Abstract
Hirsutella thompsonii (Ht) is a fungal pathogen of acarines and the primary cause of epizootics among mites. The draft genomes of two isolates of Ht (MTCC 3556: Ht3, 34.6 Mb and MTCC 6686: Ht6, 34.7 Mb) are presented and compared with the genomes of Beauveria bassiana (Bb) ARSEF 2860 and Ophiocordyceps sinensis (Os) CO18. Comparative analysis of carbohydrate active enzymes, pathogen–host interaction genes, metabolism-associated genes, and genes involved in biosynthesis of secondary metabolites in the four genomes was carried out. Reduction in gene family sizes in Ht3 and Os as compared with Ht6 and Bb is observed. Analysis of the mating type genes in Ht reveals the presence of MAT idiomorphs which is suggestive of cryptic sexual traits in Ht. We further identify and classify putative chitinases that may function as virulence factors in fungal entomopathogens due to their role in degradation of arthropod cuticle.
Keywords: glycoside hydrolase family 18; mycoinsecticide; pathogen–host interactions; phylogenetic classification; subgroups A, B, and C
Introduction
Hirsutella thompsonii (Ht) (Ophiocordycipitaceae, Hypocreales, and Sordariomycetes) is a pathogenic fungal species that infects arthropod hosts and causes natural epizootics in populations of blueberry bud mites (Acalitus vaccinii), citrus rust mites (Phyllocoptruta oleivora), and coconut fruit mites (Aceria guerreronis) (Samson et al. 1980; Weibelzahl and Liburd 2009). It produces an insecticidal protein Hirsutellin A, which is ribotoxic to a variety of pest populations (Herrero-Galán et al. 2008, 2013). The insecticidal property of Ht has been exploited, for example, spore suspension of Ht is used against Varroa mites and promoted under the trade name Mycar, but this product has limited commercial success due to efficacy issues (Haragsim and Ruzicka 2001; Weinzierl et al. 2005). The genus Hirsutella includes some other well-known insect-pathogenic fungi, such as Hirsutella rhossiliensis and Hirsutella sinensis (Sung et al. 2007). A range of bioactive secondary metabolites have been reported in Hirsutella species, such as linear and cyclic nonribosomal peptides: Hirsutellic acid A, hirsutide, hirsutatins A and B; polyketide synthase–nonribosomal peptide synthase (PKS–NRPS) hybrid metabolites: Cytostatic cytochalasin Q (Molnar et al. 2010). Some strains of Hirsutella are also used in traditional Chinese medicines and neutraceutical preparations (Paterson 2008; Molnar et al. 2010).
The dynamics of insect–fungal interactions is complex and has critical implications in biocontrol of insect pests. Recent genome-based studies are providing interesting insights into genetic and pathogenic mechanisms involved in insect–fungal interactions (Gao et al. 2011; Kubicek et al. 2011; Zheng et al. 2011; Xiao et al. 2012; Bushley, Raja, et al. 2013; Wang et al. 2014). For example, phylogenomic analyses of insect-pathogenic fungi Beauveria bassiana (Bb) (Xiao et al. 2012), Cordyceps militaris (Zheng et al. 2011), and Metarhizium anisopliae (Gao et al. 2011) indicate similarity in evolutionary expansion of gene families such as chitinases, lipases, proteases, etc. within insect-pathogenic fungi that could be attributed to their insect killing strategies and host ranges. Such genome-wide studies are the leading steps for comprehensive understanding of insect–fungal associations in the context of fungal evolution and ecology. This study was thus initiated to decipher the genome sequence of Ht and compare it with two phylogenetically closely related species within the fungal order Hypocreales: Ophiocordyceps sinensis (Os) (Ophiocordycipitaceae) (Liu et al. 2002; Hu et al. 2013), and Bb (Cordycipitaceae), an effective mycoinsecticide (Xiao et al. 2012), so as to reveal similarities and differences in their genetic make-up and its possible implications in insect–fungal interactions and pathogenicity.
Insect-pathogenic fungi disintegrate the chitin-rich insect cuticle by the action of a variety of chitinases (Charnley 2003). Fungal chitinases (EC 3.2.1.14) have been exploited in the development of biocontrol strategies against insects, fungi, or nematodes (Seidl 2008; St. Leger and Wang 2010). Pathogenic fungi contain various chitin-degrading enzymes with an average of 10–20 different chitinases, where chitinases play diverse biological roles, such as cell-wall remodeling, nutrient scavenging and aggressive functions (Seidl 2008; Gao et al. 2011; Hartl et al. 2012; Hamid et al. 2013). Genome surveys of chitinases from pathogenic fungi suggest high variability in gene size and modular structure of chitinases that could be related to their diverse lifestyle pattern (Seidl et al. 2005; Karlsson and Stenlid 2009; Hartl et al. 2012). However, there has been no report on diversity and phylogeny of chitinases from Hirsutella species. Our study focuses on the evolutionary relationships of Bb, Ht, and Os chitinases.
Materials and Methods
Fungal Isolates and Maintenance
Hirsutella thompsonii isolates MTCC 3556 (Ht3) and MTCC 6686 (Ht6) were accessed from the Microbial Type Culture Collection (MTCC), located at CSIR-Institute of Microbial Technology, Chandigarh, India. The Ht3 strain, isolated from coconut perianth mite (Aceria guerreronis), was deposited in MTCC by S. Ramarethinam from J. Stanes and Co. Ltd., Coimbatore, Tamil Nadu. The Ht6 strain, isolated from red spider mite, was deposited in MTCC by S. Debnath of the Tea Research Association, P.O. Cinnamara, Jorhat, Assam.
The fungal cultures were grown on potato-dextrose agar medium and incubated at 25 °C for 6–8 days. Glycerol stocks were prepared from fresh mycelia in 10% glycerol and stored at −70 °C for further use. Genomic DNA was isolated from fresh mycelia using ZR Fungal/Bacterial DNA kit (Zymo Research, catalog number D6005) following manufacturer’s protocol. The concentration and quality of DNA extracted were assessed by Nanodrop Spectrophotometer ND-1000 (Thermo Scientific, Wilmington) and 0.8% agarose gel electrophoresis, respectively.
Morphological Characterization of Ht3 and Ht6
Fungal morphological features were observed under Olympus BX51 microscope. The microscopic images were captured using Olympus XC10 digital camera and analyzed using CellB image analysis software (Olympus, Tokyo, Japan).
Phylogenetic Position of Ht3 and Ht6
The nuclear ribosomal small and large subunits (nrSSU and nrLSU), elongation factor 1α (tef1), the largest subunit of RNA polymerase ІІ (rpb1), and β-tubulin (tub) genes were retrieved from the Ht3 and Ht6 genomes. The gene sequences were included in the concatenated five gene data set of hypocrealean fungi (Sung et al. 2007). Maximum parsimony analysis was performed in PAUP v4.0 (Swofford 2003). Ambiguously aligned regions were excluded from the analysis. Gaps were treated as missing data. Trees were inferred using the heuristic search option with 1,000 random sequence additions.
Genome Sequencing, Assembly, and Annotation
Paired-end (PE) (insert size of 350 bp and length 101 bp) shotgun sequencing of Ht genomes was performed using Illumina HiSeq 1000 technology at the Centre for Cellular and Molecular Platforms (C-CAMP) Bangalore, India. Mate-pair (MP) (insert size of 2 kb and length 101 bp) information was also obtained for Ht3. De novo assembly was performed using CLCbio wb6.0 (http://www.clcbio.com/, last accessed March 10, 2015). The data sets were scanned and filtered for the presence of any reads from bacterial DNA that may be present as contaminants. The DNA of Ht6 was found to likely be contaminated by some of the bacterial strains, mainly belonging to the genera, Bordetella, Burkholderia, Escherichia, and Phyllobacterium. The genomes of all available species of these bacteria were retrieved from National Centre for Biotechnological Information (NCBI) and the reads of Ht6 were aligned to these genomes. The contigs obtained were also subjected to BLAST against nucleotide collection database at NCBI, and the reads that showed significant matches to only bacterial sequences but no hits to fungal sequences were discarded in the final assembly. The refined contigs obtained were scaffolded using SSPACE v2.0 (Boetzer et al. 2011) and gaps were filled using GapFiller v1.10 (Boetzer and Pirovano 2012).
Genome annotation was performed using the MAKER pipeline v2.0 with Augustus as the gene predictor (Stanke and Morgenstern 2005; Cantarel et al. 2008). Bb ARSEF 2860 (PRJNA38719) and Os CO18 (PRJNA59569) genomes were reannotated using the same methodology. Combinations of both the original and reannotated set of genes/proteins from all genomes were used for further computational analyses. The predicted proteins were subjected to BLASTp (Altschul et al. 1990) against the nonredundant protein sequences database at NCBI. The tRNAs in Ht3 and Ht6 were identified using the tRNAscan-SE v1.3 (Lowe and Eddy 1997). The draft genomes of Ht3 and Ht6 were deposited at NCBI-GenBank under the accession number APKB00000000 and APKU00000000, respectively.
