Abstract
Objective
Angiotensin II (AngII) has been shown to regulate angiogenesis and at high pathophysiological doses to cause vasoconstriction through the AngII receptor type 1 (AT1R). Angiotensin 1-7 (Ang-(1-7)) acting through the Mas1 receptor can act antagonistically to high pathophysiological levels of AngII by inducing vasodilation, while the effects of Ang-(1-7) signaling on angiogenesis are less defined. To complicate the matter, there is growing evidence that a subpressor dose of AngII produces phenotypes similar to Ang-(1-7).
Approach and Results
This study shows that low dose Ang-(1-7), acting through the Mas1 receptor, promotes angiogenesis and vasodilation similarly to a low, subpressor dose of AngII acting through AT1R. Additionally, we show through in vitro tube formation that Ang-(1-7) augments the angiogenic response in rat microvascular endothelial cells. Utilizing proteomic and genomic analyses, downstream components of Mas1 receptor signaling were identified, including Rho Family GTPases, phosphatidylinositol 3-kinase, protein kinase D1, mitogen activated protein kinase (MAPK), and extracellular signal-related kinase (ERK) signaling. Further experimental antagonism of ERK1/2 and p38MAPK signaling inhibited endothelial tube formation and vasodilation when stimulated with equimolar, low doses of either AngII or Ang-(1-7).
Conclusions
These results significantly expand the known Ang-(1-7)/Mas1 receptor signaling pathway and demonstrate an important distinction between the pathological effects of elevated and suppressed AngII as compared to the beneficial effects of AngII normalization and Ang-(1-7) administration. The observed convergence of Ang-(1-7)/Mas1 and AngII/AT1R signaling at low ligand concentrations suggests a nuanced regulation in vasculature. These data also reinforce the importance of MAPK/ERK signaling in maintaining vascular function.
Keywords: angiogenesis, vascular endothelium, renin-angiotensin system, vasodilation, signaling pathways
Introduction
The renin-angiotensin system is crucial for regulation of sodium homeostasis, vascular tone, angiogenesis, and overall cardiovascular function. Within this complex system, angiotensinogen is released into circulation from the liver and is cleaved into the decapeptide angiotensin I by renin1, 2. Angiotensin I is further cleaved into the active octapeptide angiotensin II (AngII) by angiotensin converting enzyme and the heptapeptide angiotensin-(1-7) (Ang-(1-7)) by either angiotensin converting enzyme 2 or various endopeptidases2–4. AngII is the most characterized of the renin-angiotensin system peptides and has been shown to mediate the majority of its physiological functions through the AngII type 1 receptor (AT1R)2, 5. AT1R activation occurs through heterotrimeric G proteins (Gq/11, Gi/o and G12/13), which in turn activate phosopholipase C, inhibit adenylate cyclase and activate RHO GTPases2. While normal AngII signaling contributes to vascular homeostasis, elevated levels of AngII are deleterious, causing vasoconstriction and oxidative stress, while insufficient levels like those seen in the absence of renin activity result in endothelial dysfunction2, 5–7.
Ang-(1-7) signaling, mediated by the Mas1 receptor8, has been shown to exhibit several protective cardiovascular effects that act antagonistically to elevated pathophysiological levels of AngII9–12. These antagonistic effects include the induction of vasodilation, preservation of endothelial function, the promotion of antifibrotic conditions, and facilitating antihypertrophic effects9, 11, 13–17. However, under conditions of renin-angiotensin system suppression, such as in low renin forms of hypertension and during periods of elevated sodium intake, low-dose AngII infusion, which normalizes levels of circulating AngII to physiological concentrations, also improves endothelial function and restores impaired vasodilator ability6, 7. These studies suggest that there is a delicate balance of the levels of AngII and Ang-(1-7) in the renin-angiotensin system that is more complex than once thought.
