulmonary fibroblasts that deposit excessive extracellular matrix in the lung interstitium.6,7 The aberrant function of these fibroblasts is the result of a dysregulated wound healing response.6 Complex signaling between fibroblasts, epithelial cells, and immune mediators, results in the pro-fibrotic phenotype observed.8 Additionally, IPF fibroblasts have been shown to have aberrant metabolic profiles characterized by increased glycolytic activity. When these metabolic processes were inhibited it resulted in decreased fibrosis9,10, suggesting at the role of glycolytic reprogramming in fibrogenesis.
Investigations into fibroblasts function in LTS have demonstrated a similar hyperfunctional phenotype; however, the underlying cellular and metabolic mechanisms’ for this disease remain largely uncharacterized. In a rabbit model of iatrogenic LTS, LTS-derived fibroblasts demonstrated an increased rate of migration and increased cell contractility.11 In vitro, human LTS-derived fibroblasts showed collagen production and fibroblast proliferation were sensitive to the anti-metabolic agent rapamycin.12 Additionally, the decrease in collagen expression and proliferation after rapamycin treatment coincided with a reduction in the overall rate of oxidative phosphorylation, indicating a potential role for glycolytic reprogramming in LTS fibrogenesis.12 Despite these findings, a comparison of LTS-derived fibroblasts to normal airway fibroblasts has not been investigated.
The objective of this study is to characterize the function, proliferation, and metabolic profile of iatrogenic LTS-derived fibroblasts and compare them to normal airway fibroblasts harvested from the same patients. The focus on iatrogenic patients allows for an investigation into a specific etiology of LTS who suffered fibrosis from endotracheal tube or tracheostomy tube injury. We will perform an in vitro comparative analysis using gene expression, cell proliferation analysis, as well as oxidative phosphorylation and glycolysis assays of normal and scar-derived fibroblasts. We hypothesize that LTS-derived fibroblasts will demonstrate aberrant behavior with faster proliferation, increased collagen production, and altered metabolic allocation compared with normal fibroblasts.
Materials and Methods
Fibroblast Isolation and Cell Culture
After obtaining approval from the Johns Hopkins University Institutional Review Board and informed written consent from participants, biopsies were obtained from seven patients with iatrogenic LTS undergoing operative procedures between 2012 and 2015. Biopsies were taken from segments of lamina propria thickening in the subglottis/proximal trachea and from normal tracheal lamina propria distal to the area of scar. Four patients had subglottic stenosis extending into the proximal trachea with the other three having proximal tracheal stenosis. Medical comorbidities were relevant for obesity (n=2), diabetes mellitus (n=3), asthma (n=2), hypertension (n=3), heart failure (n=3), coronary artery disease (n=2), stroke (n=1), anxiety/depression (n=3). Immediately after excision, tissue was placed in phosphate-buffered saline (PBS; Gibco Life Technologies by Invitrogen, Grand Island, New York, USA). Biopsy specimens were cut into tissue culture petri dishes (BD Biosciences, San Jose, California, USA) using a #10 scalpel blade, and suspended in fibroblast growth medium at 37°C in 5% CO2 humidified atmosphere. Fibroblast growth medium contained Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; HyClone by Thermo Scientific, Logan, Utah, USA), 100 U/mL penicillin (Gibco), 100 ug/mL streptomycin (Gibco), and 100× nonessential amino acids (NEAA; Gibco). Once cells were confluent (20 – 30 days), they were passaged by using a short-term application of trypsin 0.25% (Gibco) and re-seeded and expanded in larger tissue culture flasks (BD Biosciences). Passages two to three of these primary fibroblast cultures were used for all experiments. Fibroblast presence was confirmed in culture with positive immunohistochemical staining of the anti-fibroblast antibody ER-TR7 (Santa Cruz Biotechnology, Inc, Dallas, Texas, USA) and negative staining for endothelial cells using ERG, muscles cells using Desmin, and epithelial cells using anti-human panCytokeratin antibody AF488 (Becton Dickenson, Franklin Lakes, New Jersey, USA).12–14 Due to the expense of immunohistochemistry, staining was performed on two representative sets of patient cells (both normal and scar) to ensure consistently negative results.
