Abstract
The infralimbic and prelimbic (IL and PL, respectively) regions of the medial prefrontal cortex regulate the control of drug-seeking behavior. However, their roles in cocaine seeking in a discriminative stimulus (DS)-based self-administration task are unclear. To address this issue, male Sprague-Dawley rats were trained on a DS task in which, on a trial-by-trial basis, a DS+ indicated that a lever press would produce a cocaine infusion, whereas a distinct DS− indicated that a lever press would produce nothing. IL and PL inactivation via GABA receptor activation decreased performance accuracy and disinhibited behavioral responding on DS− trials, resulting in greater lever pressing during the DS− presentation. This was accompanied by a decrease in cocaine infusions obtained, a finding confirmed in a subsequent experiment using a standard FR1 cocaine self-administration paradigm. We repeated the DS study using a food reward and found that inactivation of each region decreased performance accuracy but had no effect on the total number of food pellets earned. Additional experiments with the cocaine DS task found that dopamine receptor blockade in the IL, but not PL, reduced performance accuracy and disinhibited behavioral responding on DS− trials, whereas AMPA receptor blockade in the IL and PL had no effect on performance accuracy. These findings strongly suggest that, in a DS-based self-administration task in which rats must actively decide whether to engage in lever pressing (DS+) or withhold lever pressing (DS−) on a trial-by-trial basis, both the IL and PL contribute to the inhibitory control of drug-seeking behavior.
The medial prefrontal cortex (mPFC) is critically involved in the promotion and inhibition of the motivated behavior involved in drug seeking and taking. In the context of drug taking, the inhibition of behavior involves, in part, withholding drug seeking in situations in which drug rewards are not available. Considerable work has focused on understanding the roles of the infralimbic and prelimbic (IL and PL, respectively) subregions of the mPFC in such behavior, yet previous findings have produced conflicting results. Studies using self-administration paradigms indicate that PL and IL activity drive and inhibit, respectively, cocaine seeking during extinction and reinstatement or as part of an incubation of craving test (LaLumiere et al, 2012; Ma et al, 2014; McFarland and Kalivas, 2001). Yet, other work suggests more complicated pictures of the roles of each subregion in promoting or inhibiting motivated behavior (Bossert et al, 2011; Moorman and Aston-Jones, 2015a; Rogers et al, 2008; Van den Oever et al, 2008).
Drug-addicted individuals modulate their drug-seeking behavior in part by relying on specific cues, or discriminative stimuli (DSs), to predict the availability, or lack thereof, of drug reward (DS+ and DS−, respectively). Indeed, individuals with cocaine and heroin addiction have high levels of craving in contexts with a DS+ (McHugh et al, 2014; Weiss et al, 1995).
Similarly, discrete drug-paired stimuli invigorate drug-seeking and drug-taking behavior in rodents (Di Ciano and Everitt, 2003; Panlilio et al, 1996, 2000). Frequently, however, these stimuli are conditioned stimuli (CS) that coincide with delivery of the drug. In contrast, DSs precede drug delivery, providing information about the availability of the drug. With substantial training, rats are able to attend to both DS+ and DS− and to withhold drug taking in the presence of DS− (e.g., Kearns et al, 2005; Yun and Fields, 2003). This suggests the possibility of using such a design to elucidate how the IL and PL regulate the promotion and inhibition of cocaine seeking and taking in response to specific DSs.
Prior work has found that humans instructed to inhibit cigarette craving show activation of regions of the prefrontal cortex (Kober et al, 2010). In rats, withholding responding for cocaine in the presence of a long-lasting DS− enhances Fos expression in the PL and, to a lesser extent, the IL, suggesting the involvement of these regions in the inhibitory control of drug-taking behavior (Mihindou et al, 2013; Navailles et al, 2015). Other work using a DS task with a sucrose reward suggests that IL, but not PL, inactivation disinhibits behavioral responding, resulting in increased lever presses in the presence of a DS− signaling the absence of sucrose pellet availability (Ishikawa et al, 2008). In contrast, PL inactivation decreases lever pressing in the presence of a DS+.
These studies suggest the IL and PL may be involved in mediating appropriate behavioral responses during cocaine self-administration under conditions in which DSs predict drug availability and its absence. However, in light of the conflicting findings regarding how the IL and PL regulate drug seeking, the specific contribution of each region during such a task is unknown. Thus, in the present study, we employed a cocaine self-administration paradigm in which distinct DSs indicated whether a lever press would produce a cocaine infusion. Rats were given microinjections into the IL or PL and their performance in response to the DSs was assessed.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Charles River, ~300 g at the time of surgery, n = 62) were individually housed in a temperature-controlled environment and maintained on a 12-h reverse light-dark cycle. Rats were given ~20 g of rat chow per day. Methods were approved by the University of Iowa Institutional Animal Care and Use Committee and were in compliance with NIH guidelines for care of laboratory animals. Rats underwent behavioral training 6 days per week.
Surgery
Each rat underwent surgery for implantation of a double-barreled cannula aimed simultaneously at the infralimbic and prelimbic cortices (IL and PL, respectively) and, in some cases, implantation of an intravenous jugular catheter. Surgical details are provided in the Supplement.
Microinjections
Microinjection procedures are described in the Supplement. Drugs and doses for microinjections were based on previous work, as follows: GABAB/A receptor agonists baclofen and muscimol (BM, administered as a cocktail at 0.3 nmol and 0.03 nmol per side, respectively), the general dopamine receptor antagonist fluphenazine (10 nmol per side), and the AMPA/kainate glutamate receptor antagonist CNQX (1 nmol per side) (Cornish and Kalivas, 2000; Cosme et al, 2015; LaLumiere and Kalivas, 2008; McFarland and Kalivas, 2001). These drugs have been shown to act as agonists/antagonists for their respective receptors (Baufreton et al, 2001; Honore et al, 1988; O’Neil 2006; Morgan and Finch, 1986; Wang et al, 2003). All drugs were dissolved into artificial cerebrospinal fluid (aCSF), which also served as the vehicle control. A volume of 0.3 μL of aCSF or the drugs (BM, CNQX, or fluphenazine) was infused at a rate of 0.3 μL/min, and microinjectors remained in place for 3 minutes to permit diffusion. All rats received microinjections of aCSF and drug in both brain regions in a counterbalanced fashion, except when catheter patency was lost or rats fell ill before completion of microinjections. Thus, in most cases, rats received four microinjections. At least two training days occurred between each microinjection.
