Abstract
Acontia, located in the gastrovascular cavity of anemone, are thread-like tissue containing numerous stinging cells which serve as a unique defense tissue against predators of the immobile acontiarian sea anemone. Although its morphology and biological functions, such as defense and digestion, have been studied, the defense behavior and the specific events of acontia ejection and retraction are unclear. The aim of this study is to observe and record the detailed process of acontia control in anemones. Observations reveal that the anemone, Exaiptasia pallida, possibly controls a network of body muscles and manipulates water pressure in the gastrovascular cavity to eject and retract acontia. Instead of resynthesizing acontia after each ejection, the retraction and reuse of acontia enables the anemone to respond quickly at any given time, thus increasing its overall survivability. Since the Exaiptasia anemone is an emerging model for coral biology, this study provides a foundation to further investigate the biophysics, neuroscience, and defense biology of this marine model organism.
Keywords: Acontia, Exaiptasia pallida, Defense, Ejection, Retraction
Introduction
Like many cnidarians, sea anemones contain specialized cells, known as cnidocytes or nematocytes, in their body column, oral disk, pharynx, tentacles, mesenterial filaments, and acontia (Manuel, 1988; Shick, 1991). Cnidocytes contain nematocysts, penetrative cells that discharge toxic compounds into the target (Lotan et al., 1995) in response to certain chemical and mechanical stimulation (Mariottini & Pane, 2010). Despite being a venomous organism, Exaiptasia anemone are prey to Lysmata shrimp and nudibranch species, such as Aeolidiella stephanieae (Valdés, 2005). In order to protect themselves from predator attacks, anemones either detach and move by pedal locomotion or eject acontia by contracting the body column and extending its tentacles in self-defense (Waters, 1973; Edmunds et al., 1974; Edmunds et al., 1976; Schlesinger et al., 2009). Based on our observation, Exaiptasia anemones rarely perform detachment and pedal locomotion, but rather the ejection of acontia.
Acontia is the distinguishing feature of the actiniarian group Acontiaria (Rodríguez et al., 2012). Acontia in sea anemones usually have a white, coiled threadlike appearance and form at the end of the thickened edge of mesenteries near the pedal disk (Fig. 1). These thread-like extensions of the mesenterial filaments are filled with nematocyst-containing cnidocytes (Stephenson, 1935). Nematocyst discharge can be induced by physical contact, specific molecules, or chemical markers that are possibly recognized by a cellular recognition system of the anemone (Yanagita, 1959; Yanagita, 1960; Blanquet & Lenhoff, 1966; Ishihara, 1967; Blanquet, 1970; Lubbock, 1980). Once stimulated, the dart-like tubules are propelled from the nematocysts with enough force to penetrate the exoskeleton of the predator to sting its target with cytolytic peptide and protein toxins that cause paralysis (Conklin, Bigger & Mariscal, 1977; Kem, 1988; Bernheimer, 1990; Turk, 1991; Maček, 1992; Anderluh & Maček, 2002). Acontial defenses of the anemone can sometimes dissuade Aeolidia papillosa from feeding and potentially result in the death of this nudibranch (Harris, 1973). Since detachment and pedal locomotion have rarely been observed in Exaiptasia anemones, acontia ejection serves as an important defense mechanism against predator attack.