Cluster of Orthologous Groups
In order to classify the proteins into orthologous groups of various cellular and metabolic functions, clusters of orthologous groups (COG) were generated by subjecting the predicted proteomes to BLASTp against the COG database (Tatusov et al. 2000) (http://www.ncbi.nlm.nih.gov/COG/, last accessed March 10, 2015) with an E value cutoff of 1e−5. For each COG mapping, functional assignment was provided based on COG general category letter associations. Orthologous proteins among all the four genomes were characterized by reciprocal best BLAST hits (E value cutoff of 1e−10) obtained using a PERL script of Proteinortho v2.0 (Lechner et al. 2011). Gene family expansions and contractions were estimated using the Computational Analysis of gene Family Evolution (CAFE) v3.0 (Han et al. 2013). Molecular Evolutionary Genetics Analysis (MEGA) v6.0 (Tamura et al. 2013) was used to prepare Maximum Likelihood Newick tree that was used as an input for CAFE. Significant changes in gene numbers were analyzed at P value cutoff of 0.01.
Carbohydrate Active Enzymes
Structurally related catalytic enzymes belonging to classes, glycoside hydrolases (GHs), glycosyltransferases (GTs), polysaccharide lyases (PLs), carbohydrate esterases (CEs) and auxiliary activities (AAs), and associated carbohydrate-binding modules (CBMs) were searched in the predicted proteome of all the four genomes. The HMM profiles of CAZy database (Lombard et al. 2014) (http://www.cazy.org/, last accessed March 10, 2015) on dbCAN web server (Yin et al. 2012) were used to assign the CAZy families to the predicted protein sequences of Bb, Ht3, Ht6, and Os.
Protein Family Classification
The gene clusters and the proteins responsible for the biosynthesis of secondary metabolites were identified by subjecting the predicted proteome from all the four genomes to antiSMASH server (Medema et al. 2011). Pathogen–host interacting (PHI) partners were identified using PHI database (Winnenburg et al. 2006) with an E value cutoff of 1e−5. In order to identify ribonuclease (RNase) sequences from all the four genomes, “Ribonucleases fungi” was used as keyword search in NCBI and 817 RNase sequences were retrieved. These retrieved RNase sequences were searched against protein databases for all the four genomes using BLASTp at E value cutoff of 1e−5. Transporter Classification Database (Saier et al. 2014) was used to identify membrane transport proteins. The cytochrome P450 (CYP) superfamily was classified according to P450 classification database (Nelson 2009). The mating type genes for fruiting body development, karyogamy and meiosis, mating process and mating signaling in Aspergillus nidulans, Metarhizium acridum, M. anisopliae, and Neurospora crassa (Gao et al. 2011) were retrieved from NCBI and the predicted protein sequences of all the four genomes were subjected to BLASTp against these proteins with an E value cutoff of 1e−5.
Identification of MAT Idiomorphs
Sexual development in fungi is regulated through the mating type genes (Lee et al. 2010). The presence of mating type loci in the four genomes was assessed by sequence similarities to the corresponding MAT genes in other filamentous fungi (Gao et al. 2011). MAT1-1-1 idiomorphic region encodes for alpha domain, MAT1-1-2 for conserved Proline–Proline–Phenylalanine domain whereas MAT1-1-3 for High Mobility Group (HMG) domain with a DNA-binding site (Bushley, Li, et al. 2013; Klix et al. 2010). Likewise, MAT1-2-1 idiomorph encodes for MatA HMG box with a DNA-binding site (Bushley, Li, et al. 2013). The presence of these conserved domains was used to identify the mating type loci. Synteny among all the four genomes was analyzed for the scaffolds containing the mating type locus by locating the position of MAT locus and their flanking genes. The absence of any gene was rechecked through mapping back the reads on these genes.
Transposable Elements and Repeat-Induced Point Mutation Analysis
The transposable elements (TEs) were classified by subjecting genome sequences of Bb, Ht3, Ht6, and Os to BLASTn against the Repbase (http://girinst.org, last accessed March 10, 2015) libraries of RepeatMasker (http://www.repeatmasker.org, last accessed March 10, 2015). Repeat-induced point mutations (RIPs) were estimated using the RIPCAL v1.0 (Hane and Oliver 2008).
Phylogenetic Analysis of GH Family 18 Chitinases
Chitinase sequences were retrieved from the predicted proteomes of all the four genomes using ProfileScan (http://www.csd.hku.hk/bruhk/gcgdoc/profilescan.html, last accessed March 10, 2015) by identifying the conserved active site signature motif [LIVMF]-[DN]-G-[LIVMF]-[DN]-[LIVMF]-[DN]-x-E (Jablonowski et al. 2001) (Prosite No. PS01095). The retrieved chitinase sequences were aligned using PCMA (Pei et al. 2003) and a hidden Markov model profile was built using HMMER v3.1b1 (Finn et al. 2011). This HMMER profile hidden Markov model was further used to scan the four predicted proteomes to identify additional homologs of GH18 chitinases that may have a mutated active site motif. This list of GH18 chitinase sequences was found to be identical to what we retrieved through CAZy GH searches (supplementary table S1, Supplementary Material online) with the exception of one chitinase sequence (Bb GH18-3) that was observed to be shorter in length.
A total of 140 fungal GH18 chitinase sequences were selected from the chitinase data set reported previously (Karlsson and Stenlid 2009). The 74 chitinase sequences from the four genomes were classified and aligned with these fungal GH18 family chitinase sequences using MEGA. Three alignment files for subgroups A, B, and C fungal chitinases were prepared as previously described (Seidl et al. 2005; Karlsson and Stenlid 2009) (supplementary sets S1–S3, Supplementary Material online). Only the sequences of the catalytic domains were included for the phylogenetic classification (Saito et al. 2003). Each alignment was manually adjusted to allow maximum alignment of residues and minimize gaps. Maximum parsimony analysis was performed using PAUP.
Elucidation of Domains Architecture of GH Family 18 Chitinases
The domain architecture of the putative chitinases was obtained using the InterProScan, Pfam, and SMART databases (Schultz et al. 1998; Quevillon et al. 2005; Letunic et al. 2012; Punta et al. 2012). Also these sequences were analyzed to evaluate their theoretical pI and molecular weight using the Protparam tool (Gasteiger et al. 2005).
Results
General Features
Ht3 and Ht6 were observed to have typical Hirsutella-morphologies (Kirk et al. 2008) (supplementary fig. S1, Supplementary Material online). In the multigene phylogenetic analysis, Ht3 and Ht6 were found to be clustered within Ophiocordycipitaceae (Hypocreales) (supplementary fig. S2, Supplementary Material online). The genome sequencing, assembly, and annotated features for Ht3 and Ht6 genomes are presented in table 1. About 75% of predicted proteins in Ht3 and Ht6 were found to have homologs in Bb ARSEF 2860 (Cordycipitaceae, Hypocreales), whereas about 44% of predicted proteins in Ht3 and 38% in Ht6 were found to have homologs with M. anisopliae ARSEF 23 (Clavicipitaceae, Hypocreales). Table 2 presents the highlights of the comparative analyses for the four genomes. A total of 10,714, 9,756, 10,259, and 9,130 predicted proteins were identified in Bb, Ht3, Ht6, and Os genomes, respectively. A comparative distribution of functional attributes of the predicted proteins is presented in supplementary figure S3A, Supplementary Material online. Reciprocal BLAST analysis identified 4,989 proteins as the core proteome of the four genomes contributing almost 50% of their distinct proteomic content (supplementary fig. S3B, Supplementary Material online). Ht3 and Ht6 were found to share 8,182 proteins, exhibiting maximum orthology as expected. The unique protein content of each genome varied in the order Ht3 (15%) < Ht6 (19%) < Os (36%) < Bb (42%) (supplementary fig. S3B, Supplementary Material online).
Table 1.
Sequencing, Assembly, and Annotated Features for the Ht Genomes
| General genome Features | Ht3 | Ht6 |
|---|---|---|
| Genome size | 34.6 Mb | 34.7 Mb |
| PE reads | 58,288,788 | 63,296,052 |
| High-quality reads | 57,988,713 | 56,897,996 |
| High-quality mapped reads | 44,357,832 | 48,273,170 |
| MP reads | 192,572 | — |
| N50 contigs | 180,407 | 214,019 |
| Scaffolds | 474 | 981 |
| Contigs | 621 | 1,003 |
| Coverage (mapped) | 169× (121×) | 165× (116×) |
| ORFs | 9,756 | 10,259 |
| Annotated ORFs | 8,699 | 8,705 |
| NCBI-GenBank accession no. | APKB00000000 | APKU00000000 |
Table 2.