Unless otherwise noted, cells were seeded at a concentration of 5×103 cells per well. All experiments were run in triplicate, except cell proliferation, which was run in duplicate.
Cell Proliferation by Cell Count
Cells were trypsinized and counted daily using a hemocytometer allowing generation of a proliferation curve over five days.
Cellular Histology and Trichrome Staining
Fibroblasts were grown on microscope cover glass slips (Fisher Scientific, Hampton, NH) pretreated with 0.01% gelatin solution. The cells were stained with Masson’s Trichrome #HT15 kit (Sigma-Aldrich, St. Louis, MO) as per manufacturer’s protocol. Microscopic images of stained coverslips were captured using a Zeiss AX10 microscope (Carl Zeiss, Oberkochen, Germany). Stereological cellular area measurements were obtained using ImageJ (National Institute of Health, Bethesda, MD) in conjunction with a Wacom Tablet (Wacom, Kazo, Japan). 15
Gene Expression Analysis by Real-Time Polymerase Chain Reaction
Fibroblasts and fibrosarcoma cells were seeded at a concentration of 2×104 cells per well. RNA extraction, cDNA synthesis and RT-PCR were performed as per published methods.16 A panel of fibrosis genes including collagen-1, collagen-3, fibronectin, MMP-2, aSMA and TGF-b were assessed and normalized against the housekeeping gene Beta Actin. The level of expression was calculated as 2−ΔΔCt equaling a fold change from each “scar” sample compared to each patient sets’ respective “normal” fibroblast fold change.
Cellular Oxygen Consumption Rate
Mitochondrial function was analyzed by measuring the oxygen consumption rate (OCR) via a XF24 Flux Analyzer and XF Cell Mito Stress Test (Seahorse Bioscience, Billerica, MA). The cells were seeded in DMEM at 3×104 cells per well a day prior to analysis. Mitochondrial stress test protocol was followed as per methods reported in Namba et al.12
Cellular Extracellular Acidification Rates
Glycolytic function measurements were analyzed by evaluating the extracellular acidification rate (ECAR) using a XF24 Flux Analyzer and XF Glycolysis Stress Test (Seahorse Bioscience, Billerica, MA). The cells were seeded in DMEM at 4×104 cells per well a day prior to analysis. Glycolysis stress test was performed the following day as per manufacturer’s protocol.
Statistical Analysis
Results were expressed as mean ± SEM. For cellular area measurements a Mann-Whitney Test was utilized. For proliferation, gene expression and metabolic studies, a Wilcox Signed Rank Test was utilized. Statistically significant values were those with a P value < 0.05. Statistics were conducted using Prism (GraphPad; La Jolla, California).
Results
LTS-Derived fibroblasts showed increased cell proliferation when compared to normal
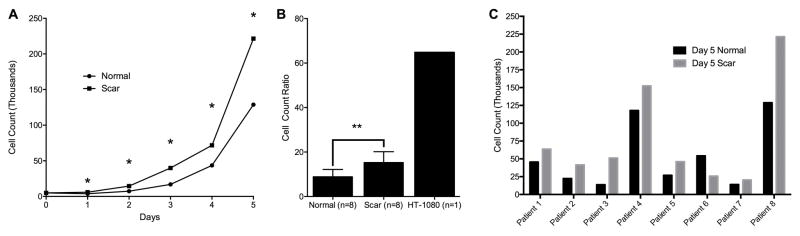
When cultured and grown under the same conditions in vitro, LTS-derived fibroblasts demonstrated increased cell proliferation rate by cell counting across all seven patient cell lines (p=0.0078) (Figure 1B). Representative patient results are shown in Figure 1A. Figure 1C demonstrates the heterogeneity of LTS by displaying paired LTS-scar and normal fibroblasts cell proliferation counts at day five for each of the seven patients. Seven of seven (100%) patients tested had an increase in scar fibroblast proliferation at day five.