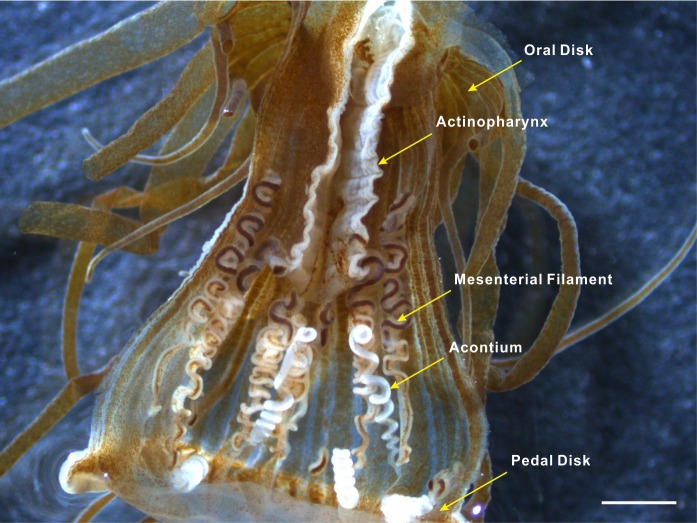
Figure 1. Acontia tissue within the body column of Exaiptasia anemone.
Acontia tissue is comprised of long white thread-like organs that have a simple coiling morphology; this tissue is densely lined with nematocysts and form from the mesentery edge near the pedal disk of the anemone. Scale bar: 1 mm.
Early reports have briefly described the phenomenon of acontia ejection, whereby the threatened anemone strongly contracts its body column, forcing water out of the cinclides. This causes the ejection of acontia, which are carried by the water currents (Stephenson, 1928; Manuel, 1988). However, the details of acontia control, specifically retraction, are unclear. With merely the presence of nervous elements but no nerve net or brain (Wada, 1972), the anemone is able to control the ejection and retraction of acontia using the shortening or elongation of the body column in response to mechanical stimulation. These simple but effective control mechanisms of acontia enable the anemone to readily respond to predation at any given time. Through detailed observation, this study presents meticulous detail of acontia ejection and retraction during the defensive and recovering states of anemones.
Material and Methods
The sea anemones, Exaiptasia pallida, were collected from the tanks in the Husbandry Centre of the National Museum of Marine Biology and Aquarium in Pingtung, located in Southern Taiwan. The origin of the anemones comes from the wild population, since the unfiltered seawater was pumped from the location beneath the native habitat (N22 03 00.08 E120 41 42.88) of Exaiptasia pallida (the scientific name was recently changed from Aiptasia pulchella by Grajales & Rodríguez 2014). Collected anemones were cultured in tanks with filtered seawater at an ambient temperature (25 °C) with a 12 h light (34 µmol m−2s−1): 12 h dark photoperiod in laboratory. Anemones were fed Artemia nauplii weekly. Samples with a body column height greater than 20 mm were chosen for this study due to the positive correlation of the effectiveness of acontial ejection with the size of the anemone (Harris, 1986). Samples were removed by scraping beneath the pedal disk to detach the anemone from the tank. The anemones were cultured in tanks separately for one week before observation. During observation, a plastic dropper was used as a seemingly threatening stimulus to provoke the anemone to exhibit defensive behavior. For each stimulation, we probed the anemone between the cinclides and oral disk several times for about two to three seconds until the contraction of the body column into a ball-like shape. Then, we waited for the anemone to relax by extending its tentacles, before continuing with the next stimulation. After allowing the anemone to recover slightly, we proceeded with the second stimulation to the target region, inducing the ejection of acontia. Repeated stimulations were performed to induce more acontia ejection from the cinclides. The ejection and retraction process of six specimen were observed and recorded. High quality videos (Video S1) were recorded using a high definition camcorder (HDR CX-550; Sony, Tokyo, Japan). Snapshot images were taken from the recorded video materials, and measurements were made using the open source, Java-based software package Image J (National Institutes of Health, Bethesda, MD, USA).