Major Highlights of the Comparative Analyses for the Four Genomes: Ht3, Ht6, Os, and Bb
| Category | Ht3 | Ht6 | Os | Bb | Range |
|---|---|---|---|---|---|
| Total no. of predicted proteins | 9,756 | 10,259 | 9,130 | 10,714 | 9,130–10,714 |
| Total no. of GHs | 149 | 149 | 108 | 186 | 108–186 |
| Total no. of GTs | 99 | 103 | 79 | 107 | 79–107 |
| Total no. of PLs | 1 | 2 | 2 | 3 | 1–3 |
| Total no. of CEs | 78 | 82 | 38 | 90 | 38–90 |
| Total no. of AAs | 58 | 69 | 28 | 66 | 28–69 |
| Total no. of CBMs | 25 | 31 | 33 | 60 | 25–60 |
| No. of CBM1 proteins | 0 | 0 | 0 | 4 | 0–4 |
| No. of CBM18 proteins | 5 | 7 | 6 | 10 | 5–10 |
| No. of CBM50 (LysM) proteins | 10 | 13 | 15 | 19 | 10–19 |
| No. of PHI proteins | 1,931 | 1,995 | 1,572 | 1,979 | 1,572–1,995 |
| No. of membrane transporters | 568 | 610 | 743 | 764 | 568–764 |
| No. of G-protein coupled receptors | 15 | 14 | 15 | 9 | 9–15 |
| No. of RNase genes | 24 | 25 | 10 | 18 | 10–25 |
| No. of genes involved in biosynthesis of sec. metabolites | 56 | 59 | 32 | 43 | 32–59 |
| No. of CYPs | 137 | 140 | 92 | 132 | 92–140 |
| No. of chitinase sequences | 19 | 15 | 17 | 23 | 15–23 |
| Subgroup-A chitinases | 12 | 9 | 9 | 5 | 5–12 |
| Subgroup-B chitinases | 4 | 3 | 3 | 11 | 3–11 |
| Subgroup-C chitinases | 3 | 3 | 5 | 7 | 3–7 |
Survey of Carbohydrate Active Enzymes
A total of six classes: GHs (supplementary table S1, Supplementary Material online), GTs (supplementary table S2, Supplementary Material online), PLs (supplementary table S3, Supplementary Material online), CEs (supplementary table S4, Supplementary Material online), AAs (supplementary table S5, Supplementary Material online), and CBMs (supplementary table S6, Supplementary Material online) as provided in CAZy database were compared in this study. The significantly expanded/contracted gene families are listed in table 3. All the four genomes were found to contain genes putatively encoding enzymes of the GH16 (xyloglucan: xyloglucosyltransferase), GH18 (chitinase), and GH76 (α-1,6-mannanase) families. GT4 family enzyme identified as N-acetylglucosaminyltransferase (CAFE P value, 0.001) was observed in all four species, showing its expansion in Ht3, Ht6, and Bb genomes. GT41 was observed in Ht6 and Bb. Further, Ht6 and Bb were found to contain CBM66 (CAFE P value is 0.01) that is implicated in β-fructosidase activity, usually in bacteria (Cuskin et al. 2012). Both GT41 and CBM66 are expanded in Ht6 and Bb. CBM18 and CBM50 (LysM) family modules implicated in chitin-binding function in association with chitinase catalytic domains were found to be predominantly present in all the four genomes. Moreover, CBM19 and CBM55 also implicated in chitin-binding functions were observed in Bb and Os, respectively.
Table 3.
List of Statistically Significant Expanded/Contracted Gene Families
| Family | Ht3 | Ht6 | Os | Bb | Gene Family Expansion |
|---|---|---|---|---|---|
| GH15 | 1 | 3 | 1 | 2 | Ht6, Bb |
| GH18 | 19 | 15 | 17 | 22 | Ht3, Bb |
| GH74 | 2 | 4 | 5 | 1 | Os, Ht6 |
| GT4 | 5 | 8 | 2 | 4 | Ht3, Ht6, Bb |
| GT26 | 1 | 0 | 0 | 0 | Ht3 |
| GT41 | 0 | 2 | 0 | 1 | Ht6, Bb |
| AA7 | 17 | 23 | 11 | 28 | Ht3, Ht6, Bb |
| CBM66 | 0 | 2 | 0 | 5 | Ht6, Bb |
| CoA_Ligase | 22 | 29 | 9 | 16 | Ht3, Ht6, Bb |
| Intermediate_filament_protein_MDM1 | 1 | 0 | 1 | 1 | Ht3, Os, Bb |
| Oxidoreductase FAD/NAD(P)-binding | 28 | 35 | 4 | 27 | Ht3, Ht6, Bb |
PHI Genes
The virulence-associated genes that are likely to be involved in a variety of fungal–host interactions were identified in Bb, Ht3, Ht6, and Os (table 4 and supplementary table S7, Supplementary Material online). We identified 10.3%, 10.1%, 10.4%, and 8.2% of the 19,162 experimentally verified PHI genes (Winnenburg et al. 2006) involved in virulence from Bb, Ht3, Ht6, and Os, respectively. List of significantly expanded/contracted PHI families is provided in table 3.
Table 4.
Pathogen–Host Interacting Genes and Their Distribution in Ht3, Ht6, Os, and Bb Genomes
| Crucial Steps in Pathogen–Host Interactions | Interacting Virulence-Associated Genes | Ht3 | Ht6 | Os | Bb | References |
|---|---|---|---|---|---|---|
| 1. Adhesion | Hydrophobins | 3 | 3 | 1 | 4 | St Leger et al. (1992) |
| Cell-wall protein genes (Mad1, Mad2) | 1 | 1 | 2 | 0 | Wang and St. Leger (2007) | |
| 2. Appresorium formation | Protein kinases (MAPK/hog1) | 123 | 129 | 128 | 139 | Zhang et al. (2010); Zhang et al. (2009) |
| 3. Epicuticle Penetration | CYP | 69 | 72 | 42 | 71 | Pedrini et al. (2010) |
| Vitamin H: biotin transporter | 2 | 1 | 1 | 1 | Fan et al. (2011) | |
| 4. Cuticle Penetration | Glycosyl transferases (carboxylate transporter genes: increase pH) | 37 | 37 | 31 | 35 | Jin et al. (2009) |
| Subtilisin protease: protein lysis in insect epidermis | 12 | 13 | 9 | 15 | Donatti et al. (2008) | |
| Glutaminase and NADH-specific glutamate dehydogenase: catabolism of aminoacids | 4 | 4 | 7 | 2 | Freimoser et al. (2005) | |
| GHs | 22 | 20 | 15 | 26 | Fang et al. (2005) |
Genes involved in signal transduction were identified in all the four genomes (supplementary table S8, Supplementary Material online). These included several multidrug resistance transporters, responsible for protection against a variety of toxic compounds (Morschhauser 2010). We also identified RNases (supplementary table S9, Supplementary Material online) in the four genomes that catalyze the cleavage of phosphodiester bonds in RNA and play important role in RNA processing and nonspecific RNA degradation (Mishra and Nawin 1995).
Gene Clusters Involved in Biosynthesis of Secondary Metabolites
In the present genome analysis, genes involved in the biosynthesis of secondary metabolites from the four genomes were found to mainly consist of NRPS, PKS, and terpene gene clusters (supplementary table S10, Supplementary Material online). The contraction of these gene clusters was observed in Os, except for unusual heterocyst glycolipid synthase (HglD/E)- like PKS known for production of unsaturated fatty acids and lipids (Oßwald et al. 2014) that were expanded in Os, and is perhaps related to their psychrophilic adaptation.
Metabolism-Associated Genes
CYP family enzymes, CYP51 (lanosterol 14 alpha-demethylase) which is involved in ergosterol biosynthesis and in membrane permeabilization (Ferreira et al. 2005) and CYP53 involved in detoxification of toxic benzoate molecules (Jawallapersand et al. 2014), were observed in all the four genomes. In addition to these, CYP504, involved in detoxification of phenylacetate derivatives (Ferrer-Sevillano and Fernandez-Canon 2007), was observed in maximum copy numbers as compared with the other CYPs in the four genomes. CYP52 family proteins, known to participate in host–cuticle penetration by metabolizing host epicuticle lipids (Zhang et al. 2012), were also observed in all the four genomes, with comparatively more proteins in Bb. Ht3, Bb, and Os showed contraction (negative CAFE average expansion values, Ht3: −0.03, Bb: −0.32, Os: −0.44) of CYPs whereas expansion of CYPs was observed in Ht6 (CAFE average expansion is 0.02) (supplementary table S11, Supplementary Material online). Interestingly, a protein belonging to the CYP55/PNOR family of nitric oxide reductases was detected in the Ht genomes. This protein was observed to share synteny in Ht3 and Ht6 with conserved flanking regions (supplementary fig. S4, Supplementary Material online).