Figure 1. LTS-Scar Fibroblasts Proliferate More than Normal Fibroblasts.
(A) Proliferation rates of a typical patient set. (B) Cell count growth ratio between day five and the initial seeding. (C) Paired LTS-scar and normal fibroblasts cell proliferation counts at day 5 demonstrate variability in fibroblast proliferation between individual patients. (LTS – laryngotracheal stenosis, * = p < 0.05, ** = p < 0.01)
Mean surface area of LTS-derived fibroblasts is significantly larger
The surface area of LTS-derived fibroblasts is compared to normal fibroblasts using Masson’s Trichrome staining (Figure 2). Normal and scar mean cell surface area were 1164.70 ± 995.41 μm2 and 1549.69 ± 976.21 μm2, respectively (p=0.0167).
Figure 2. Morphological and Proliferative Differences Between LTS-Scar and Normal Human Laryngotracheal Fibroblasts.
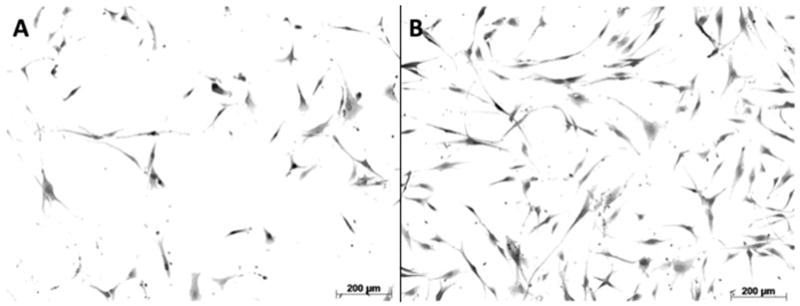
Normal (A) and LTS-scar (B) were imaged at Day 6 at 10× magnification. Scar cells demonstrated an increased cell surface area compared with normal cells (p < 0.05).
Collagen gene expression is elevated in LTS-derived fibroblasts
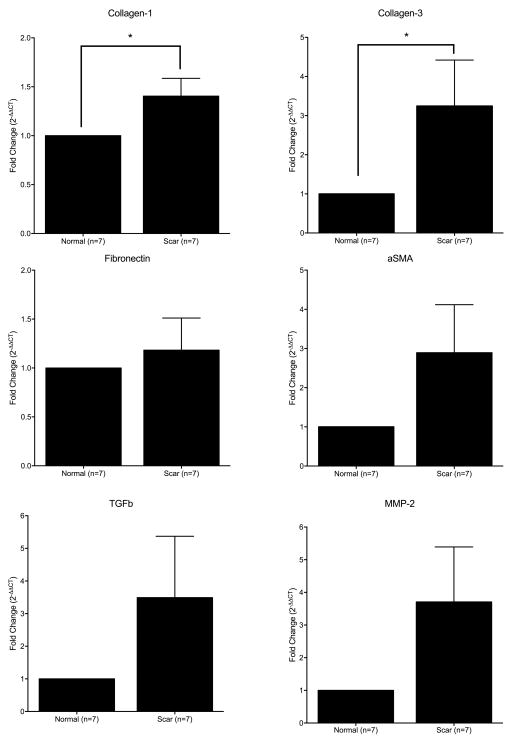
Seven of the seven patients demonstrated elevated collagen-1 gene expression when scar fibroblasts were compared to normal fibroblasts. Six of the seven patients demonstrated elevated collagen-3 gene expression when scar fibroblasts were compared to normal fibroblasts. When averaged, LTS-derived fibroblasts demonstrated a significant increase in collagen-1 (p = 0.0156) and collagen-3 (p = 0.0469) gene expression when compared to normal (Figure 3). The fold change in genes fibronectin, MMP-2, aSMA and TGFb was not significantly different from that in normal fibroblasts.
Figure 3. Collagen-1 and Collagen-3 Gene Expression is Greater in LTS-Scar Fibroblasts.