Behavioral Training
All experiments were carried out in operant chambers (Med Associates, Fairfield, VT) equipped with a central food trough flanked by retractable levers, as well as a house light and tone generator. Experiments 1, 4, and 5 used a discriminative stimulus (DS) cocaine self-administration task, whereas Experiment 2 used a Food DS task. The DS task procedures are described under Experiment 1 but apply to all four DS experiments. The procedures for Experiments 3 and 6 are described in their respective sections. In the final form of each task, sessions were 2 h in length.
Experiment 1
For all DS experiments, rats underwent a series of food training procedures to learn the DS task. For rats that performed the Cocaine DS task, cocaine was slowly substituted for the food reward. These initial training procedures are described in detail in the Supplement, whereas the following section describes the final parameters of the task in which microinjections were given.
The house light and tone generator served as the DSs, and the assignment as DS+ and DS− was counterbalanced across subjects. Sessions began with a 5 min “load-up” period, during which the DS+ was continuously presented except for a 20 s time out period following each lever press, and any lever presses on the active lever resulted in a cocaine infusion (Yun et al, 2003). During the full task described in Figure 1A, active lever presses during DS+ presentation resulted in the delivery of either a 50 μL infusion of 150 μg cocaine (Cocaine DS task) or a 45 mg food pellet (Food DS task) into the central reward receptacle, whereas lever presses during the DS− presentation had no outcome. Following reinforced lever presses or 10 s of cue presentation, whichever came first, both levers were retracted and the DS was extinguished for an intertrial interval of 40–70 s, pseudorandomly selected. During the Cocaine DS task used in Experiments 1, 4, and 5, the DS+ and the DS− were each presented 53–57 times per session, and the total number of each trial type presented differed by no more than three. We selected an intertrial interval and a cocaine dose that would produce more DS+ presentations than rats would respond to in order to avoid a ceiling effect in the event that a drug manipulation increased responding during DS+ trials. Lever retraction was used to ensure that all lever presses occurred in the presence of a DS+ or a DS−. Lever presses on the inactive (left) lever had no consequence. Accuracy in the task was assessed using the following formula: Rewards earned / total active lever responses * 100. In Experiments 1, 2, 4, and 5, rats were required to achieve 85% accuracy and at least 15 infusions or pellets for three consecutive days before the first microinjection. At least two consecutive days of 85% accuracy was required between microinjections.
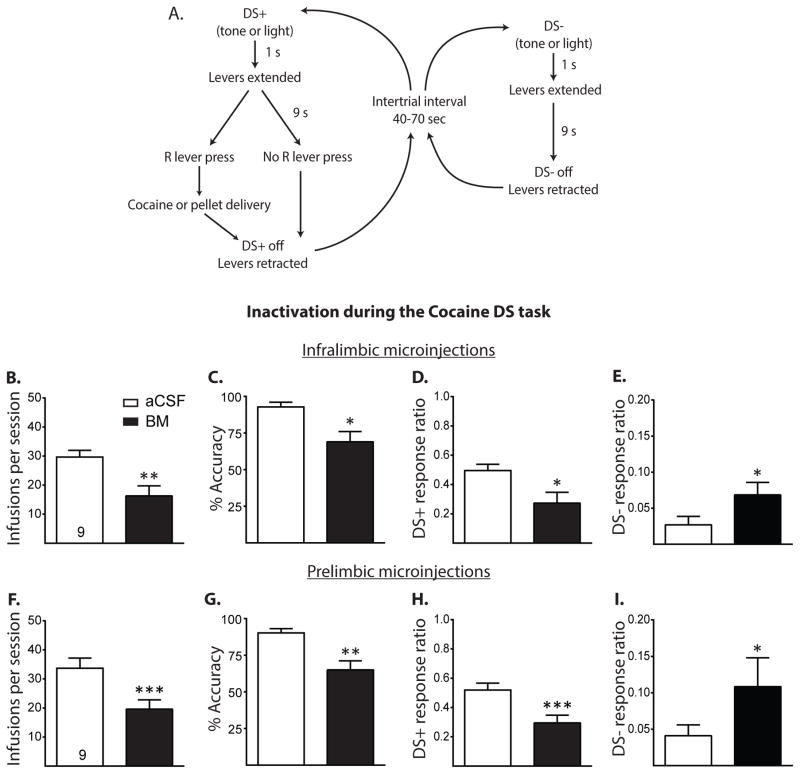
Figure 1.
A. Flowchart for the final form of the DS task. Trials began with the presentation of a house light or tone that served as the DSs (either the DS+ or the DS−), followed 1 s later by the extension of both levers. A right lever press within 9 s of lever extension resulted in the infusion of cocaine or delivery of a food pellet, termination of the DS+, and retraction of the levers. Lever presses during DS− trials had no programmed consequence. Intertrial intervals were 40–70 s long. B–E, Total cocaine infusions, accuracy, DS+ response ratio, and DS− response ratio of those rats receiving either aCSF or baclofen/muscimol (BM, 0.3 nmol and 0.03 nmol per side, respectively) microinjections into the IL during the Cocaine DS task, respectively (all given as mean +/− SEM). IL inactivation decreased total infusions, accuracy, and DS+ response ratio and increased DS− response ratio. F–I, Same measures as above, respectively, with rats receiving intra-PL microinjections. PL inactivation significantly decreased total infusions, accuracy, and DS+ response ratio, and increased DS− response ratio. #p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.0001.