RIP and Diversity of TEs
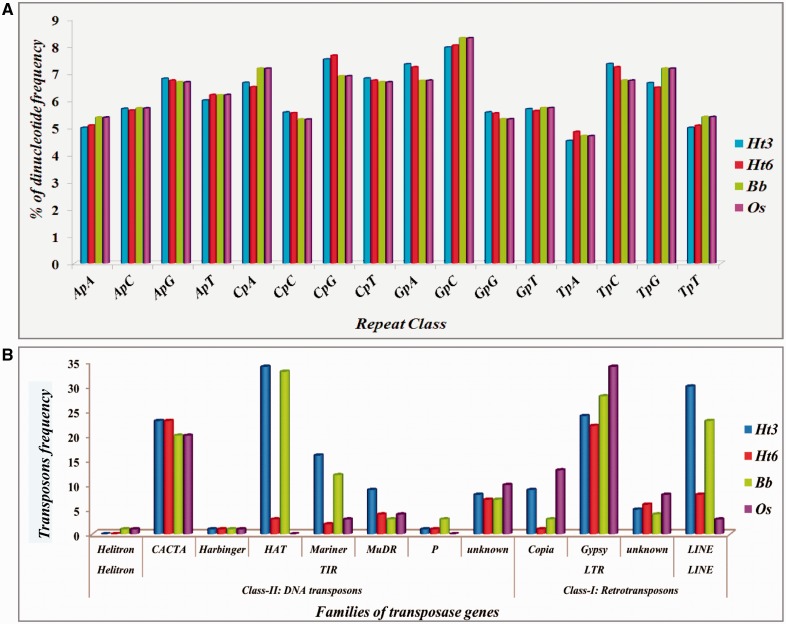
Repeat-induced point mutation (RIP) is a genome defense mechanism in fungi that acts on repetitive DNA to control the progression of TEs through hypermutation (Hood et al. 2005). The RIP frequency index in all the four genomes was found to be almost similar whereas the distribution of TEs differed (fig. 1). Overall, an irregular pattern of TEs was observed in all the four genomes.
Fig. 1.—
(A) Estimation of RIPs and (B) Diversity of transposase genes in Ht3, Ht6, Bb, and Os genomes. RIPs were estimated using the RIPCAL. RepeatMasker was used to search for the TEs.
Mating Type
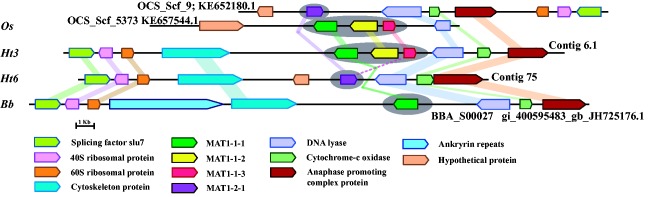
A number of mating type genes common in fungi were observed in the Ht3, Ht6, Bb, and Os genomes (supplementary table S12, Supplementary Material online). Based on the presence of conserved domains and sequence similarity with mating type genes of hypocrealean entomopathogenic and plant pathogenic fungi, six mating type genes, that is, MAT1-1-1, MAT1-1-2, MAT1-1-3 (MAT1-1 locus), MAT1-2-1 (MAT1-2 locus), matA/MAT A3 (MAT2 locus: Two copies) were identified in the Ht3 and Os genomes, whereas only four mating type genes were identified in the Ht6 and Bb genomes, including MAT1-2-1 (MAT1-2 locus: Two copies) and matA/MAT A3 (MAT2 locus: Two copies) in Ht6 genome; and MAT1-1-1 (MAT1-1 locus), MAT1-2-1 (MAT1-2 locus), and matA/MAT A3 (MAT2 locus: Two copies) in Bb genome. Interestingly, syntenic analysis revealed the presence of MAT1-2 locus in place of MAT1-1 locus and the conservation of genes flanking the MAT1-1 locus in Ht6 (fig. 2). Bb showed the presence of only MAT1-1-1 gene (fig. 2). Synteny was also analyzed for other MAT loci, MAT1-2 and MAT2, and the genes flanking these MAT loci were found to be mostly conserved in the four genomes (supplementary fig. S5, Supplementary Material online). The genomic location of these mating type genes is provided in supplementary table S13, Supplementary Material online.
Fig. 2.—
Synteny of mating-type loci (MAT1-1-1, MAT1-1-2, and MAT1-1-3) and their flanking regions in Os, Ht3, Ht6, and Bb genomes. MAT loci and their flaking gene regions showing orthologous relationships are labeled in the same color.
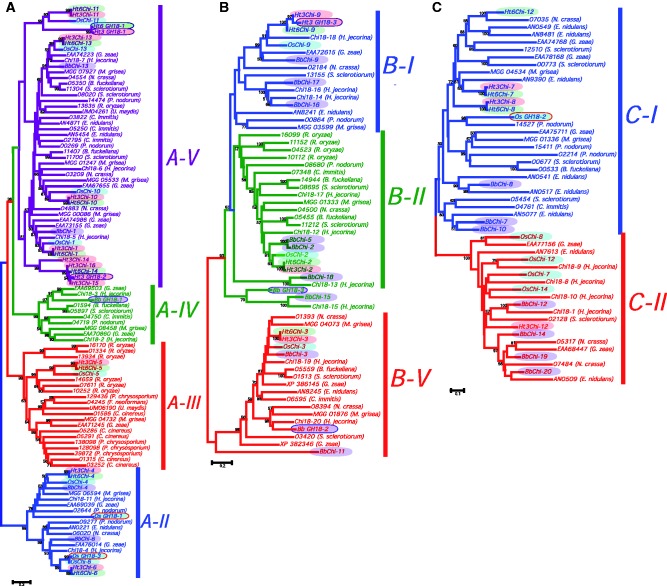
Phylogenetic Analysis of GH Family 18 Chitinase Sequences
Fungal chitinases have previously been classified into three subgroups A, B, and C on the basis of amino acid (aa) sequence similarity of their GH18 catalytic domains (Seidl et al. 2005; Seidl 2008). In our analysis, the three data sets, through, subgroups A, B, and C were individually analyzed to reconstruct the phylogeny of fungal chitinases. Uniformity in the sequence profile of Ht3 and Ht6 chitinases was observed. These Ht3 and Ht6 chitinase sequences have been named based on their increasing molecular weight values (supplementary table S14, Supplementary Material online). Bb and Os chitinases have been named on the basis of sequence similarity with Ht chitinases. These chitinases are represented as XChi-z, where X is the organism label (Ht3, Ht6, Bb, or Os) and z is the designated number. GH18 chitinase sequences with mutations in the catalytic site are referred to with a different nomenclature, where X GH18-z is used for demarcating it from the putative chitinolytically active proteins. Phylogenetic trees are shown in figure 3A–C and the PAUP statistical information on data sets is presented in supplementary table S15, Supplementary Material online.
Fig. 3.—
Phylogenetic relationships of catalytic domains of GH family 18 chitinases subgroups A, B, and C (A–C). MP tree showing evolutionary history (PAUP* 4.0 b win). Bootstrap values greater than 50% are shown. Shaded region shows the chitinase sequences obtained from Ht3, Ht6, Bb, and Os genomes.
In this study, 23, 19, 15, and 17 chitinase sequences from Bb, Ht3, Ht6, and Os genomes, respectively, were identified. Among four additional chitinase sequences identified in Ht3 as compared with Ht6, three belong to subgroup-A and one to subgroup-B (fig. 3). The three chitinase sequences (Ht3Chi-15, Ht3Chi-16, and Ht3 GH18-2) belonging to subgroup-A are observed to cluster with Ht6Chi-14. Further, all these chitinase sequences show close similarity with Ht3Chi-14 (fig. 3A). Ht3Chi-15 and Ht3Chi-16 are identical in length (278 aa) and with a predicted molecular weight of 31 kDa. Their sequences are 97% identical, differing only at 7 aa positions. Ht3 GH18-2 sequence is 208 aa long and 23 kDa in weight. Ht3Chi-14 sequence differs from Ht3Chi-15, Ht3Chi-16, and Ht3GH18-2 in having a higher predicted molecular weight (38 kDa) and length (338 aa).
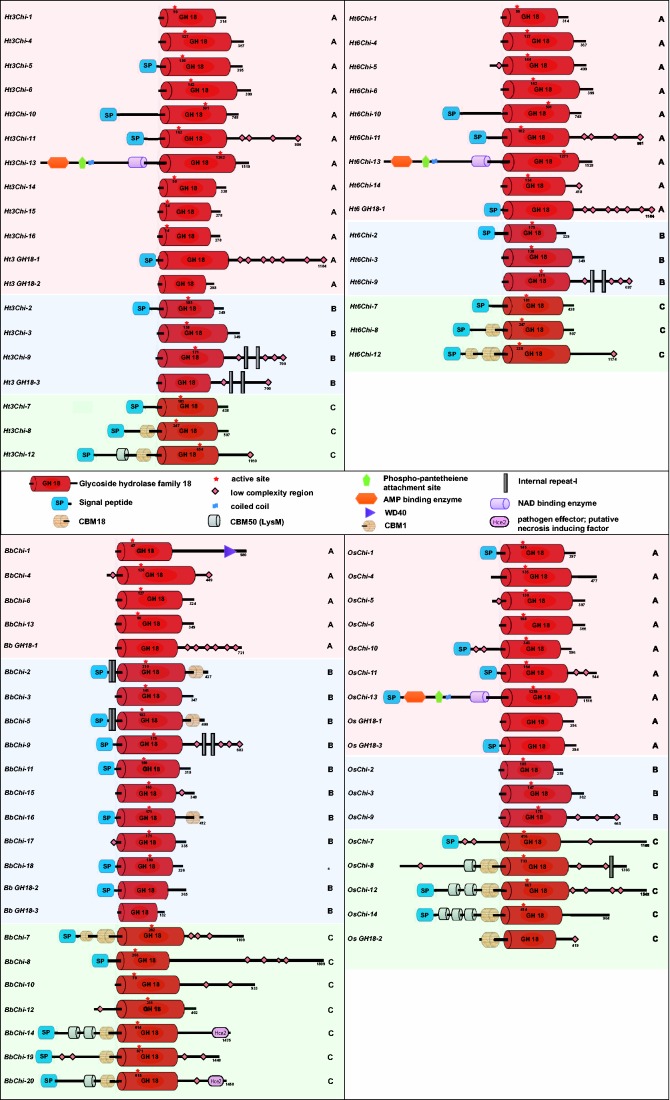
Domain Architecture of GH Family 18 Chitinases
The domain organization for all the chitinases identified in this study is presented in figure 4. Ht3 and Ht6 were found to have a similar domain pattern with the exception of Chi-12. Ht3Chi-12 was observed to have a single chitin-binding domain belonging to CBM18 family, whereas Ht6Chi-12 had two CBM18 domains. Additional domains for AMP-binding enzyme, phosphopantetheine attachment site and nicotinamide adenine dinucleotide (NAD)-binding enzyme were observed in HtChi-13 and OsChi-13. Similarly, WD40 domain was observed in BbChi-1 and Hce2 domain was detected in BbChi-14 and BbChi-20.