When normalized to respective patient set, LTS-scar fibroblasts express an increase in collagen-1 and collagen-3 gene expression compared with normal laryngotracheal fibroblasts. (* = p < 0.05)
Oxidative phosphorylation is greater in normal fibroblasts
Normal laryngotracheal fibroblasts were more metabolically active in oxidative phosphorylation than scar fibroblasts. Figure 4A shows a representative graph of a normal and scar mitochondrial stress test to measure oxidative phosphorylation and were used to calculate the indicators of oxidative phosphorylation: basal metabolism, ATP production and maximal respiratory capacity. Basal metabolism (Figure 4B) was calculated by taking the difference in the peak OCR prior to oligomycin and the base OCR after the rotenone & antimycin injection (79.94 ± 13.72, 47.61 ± 9.66 for normal and scar, respectively). ATP production (Figure 4C) was calculated by evaluating the difference pre and post oligomycin injection (65.59 ± 10.87, 35.75 ± 6.76 for normal and scar, respectively). Maximal respiratory capacity (Figure 4D) was determined by the difference between the peak OCR post-FCCP injection and the base OCR post rotenone & antimycin injection (117.48 ± 26.17, 55.67 ± 11.86 for normal and scar, respectively). Basal metabolism (p = 0.0469) and maximal respiratory capacity (p = 0.0156) were significantly greater in normal fibroblasts than in scar fibroblasts.
Figure 4. Increased Oxidative Phosphorylation Capacity in Normal Laryngotracheal Fibroblasts.
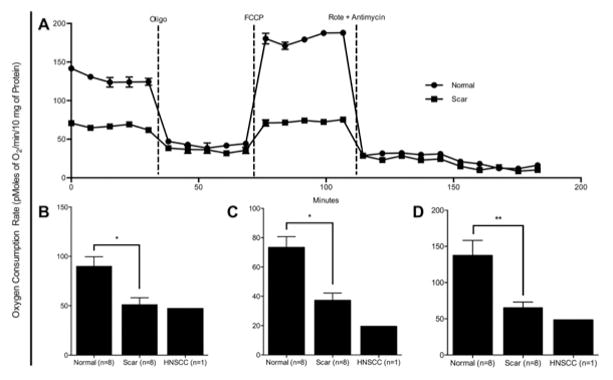
Panel (A) demonstrates a mitochondrial stress test of a patient set conducted in a XF24 analyzer. Normal laryngotracheal fibroblasts show a significant increase in (B) basal respiration, (C) ATP production and (D) maximum respiration. (* = p < 0.05, ** = p < 0.01)
LTS-derived fibroblasts demonstrate increased reliance on aerobic glycolysis
Figure 5A demonstrates a representative glycolytic stress test chart that is used to measure glycolysis and calculate glycolytic capacity. The difference between the peak ECAR post oligomycin and the base ECAR value post 2-DG is used to calculate the glycolytic capacity. Scar fibroblasts demonstrated no significant difference in glycolytic capacity (Figure 5B) compared with normal (13.23 ± 7.90, 12.67 ± 7.93 for normal and scar, respectively). The ratio of glycolytic capacity to maximum respiratory capacity, determines the cellular metabolic allocation (Figure 5C). The cellular metabolic allocation was significantly different between normal and LTS-scar fibroblasts (p=0.0469) with LTS-scar fibroblasts demonstrating a higher ECAR/OCR ratio.
Figure 5. LTS-Derived Fibroblasts Dedicates More Metabolic Activity to Glycolysis than Normal Fibroblasts.