Experiment 1 examined whether IL and PL inactivation altered performance in the Cocaine DS task. For this experiment, rats were given BM or aCSF microinjections into the respective brain region (IL or PL) immediately prior to undergoing the DS task on that day.
Experiment 2
To determine whether the results of Experiment 1 extended to a food pellet reward, Experiment 2 examined the effects of PL and IL inactivation during a Food DS task. Rats tended to respond on more behavioral trials during the Food DS task relative to the Cocaine DS task, and as such, the full 10 s between the DS+ presentation and lever retraction passed on fewer trials. Therefore, the DS+ and DS− were each presented 53 to 63 times per session in the Food DS task.
Experiment 3
Because both IL and PL inactivation decreased the number of cocaine infusions in Experiment 1, this experiment investigated whether IL and PL inactivation affected cocaine self-administration on a fixed ratio 1 (FR1) schedule of reinforcement, using self-administration procedures used previously (Cosme et al, 2015).
Experiment 4
As prior work indicates that dopamine input to the mPFC is necessary for at least some of the reinforcing effects of psychostimulants (Ecke et al, 2012; McGregor and Roberts, 1995), Experiment 4 investigated whether dopamine receptor blockade via microinjections of the dopamine receptor antagonist fluphenazine into the PL and IL alter behavior during the Cocaine DS task.
Experiment 5
To determine whether AMPA/kainate receptor blockade in the PL and IL alters behavior during the Cocaine DS task, rats received microinjections of the AMPA/kainate receptor antagonist CNQX into the IL and PL in the Cocaine DS task.
Experiment 6
The results from Experiments 1 and 2 indicated that PL inactivation decreased the number of cocaine infusions earned but, surprisingly, had no effect on the number of food pellets earned. Rats can immediately consume a food pellet upon delivery, whereas prior work indicates that an intravenous cocaine infusion requires approximately 10 s to increase dopamine levels in the brain (Aragona et al, 2008). Therefore, we considered the possibility that PL inactivation differentially affects motivation to earn immediate vs. delayed rewards or impairs rats’ ability to make an association between a lever press and the delivery of a delayed reward (cocaine). To address this issue, rats underwent training procedures for the Immediate vs. Delayed Food Reward task described in detail in the Supplement. In brief, rats learned that one cue predicted the extension of a lever associated with an immediate food reward, and the other cue predicted the extension of a different lever associated with a delayed (20 s) food reward. A lever press resulted in the delivery of the associated reward. The effects of IL and PL inactivation on immediate and delayed pellets earned during the task were then examined.
Histological Analysis
Rats were administered an overdose of sodium pentobarbital (100 mg/kg, i.p.) and intracardially perfused with saline. Brains were placed in 3.7% formaldehyde for a minimum of 48 h prior to sectioning. Coronal slices (75 μm) were taken and mounted on gelatin-coated slides. Sections were stained with Cresyl violet and coverslipped. Microinjector track marks were visualized using a light microscope, and rats with termination points that fell outside the IL or PL were excluded from analysis.
Data Analysis
Several measures of behavior were assessed. Calculation of total infusions included the 5 min load-up period at the beginning of the session, whereas all other analyses excluded this period. Total rewards earned (cocaine or food pellets) and percent accuracy were analyzed using a paired t-test to compare aCSF to drug microinjections. Because a change in accuracy can reflect a change in either the total rewards earned, the incorrect lever presses, or both, DS+ and DS− response ratios are also reported. DS+ and DS− response ratios are presented as the proportion of DS presentations to which the rats responded. The DS+ response ratio was calculated as: Total Infusions / Total DS+ presentations. The DS− response ratio was calculated as: DS− trials during which rats pressed the active lever / Total DS− presentations. Response ratios were analyzed using a paired t-test. Rats with < 70% accuracy on the aCSF day were excluded from analyses. For all analyses, p < 0.05 was considered statistically significant. Measures are expressed as mean ± SEM.
Results
Supplemental Figure 1 shows histological confirmation of correct cannula placements, with a detailed description of the included animals described in the Supplement. Supplemental Table 1 summarizes the main findings from Experiments 1–5. In all experiments, microinjections had no effect on the number of inactive lever presses (data not shown).
Experiment 1
Figure 1 shows the results of Experiment 1, in which rats received BM microinjections into the IL and PL during the Cocaine DS task. Figures 1B and C show that IL inactivation significantly decreased the number of cocaine infusions in the Cocaine DS task (t(8) = 3.67, p < 0.01) and decreased performance accuracy (t(8) = 3.07, p < 0.05). Figures 1D and E show that IL inactivation significantly decreased DS+ response ratio (t(8) = 2.50, p < 0.05), while increasing DS− response ratio (t(8) = 2.27, p < 0.05). Because the levers did not retract following responses on DS− trials, rats could make more than one response per trial. An examination of total active lever presses during both types of trials showed that rats made fewer active lever presses on DS+ trials following IL inactivation (mean ± SEM: 27.67 ± 2.56 for aCSF vs. 14.89 ± 4.03 for BM, t(8) = 2.56, p < 0.05) but made more lever presses on DS− trials following IL inactivation (mean ± SEM: 1.44 ± 0.59 for aCSF vs. 6.22 ± 1.59 for BM, t(8) = 2.32, p < 0.05). Figures 1F and G show that PL inactivation significantly decreased both the number of cocaine infusions t(8) = 6.07, p < 0.001) and performance accuracy (t(8) = 3.96, p < 0.01). Figures 1H and I show that PL inactivation significantly decreased DS+ response ratio (t(8) = 5.54, p < 0.001) and increased DS− response ratio (t(8) = 2.38, p < 0.05). An examination of total active lever presses showed that IL inactivation decreased total active lever presses during DS+ trials (mean ± SEM: 28.78 ± 2.64 for aCSF vs. 16.11 ± 2.85 for BM, t(8) = 5.80, p < 0.001), while marginally increasing active lever presses during DS− trials (mean ± SEM: 3.44 ± 1.04 for aCSF vs. 10.78 ± 4.16 for BM, t(8) = 1.92, p = 0.09).