Results
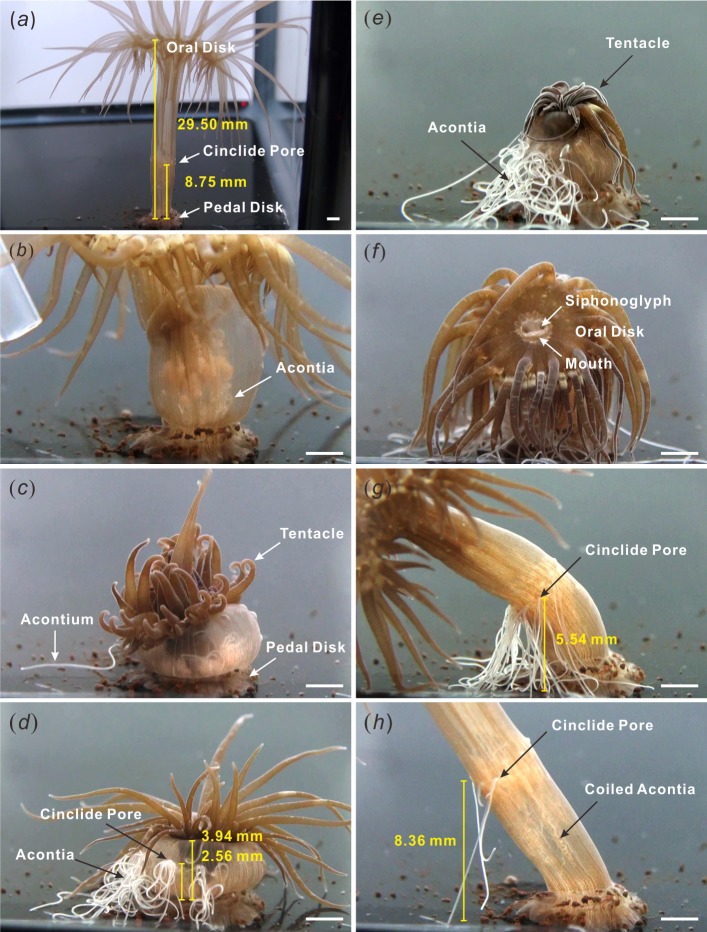
In this study, the relaxed adult anemone (Exaiptasia pallida) displayed an elongated body column with tentacles evenly spread out at the oral disk. In Fig. 2A, the length of the body column from the oral disk to the pedal disk of the representative anemone was 29.50 mm (average =30.65 ± 2.90 mm (SEM for this and all other error terms), n = 6 specimens, Table 1), and the approximate distance between the cinclides and the pedal disk was 8.75 mm (average =7.73 ± 0.73 mm (SEM), n = 6 specimens, Table 1). When the anemone was gently probed with a plastic dropper, no acontia were released. The anemone only withdrew by shortening its body length (Fig. 2B).
Figure 2. Acontia ejection and retraction.
(A) relaxed anemone, fully elongated; (B) initial stimulation made the anemone contract but did not release of acontia; (C) the second stimulation caused slight acontia ejection; (D) multiple stimulations provoked defensive state with the profuse release of acontia and upright tentacles; (E) anemone displayed deflated morphology and withdrawn oral disk; (F) start of recovery, anemone mouth remained closed while siphonoglyphs were opened for the intake of water; (G) column elongation and acontia retraction; (H) approximately 40–50 min for the retraction of all acontia until next ejection (Video S1). Scale bar: 2 mm.
Table 1. The height of cinclides during acontia ejection and retraction.