Despite the growing evidence that Ang-(1-7)/Mas1 signaling is biologically important to the renin-angiotensin system, studies on the downstream signaling mechanisms have been limited, especially compared to the more studied AngII/AT1R-axis. It has been shown that attenuation of extracellular signal-related kinases 1/2 (ERK1/2) activation through Ang-(1-7) induced Mas1 receptor signaling in glomerular mesangial cells occurs through both cyclic adenosine monophosphate and protein kinase A3, 18. Additionally, Ang-(1-7) treatment preserves endothelial function and regulates vascular oxidative stress through mechanisms involving endothelial nitric oxide synthase (eNOS) and nitric oxide bioavailability3, 6, 12. Studies involving human endothelial cells constitutively expressing the Mas1 receptor also suggest that Ang-(1-7) regulation of eNOS signaling may be occurring through the regulation of phosphatidylinositol 3-kinase (PI3K) and serine/threonine protein kinase AKT pathways3, 10. In addition, a study using quantitative phosphoproteomics provided insights into Ang-(1-7) induced phosphorylation changes19 and a second study using an antibody-based protein assay to monitor Ang-(1-7) altered protein expression20 in human endothelial cells. Both of these studies indicated global changes in signal transduction, apoptosis, cell cycle, and gene expression regulation. These studies provide a strong initial basis for understanding certain aspects of Ang-(1-7) mediated Mas1 receptor signaling; however, they do not reveal the components of the proximal signaling complex generated from early Ang-(1-7) stimulation of the Mas1 receptor.
In order to improve our understanding of the signaling mechanisms behind the protective effects of Ang-(1-7) in the vasculature, we characterized the effects of Ang-(1-7) and compared them to the vascular phenotypes resulting from a low, subpressor dose AngII treatment in renin suppressed rat models on high salt diet. We hypothesized that 1) Ang-(1-7) would display similar effects on angiogenesis and vasodilation compared to the low, subpressor dose of AngII, 2) that Ang-(1-7)/Mas1 and AngII/AT1R signaling pathways would overlap, and 3) that these pathways converge upon a common mechanistic pathway regulating both endothelium-dependent vasodilation and angiogenesis. In order to test these hypotheses, this study utilized a combination of investigative techniques including an in vivo electrical stimulation rat angiogenesis model, ex vivo middle cerebral artery (MCA) vasodilation in response to acetylcholine (ACh), and in vitro endothelial tube formation. These functional analyses were supplemented with proteomic and genomic pathway comparisons, followed by pharmacological targeting of implicated pathways contributing to Ang-(1-7) mediated angiogenesis and vasodilation in the functional assays. This combined systematic approach provided a detailed insight into the intersection between Ang-(1-7)/Mas1 receptor signaling and low dose AngII/AT1R signaling in the vascular endothelium.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
AngII and Ang-(1-7) Enabled Angiogenesis in Response to Electrical Stimulation
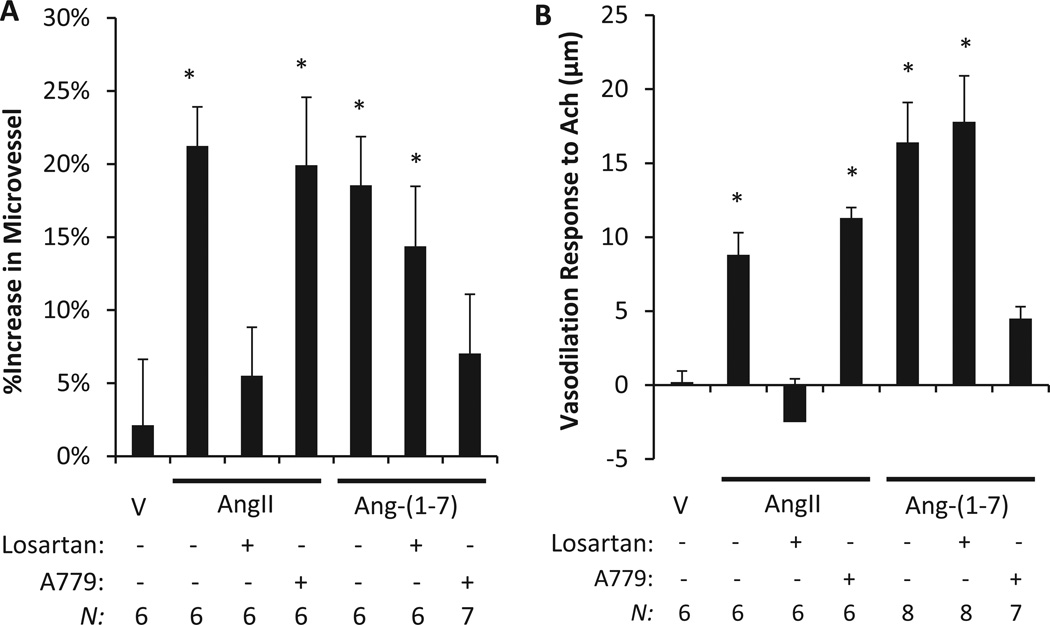