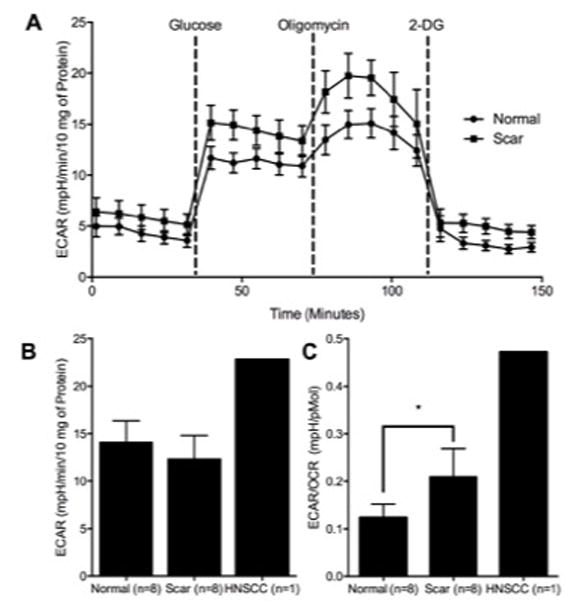
Panel (A) demonstrates a glycolytic test conducted on an XF24 analyzer of a patient set. While normal and LTS-scar fibroblasts are similar in glycolytic capacity (B), LTS-derived fibroblasts demonstrate a significantly increased ECAR/OCR Ratio (C). (* = p < 0.05)
Discussion
The results of this study demonstrate that human LTS scar-derived fibroblasts have distinct behavior in vitro when compared to normal fibroblasts isolated from the same patients with iatrogenic LTS. Fibroblasts were isolated in vitro and verified as fibroblasts with positive immunohistochemical staining for the fibroblast maker, ER-TR7, and negative staining for epithelial, endothelial, and muscle cell markers. Iatrogenic scar-derived fibroblasts were shown to have greater proliferation, more functionally active, and have a metabolic profile that favors glycolysis over oxidative phosphorylation. These biologic differences help explain the histologic findings of a thickened lamina propria in iatrogenic LTS. Furthermore, the shift toward glycolytic metabolism fueling increased cell proliferation reveals a Warburg-like effect in iatrogenic laryngotracheal fibrosis.
The distinct cellular changes in LTS fibroblasts compared to normal airway fibroblasts demonstrated in this study provide an explanation for the histologic finding of increased collagen deposition in the lamina propria of iatrogenic LTS specimens. Fibroblasts showed increased proliferation, higher collagen gene expression, and distinct morphologic changes when compared to normal fibroblasts harvested from uninjured trachea in the same patients. These changes indicate a hyperfunctional and pro-fibrotic phenotype for these cells and further implicate fibroblasts as the main effector cell in LTS. In physiologic wound repair fibroblasts deposit collagen and extra-cellular matrix, which is balanced by matrix remodeling to restore normal physiologic function.17 However, in LTS the collagen and extra-cellular matrix accumulates leading the development of pathologic scar.3 This study’s results show increased collagen production without a matched increase in matrix remodeling, as evidenced by unchanged gene expression of the collagenase MMP-2, suggesting mechanisms for collagen breakdown and matrix remodeling are not compensating for the increased extracellular matrix protein production.
Bioenergetic analysis uses oxygen consumption and extracellular acidification to determine cellular metabolism. This provides critical information on the specialized metabolic demands of individual cells that may explain their function and behavior. In this study, the cellular metabolic allocation was significantly different between normal and LTS-scar fibroblasts. Normal cells, represented by normal fibroblasts in this study, fueled their physiologic proliferation with energy derived from the more efficient oxidative phosphorylation. In contrast, the more proliferative LTS-derived fibroblasts are utilizing the less efficient metabolic pathway of aerobic glycolysis in the oxygen rich environment of the trachea. The biological phenomenon “the Warburg effect” is the observation that cells rely primarily on aerobic glycolysis to drive proliferation rather than oxidative phosphorylation.18 The Warburg effect is typically seen in cancer cells and may be encountered in some T-lymphocytes; however, the pathophysiologic process is not clearly understood.19–22 While there are a number of theories, it has been proposed that the Warburg effect is advantageous for proliferation. In mammalian cells, glycolysis and glutamine are the fundamental building blocks for energy creation and a necessary source of carbons, nitrogen, and reducing equivalents, which are important components in cellular proliferation. Although oxidative phosphorylation optimizes the creation of ATP, it does so at the expense of carbons via CO2. Aerobic glycolysis converts glucose to pyruvate, of which the majority is then converted into lactate, with a minority entering the TCA cycle to create ATP.22 Therefore, aerobic glycolysis is better able to conserve carbon atoms, which is advantageous for cells that are highly proliferative. Combined, the metabolic and proliferative data suggest that LTS has a “Warburg-like effect” in vitro.