Fig. 4.—
Domain organization of Ht3, Ht6, Bb, and Os chitinases. Protein domains, as identified with InterProScan, Pfam, and SMART databases are shown.
Discussion
Comparative Distinctions among Bb, Ht, and Os Genomes
Genome comparisons of Bb, Ht3, Ht6, and Os suggest overall reduced gene family sizes in Ht3 and Os as compared with Ht6 and Bb genomes. It is known that RIP is a maintenance force of evolutionary diversity and genome organization during sexual cycle (Selker et al. 1987; Galagan et al. 2003). We speculate that the differences in gene family sizes in the four fungi may perhaps be related to the frequency of meiotic/recombination events linked to the active RIP mechanism (Meerupati et al. 2013), in addition to the other selective forces.
Hypocrealean fungi are known to exhibit diversity in their mating behavior. Some of these fungi are self-fertile/homothallic, whereas some are self-sterile/heterothallic (Ni et al. 2011). Genotypically, their mating response has been indicated to be regulated by mating type genes (Lee et al. 2010; Ni et al. 2011). The presence of two compatible MAT idiomorphs, MAT1-1 and MAT1-2, is suggested to decide homothallism and heterothallism in ascomycetes (Lee et al. 2010; Ni et al. 2011). As of now, there has been no report on mating/teleomorph in Ht. It is quite possible that Ht undergoes recombination/meiosis but it is uncertain yet. Interestingly, in this study, MAT1-1-1, MAT1-1-2, and MAT1-1-3 mating type genes (MAT1-1 idiomorph) are identified in Ht3, whereas synteny analysis reveals the presence of MAT1-2-1 gene (MAT1-2 idiomorph) in place of MAT1-1 idiomorph in Ht6 (fig. 2). This indicates at cryptic sexuality in Ht. Similar to this study, isolate-specific differences in mating genes were observed in two isolates of Metarhizium robertsii (Previous name: M. anisopliae) (Pattemore et al. 2014). However, fungi can switch between homo- and heterothallic mode (Ni et al. 2011); therefore, additional gene expression and experimental studies are required to investigate their homo-/heterothallic mode of mating.
Entomopathogenic fungi have a number of virulence-associated genes in their genome to adapt to the diverse ecological niches (Klosterman et al. 2011; Gardiner et al. 2013; Zhao et al. 2014). Therefore, the expansion of virulence genes is suggested to affect the pathogenicity potential and evolution of host affiliation. Os genome (size: 78.5 Mb) is about more than double the size of other three fungal genomes but shows a comparatively reduced set of protein-coding genes and is rich in repeats similar to that observed for many obligatory pathogens such as powdery mildew fungi (Spanu et al. 2010) and rust fungi (Duplessis et al. 2011). It shows peculiar behavioral traits, such as a biphasic pathogenic lifestyle, obligatory mycoparasitism, and psychrophilic adaptation. Unlike the other three fungi in this study, Os has a unique lifestyle exhibiting concealed virulence in early larval developmental stages of ghost moths and a fatal virulence in late caterpillar instars (Hu et al. 2013). Interestingly, Os contains several unusual PKS that participate in accumulation of lipids and unsaturated fatty acids (Oßwald et al. 2014) that perhaps help it to adapt to extreme cold environments.
CYPs represent one of the largest protein families which are involved in a number of biochemical reactions associated with primary, secondary, and xenobiotic metabolism (Cresnar and Petric 2011). CYPs are suggested to be important for organisms to acclimatize to novel ecological niches through functional diversification (Cresnar and Petric 2011). These proteins play important roles in detoxification, biosynthesis, and other metabolic reactions (Cresnar and Petric 2011). Ht genomes contain a CYP55/PNOR family gene that is reported to participate in anaerobic denitrification and detoxification (Takaya et al. 2002; Shoun et al. 2012), possibly enabling adaptation to hypoxic/anoxic conditions in Ht. This gene was likely acquired through horizontal transfer from actinobacteria as inferred by sequence identities (about 40%) with bacterial CYP105 (Tomura et al. 1994; Shoun et al. 2012). This gene is suggested to be an important constituent of fungal denitrification system that involves reduction of toxic nitric oxide into nontoxic nitrous oxide (Shoun et al. 2012). The metabolic process of this system is not connected to the mitochondrial respiratory system and redox partners, rather electrons are directly received by CYP55 from NAD to reduce nitric oxide to nitrous oxide (Shoun et al. 2012). Therefore, the presence of CYP55 could be related to the fungal survival strategy under oxygen deficient or oxidative stress conditions in response to the antimicrobial defense of the host (Nittler et al. 2005; Shoun et al. 2012). It is thus suggested as one of the factors in Ht that could be responsible for its variable ecological niche adaptations and host affiliations.
Ht3 and Ht6 Chitinases
This study reports 19 and 15 chitinase sequences, including three and one GH18 sequences with mutations at the active site in Ht3 and Ht6 genomes, respectively. This observation is in accordance with the previous reports that there are higher numbers of chitinase genes in entomopathogenic fungi (ranging from 10 to 20) than plant-pathogenic fungi (ranging from 6 to 15) (Gao et al. 2011; Xiao et al. 2012). Ht3Chi-14 sequence is predicted to possibly share paralogous relationship with Ht3Chi-15, Ht3Chi-16, and Ht3GH18-2 chitinase sequences. We therefore consider that additional subgroup-A chitinase sequences in Ht3 strain could have been acquired through gene duplication events.
Revision of Classification of Bb Chitinases
Our study refines the classification of Bb chitinases previously proposed (Xiao et al. 2012), where they compared the occurrence of domains and modules in chitinases and classified nine Bb chitinases without CBM in subgroup-A, four Bb chitinases with CBM at C-terminal in subgroup-B, and seven Bb chitinases with CBM18 and CBM50 in subgroup-C. In our study, we have classified the chitinases based on aa sequence similarity of catalytic domains of subgroups A, B, and C chitinases, similar to previous reports (Seidl et al. 2005; Karlsson and Stenlid 2009). Interestingly, 5, 11, and 7 Bb chitinases are grouped in subgroups A, B, and C, respectively. The differences could be observed in domain distribution previously proposed (Xiao et al. 2012) (fig. 4). One of the subgroup-A chitinases (BbChi-1) contains a WD domain. Only three of the subgroup-B chitinases classified in our analysis are observed to contain CBM1. The remaining eight subgroup-B chitinases are not found to contain any CBMs. Similarly, only four of seven subgroup-C chitinase sequences are observed to contain CBM18 and CBM50 domains. Subgroup-B and -C chitinases are mainly attributed to participate in nutritional and aggressive functions such as virulence to insects in entomopathogenic fungi (de las Mercedes Dana et al. 2001; da Silva et al. 2005; Baratto et al. 2006); therefore, higher number of subgroup-B and subgroup-C chitinases in Bb genome may be related to the virulence of Bb.
Evolutionary Peculiarities in Bb Chitinases
Previous studies suggest Hypocrea jecorina Chi18-15 (GenBank: BK006088.1), an active participant in degradation of insect cuticle, to have been horizontally transferred from actinobacteria (Karlsson and Stenlid 2009; Ihrmark et al. 2010). Hypocrea jecorina Chi18-15 clusters with BbChi-15 (GenBank: KM220983), sharing 84% aa sequence similarity in the catalytic domain that suggests this chitinase was horizontally transferred prior to the split of H. jecorina and Bb (fig. 3B). The evolution of H. jecorina Chi18-15 and its fungal and bacterial orthologs have been suggested to occur under different selective forces following horizontal gene transfer where the functional divergence is proposed to be created by loop-associated regions to rapidly evolve selective modifications (Ubhayasekera and Karlsson 2012). This suggests adaptive evolution in BbChi-15 chitinase and positive selection of adaptable modifications in functional properties (Alsmark et al. 2013) that may affect the entomopathogenicity potential of Bb.