We have previously shown that 7 days of electrical stimulation in vivo produces a robust angiogenic response in rat models that is eliminated by a high salt diet and restored by a low, subpressor dose AngII infusion7, 21; this result was replicated for a control in this study (Figure 1A). In vivo administration of the AT1R antagonist Losartan abolished the restorative effect of low dose AngII infusion, whereas Mas1 receptor antagonist A779 had no effect on the ability of low dose AngII infusion to restore angiogenesis. This experiment was repeated with Ang-(1-7) infusion and co-treatment with Losartan, A779, or vehicle (Figure 1A). Vessel density was significantly increased in the Ang-(1-7) infused rats, as observed with AngII infusion; however, Losartan was unable to block the increase in vessel density resulting from Ang-(1-7) treatment. Rats infused with Ang-(1-7) plus A779 exhibited no significant increase of vessel density, suggesting Ang-(1-7) restored angiogenesis directly through the Mas1 receptor. Vehicle treatment (without low-dose AngII or Ang-(1-7) infusion) was unable to restore stimulated angiogenesis in Sprague Dawley (SD) rats on a high salt diet. These data suggest that Ang-(1-7) restores stimulated angiogenesis through a Mas1 receptor-dependent signaling pathway and does not depend on AngII activation of the AT1R axis (Figure 1A).
Figure 1.
Angiogenesis and vasodilation was restored in Sprague Dawley (SD) rats undergoing high salt diet (4% NaCl) suppression of the renin-angiotensin system. (A) Rats were unilaterally stimulated while intravenously treated with AngII, Ang-(1-7) or vehicle (V), and co-treated with AT1R antagonist (Losartan) or Mas1 antagonist (A779). Both AngII through the AT1 receptor and Ang-(1-7) through the MAS1 receptor significantly restored angiogenesis versus vehicle (*p<0.05). (B) Maximum change of isolated rat middle cerebral artery diameter in response to acetylcholine (Ach; 10−5 M) following AngII, Ang-(1-7) or vehicle co-treatment with Losartan and A779 was measured. Both AngII acting through AT1R and Ang-(1-7) acting through the Mas1 receptor restored the vasodilation response to acetylcholine (Ach; *p<0.05; N is indicated above for each condition).
AngII and Ang-(1-7) Mediated Vasorelaxation Response to ACh
The effects of low-dose AngII, Ang-(1-7), or vehicle infusion on isolated maximum MCA responses to ACh in SD rats on a high salt diet is summarized in Figure 1B. As in previous studies6, 7, this study shows that ACh does not induce MCA vasodilation in vehicle treated control rats on a high salt diet (renin suppressed). A chronic infusion of a low, subpressor dose of AngII or Ang-(1-7) in these renin suppressed rats restored endothelium-dependent vasodilation in response to ACh. AngII induced vasodilation was eliminated by co-treatment with AT1R antagonist losartan; however, it was not affected by the Mas1 receptor antagonist A779. Similarly, Ang-(1-7) induced vasodilation in response to ACh was inhibited by co-treatment with A779, but not by losartan. As observed with angiogenesis in the SD rat hind limb (Figure 1A), this data suggests that Ang-(1-7) mediated vasodilation operates exclusively through a Mas1 receptor-dependent signaling pathway, while low-dose AngII mediated vasodilation is exclusively AT1R dependent (Figure 1B).