| Specimen no. | Height of body column (mm) | Height of cinclide (mm) | Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Initiala | Ejectionb | Ratio(b/a) | Initialc | Ejectiond | Retractione | Ejection(d/c) | Retraction(e/d) | |
| 1 | 29.50 | 3.94 | 0.134 | 8.75 | 2.56 | 8.36 | 0.293 | 3.266 |
| 2 | 23.87 | 7.39 | 0.310 | 6.46 | 4.95 | 7.91 | 0.766 | 1.598 |
| 3 | 29.83 | 3.08 | 0.103 | 6.44 | 2.48 | 3.51 | 0.385 | 1.415 |
| 4 | 25.40 | 4.18 | 0.165 | 7.13 | 1.59 | 6.07 | 0.223 | 3.818 |
| 5 | 31.42 | 4.59 | 0.146 | 6.63 | 2.70 | 8.30 | 0.407 | 3.074 |
| 6 | 43.90 | 5.34 | 0.122 | 10.94 | 2.23 | 3.70 | 0.204 | 1.659 |
| Average ± SEM | 30.65 ± 2.90 | 4.75 ± 0.61 | 0.163 ± 0.031 | 7.73 ± 0.73 | 2.75 ± 0.47 | 6.31 ± 0.92 | 0.380 ± 0.084 | 2.472 ± 0.422 |
The second stimulation, during which the body column of the anemone was repeatedly probed with a dropper, resulted in the ejection of some acontia (Fig. 2C). Repeated stimulation to the target area caused the anemone to further contract into a ball-like shape with fully extended tentacles (Fig. 2D, Video S1). The length of the body column became 3.94 mm (average =4.75 ± 0.61 mm (SEM), n = 6 specimens, Table 1), and the distance between the cinclides and the pedal disk was approximately 2.56 mm (average =2.75 ± 0.47 mm (SEM), n = 6 specimens, Table 1). As the anemone contracted its body column, acontia were profusely released from cinclides (Fig. 2D, Video S1) and simultaneously resulted in the deflation of the tentacles (Fig. 2E). At this stage, the height between the cinclides and the base of a representative anemone decreased by 29.3% (average = 38 ± 8.4% (SEM), n = 6 specimens, Table 1).
After the removal of all physical stimuli, the anemone slowly recovered. The body column was restored to a relaxed state of an exposed oral disk and extended body column and tentacles (Fig. 2F). In its relaxed state, the mouth was closed and siphonoglyphs widely opened to limit and control water intake. The acontia were retracted into the anemone as the body column slowly extended to a length of 5.54 mm from the cinclides to the base (Fig. 2G). Eventually, the distance between the cinclides and the base of the representative anemone increased to 8.36 mm (average =6.31 ± 0.92 mm (SEM), n = 6 specimens, Table 1) due to the body column becoming fully elongated; at this point, the acontia recoiled inside the body (Fig. 2H). During the recovery process, the height between the cinclides and the base of the representative anemone increased by 327% (average = 247 ± 42.2% (SEM), n = 6 specimens, Table 1). The entire acontia recovery process took about 40–50 min for the representative anemone (Video S1).
Discussion
Exaiptasia anemones are simple bi-radial organisms that are exclusively polypoid with an external morphology limited to tentacles, oral disk, mouth, body column, and pedal disk (Manuel, 1988; Shick, 1991) without a central nervous system (Dahl et al., 1963; Nakanishi et al., 2012). External stimuli are sensed by a nerve net that extends throughout the body. The nerve net follows the network of contractile muscle systems, which contain sensory neurite cells between the endoderm and muscle fibers (Batham, Pantin & Robson, 1960). Passing through the mesoglea, neurites connect to muscles in the ectoderm and endoderm for coordination and rapid response (Robson, 1963).
When initially stimulated by a dropper, the Exaiptasia anemone contracted its body column (Video S1). This is a common response of anemones to external stimuli, such as water flow changes and physical contact. The degree of body contortion depends on the amount of contraction exerted by longitudinal muscles in the body column and retractor muscles in the mesenteries. Since the hydrostatic skeleton determines the body shape of the anemone (McFarlane, 1974), any change in water pressure will alter the shape of the anemone. Thus, we hypothesize that the contraction of the body column of the Exaiptasia anemone generates positive water pressure as demonstrated in the Metridium anemone (Batham & Pantin, 1950), forcing water out through the cinclides, which carries the free ends of the acontia with it (Manuel, 1988), and causes the deflation of the tentacles.