Bb GH18-3 represents a paralog of BbChi-15 and H. jecorina Chi18-15 (fig. 3B). The identified Bb GH18-3 chitinase sequence (132 aa) is almost half the length of other GH18 chitinase catalytic domain sequences. Sequence and structure-based analysis suggests a half TIM-barrel fold, containing only the N-terminal half region, for Bb GH18-3. The TIM-barrel (α/β)8 fold has been suggested previously to have evolved by tandem gene duplication and fusion of half-barrels (α/β)4 and hence it is possible that Bb GH8-3 may function as a homodimer (Lang et al. 2000; Nagano et al. 2002). Nevertheless, there is a need to experimentally validate biochemical function of Bb GH18-3 to comment on its evolutionary implications.
Protein Domain Distribution in Fungal Chitinases
This study provides interesting insights about the domain distribution in chitinases of entomopathogenic fungi (fig. 4). For example, Ht3Chi-12 is found to have domains CBM18 and CBM50 (LysM), whereas Ht6Chi-12 has two CBM18 domains. However, both CBM18 and CBM50 are implicated in chitin-binding functions (Seidl 2008). Similarly, presence of AMP+PP+NAD in subgroup-A Chi-13 of Ht and Os indicates their role in secondary metabolites (nonribosomal peptide synthetases) production, virulence, and fungal development (Molnar et al. 2010). Likewise, presence of WD40 domain in Bb-Chi1 belonging to subgroup-A could have implications in molecular recognition events through protein–protein or protein–DNA interaction (Xu and Min 2011). Subgroup-A chitinases are so far known to participate in general physiological functions related to the growth and development of the fungus (Seidl 2008). The occurrences of additional domains AMP+PP+NAD or WD40 in subgroup-A chitinases hints at a possible role of subgroup-A chitinases in pathogenicity in addition to other routine functions. However, further investigations are required to confirm the role of subgroup-A chitinases in virulence.
Though subgroup-B chitinases are expected to have CBM domains (Hartl et al. 2012), our analysis reveals inconsistent scenarios. The CBMs are found to be present in subgroup-B chitinases from Bb genome but absent in those from Ht and Os genomes. Aspergillus niger, A. nidulans, A. terreus, A. flavus,Neosartorya fischeri (Ascomycota) and Coprinus cinereus, Sporobolomyces roseus (Basidiomycota) too lack CBMs in their subgroup-B chitinases (Seidl 2008). The CBMs are involved in chitin-binding functions and their absence in Ht, Os and other ascomycete and basidiomycete genomes may affect the specific recognition events of fungal chitinases. Further, the exclusive association of CBM1 family members with subgroup-B chitinases and the association of CBM18 and CBM50 family members (possessing chitin-binding ability) with subgroup-C chitinases identified in our study are found to be consistent with the CBM distribution of mycoparasitic Trichoderma spp. that are important biocontrol agents (Karlsson and Stenlid 2009; Gruber et al. 2011).
The domain architecture of subgroup-C chitinases interestingly reveals the presence of Hce2 domains in BbChi-14 and BbChi-20. Hce2 has been identified as a homolog of the Ecp2 effector protein of plant-pathogenic fungus Cladosporium fulvus (Van den Ackerveken et al. 1993; Lauge et al. 1998; Stergiopoulos et al. 2010, 2012). It has been recognized as a necrosis-inducing factor in plants that is involved in pathogenicity against the host (Bolton et al. 2008; Stergiopoulos et al. 2010, 2012). In silico characterization has revealed that in conjugation with GH18 chitinases, Hce2 shows high similarity with killer toxin Zymocin from Kluyveromyces lactis with possible antagonistic functions (Stergiopoulos et al. 2012). Similarly, a recent functional study of A. nidulans subgroup-C killer toxin-like chitinases has shown the effect of deleted subgroup-C chitinase associated with Hce2 domain in lowering the growth inhibitory activity of culture filtrates, implicating the role of this protein in interspecific fungal–fungal interactions (Tzelepis et al. 2014). Accordingly, the occurrence of Hce2 in Bb could be one of the factors for virulence and ecological niche adaptations.
Research Scope
Ht is known to produce the insecticidal ribotoxin hirsutellin A (HtA) (Herrero-Galán et al. 2008). Although about 20 ribotoxins have been described from filamentous fungi, HtA is the only one known from entomopathogenic fungi (Arruda et al. 1992; Lin et al. 1995; Huang et al. 1997; Kao et al. 2001; Herrero-Galán et al. 2008). We have identified a total of 77 putative RNase protein sequences in Bb, Ht3, Ht6, and Os genomes (supplementary table S9, Supplementary Material online); however, there is a need to gain more insights about their evolution and function in entomopathogenic fungi that could help in the identification of components responsible for making RNases into ribotoxins. Additionally, it will aid in the exploitation of these ribotoxins in plant protection measures and biomedical applications.
The GHs analysis shows the prevalence of GH families GH2, GH3, GH16, GH18, GH43, GH47, GH72, GH76, and GH109 in the four genomes. Among these characterization of 5 GH2, 30 GH3, 5 GH16, 13 GH18, 6 GH43, and 5 GH47 members of fungal origin have been reported (Murphy et al. 2011), but none from GH72, GH76, and GH109. Moreover, about 25% of annotated proteins in all the four genomes are with unknown/hypothetical functions.
A total of 244 PHI protein families categorized from the four genomes can further help in exploring their role in pathogenicity, such as to aid in the discovery of enzymes with potential insecticidal properties.
Conclusions
The genome sequencing of the insect pathogenic fungi Ht and its comparative analysis with Bb and Os genomes have provided interesting insights into insect–fungal associations, evolutionary insights into chitinases, and offer clues to improve the efficacy of Ht as a biological control agent.
Supplementary Material
Supplementary tables S1–S15, figures S1–S5, and data sets S1–S3 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
The authors acknowledge the Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India from where the isolates were retrieved and the C-CAMP next-generation genomics facility, Bangalore, India for help in obtaining NGS data. They thank Dr Magnus Karlsson, Department of Forest Mycology and Pathology, Swedish University of Agricultural Sciences, Uppsala, Sweden for providing the chitinase data set of 394 sequences. They also thank Ms Poonam Mual for her help in DNA isolation, Mr Gaurav Sharma, and Ms Gurmeet Kaur for useful discussions on chitinase sequence and structure analysis. They also thank Mr Tarun Narwani for help in statistical analysis. This work was supported by a project “Expansion and modernization of MTCC jointly supported by Council of Scientific and Industrial Research (CSIR) Grant No. BSC0402 and Department of Biotechnology (DBT) Government of India Grant No. BT/PR7368/INF/22/177/2012.” Y.A. and I.K. are supported by research fellowships from CSIR and University Grants Commission (UGC), respectively. B.D.S. and S.K.S. conceived the idea. I.K. generated the data. Y.A. analyzed the data and wrote the manuscript. All authors reviewed the manuscript.