Analysis of Endothelial Cell Mas1 Receptor Signaling Pathways
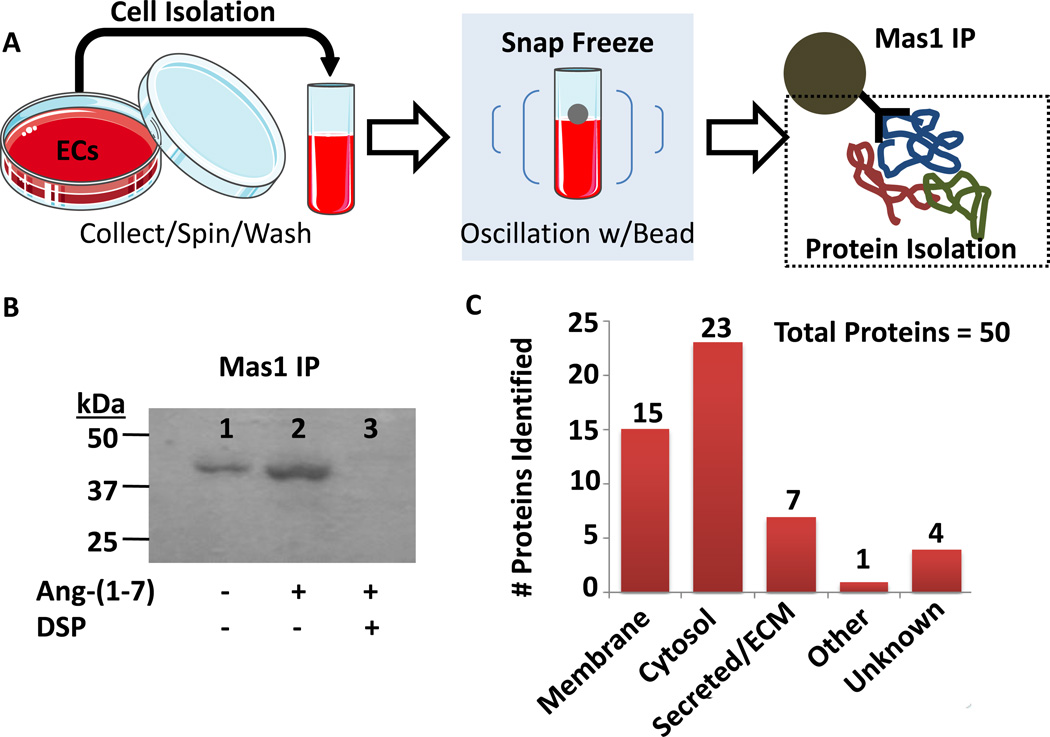
The in vivo experiments suggested an endothelium-dependent Ang-(1-7)/Mas1 receptor pro-angiogenesis and pro-vasodilator response. This endothelium-dependent result was recapitulated in preliminary tube formation assays in which rat microvascular endothelial cells (RMVECs) treated with Ang-(1-7) displayed a 16% in tube formation versus the non-treated (p<0.05; n=5). In order to directly analyze the endothelium-dependent response in relation to Mas1 receptor signaling a combination of cryolysis, immno-precipitation, and tandem mass spectrometry (MS/MS) analysis was utilized on signaling complexes isolated from RMVECs (Figure 2A). Fluorescence microscopy experiments confirmed that the Mas1 receptor antibody (Santa Cruz, sc-135063) epitope was intracellular (Supplemental Figure I). Signaling complexes were then verified for presence of Mas1 by immunoblot (Figure 2B, Supplemental Figure II) according to previous protocols22–25 using rabbit anti-rat Mas1 primary antibody (Santa Cruz, sc-135063) at 1:1000 and secondary HRP-conjugated goat anti-rabbit IgG antibody (BioRad) at 1:2000, as well as in the raw MS/MS data (Supplemental Figure II). In the Ang-(1-7) stimulated RMVEC signaling complexes, 50 proteins were identified as significantly increased (p<0.05, n=8 plus a technical replicate, 16 total MS runs) versus non-stimulated RMVECs after application of stringent filters (Figure 2C, Table 1, Supplemental Table I). Further bioinformatic pathway analysis indicated that the top represented biological signaling networks involved hematological system development/function, hematopoiesis, nervous system development/function, and tissue morphology following Ang-(1-7) stimulation of RMVECs. Within the exclusive protein signaling complexes from Ang-(1-7) stimulated RMVEC immunoprecipitations numerous G-protein signaling components were detected, including those involved in Rho Family GTPase (RHO, RAS, RAC signaling; Table 1). Calcium signaling regulators essential for G-protein and other signaling pathways were also observed, such as diacylglycerol kinase, PI3K, and protein kinase C (Table 1). AKT1 and nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) regulators, such as mTOR (mechanistic target of rapamycin) and CARD10 (caspase recruitment domain family member 10), essential for cell regulation of inflammatory response were present as well (Table 1). CARD10, an important activator of NFkB, was detected in significantly lower amounts in the stimulated receptor complex, suggesting a decrease in activation and a potential anti-inflammatory effect. Additionally, regulators of cellular reorganization and initiation of nascent protein synthesis were detected.
Figure 2.