Metabolic therapeutics including metformin, statins, and mTOR inhibitors represent a rational approach to target the deregulated cellular metabolism seen in fibrosis. Metformin and statins have suggested a reduced risk in cancer and/or cancer related mortality, while the mTOR inhibitors, everolimus and temsirolimus, have been approved as anticancer therapy.23–25 In fibrosis, multiple studies demonstrated rapamycin to mitigate aberrant wound healing and collagen deposition, presumably through mTOR-mediated immunosuppressive and anti-fibroblast mechanism.12,26–28 Furthermore, the mTOR inhibitor, rapamycin, is FDA-approved as topical elution from vascular stents to prevent re-stenosis of coronary arteries.23 These immunosuppressive and anti-fibroblast effects suggest at the potential of rapalogues, and other metabolic inhibitors, as adjuvant therapy for LTS.29
While our results are encouraging, our study has limitations. In vitro studies are not representative or indicative of fibroblasts in vivo but rather serve as a model to help bridge our understanding between the differences between the scar and normal cell types. In order to prevent changes to the primary harvested fibroblasts due to tissue culture plastic (in vitro environment), we limited the study to only passage two or three cells and minimized repeated passaging and freeze-thawing of cells. We do realize that there is dedifferentiation of the fibroblasts cultured in an in vitro environment, that would likely result in differences in gene expression when compared to analysis performed on in vivo tissue. While this is the imperfect nature of an in vitro model, we feel that culture artifact affected normal and scar LTS fibroblasts equally, and are therefore representative of the respective fibroblast behavior in vivo. Furthermore, as with any primary harvested cells, there will be variability between patients in which the cells were derived. Paired statistical analyses between normal and scar fibroblasts were performed in order to account for the heterogeneity of the disease process in different patients. When all patients were averaged there were significant differences between normal and scar fibroblasts across all outcomes measured. Further elucidation of genetic abnormalities and metabolic allocation of diseased fibroblasts would be prudent moving forward.
Conclusion
Laryngotracheal stenosis is a benign disease that has the potential to generate significant impairment of breathing and communication with few medical treatment options. This study demonstrates a Warburg-like effect in iatrogenic human LTS-derived fibroblasts in vitro with a shift toward glycolytic reprogramming. The metabolic, functional, and proliferative differences between diseased and normal fibroblasts improve our understanding of the cellular biology of iatrogenic laryngotracheal stenosis. Highlighting metabolic and biologic abnormalities of fibroblasts may instigate shifts in scientific perspective on fibrosis while targeting these differences may be beneficial in treating laryngotracheal stenosis and fibrosis in general.
Acknowledgments
Funding Source: Research reported in this publication was supported by National Institute of Deafness and Other Communication Disorders of the National Institutes of Health under award number 1K23DC0140823, as well as by a T32 NIH training grant (Kevin Motz). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Research reported in this publication was supported by National Institute of Deafness and Other Communication Disorders of the National Institutes of Health under award number 1K23DC0140823, as well as by a T32 NIH training grant (Kevin Motz). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, research reported in this publication was supported by the Triological Society and American College of Surgeons.
Footnotes
Conflict of Interest: The authors have no financial relationships or conflicts of interest to disclose.