Literature Cited
- Alsmark C, et al. Patterns of prokaryotic lateral gene transfers affecting parasitic microbial eukaryotes. Genome Biol. 2013;14:R19. doi: 10.1186/gb-2013-14-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arruda LK, Mann BJ, Chapman MD. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J Immunol. 1992;149:3354–3359. [PubMed] [Google Scholar]
- Baratto CM, et al. Isolation, characterization, and transcriptional analysis of the chitinase chi2 Gene (DQ011663) from the biocontrol fungus Metarhizium anisopliae var. anisopliae. Curr Microbiol. 2006;53:217–221. doi: 10.1007/s00284-006-0078-6. [DOI] [PubMed] [Google Scholar]
- Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27:578–579. doi: 10.1093/bioinformatics/btq683. [DOI] [PubMed] [Google Scholar]
- Boetzer M, Pirovano W. Toward almost closed genomes with GapFiller. Genome Biol. 2012;13:R56. doi: 10.1186/gb-2012-13-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton MD, et al. The novel Cladosporium fulvum lysin motif effector Ecp6 is a virulence factor with orthologues in other fungal species. Mol Microbiol. 2008;69:119–136. doi: 10.1111/j.1365-2958.2008.06270.x. [DOI] [PubMed] [Google Scholar]
- Bushley KE, Li Y, et al. Isolation of the MAT1-1 mating type idiomorph and evidence for selfing in the Chinese medicinal fungus Ophiocordyceps sinensis. Fungal Biol. 2013;117:599–610. doi: 10.1016/j.funbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Bushley KE, Raja R, et al. The genome of Tolypocladium inflatum: evolution, organization, and expression of the cyclosporin biosynthetic gene cluster. PLoS Genet. 2013;9:e1003496. doi: 10.1371/journal.pgen.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel BL, et al. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 2008;18:188–196. doi: 10.1101/gr.6743907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnley AK. Fungal pathogens of insects: cuticle degrading enzymes and toxins. Adv Bot Res. 2003;40:241–321. [Google Scholar]
- Cresnar B, Petric S. Cytochrome P450 enzymes in the fungal kingdom. Biochim Biophys Acta. 2011;1814:29–35. doi: 10.1016/j.bbapap.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Cuskin F, et al. How nature can exploit nonspecific catalytic and carbohydrate binding modules to create enzymatic specificity. Proc Natl Acad Sci U S A. 2012;109:20889–20894. doi: 10.1073/pnas.1212034109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva MV, et al. Cuticle-induced endo/exoacting chitinase CHIT30 from Metarhizium anisopliae is encoded by an ortholog of the chi3 gene. Res Microbiol. 2005;156:382–392. doi: 10.1016/j.resmic.2004.10.013. [DOI] [PubMed] [Google Scholar]
- de las Mercedes Dana M, et al. Regulation of chitinase 33 (chit33) gene expression in Trichoderma harzianum. Curr Genet. 2001;38:335–342. doi: 10.1007/s002940000169. [DOI] [PubMed] [Google Scholar]
- Donatti AC, Furlaneto-Maia L, Fungaro MH, Furlaneto MC. Production and regulation of cuticle-degrading proteases from Beauveria bassiana in the presence of Rhammatocerus schistocercoides cuticle. Curr Microbiol. 2008;56:256–260. doi: 10.1007/s00284-007-9071-y. [DOI] [PubMed] [Google Scholar]
- Duplessis S, et al. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc Natl Acad Sci U S A. 2011;108:9166–9171. doi: 10.1073/pnas.1019315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zhang S, Kruer N, Keyhani NO. High-throughput insertion mutagenesis and functional screening in the entomopathogenic fungus Beauveria bassiana. J Invertebr Pathol. 2011;106:274–279. doi: 10.1016/j.jip.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Fang W, et al. Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl Environ Microbiol. 2005;71:363–370. doi: 10.1128/AEM.71.1.363-370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ME, et al. The ergosterol biosynthesis pathway, transporter genes, and azole resistance in Aspergillus fumigatus. Med Mycol. 2005;43:S313–S319. doi: 10.1080/13693780400029114. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sevillano F, Fernandez-Canon JM. Novel phacB-encoded cytochrome P450 monooxygenase from Aspergillus nidulans with 3-hydroxyphenylacetate 6-hydroxylase and 3,4-dihydroxyphenylacetate 6-hydroxylase activities. Eukaryot Cell. 2007;6:514–520. doi: 10.1128/EC.00226-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimoser FM, Hu G, St, Leger RJ. Variation in gene expression patterns as the insect pathogen Metarhizium anisopliae adapts to different host cuticles or nutrient deprivation in vitro. Microbiology. 2005;151:361–371. doi: 10.1099/mic.0.27560-0. [DOI] [PubMed] [Google Scholar]
- Galagan JE, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- Gao Q, et al. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011;7:e1001264. doi: 10.1371/journal.pgen.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner DM, Kazan K, Manners JM. Cross-kingdom gene transfer facilitates the evolution of virulence in fungal pathogens. Plant Sci. 2013;210:151–158. doi: 10.1016/j.plantsci.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, et al. Protein identification and analysis tools on the ExPASy server. In: John MW, editor. The proteomics protocols handbook. Totowa (NJ): Humana Press; 2005. pp. 571–607. [Google Scholar]
- Gruber S, et al. Analysis of subgroup C of fungal chitinases containing chitin-binding and LysM modules in the mycoparasite Trichoderma atroviride. Glycobiology. 2011;21:122–133. doi: 10.1093/glycob/cwq142. [DOI] [PubMed] [Google Scholar]
- Hamid R, et al. Chitinases: an update. J Pharm Bioallied Sci. 2013;5:21–29. doi: 10.4103/0975-7406.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MV, Thomas GW, Lugo-Martinez J, Hahn MW. Estimating gene gain and loss rates in the presence of error in genome assembly and annotation using CAFE 3. Mol Biol Evol. 2013;30:1987–1997. doi: 10.1093/molbev/mst100. [DOI] [PubMed] [Google Scholar]
- Hane JK, Oliver RP. RIPCAL: a tool for alignment-based analysis of repeat-induced point mutations in fungal genomic sequences. BMC Bioinformatics. 2008;9:478. doi: 10.1186/1471-2105-9-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haragsim O, Ruzicka V. US patent (United States); 2001. Biological control of Varroa mites in honeybee hives with Hirsutella thompsonii. . Patent No. 6.277.371 b1. Application No. 09/574,933. [Google Scholar]
- Hartl L, Zach S, Seidl-Seiboth V. Fungal chitinases: diversity, mechanistic properties and biotechnological potential. Appl Microbiol Biotechnol. 2012;93:533–543. doi: 10.1007/s00253-011-3723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Galán E, et al. The insecticidal protein hirsutellin A from the mite fungal pathogen Hirsutella thompsonii is a ribotoxin. Proteins. 2008;72:217–228. doi: 10.1002/prot.21910. [DOI] [PubMed] [Google Scholar]
- Herrero-Galán E, et al. Hirsutellin A: a paradigmatic example of the insecticidal function of fungal ribotoxins. Insects. 2013;4:339–356. doi: 10.3390/insects4030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood ME, Katawczik M, Giraud T. Repeat-induced point mutation and the population structure of transposable elements in Microbotryum violaceum. Genetics. 2005;170:1081–1089. doi: 10.1534/genetics.105.042564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, et al. Genome survey uncovers the secrets of sex and lifestyle in caterpillar fungus. Chin Sci Bull. 2013;58:2846–2854. [Google Scholar]
- Huang KC, Hwang YY, Hwu L, Lin A. Characterization of a new ribotoxin gene (c-sar) from Aspergillus clavatus. Toxicon. 1997;35:383–392. doi: 10.1016/s0041-0101(96)00170-5. [DOI] [PubMed] [Google Scholar]
- Ihrmark K, et al. Comparative molecular evolution of Trichoderma chitinases in response to mycoparasitic interactions. Evol Bioinform Online. 2010;6:1–26. doi: 10.4137/ebo.s4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonowski D, et al. Saccharomyces cerevisiae cell wall chitin, the Kluyveromyces lactis zymocin receptor. Yeast. 2001;18:1285–1299. doi: 10.1002/yea.776. [DOI] [PubMed] [Google Scholar]
- Jawallapersand P, et al. Cytochrome P450 monooxygenase CYP53 family in fungi: comparative structural and evolutionary analysis and its role as a common alternative anti-fungal drug target. PLoS One. 2014;9:e107209. doi: 10.1371/journal.pone.0107209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Carboxylate transporter gene JEN1 from the entomopathogenic fungus Beauveria bassiana is involved in conidiation and virulence. Appl Environ Microbiol. 2009;76:254–263. doi: 10.1128/AEM.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao R, Martinez-Ruiz A, Martinez del Pozo A, Crameri R, Davies J. Mitogillin and related fungal ribotoxins. Methods Enzymol. 2001;341:324–335. doi: 10.1016/s0076-6879(01)41161-x. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Stenlid J. Evolution of family 18 glycoside hydrolases: diversity, domain structures and phylogenetic relationships. J Mol Microbiol Biotechnol. 2009;16:208–223. doi: 10.1159/000151220. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. Dictionary of the fungi. 10th ed. Wallingford (United Kingdom): CAB International; 2008. [Google Scholar]
- Klix V, et al. Functional characterization of MAT1-1-specific mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and nonessential sexual regulators. Eukaryot Cell. 2010;9:894–905. doi: 10.1128/EC.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterman SJ, et al. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 2011;7:e1002137. doi: 10.1371/journal.ppat.1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek CP, et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011;12:R40. doi: 10.1186/gb-2011-12-4-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Thoma R, Henn-Sax M, Sterner R, Wilmanns M. Structural evidence for evolution of the beta/alpha barrel scaffold by gene duplication and fusion. Science. 2000;289:1546–1550. doi: 10.1126/science.289.5484.1546. [DOI] [PubMed] [Google Scholar]
- Lauge R, et al. Successful search for a resistance gene in tomato targeted against a virulence factor of a fungal pathogen. Proc Natl Acad Sci U S A. 1998;95:9014–9018. doi: 10.1073/pnas.95.15.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner M, et al. Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics. 2011;12:124. doi: 10.1186/1471-2105-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Ni M, Li W, Shertz C, Heitman J. The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev. 2010;74:298–340. doi: 10.1128/MMBR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40:D302–D305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Huang KC, Hwu L, Tzean SS. Production of type II ribotoxins by Aspergillus species and related fungi in Taiwan. Toxicon. 1995;33:105–110. doi: 10.1016/0041-0101(94)00140-4. [DOI] [PubMed] [Google Scholar]