Mas1 protein signaling complexes were isolated by immunoprecipitation (IP). (A) Indicated is an overview of the identification of Ang-(1-7) activated Mas1 receptor signaling complexes. (B) Isolated signaling complexes from RMVECs were immuno-blotted for Mas1 in unstimulated, stimulated with 100 nM Ang-(1-7), and stimulated with 100 nM Ang-(1-7) plus 1 mM DSP cross-linker. Immunoblots revealed that Ang-(1-7) stimulated without cross-linker was optimal. (C) Mass spectrometry analysis of Ang-(1-7) stimulated Mas1 IP complexes revealed a total of 50 associated proteins (N=8 plus techinical replicate, 16 total runs; see methods for filtering criteria).
Table 1.
Ang-(1-7) Stimulated MAS1 Receptor Immuno-precipitation ‘Top Proteomic Hits’
| Accession Number |
Annotated Protein | Ang-(1-7) IP Peptides/Scans |
NormLog2Ratio* (norm. p-value) |
Notable Signaling Involvement |
|---|---|---|---|---|
| Q99MV5 | Putative Helicase Mov10l1 (M10L1) | 9 / 63 | 2.34 (4.27E-8) | Cell Cycle Regulation (−) |
| P25095 | Type-1 Angiotensin II Receptor (AGTR1) | 2 / 19 | 0.9173 (0.034) | G-protein and Renin-Angiotensin System Signaling |
| Q64438 | Angiogenin-2 (ANG2) | 4 / 102 | Unique (5.41E-26) | Transcription Regulation |
| P70478 | Adenomatous polyposis coli protein (APC) | 38 / 62 | 0.63 (0.046) | WNT Signaling (−) |
| Q80YF9 | Rho GTPase-activating protein 33 (RHG33) | 5 / 57 | 0.72 (0.031) | G-protein Signaling (CDC42, RHO/RAC) |
| P58660 | Caspase recruitment domain-containing protein 10 (CARD10) |
13 / 35 | 0.86 (0.051) | IKK/NFkB Signaling |
| Q66K08 | Cartilage intermediate layer protein 1 (CILP-1) | 13 / 60 | 1.63 (2.34E-5) | TGFβ1 and IGF1Signaling (−) |
| P49025 | Citron Rho-interacting kinase (CRIK) | 28 / 54 | 0.71 (0.041) | G-protein Signaling (RHO/RAC1) |
| P08081 | Clathrin light chain A | 7 / 57 | 0.91 (0.0090) | Endosomal Pathway |
| Q5PPG7 | Eukaryotic translation initiation factor 2D (EIF2D) | 11 / 27 | 1.29 (0.017) | Translation Initiation |
| Q69ZL1 | FYVE, RhoGEF and PH domain-containing protein 6 (FGD6) |
14 / 51 | 1.51 (0.00023) | G-protein Signaling (CDC42, +) |
| Q00342 | Receptor-type tyrosine-protein kinase FLT3 | 6 / 60 | 0.63 (0.049) | SHC1(+), AKT1(+), MTOR (+), RAS(+), MAPK/ERK(+), PLCG1(+), and STAT5 Signaling |
| P35439 | Glutamate receptor ionotropic, NMDA 1 (GluN1) | 11 / 43 | 1.15 (0.0058) | NMDA Signaling |
| P97879 | Glutamate receptor-interacting protein 1 (GRIP-1) | 13 / 28 | 1.02 (0.044) | Signaling Complex Scaffold |
| Q61754 | Glandular kallikrein K24 (mGK-24) | 2 / 37 | Unique (2.15E-10) | Kallikrein/Bradykinin Signaling |
| Q60682 | Killer cell lectin-like receptor 8 | 6 / 64 | 0.83 (0.010) | Class I MHC Signaling |
| D3ZBP4 | Protein-methionine sulfoxide oxidase MICAL1 | 7 / 67 | 1.08 (0.0011) | Apoptosis Signal Regulation (−) |
| P12526 | Proto-oncogene Mas (MAS1) | 4 / 13 | Unique (1.67E-4) | G-protein and Renin-Angiotensin System Signaling |
| P42346 | Mammalian target of rapamycin (MTOR) | 24 / 53 | 1.26 (0.0010) | G-protein (RHO/RAC1) and PI3K-AKT Signaling |
| Q99466 | Neurogenic locus notch homolog protein 4 (NOTCH4) |
27 / 33 | 1.58 (0.0022) | Cell Survival Signaling (+) |
| Q99MR9 | Protein phosphatase 1 regulatory subunit 3A | 7 / 93 | 0.55 (0.031) | Glycogen Synthesis |
| Q9WTQ1 | Serine/threonine-protein kinase D1 (PRKD1) | 10 / 25 | 1.86 (0.0029) | PKC (+), DAG (+), ERK1/2 (+), IKK/NFkB (+), p38MAPK (+), and EGF (−) Signaling |