Level of Evidence: NA
References
- 1.Hillel AT, Karatayli-Ozgursoy S, Benke JR, et al. Voice quality in laryngotracheal stenosis: impact of dilation and level of stenosis. Ann Otol Rhinol Laryngol. 2015;124(5):413–418. doi: 10.1177/0003489414564249. [DOI] [PubMed] [Google Scholar]
- 2.Herrington HC, Weber SM, Andersen PE. Modern management of laryngotracheal stenosis. Laryngoscope. 2006;116(9):1553–1557. doi: 10.1097/01.mlg.0000228006.21941.12. [DOI] [PubMed] [Google Scholar]
- 3.Hillel AT, Namba D, Ding D, Pandian V, Elisseeff JH, Horton MR. An in situ, in vivo murine model for the study of laryngotracheal stenosis. JAMA Otolaryngol Head Neck Surg. 2014;140(10):961–966. doi: 10.1001/jamaoto.2014.1663. [DOI] [PubMed] [Google Scholar]
- 4.Jette ME, Hayer SD, Thibeault SL. Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope. 2013;123(3):738–745. doi: 10.1002/lary.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockey DC, Bell PD, Hill JA. Fibrosis--A Common Pathway to Organ Injury and Failure. N Engl J Med. 2015;373(1):96. doi: 10.1056/NEJMc1504848. [DOI] [PubMed] [Google Scholar]
- 6.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 7.Ramos C, Montano M, Garcia-Alvarez J, et al. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24(5):591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 8.Sakai N, Tager AM. Fibrosis of two: Epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta. 2013;1832(7):911–921. doi: 10.1016/j.bbadis.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie N, Tan Z, Banerjee S, et al. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. Am J Respir Crit Care Med. 2015;192(12):1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milara J, Morcillo E, Monleon D, Tenor H, Cortijo J. Roflumilast Prevents the Metabolic Effects of Bleomycin-Induced Fibrosis in a Murine Model. PLoS One. 2015;10(7):e0133453. doi: 10.1371/journal.pone.0133453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh T, Sandulache VC, Otteson TD, et al. Subglottic stenosis examined as a fibrotic response to airway injury characterized by altered mucosal fibroblast activity. Arch Otolaryngol Head Neck Surg. 2010;136(2):163–170. doi: 10.1001/archoto.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namba DR, Ma G, Samad I, et al. Rapamycin inhibits human laryngotracheal stenosis-derived fibroblast proliferation, metabolism, and function in vitro. Otolaryngol Head Neck Surg. 2015;152(5):881–888. doi: 10.1177/0194599815573708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miettinen M, Wang ZF, Paetau A, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35(3):432–441. doi: 10.1097/PAS.0b013e318206b67b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangdaeng S, Truong LD. Comparative immunohistochemical staining for desmin and muscle-specific actin. A study of 576 cases. Am J Clin Pathol. 1991;96(1):32–45. doi: 10.1093/ajcp/96.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillel AT, Samad I, Ma G, et al. Dysregulated Macrophages Are Present in Bleomycin-Induced Murine Laryngotracheal Stenosis. Otolaryngol Head Neck Surg. 2015;153(2):244–250. doi: 10.1177/0194599815589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 18.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 19.Chang CH, Curtis JD, Maggi LB, Jr, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall A, Meyle KD, Lange MK, et al. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget. 2013;4(4):584–599. doi: 10.18632/oncotarget.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66(18):8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 22.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 24.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367(19):1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Chen H, Wang C, et al. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One. 2012;7(3):e33626. doi: 10.1371/journal.pone.0033626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamaki Z, Asano Y, Kubo M, et al. Effects of the immunosuppressant rapamycin on the expression of human alpha2(I) collagen and matrix metalloproteinase 1 genes in scleroderma dermal fibroblasts. J Dermatol Sci. 2014;74(3):251–259. doi: 10.1016/j.jdermsci.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Yu SY, Liu L, Li P, Li J. Rapamycin inhibits the mTOR/p70S6K pathway and attenuates cardiac fibrosis in adriamycin-induced dilated cardiomyopathy. Thorac Cardiovasc Surg. 2013;61(3):223–228. doi: 10.1055/s-0032-1311548. [DOI] [PubMed] [Google Scholar]
- 29.Hillel AT, Gelbard A. Unleashing rapamycin in fibrosis. Oncotarget. 2015;6(18):15722–15723. doi: 10.18632/oncotarget.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]