- Liu Z-Y, et al. Molecular evidence for teleomorph-anamorph connections in Cordyceps based on ITS-5.8S rDNA sequences. Mycol Res. 2002;106:1100–1108. [Google Scholar]
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema MH, et al. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011;39:W339–W346. doi: 10.1093/nar/gkr466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerupati T, et al. Genomic mechanisms accounting for the adaptation to parasitism in nematode-trapping fungi. PLoS Genet. 2013;9:e1003909. doi: 10.1371/journal.pgen.1003909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra NC. Molecular biology of nucleases. Boca Raton (FL): CRC Press; 1995. pp. 46–52. [Google Scholar]
- Molnar I, Gibson DM, Krasnoff SB. Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat Prod Rep. 2010;27:1241–1275. doi: 10.1039/c001459c. [DOI] [PubMed] [Google Scholar]
- doi: 10.1016/j.fgb.2009.08.002. Morschhauser J. 2010. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol. 47: 94–106. [DOI] [PubMed] [Google Scholar]
- Murphy C, Powlowski J, Wu M, Butler G, Tsang A. Curation of characterized glycoside hydrolases of fungal origin. Database (Oxford) 2011;2011:bar020. doi: 10.1093/database/bar020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano N, Orengo CA, Thornton JM. One fold with many functions: the evolutionary relationships between TIM barrel families based on their sequences, structures and functions. J Mol Biol. 2002;321:741–765. doi: 10.1016/s0022-2836(02)00649-6. [DOI] [PubMed] [Google Scholar]
- Nelson DR. The cytochrome p450 homepage. Hum Genomics. 2009;4:59–65. doi: 10.1186/1479-7364-4-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Feretzaki M, Sun S, Wang X, Heitman J. Sex in fungi. Annu Rev Genet. 2011;45:405–430. doi: 10.1146/annurev-genet-110410-132536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittler MP, Hocking-Murray D, Foo CK, Sil A. Identification of Histoplasma capsulatum transcripts induced in response to reactive nitrogen species. Mol Biol Cell. 2005;16:4792–4813. doi: 10.1091/mbc.E05-05-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oßwald C, et al. A highly unusual polyketide synthase directs dawenol polyene biosynthesis in Stigmatella aurantiaca. J Biotechnol. 2014 doi: 10.1016/j.jbiotec.2014.07.447. 191:54–63. [DOI] [PubMed] [Google Scholar]
- Paterson RR. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69:1469–1495. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattemore J, et al. The genome sequence of the biocontrol fungus Metarhizium anisopliae and comparative genomics of Metarhizium species. BMC Genomics. 2014;15:660. doi: 10.1186/1471-2164-15-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini N, Zhang S, Juarez MP, Keyhani NO. Molecular characterization and expression analysis of a suite of cytochrome P450 enzymes implicated in insect hydrocarbon degradation in the entomopathogenic fungus Beauveria bassiana. Microbiology. 2010;156:2549–2557. doi: 10.1099/mic.0.039735-0. [DOI] [PubMed] [Google Scholar]
- Pei J, Sadreyev R, Grishin NV. PCMA: fast and accurate multiple sequence alignment based on profile consistency. Bioinformatics. 2003;19:427–428. doi: 10.1093/bioinformatics/btg008. [DOI] [PubMed] [Google Scholar]
- Punta M, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevillon E, et al. InterProScan: protein domains identifier. Nucleic Acids Res. 2005;33:W116–W120. doi: 10.1093/nar/gki442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Reddy VS, Tamang DG, Vastermark A. The transporter classification database. Nucleic Acids Res. 2014;42:D251–D258. doi: 10.1093/nar/gkt1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Fujii T, Miyashita K. Distribution and evolution of chitinase genes in Streptomyces species: involvement of gene-duplication and domain-deletion. Antonie Van Leeuwenhoek. 2003;84:7–15. doi: 10.1023/a:1024463113606. [DOI] [PubMed] [Google Scholar]
- Samson RA, McCoy CW, O’Donnell KL. Taxonomy of the acarine parasite Hirsutella thompsonii. Mycologia. 1980;72:359–377. [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl V. Chitinases of filamentous fungi: a large group of diverse proteins with multiple physiological functions. Fungal Biol Rev. 2008;22:36–42. [Google Scholar]
- Seidl V, Huemer B, Seiboth B, Kubicek CP. A complete survey of Trichoderma chitinases reveals three distinct subgroups of family 18 chitinases. FEBS J. 2005;272:5923–5939. doi: 10.1111/j.1742-4658.2005.04994.x. [DOI] [PubMed] [Google Scholar]
- Selker EU, Cambareri EB, Jensen BC, Haack KR. Rearrangement of duplicated DNA in specialized cells of Neurospora. Cell. 1987;51:741–752. doi: 10.1016/0092-8674(87)90097-3. [DOI] [PubMed] [Google Scholar]
- Shoun H, Fushinobu S, Jiang L, Kim SW, Wakagi T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos Trans R Soc Lond B Biol Sci. 2012;367:1186–1194. doi: 10.1098/rstb.2011.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu PD, et al. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science. 2010;330:1543–1546. doi: 10.1126/science.1194573. [DOI] [PubMed] [Google Scholar]
- St. Leger RJ, Staples RC, Roberts DW. Cloning and regulatory analysis of starvation-stress gene, ssgA, encoding a hydrophobin-like protein from the entomopathogenic fungus, Metarhizium anisopliae. Gene. 1992;120:119–124. doi: 10.1016/0378-1119(92)90019-l. [DOI] [PubMed] [Google Scholar]
- St. Leger RJ, Wang C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl Microbiol Biotechnol. 2010;85:901–907. doi: 10.1007/s00253-009-2306-z. [DOI] [PubMed] [Google Scholar]
- Stanke M, Morgenstern B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005;33:W465–W467. doi: 10.1093/nar/gki458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos I, et al. Tomato Cf resistance proteins mediate recognition of cognate homologous effectors from fungi pathogenic on dicots and monocots. Proc Natl Acad Sci U S A. 2010;107:7610–7615. doi: 10.1073/pnas.1002910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos I, et al. In silico characterization and molecular evolutionary analysis of a novel superfamily of fungal effector proteins. Mol Biol Evol. 2012;29:3371–3384. doi: 10.1093/molbev/mss143. [DOI] [PubMed] [Google Scholar]
- Sung GH, et al. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (* and other methods). Version 4.0. Sunderland (MA): Sinauer Associates; 2003. [Google Scholar]
- Takaya N, Uchimura H, Lai Y, Shoun H. Transcriptional control of nitric oxide reductase gene (CYP55) in the fungal denitrifier Fusarium oxysporum. Biosci Biotechnol Biochem. 2002;66:1039–1045. doi: 10.1271/bbb.66.1039. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura D, Obika K, Fukamizu A, Shoun H. Nitric oxide reductase cytochrome P-450 gene, CYP 55, of the fungus Fusarium oxysporum containing a potential binding-site for FNR, the transcription factor involved in the regulation of anaerobic growth of Escherichia coli. J Biochem. 1994;116:88–94. doi: 10.1093/oxfordjournals.jbchem.a124508. [DOI] [PubMed] [Google Scholar]
- Tzelepis GD, Melin P, Stenlid J, Jensen DF, Karlsson M. Functional analysis of the C-II subgroup killer toxin-like chitinases in the filamentous ascomycete Aspergillus nidulans. Fungal Genet Biol. 2014;64:58–66. doi: 10.1016/j.fgb.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Ubhayasekera W, Karlsson M. Bacterial and fungal chitinase chiJ orthologs evolve under different selective constraints following horizontal gene transfer. BMC Res Notes. 2012;5:581. doi: 10.1186/1756-0500-5-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ackerveken GF, et al. Characterization of two putative pathogenicity genes of the fungal tomato pathogen Cladosporium fulvum. Mol Plant Microbe Interact. 1993;6:210–215. doi: 10.1094/mpmi-6-210. [DOI] [PubMed] [Google Scholar]
- Wang Z-X, et al. Comparative transcriptomic analysis of the heat stress response in the filamentous fungus Metarhizium anisopliae using RNA-Seq. Appl Microbiol Biotechnol. 2014;98:5589–5597. doi: 10.1007/s00253-014-5763-y. [DOI] [PubMed] [Google Scholar]
- Wang C, St. Leger RJ. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot Cell. 2007;6:808–816. doi: 10.1128/EC.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibelzahl E, Liburd OE. Epizootic of Acalitus vaccinii (Acari: Eriophyidea) caused by Hirsutella thompsonii on southern highbush blueberry in north-central Florida. Fla Entomol. 2009;92:601–607. [Google Scholar]
- Weinzierl R, Henn T, Koehler PG, Tucker CL. Microbial insecticides. Gainesville (FL): University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS; 2005. [Google Scholar]
- Winnenburg R, et al. PHI-base: a new database for pathogen host interactions. Nucleic Acids Res. 2006;34:D459–D464. doi: 10.1093/nar/gkj047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep. 2012;2:483. doi: 10.1038/srep00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Min J. Structure and function of WD40 domain proteins. Protein Cell. 2011;2:202–214. doi: 10.1007/s13238-011-1018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, et al. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40:W445–W451. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Mitogen-activated protein kinase hog1 in the entomopathogenic fungus Beauveria bassiana regulates environmental stress responses and virulence to insects. Appl Environ Microbiol. 2009;75:3787–3795. doi: 10.1128/AEM.01913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Requirement of a mitogen-activated protein kinase for appressorium formation and penetration of insect cuticle by the entomopathogenic fungus Beauveria bassiana. Appl Environ Microbiol. 2010;76:2262–2270. doi: 10.1128/AEM.02246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, et al. CYP52X1, representing new cytochrome P450 subfamily, displays fatty acid hydroxylase activity and contributes to virulence and growth on insect cuticular substrates in entomopathogenic fungus Beauveria bassiana. J Biol Chem. 2012;287:13477–13486. doi: 10.1074/jbc.M111.338947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, et al. Host-to-pathogen gene transfer facilitated infection of insects by a pathogenic fungus. PLoS Pathog. 2014;10:e1004009. doi: 10.1371/journal.ppat.1004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol. 2011;12:R116. doi: 10.1186/gb-2011-12-11-r116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.