| Q91YA2 | Serine/threonine-protein kinase H1 | 7 / 52 | 0.91 (0.012) | Trafficking/ pre-mRNA Processing |
| Q9Z268 | RasGAP-activating-like protein 1 (RASL1) | 10 / 66 | 0.58 (0.057) | Ras-cAMP Pathway |
| P27671 | Ras-specific guanine nucleotide-releasing factor 1 (Ras-GRF1) |
23 / 26 | 1.07 (0.044) | G-Protein Signaling (RHO/RAC/RAS) |
| P05545 | Serine protease inhibitor A3K | 5 / 32 | 2.21 (1.61E-4) | Kallikrein Signaling (−) |
| P84551 | SKI family transcriptional corepressor 1 (SKOR1) | 12 / 34 | 1.04 (0.024) | BMP Signaling (−) |
| P50592 | TNF ligand superfamily member 10 | 2 / 68 | 1.92 (4.9E-7) | TNFα Signaling |
| Q8CIR4 | Transient receptor potential cation channel subfamily M member 6 (TPRM6) |
17 / 26 | 1.43 (0.012) | Ion Channel and Kinase |
| P68255 | 14-3-3 protein theta (1433T) | 6 / 47 | Unique (8.22E-13) | G-Protein Signaling (RHO/RAC), PDK1, and PI3K-AKT Signaling |
| Q80U44 | Zinc finger FYVE domain-containing protein 16 | 14 / 36 | 2.12 (9.70E-5 | Endosomal Pathway |
Note: Signling involvement includes but is not limited to those above. All proteins indicated passed all stringent filters indicated in the Methods; a pre-filtered protein list can be found in Supplementary Table 1.
100 nM Ang-(1-7) stimulated versus unstimulated Mas1 IP (N=8; 16 total runs).
Analysis of Gene Expression in Ang-(1-7)-Treated RMVECs
In order to add depth to the Ang-(1-7) stimulated MAS1 receptor signaling pathway data from the proteomic MS/MS analysis of the immunoprecipitation results, expression of common angiogenesis-related genes in response to 100 nM Ang-(1-7) stimulation of RMVECs was examined according to previous literature26. The response to Ang-(1-7) treatment of RMVECs were assessed (Table 2) and related to the proteomic MS/MS data. Significant gene expression changes (p<0.05) were observed in 25 of the 84 genes (30%) analyzed (Table 2), including in growth factor/G-protein signaling, signal transduction regulation, and transcriptional regulation. Interestingly, vascular endothelial growth factor receptor 1 (VEGFR1, Vegfr1/Flt1, 3.02-fold, p=0.050) and vascular endothelial growth factor receptor 2 (VEGFR2, Vegfr2/Kdr, 7.65-fold, p=0.011) were up-regulated, suggesting a link to components of RAS signaling identified in the proteomic analyses that have been shown to mediate angiogenesis through vascular endothelial growth factor (VEGF) family signaling27. Numerous other significant changes in gene expression of important angiogenesis and vasoreactive signal molecules were observed related to G-protein signaling including cell division control protein 42 (CDC42, Cdc42, 1.22-fold, p=0.047) and RAC2 (Rac2, 6.65-fold, p=0.009), ERK1/2 and MAPK signaling including MEK2 (Map2k2, 1.57-fold, p=0.005) and p38MAPK (Mapk13, 5.57-fold, p=0.002), phosphoinositol signaling, and phospholipase signaling (Table 2, p<0.05). As with Ang-(1-7) stimulation, the low dose AngII stimulation upregulated CDC42 (Cdc42, 1.26-fold, p=0.028), VEGFR1 (Vegfr1/Flt1, 1.17-fold, p=0.020), p38MAPK (Mapk13, 4.83-fold, p=0.026), and RAC signaling (Rac1, 1.23-fold, p=0.022). Interestingly, the high dose AngII (10× low dose AngII) stimulation of the RMVECs exhibited a marked decrease in AKT, VEGFR, MAPK, phosphoinositol and phospholipase signaling molecules (p<0.05) that were upregulated by Ang-(1-7) (Table 2) or low dose AngII stimulation (Table 3).