Experiment 2
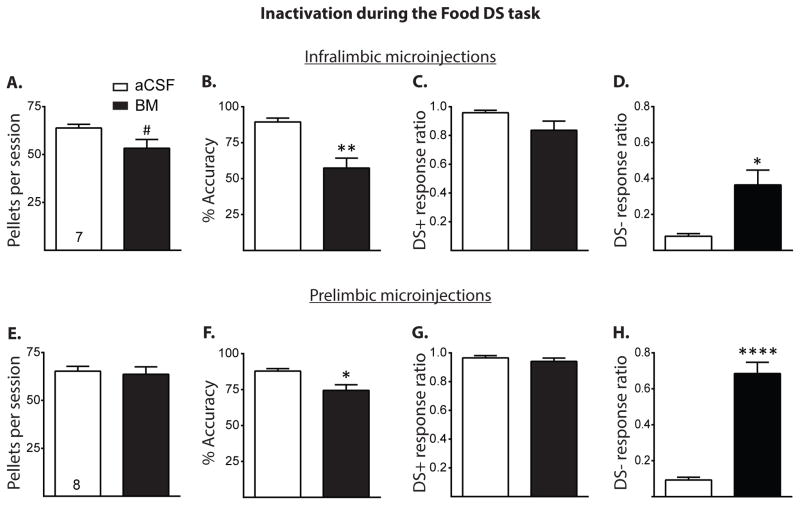
Figure 2 shows the results of Experiment 2, in which rats received BM microinjections into the IL and PL during a Food DS task. IL inactivation resulted in a trend for a decrease in the number of food pellets earned per behavioral session (Figure 2A, t(6) = 2.08, p = 0.08) and significantly decreased performance accuracy (Figure 2B, t(6) = 4.19, p < 0.01). Figures 2C and D show that IL inactivation had no effect on DS+ response ratio (t(6) = 1.69, p > 0.05) but resulted in an increase in the DS− response ratio (t(6) = 3.60, p < 0.05). IL inactivation had no effect on active lever presses during DS+ trials (mean ± SEM: 56.43 ± 0.72 for aCSF vs. 48.29 ± 3.89 for BM, t(6) = 1.89, p > 0.05) and marginally increased active lever presses during DS− trials (mean ± SEM: 7.0 ± 1.94 for aCSF vs. 44.86 ± 16.46 for BM, t(6) = 2.26, p = 0.06). PL inactivation had no effect on the number of food pellets earned (Figure 2E, t(7) = 0.76, p > 0.05) but significantly decreased performance accuracy in this task (Figure 2F, t(7) = 2.80, p < 0.05). Figures 2G and H show that PL inactivation had no effect on DS+ response ratio (t(7) = 1.35, p > 0.05) but significantly increased the DS− response ratio (t(7) = 11.05, p < 0.0001). PL inactivation had no effect on active lever presses during DS+ trials (mean ± SEM: 53.00 ± 1.32 for aCSF vs. 51.63 ± 2.14 for BM, t(7) = 0.82, p > 0.05) and significantly increased active lever presses during DS− trials (mean ± SEM: 7.50 ± 1.22 for aCSF vs. 18.38 ± 3.49 for BM, t(7) = 2.62, p < 0.05).
Figure 2.
Results of IL and PL inactivation during the Food DS task. A–D, Total food pellets, accuracy, DS+ response ratio, and DS− response ratio of those rats receiving either aCSF or baclofen/muscimol (BM, 0.3 nmol and 0.03 nmol per side, respectively) microinjections into the IL during the Food DS task, respectively (all given as mean +/− SEM). IL inactivation marginally decreased food pellets obtained, significantly decreased accuracy, and increased the DS− response ratio, without affecting the DS+ response ratio. E–H, Same measures as above, respectively, with rats receiving intra-PL microinjections. PL inactivation decreased accuracy and increased DS− response ratio but had no effect on food pellets obtained or DS+ response ratio. #p = < 0.1, *p < 0.05, **p < 0.01, ****p < 0.0001.
Experiment 3
Figure 3 shows the results of Experiment 3, in which rats received BM microinjections into the IL and PL during cocaine self-administration on an FR1 schedule of reinforcement (Cocaine FR1 task). IL and PL inactivation decreased the total number of cocaine infusions (Figure 3A and B, t(6) = 3.90, p < 0.01; t(6) = 4.47, p < 0.01, respectively).
Figure 3.
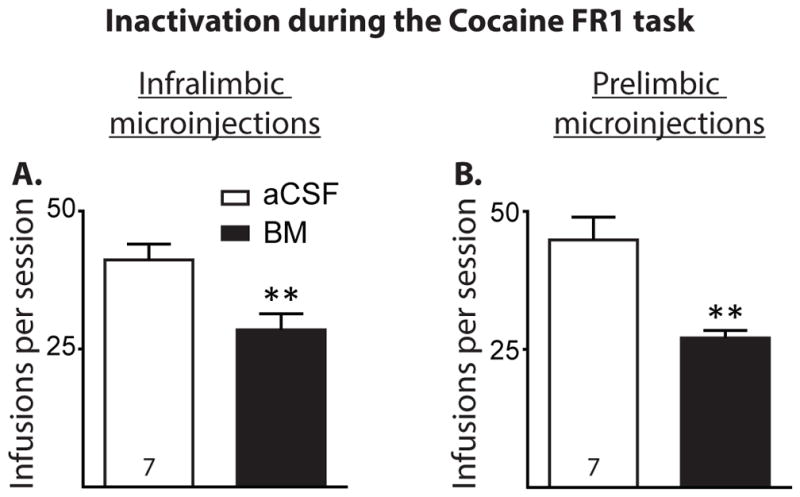
Results of IL and PL inactivation during the Cocaine FR1 task. A and B, Cocaine infusions taken with IL and PL inactivation with baclofen/muscimol (BM) microinjections, respectively (number of infusions shown as mean +/− SEM). IL and PL inactivation decreased the number of cocaine infusions in the FR1 cocaine self-administration task. **p < 0.01.