Mimicking the possible attack of a predator, repeated mechanical stimulation provoked the anemone to exhibit defensive behavior, which resulted in an average decrease of body length by 0.163 ± 0.031 fold (Table 1). The defensive behavior is characterized by the compression of the body, extension of tentacles, and ejection of acontia. This form enables the anemone to shield the oral disk using cnidocytes in the tentacles and protect the body using acontial stinging cells. This behavior may deter the predator, while the ejection of acontia act as a first line of defense by stinging the predator before close contact (Edmunds et al., 1974; Edmunds et al., 1976). Since in its natural environment, Exaiptasia anemones are attacked by Aeolidia papillosa and Lysmata wurdemanni, which feed by repeatedly poking, prodding, or biting the body column close to the pedal disk of the anemone (Edmunds et al., 1974; Edmunds et al., 1976; Rhyne & Lin, 2006), the control mechanism of acontia is a crucial factor for its survival.
Based on this study, we propose that the control of rapid acontia ejection potentially involves two factors: the contraction of the body column and the generation of positive water pressure in the gastrovascular cavity. The relaxation of circular muscles allows a wider body, while the contraction of longitudinal muscles contorts the body column. Next, the contraction of the retractor muscles withdraws the oral disk. The compressed oral disk halts water intake causing the anemone to become a closed system (Josephson, 1966). The contraction of muscles forces the body to contort vertically and effectively propel water out and eject acontia via cinclides for defense (Fig. 2D, Video S1). Since muscle fibers within the acontia do not have directed movement or sensory mechanisms (Wada, 1972), it cannot eject effectively without this mechanism.
During the recovery period, the anemone initially compresses the tentacles into a shriveled shape for maximal expulsion of water from the anemone body. Later, the anemone exposes the oral disk and extends the tentacles. Although the mouth is closed, the siphonoglyphs remain open for slow water intake (Video S1). With the relaxation of the longitudinal muscles and contraction of the circular muscles, water fills the gastrovascular cavity and extends the body column vertically (Batham & Pantin, 1950). This suggests that the control of acontia retraction involves two critical factors: the elongation of the body column and the generation of negative water pressure. Negative water pressure was observed in the Metridium anemone when recovering from a state of extreme contraction (Batham & Pantin, 1950). This negative water pressure in the gastrovascular cavity results in the intake of water through the siphonoglyphs and cinclides (Figs. 2E and 2F). The slow suction of water elongates the body column by filling up the total water volume. Although acontia can extend for many centimeters beyond the column wall (Manuel, 1988), acontia have a limited length. Thus, the elongation of the body column helps the retraction of acontia into the body, especially when the acontia is adhered to an immotile surface. This elongation is evident by the distance measured from the cinclides to the base of the tank, which increased by an average of 2.472 ± 0.422 fold (Table 1). As acontia are retracted, they recoil due to the unique position of acontial longitudinal muscles (Wada, 1972). In its natural environment, however, some of the anemone’s acontia may not be retracted when they are ripped off by the predator.
Acontia make up a unique defense tissue in acontiarian sea anemones. The ejection of acontia is an interesting process; acontia protrude through the body wall of cnidarian species upon the attack of predators or artificial stimulation. This study increases knowledge of the anemone’s defense behavior. For the first time, this study shows that the sea anemone possibly controls a network of body muscles and manipulates water pressure in the gastrovascular cavity to eject and retract acontia as a defense response. This study also provides insight to the ingenious control and economical reuse of acontia (Fig. 3). Furthermore, as an emerging model animal for coral biology, the observations and supplementary video of this study provide important information that fuels the future study of biophysics, neuroscience and defense biology of the Exaiptasia anemone to unmask the amazing behavior of this marine model organism.