Table 2.
Analysis of an angiogenesis RT-PCR gene expression array following Ang-(1-7) stimulation of rat microvascular endothelial cells
| Gene | Protein Annotation | Fold Regulation* |
p-value | Signaling Pathway Involvement* |
|---|---|---|---|---|
| Cdc42 | Cell division control protein 42 homolog (CDC42) | 1.22 | 0.047 | Rho GTPase, p21 signaling |
| Flt-1 | Vascular Endothelial Growth Factor Receptor 1 (VEGFR1) | 3.02 | 0.050 | VEGF(+), PLCG(+), PKC(+), MAPK/ERK(+), AKT1(+) signaling |
| Flt-4 | Vascular Endothelial Growth Factor Receptor 4 (VEGFR4) | −5.97 | 0.050 | VEGF(+), PLCG(+), PKC(+), MAPK/ERK(+), AKT1(+) signaling |
| Hspb1 | Heat shock protein beta-1 (HSPB1) | 2.25 | 0.015 | Chaperone, thermotolerance, apoptosis(−), NF-κB(+) |
| Kdr | Vascular endothelial growth factor receptor 2 (VEGFR2) | 7.65 | 0.011 | angiogenesis(+), mitogenesis(+), cell migration(+) signaling |
| Map2k2 | Dual specificity mitogen-activated protein kinase kinase 2 (MEK2) |
1.57 | 0.005 | MAPK/ERK(+) signaling |
| Mapk1 | Mitogen-activated protein kinase 1 (MAPK1) | −1.16 | 0.031 | integration of signaling |
| Mapk12 | Mitogen-activated protein kinase 12 (MAPK12) | 2.57 | 0.017 | p38 MAPK, inflammatory(+) signaling |
| Mapk13 | Mitogen-activated protein kinase 11 (MAPK13) | 5.57 | 0.002 | p38 MAPK, ATF2(+), ELK1(+), PRKD1(−) signaling |
| Pgf | Placental Growth Factor (PGF) | −4.38 | 0.004 | FLT-1(+) signaling |
| Pik3cb | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K beta isoform) |
−1.46 | 0.007 | PIP3(+), AKT1(+), PDPK1(+) signaling |
| Pik3cd | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (PI3K delta isoform) |
−2.68 | 0.044 | PIP3(+), AKT1(+), PDPK1(+), RAS(+), MAPK/ERK(+), PI3K(+) signaling |
| Pik3cg | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K gamma isoform) |
1.28 | 0.022 | PIP3(+), AKT1(+), PDPK1(+), cAMP(−), beta-adrenergic(−) signaling |
| Pla2g12a | Group XIIA secretory phospholipase A2 | 1.65 | 0.007 | phospholid(+), PKC(+), inflammatory(+) signaling |
| Pla2g2e | Group IIE secretory phospholipase A2 | 2.73 | 0.002 | phospholid(+), PKC(+), inflammatory(+) signaling |
| Pla2g4a | Cytosolic phospholipase A2 | −1.46 | 0.014 | phospholid(+), PKC(+), inflammatory(+) signaling |
| Ppp3ac | Calcineurin subunit B type 2 (CANB2) | 2.37 | 0.027 | calcium signaling(+) |
| Prkca | Protein kinase C alpha (PKCα) | −2.19 | 0.003 | calcium(+), DAG(+), RAF1(+), MAPK/ERK(+), proliferation(−), apoptosis(−) signaling |
| Ptgs2 | Prostaglandin G/H synthase 2 (cycloxygenase-2, COX-2) | −4.59 | 0.043 | prostaglandin signaling (+) |
| Ptk2 | Focal adhesion kinase 1 | 1.76 | 0.033 | migration(+), PI3K(+), AKT1(+), MAPK/ERK(+), Rho GTPase signaling |
| Rac2 | Ras-related C3 botulinum toxin substrate 2 | 6.65 | 0.009 | Rho GTPase signaling |
| Sh2d2a | SH2 domain-containing protein 2A (SH2D2A) | 7.18 | 0.017 | VEGFR2(+), MAPK(+), PKC(+) IKK/NFkB(+) signaling |
| Sphk1 | Sphingosine kinase 1 (SPHK1) | 1.78 | 0.023 | sphingosine(+), TNFα(+), NF-κB(+) signaling |
| Sphk2 | Sphingosine kinase 2 (SPHK2) | 1.62 | 0.045 | sphingosine(+), DAG(+), PKC(+), VEGF/MAPK/RAS(+) signaling |
| Vegfa | Vascular endothelial growth factor A (VEGF-A) | −2.93 | 0.014 | FLT1(+), KDR(+) signaling |
Note: All genes indicated significant fold change in gene expression (p≤0.05); biologic processes include but are not limited to those above (N=3).