Experiment 4
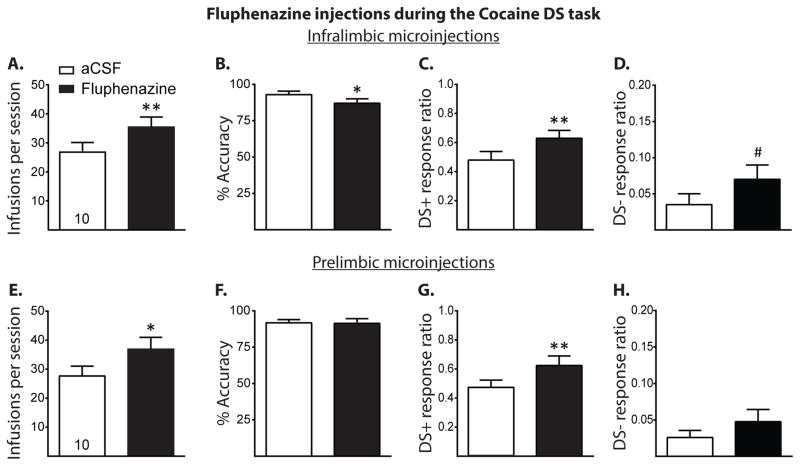
Figure 4 shows the results of Experiment 4, in which rats received fluphenazine microinjections into the IL and PL during the Cocaine DS task. Dopamine receptor blockade in the IL significantly increased the total number of infusions (Figure 4A, t(9) = 3.46, p < 0.01) and decreased performance accuracy (Figure 4B, t(9) = 2.45, p < 0.05). Figures 4C and D show that dopamine receptor blockade significantly increased the DS+ response ratio (t(9) = 3.28, p < 0.01) and marginally increased the DS− response ratio (t(9) = 1.99, p = 0.07). IL dopamine receptor blockade increased active lever presses during DS+ trials (mean ± SEM: 26.50 ± 3.32 for aCSF vs. 35.00 ± 3.18 for fluphenazine, t(9) = 3.20, p < 0.05) and marginally increased active lever presses during DS− trials (mean ± SEM: 1.60 ± 1.09 for aCSF vs. 5.60 ± 1.56 for fluphenazine, t(9) = 2.02, p = 0.07). As shown in Figures 4E and F, respectively, dopamine receptor blockade in the PL significantly increased the total number of cocaine infusions (t(9) = 3.23, p < 0.05), but had no effect on accuracy (t(9) = 0.35, p > 0.05). Intra-PL microinjections of fluphenazine increased DS+ response ratio (Figure 4G, t(9) = 4.02, p < 0.01) and had no effect on DS− response ratio (Figure 4H, t(9) = 1.21, p > 0.05). PL dopamine receptor blockade increased active lever presses during the DS+ trials (mean ± SEM: 25.70 ± 2.79 for aCSF vs. 34.40 ± 3.76 for fluphenazine, t(9) = 4.24, p < 0.01) but had no effect on active lever presses during DS− trials (mean ± SEM: 2.0 ± 0.68 for aCSF vs. 4.4 ± 1.78 for fluphenazine, t(9) = 1.29, p = 0.23).
Figure 4.
Results of dopamine receptor blockade in the IL and PL during the Cocaine DS task. A–D, Cocaine infusions, accuracy, DS+ response ratio, and DS− response ratio of those rats receiving either aCSF or fluphenazine (10 nmol/side) microinjections into the IL during the Cocaine DS task, respectively (all given as mean +/− SEM). Intra-IL microinjections of fluphenazine significantly increased cocaine infusions and DS+ response ratio, significantly decreased accuracy, and marginally increased DS− response ratio. E–H, Same measures as above, respectively, with rats receiving intra-PL microinjections. Dopamine receptor blockade in the PL significantly increased the number of cocaine infusions taken and increased the DS+ response ratio but otherwise had no effect on any other measure. #p < 0.1, *p < 0.05, ** p < 0.01.
Experiment 5
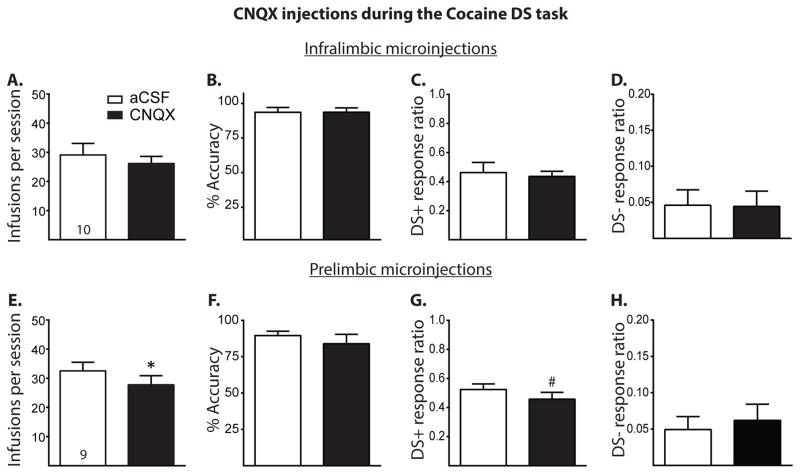
Figure 5 shows the results of Experiment 5, in which rats received intra-IL and -PL microinjections of the AMPA/kainate receptor antagonist CNQX during the Cocaine DS task. As shown in Figures 5A and B, respectively, IL AMPA receptor blockade did not alter the total number of infusions (t(8) = 0.90, p > 0.05) or performance accuracy (t(8) = 0.47, p > 0.05). Similarly, IL AMPA receptor blockade had no effect on DS+ response ratio (Figure 5C, t(8) = 0.40, p > 0.05) or DS− response ratio (t(8) = 0.15, p > 0.05). IL AMPA receptor blockade had no effect on active lever presses during DS+ trials (mean ± SEM: 25.60 ± 4.01 for aCSF vs. 23.80 ± 1.93 for fluphenazine, t(9) = 0.57, p > 0.05) or during DS− trials (mean ± SEM: 3.60 ± 1.69 for aCSF vs. 3.1 ± 1.48 for CNQX, t(9) = 0.42, p > 0.05). As shown in Figures 5E and F, respectively, PL AMPA receptor blockade significantly decreased the total number of cocaine infusions earned (t(8) = 2.84, p < 0.05) but did not affect performance accuracy (t(8) = 0.85, p > 0.05). Figures 5G and H show that CNQX microinjection into the PL marginally decreased the DS+ response ratio (t(8) = 2.13, p = 0.07) without affecting the DS− response ratio (t(8) = 0.96, p > 0.05). PL AMPA receptor blockade marginally decreased active lever presses during DS+ trials (28.78 ± 2.20 for aCSF vs. 25.11 ± 2.65 for CNQX, t(8) = 2.23, p = 0.06) but had no effect on active lever presses during DS− trials (mean ± SEM: 3.78 ± 1.25 for aCSF vs. 6.44 ± 3.92 for CNQX, t(8) = 0.91, p > 0.05).