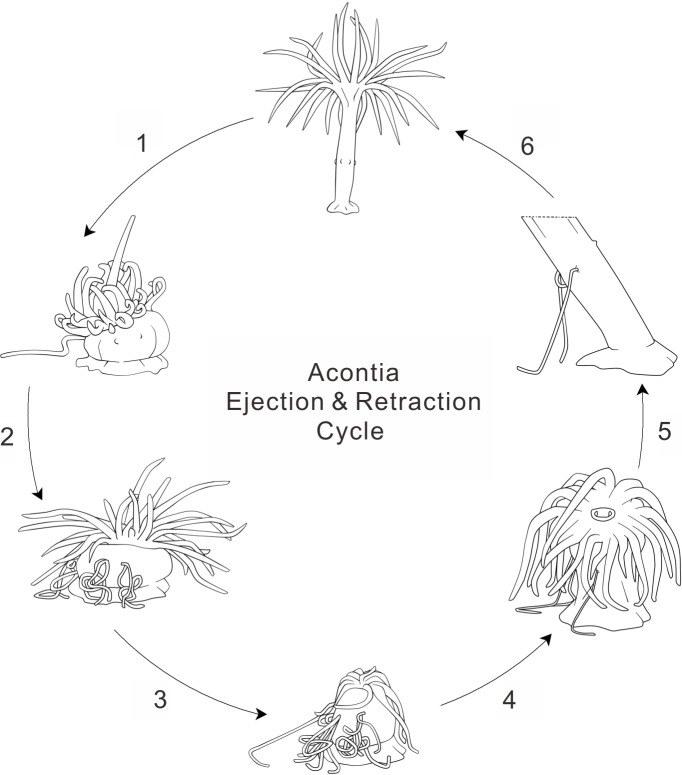
Figure 3. Anemone acontia ejection and retraction cycle.
(1) first stimulation caused body contraction and increased water pressure; (2) repeated stimulations prompted anemone defensive state and acontia ejection; (3) deflated oral disk and tentacles for maximum water expulsion; (4) anemone entered recovery state when stimulations ceased; (5) open siphonoglyphs intake water and closed mouth hold water volume, tentacles inflated and oral disk exposed; (6) generation of negative water pressure and elongation of body column pulled acontia back into body, process lasted for 40–50 min.
Supplemental Information
Acknowledgments
We express appreciation to Ms. Cherilyn Gan for edits on this manuscript.
Funding Statement
This work was supported by grants from the National Science Council (NSC 101-2311-B-291-001 and NSC 101-2311-B-291-MY3) and intramural funding from the National Museum of Marine Biology & Aquarium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Julie Lam analyzed the data, wrote the paper, reviewed drafts of the paper.
Ya-Wen Cheng performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Wan-Nan U. Chen contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Hsing-Hui Li wrote the paper, reviewed drafts of the paper.
Chii-Shiarng Chen conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Shao-En Peng conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Data Availability
References
- Anderluh & Maček (2002).Anderluh G, Maček P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria) Toxicon. 2002;40(2):111–124. doi: 10.1016/S0041-0101(01)00191-X. [DOI] [PubMed] [Google Scholar]
- Batham & Pantin (1950).Batham EJ, Pantin CFA. Muscular and hydrostatic action in the sea-anemone Metridium senile (L.) Journal of Experimental Biology. 1950;27:264–289. doi: 10.1242/jeb.27.3.264. [DOI] [PubMed] [Google Scholar]
- Batham, Pantin & Robson (1960).Batham EJ, Pantin CFA, Robson EA. The nerve-net of the sea-anemone Metridium senile: the mesenteries and the column. Journal of Cell Science. 1960;3:487–510. [Google Scholar]
- Bernheimer (1990).Bernheimer AW. Cytolytic peptides of sea anemones. In: Hall S, Strichartz G, editors. Marine toxins: origin, structure and molecular pharmacology. ACS; Washington, D.C.: 1990. pp. 304–311. [Google Scholar]
- Blanquet (1970).Blanquet R. Ionic effects on discharge of the isolated and in situ nematocysts of the sea anemone, Aiptasia pallida: a possible role of calcium. Comparative Biochemistry and Physiology. 1970;35(2):451–461. doi: 10.1016/0010-406X(70)90608-0. [DOI] [Google Scholar]
- Blanquet & Lenhoff (1966).Blanquet R, Lenhoff HM. A disulfide-linked collagenous protein of nematocyst capsules. Science. 1966;166:152–153. doi: 10.1126/science.154.3745.152. [DOI] [PubMed] [Google Scholar]
- Conklin, Bigger & Mariscal (1977).Conklin EJ, Bigger CH, Mariscal RN. The formation and taxonomic status of the microbasic q-mastigophore nematocyst of sea anemones. Biological Bulletin. 1977;152(2):159–168. doi: 10.2307/1540556. [DOI] [Google Scholar]