Figure 5.
A, Results of AMPA receptor blockade in the IL and PL during the Cocaine DS task. A–D, Cocaine infusions, accuracy, DS+ response ratio, and DS− response ratio of those rats receiving either aCSF or CNQX (1 nmol/side) microinjections into the IL during the Cocaine DS task, respectively. Intra-IL microinjections of CNQX had no effects on any measure during the task (all given as mean +/− SEM). E–H, Same measures as above, respectively, with rats receiving intra-PL microinjections. AMPA receptor blockade in the PL significantly decreased the number of cocaine infusions taken and marginally decreased the DS+ response ratio but had no effect on accuracy or DS− response ratio. #p < 0.1, *p < 0.05.
Experiment 6
Supplemental Figure 2 shows the results of Experiment 6, in which rats received BM microinjections into the IL and PL in the Immediate vs. Delayed Food Reward task. IL and PL inactivation had no effect on the total number of immediate or delayed pellets earned.
Discussion
The present study extends prior work examining how the IL and PL subregions of the mPFC control reward-seeking behavior, particularly with regard to the promotion and inhibition of the instrumental behavior involved in obtaining cocaine. In an effort to use a different approach to address this issue, these experiments used tasks in which distinct discriminative stimuli (DSs) signaled the availability, or lack thereof, of a reward. Our findings suggest that the IL and PL both contribute to the inhibitory control of cocaine and food seeking. Specifically, IL and PL inactivation decreased accuracy and increased DS− responses for the Cocaine and Food DS tasks. IL and PL inactivation also reduced DS+ responses and the number of cocaine infusions earned during the DS task, though only IL inactivation marginally decreased the number of rewards earned during the Food DS task. Finally, the present results indicate that a dopamine receptor antagonist increased the total number of cocaine infusions when microinjected into the IL or PL but impaired performance accuracy and marginally increased DS− responding only when given into the IL.
IL vs. PL control of drug and non-drug reward seeking
The goal of the present study was to examine the roles of the IL and PL in a cocaine self-administration task in which both promotion and inhibition of instrumental behavior could be simultaneously observed by using DSs to indicate reward availability. The decreased performance accuracy in the Cocaine and Food DS tasks with PL and IL inactivation reflects, at least in part, increased active lever pressing in the presence of the DS−, suggesting activity in these brain regions contributed to behavioral inhibition in the presence of the DS−. IL and PL inactivation appeared to produce greater levels of disinhibition in the Food DS task relative to the Cocaine DS, as evidenced by the DS− response ratios following IL and PL inactivation. However, it is important to note that this likely reflects differences in the nature of the reinforcement. As a post-injection pause following intravenous cocaine infusions at this dose is estimated to be > 120 s (Tsibulsky and Norman, 1999), rats may not engage in cocaine-seeking in the presence of DS− due to cocaine satiation. In contrast, rats are unlikely to be sated by a 45 mg pellet of food, and therefore, may be more likely to engage in food seeking when a DS− trial follows a DS+ trial. Thus, the substantial difference in the DS+ response ratios between the two tasks raises a critical caveat in making direct comparisons.
Many findings suggest that the PL and IL play opposing roles, driving and inhibiting reward seeking, respectively. Pharmacology studies using a DS-based sucrose-seeking task indicate that the IL inhibits behavioral responding when a DS− is present, whereas the PL drives such responding in the presence of a DS+ (Ishikawa et al, 2008). Consistent with opposing roles for these regions, IL activity suppresses cocaine seeking following extinction training, whereas PL activity drives the reinstatement of drug-seeking behavior (LaLumiere et al, 2012; McFarland and Kalivas, 2001; McLaughlin and See, 2003; Peters et al, 2008; Willcocks and McNally, 2013). Similarly, recent work using an incubation of craving paradigm found that plasticity in the IL-to-nucleus accumbens pathway is responsible for inhibiting the incubation of cocaine craving, whereas plasticity in the PL-to-accumbens pathway does the opposite (Ma et al, 2014).
Nonetheless, other research suggests the IL and PL do not serve strictly dichotomous roles, and in many studies, the PL exerts inhibitory control over motivated behavior (Moorman et al, 2015b). Consistent with this, previous work has found that disruption of PL activity impairs inhibitory control of behavior in stop-signal and waiting tasks (Broersen and Uylings, 1999; Jonkman et al, 2009; Narayanan et al, 2006) and, in one case, has found that IL inactivation does not alter inhibitory control in a stop-signal task (Bari et al, 2011). In other work, the PL, and to a lesser extent the IL, shows enhanced c-Fos expression when rats engage in the inhibitory control of cocaine-taking behavior during a 30-min session in which a DS− is continuously presented and lever presses on a previously active lever are no longer reinforced (Mihindou et al, 2013; Navailles et al, 2015). In the study by Mihindou and colleagues, PL, but not IL, inactivation disinhibited cocaine-seeking behavior in the presence of the DS−. Of particular interest, in a recent electrophysiology study that used a sucrose-seeking task in a paradigm similar to the present one, IL and PL activity did not show appreciable differences compared to each other during the promotion vs. extinction of the sucrose-seeking behavior (Moorman et al, 2015a).
Although speculative, it is likely that the discrepancies among these studies, including the current one, are accounted for by differences in the behaviors under investigation (reinstatement vs. self-administration), the task design, and the type of reward. Indeed, the mPFC likely engages in relatively complex processing in its control over motivated behavior and, thus, it is not surprising that the roles of the mPFC and its subregions are highly dependent on subtle task differences and demands. For example, in contrast to the study by Mihindou and colleagues noted above, the present study required rats to attend to the DS presentation on each trial and found that inactivation of either region disinhibited cocaine seeking on DS− trials. Thus, the nature of the DS – i.e., serving as either a long-lasting contextual cue or as a discrete trial-by-trial cue – likely involves rather different neural processing, as the demands required for each DS are distinct and, therefore, would seem to recruit the mPFC subregions in different ways.
Because IL and PL inactivation increased DS− response ratio while decreasing DS+ response ratio, response withholding in the presence of DS− versus a DS+ would appear to be mediated by different neural processes. However, a distinct possibility is that the neural processes underlying these phenomena are linked or even the same. Indeed, IL/PL inactivation may simply induce a reduction in discriminative abilities of the rats. However, as inactive lever presses did not change, such a reduction must not be global in its effects on discrimination. Moreover, it should be noted that dopamine receptor blockade in the IL increased the DS+ response ratio as well as marginally increased the DS− response ratio, a distinct effect compared to the other experiments. This suggests that these processes are either independent or can be independently influenced, even if they are in fact linked.
Previous work indicates that the PL critically contributes to instrumental responding and PL lesions impair action-outcome associations (Balleine and Dickinson 2005; Corbit and Balleine 2003), suggesting the possibility that PL inactivation has effectively disrupted the rat’s knowledge of the task rules. Other work using a sucrose-seeking task reports that the IL and PL both encode contextually appropriate behavioral initiation or withholding during a DS task, suggesting a more general role of the IL and PL in encoding information that is used to guide behaviorally appropriate responses (Moorman et al, 2015a). Thus, impaired performance accuracy resulting from IL and PL inactivation in the present task may result from disruption of encoding appropriate external information (e.g., discrete cues). In the absence of the successful encoding of the information provided by the DS−, rats would be expected to engage in cocaine-seeking behavior, ultimately resulting in a decrease in performance accuracy.
Changes in earned rewards
The decrease in accuracy following IL and PL inactivation in the Cocaine DS task was accompanied by a decrease in the total number of cocaine infusions earned, as well as a decrease in the DS+ response ratio. As IL and PL inactivation similarly decreased cocaine infusions under an FR1 schedule of reinforcement, the decrease in infusions in the Cocaine DS task may reflect a change in the reinforcing properties of cocaine or a decrease in motivation to self-administer cocaine. However, if that is the case, it is unclear why this was accompanied by an increase in DS− responding, again raising the question about potential linkage in the neural processing underlying these behaviors. One possibility is that, even with the decrease in reinforcing properties or motivation, there was a simultaneous disruption in discriminative ability, leading to impaired accuracy. Presumably, if these could be separated, IL and PL inactivation could produce an even greater level of responding during the DS− than was currently observed. Our findings showing a decrease in cocaine intake are consistent with previous work showing that PL inactivation reduces drug taking in a paradigm in which contextual cues predict drug availability (Di Pietro et al, 2006). However, they are in discordance with two previous studies that report that the PL inhibits cocaine taking. Chen et al reported that optical stimulation of the PL attenuates earned cocaine infusions in rats that showed compulsive cocaine-taking behavior, suggesting a role for the PL in inhibiting drug taking (Chen et al, 2013). In this study, rats were required to press a cocaine-seeking lever, followed by a cocaine-taking lever, and the compulsivity of cocaine seeking was later examined by exposing the rats to footshock. In another study, optical inhibition of the PL increased cocaine self-administration in rats that had short, but not long, interinfusion intervals on an FR5 schedule (Martin-Garcia et al, 2014). In both cases, notable differences in task design make direct comparison difficult and likely underlie the discrepancy.
Receptor-specific manipulations
Based on the initial findings using GABAergic agonists to inactivate the mPFC, the present study then examined the roles of dopaminergic and fast excitatory neurotransmission in the mPFC in the Cocaine DS task. In contrast to IL and PL inactivation, dopamine receptor blockade in the IL, but not PL, decreased accuracy, suggesting the importance of dopamine receptors in the IL in the inhibitory control of cocaine seeking. However, the decrease in performance accuracy with fluphenazine in the IL, while statistically significant, was lower in magnitude compared to that observed with IL inactivation (6% vs. 19%, respectively), suggesting that the IL dopamine receptors only partially contribute to the role of the IL in withholding responses in this task. Unexpectedly, AMPA receptor blockade in the PL and IL had no effect on performance accuracy or DS− response ratio, indicating that AMPA receptor-mediated transmission played little-to-no role in the inhibition of such behavior.
Additionally, the present experiments found that dopamine receptor blockade in both structures increased cocaine self-administration, as evidenced by the increase in number of cocaine infusions obtained, as well as the increase in the DS+ response ratio. This is consistent with previous work showing that dopamine receptor blockade in the medial prefrontal cortex increased cocaine self-administration on an FR1 schedule of reinforcement (Ecke et al, 2012; McGregor et al, 1995). These findings suggest that dopamine receptor activation in the mPFC plays a role in the reinforcing effects of cocaine, as the rats take more cocaine in order to increase dopamine levels even further and “overcome” the dopamine receptor blockade.