- Dahl et al. (1963).Dahl E, Falck B, Von Mecklenburg C, Myhrberg H. An adrenergic nervous system in sea anemones. Quarterly Journal of Microscopial Science. 1963;104:531–534. [Google Scholar]
- Edmunds et al. (1974).Edmunds M, Potts GW, Swinfen RC, Waters VL. The feeding preferences of Aeolidia papillosa (L.) (Mollusca, Nudibranchia) Journal of the Marine Biological Association of the United Kingdom. 1974;54(4):939–947. doi: 10.1017/S0025315400057660. [DOI] [Google Scholar]
- Edmunds et al. (1976).Edmunds M, Potts GW, Swinfen RC, Waters VL. Defensive behavior of sea anemones in response to predation by the Opisthobranch Mollusc Aeolidia papillosa (L.) Journal of the Marine Biological Association of the United Kingdom. 1976;56(1):65–83. doi: 10.1017/S0025315400020440. [DOI] [Google Scholar]
- Grajales & Rodríguez (2014).Grajales A, Rodríguez E. Morphological revision of the genus Aiptasia and the family Aiptasiidae (Cnidaria, Actiniaria, Metridioidea) Zootaxa. 2014;3826(1):55–100. doi: 10.11646/zootaxa.3826.1.2. [DOI] [PubMed] [Google Scholar]
- Harris (1973).Harris LG. Nudibranch associations. In: Cheng TC, editor. Current topics in comparative pathobiology, vol. 2. Academic Press; New York: 1973. pp. 213–315. [Google Scholar]
- Harris (1986).Harris LG. Size-selective predation in a sea anemone, nudibranch, and fish food chain. The Veliger. 1986;29:38–47. [Google Scholar]
- Ishihara (1967).Ishihara R. Stimuli causing extrusion of polar filaments of Glugea fumiferanae spores. Canadian Journal of Microbiology. 1967;13:1321–1332. doi: 10.1139/m67-178. [DOI] [PubMed] [Google Scholar]
- Josephson (1966).Josephson PK. Neuromuscular transmission in a sea anemone. Journal of Experimental Biology. 1966;45:305–319. [Google Scholar]
- Kem (1988).Kem WR. Sea anemone toxins: structure and action. In: Hessinger DA, Lenhoff HM, editors. The biology of nematocysts. Academic Press, Inc; San Diego: 1988. pp. 375–405. [Google Scholar]
- Lotan et al. (1995).Lotan A, Fishman L, Loya F, Zlotkin E. Delivery of a nematocyst toxin. Nature. 1995;375:456. doi: 10.1038/375456a0. [DOI] [PubMed] [Google Scholar]
- Lubbock (1980).Lubbock R. Clone-specific cellular recognition in a sea anemone. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(11):6667–6669. doi: 10.1073/pnas.77.11.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maček (1992).Maček P. Polypeptide cytolytic toxins from sea anemones (Actiniaria) FEMS Microbiology Immunology. 1992;5(1–3):121–130. doi: 10.1111/j.1574-6968.1992.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Manuel (1988).Manuel RL. British Anthozoa. Academic Press; London: 1988. [Google Scholar]
- Mariottini & Pane (2010).Mariottini GL, Pane L. Mediterranean jellyfish venoms: a review on scyphomedusae. Marine Drugs. 2010;8(4):1122–1152. doi: 10.3390/md8041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane (1974).McFarlane ID. Excitatory and inhibitory control of inherent contractions in the Sea Anemone Calliactis parasitica. Journal of Experimental Biology. 1974;60:397–422. doi: 10.1242/jeb.60.2.397. [DOI] [PubMed] [Google Scholar]