Experimental Design Considerations
As noted in the Introduction, CSs and DSs may both be important aspects of drug use that help to drive drug-seeking behavior in humans, and, indeed, CSs acting as conditioned reinforcers have been widely used in self-administration and reinstatement paradigms to drive lever-pressing behavior. Though less common, paradigms using DSs permit interrogation of the systems that utilize external cues to both promote and inhibit drug-seeking behavior. Although some previous studies have used a DS− to indicate that lever presses on a previously rewarded or active lever will no longer result in drug delivery, these have often used a long-lasting DS− (e.g., a house light turning on or off for a length of time) indicating drug availability. In contrast, the present experiments used a modified version of a DS task used by Yun and colleagues, which employed discrete, short presentations of DSs (Yun et al, 2003). Thus, rats were required to engage in or withhold lever-pressing behavior on a trial-by-trial basis depending on the specific stimulus presented (DS+ vs. DS−, respectively). Because this requires attention at each trial and the DS+ and DS− trials are interspersed over the 2 h test period, concerns about the time course of animal behavior and/or half-life of pharmacological agents are mitigated. Moreover, the present task and its associated measures enable dissociation between neural activity responsible for driving behavior during the DS+ and withholding behavior during the DS−. It should be noted, however, that the Cocaine DS task used herein required extensive training relative to a standard FR1 self-administration task, a challenge that may preclude its widespread use.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Edward Wasserman for insightful discussion regarding the task design.
Footnotes
Author Contributions
ALG and RTL conceptualized and designed the experiment. ALG, VAE, CVC, and WRW carried out the experiments. ALG analyzed data. ALG and RTL wrote the manuscript, and VAE, WRW, and CVC provided manuscript revisions.
Funding and Disclosure
This research was supported by NIH grant USPHS DA034684 (RTL). The authors declare no conflict of interest.
References
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28(35):8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4–5):407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31(25):9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Garret M, Dovero S, Dufy B, Bioulac B, Taupignon A. Activation of GABA(A) receptors in subthalamic neurons in vitro: properties of native receptors and inhibition mechanisms. J Neurophysiol. 2001;86(1):75–85. doi: 10.1152/jn.2001.86.1.75. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14(4):420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen LM, Uylings HB. Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience. 1999;94(1):47–57. doi: 10.1016/s0306-4522(99)00312-7. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496(7445):359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146(1–2):145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20(15):RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme CV, Gutman AL, LaLumiere RT. The dorsal agranular insular cortex regulates the cued reinstatement of cocaine-seeking, but not food-seeking, behavior in rats. Neuropsychopharm. 2015;40(10):2425–2433. doi: 10.1038/npp.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci. 2003;117(5):952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24(11):3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Ecke LE, Elmer GI, Suto N. Cocaine self-administration is not dependent upon mesocortical alpha1 noradrenergic signaling. Neuroreport. 2012;23(5):325–330. doi: 10.1097/WNR.0b013e3283517628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, et al. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155(3):573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Mar AC, Dickinson A, Robbins TW, Everitt BJ. The rat prelimbic cortex mediates inhibitory response control but not the consolidation of instrumental learning. Behav Neurosci. 2009;123(4):875–885. doi: 10.1037/a0016330. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ, Schindler CW, Panlilio LV. Conditioned inhibition of cocaine seeking in rats. J Exp Psychol Anim Behav Process. 2005;31(2):247–253. doi: 10.1037/0097-7403.31.2.247. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107(33):14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. The European journal of neuroscience. 2012;35(4):614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Garcia E, Courtin J, Renault P, Fiancette JF, Wurtz H, Simonnet A, et al. Frequency of cocaine self-administration influences drug seeking in the rat: Optogenetic evidence for a role of the prelimbic cortex. Neuropsychopharm. 2014;39(10):2317–2330. doi: 10.1038/npp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behav Brain Res. 1995;67(1):75–80. doi: 10.1016/0166-4328(94)00106-p. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Park S, Weiss RD. Cue-induced craving in dependence upon prescription opioids and heroin. Am J Addict. 2014;23(5):453–458. doi: 10.1111/j.1521-0391.2014.12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168(1–2):57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Mihindou C, Guillem K, Navailles S, Vouillac C, Ahmed SH. Discriminative inhibitory control of cocaine seeking involves the prelimbic prefrontal cortex. Biol Psychiatry. 2013;73(3):271–279. doi: 10.1016/j.biopsych.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc Natl Acad Sci U S A. 2015a;112(30):9472–9477. doi: 10.1073/pnas.1507611112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015b;1628(Pt A):130–146. doi: 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DG, Finch CE. [3H]Fluphenazine binding to brain membranes: simultaneous measurement of D-1 and D-2 receptor sites. J Neurochem. 1986;46(5):1623–1631. doi: 10.1111/j.1471-4159.1986.tb01785.x. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139(3):865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Navailles S, Guillem K, Vouillac-Mendoza C, Ahmed SH. Coordinated Recruitment of Cortical-Subcortical Circuits and Ascending Dopamine and Serotonin Neurons During Inhibitory Control of Cocaine Seeking in Rats. Cereb Cortex. 2015;25(9):3167–3181. doi: 10.1093/cercor/bhu112. [DOI] [PubMed] [Google Scholar]
- O’Neil MJ. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Whitehouse Station, N.J: Merck; 2006. [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacology (Berl) 1996;125(3):202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Effects of compounding drug-related stimuli: escalation of heroin self-administration. J Exp Anal Behav. 2000;73(2):211–224. doi: 10.1901/jeab.2000.73-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151(2):579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839(1):85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nature neuroscience. 2008;11(9):1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- Wang D, Cui LN, Renaud LP. Pre- and postsynaptic GABA(B) receptors modulate rapid neurotransmission from suprachiasmatic nucleus to parvocellular hypothalamic paraventricular nucleus neurons. Neuroscience. 2003;118(1):49–58. doi: 10.1016/s0306-4522(02)00906-5. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Hufford C. Craving in hospitalized cocaine abusers as a predictor of outcome. Am J Drug Alcohol Abuse. 1995;21(3):289–301. doi: 10.3109/00952999509002698. [DOI] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37(2):259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121(3):747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.