- Nakanishi et al. (2012).Nakanishi N, Renfer E, Technau U, Rentzsch F. Nervous systems of the sea anemone Nematostella vectensis are generated by ectoderm and endoderm and shaped by distinct mechanisms. Development. 2012;139:347–357. doi: 10.1242/dev.071902. [DOI] [PubMed] [Google Scholar]
- Rhyne & Lin (2006).Rhyne AL, Lin J. A Western Atlantic peppermint shrimp complex: redescription of Lysmata wurdemanni, description of four new species, and remarks on Lysmata rathbunae (Crustacea: Decapoda: Hippolytidae) Bulletin of Marine Science. 2006;79:165–204. [Google Scholar]
- Robson (1963).Robson EA. The nerve-net of a swimming anemone, Stomphia coccinea. Quarterly Journal of Microscopical Science. 1963;104:535–549. [Google Scholar]
- Rodríguez et al. (2012).Rodríguez E, Barbeitos M, Daly M, Guismao LC, Häussermann V. Toward a natural classification: phylogeny of acontiate sea anemones (Cnidaria, Anthozoa, Actiniaria) Cladistics. 2012;28:375–392. doi: 10.1111/j.1096-0031.2012.00391.x. [DOI] [PubMed] [Google Scholar]
- Schlesinger et al. (2009).Schlesinger A, Zlotkin E, Kramarsky-Winter E, Loya Y. Cnidarian internal stinging mechanism. Proceedings of the Royal Society of London B: Biological Sciences. 2009;276:1063–1067. doi: 10.1098/rspb.2008.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shick (1991).Shick JM. A functional biology of sea anemones. Chapman & Hall; UK: 1991. [Google Scholar]
- Stephenson (1928).Stephenson TA. The British sea anemones, vol. 1. The Ray Society; London: 1928. [Google Scholar]
- Stephenson (1935).Stephenson TA. The British sea anemones, vol. II. The Ray Society; London: 1935. [Google Scholar]
- Turk (1991).Turk T. Cytolytic toxins from sea anemones. Journal of Toxciology—Toxin Reviews. 1991;10(3):223–262. doi: 10.3109/15569549109053857. [DOI] [Google Scholar]
- Valdés (2005).Valdés A. A new species of Aeolidiella Bergh, 1867 (Mollusca: Nudibranchia: Aeolidiidae) from the Florida Keys, USA. The Veliger. 2005;47:218–223. [Google Scholar]
- Wada (1972).Wada T. Contractile activities of the acontial filaments of Metridium senile var. fimbriatum Verrill. Journal of the Faculty of Science, Hokkaido University: Zoology, Ser. VI. 1972;18:387–399. [Google Scholar]
- Waters (1973).Waters VL. Food-preference of the nudibranch Aeolidia papillosa, and the effect of the defenses of the prey on predation. The Veliger. 1973;15:174–92. [Google Scholar]
- Yanagita (1959).Yanagita TM. Physiological mechanisms of nematocyst responses in the sea anemone—I. Effects of trypsin and thioglycolate upon isolated nematocysts. Japanese Journal of Applied Entomology and Zoology. 1959;12:361–375. [Google Scholar]
- Yanagita (1960).Yanagita TM. Physiological mechanisms of nematocyst responses in the sea anemone—III. Excitation and anaesthetization of the nettling response system. Comparative Biochemistry and Physiology. 1960;1(2):123–139. doi: 10.1016/0010-406X(60)90045-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
All of the raw data are presented in Table 1. A supplementary video has been included as Video S1.