Indicates 100 nM Ang-(1-7) stimulated versus unstimulated endothelial cells.
Table 3.
Analysis of an angiogenesis RT-PCR gene expression array following AngII low and high dose stimulation of rat microvascular endothelial cells.
| Gene | Protein Annotation | Fold Change* | p-value | Signaling Pathway Involvement* |
|---|---|---|---|---|
| LOW | ANGII | |||
| Cdc42 | Cell division control protein 42 homolog (CDC42) | 1.26 | 0.028 | Rho GTPase, p21 signaling |
| Hif1a | Vascular Endothelial Growth Factor Receptor 1 (VEGFR1) | 1.17 | 0.020 | Master regulator of hypoxia response |
| Mapk13 | Mitogen-activated protein kinase 11 (MAPK13) | 4.83 | 0.026 | p38 MAPK, ATF2(+), ELK1(+), PRKD1(−) signaling |
| Pdgfc | Platelet derived growth factor C | 1.31 | 0.016 | proliferation(+), migration(+), cell survival(+) signaling |
| Pik3r1 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit |
1.39 | 0.007 | insulin(+), FGF, KIT, PDGF signaling |
| Ppp3cb | Calcineurin subunit B type 2 (CANB2) | 1.15 | 0.047 | calcium signaling |
| Prkcb | Protein kinase C beta (PKCβ) | 1.47 | 0.019 | calcium(+), DAG(+), NF-κB(+), ANDR(+), RAF/MAP/ERK(+) signaling |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 | 1.23 | 0.022 | Rho GTPase signaling |
| HIGH | ANGII | |||
| Akt1 | V-akt murine thymomavival oncogene homolog 1 | −3.68 | 0.019 | PDGF(+), cell survival, angiogenesis, insulin signaling |
| Akt2 | V-akt murine thymomavival oncogene homolog 2 | −4.83 | 0.015 | PDGF(+), cell survival, angiogenesis, insulin signaling |
| Arnt | Aryl hydrocarbon receptor nuclear translocator | −2.61 | 0.040 | transport ligand to nucleus |
| Bad | Bcl2 associated agonist of cell death | −4.16 | 0.037 | apoptosis(+) signaling |
| Cdc42 | Cell division control protein 42 homolog (CDC42) | 1.46 | 0.014 | Rho GTPase, p21 signaling |
| Flt-4 | Vascular Endothelial Growth Factor Receptor 4 (VEGFR1) | −5.97 | 0.050 | VEGF(+), PLCG(+), PKC(+), MAPK/ERK(+), AKT1(+) signaling |
| Map2k1 | Dual specificity mitogen-activated protein kinase kinase 1 | −4.74 | 0.009 | MAPK/ERK(+) signaling |
| Mapk11 | Mitogen-activated protein kinase 11 (MAPK11) | −13.02 | 0.005 | p38MAKP(+) signaling |
| Mapk14 | Mitogen-activated protein kinase 14 (MAPK14) | −2.52 | 0.009 | p38MAKP(+) signaling |
| Mapkapk2 | Mitogen-activated protein kinase-activated protein kinase 2 | −2.37 | 0.045 | p38MAPK/MAPK14(+), TNFα(+), HSP27(+) signaling |
| Mapkapk3 | Mitogen-activated protein kinase-activated protein kinase 3 | −4.73 | 0.018 | p38MAPK/MAPK14(+), TNFα(+), ERK(+), JNK(+) signaling |
| Nrp2 | Neuropilin 2 | −3.07 | 0.018 | VEGF(+), PLGF-2(+) signaling |
| Pgf | Placental Growth Factor (PGF) | −8.12 | 0.002 | FLT-1(+) signaling |
| Pik3cb | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K beta isoform) |
−2.03 | 0.024 | PIP3(+), AKT1(+), PDPK1(+) signaling |
| Pik3cd | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (PI3K delta isoform) |
−5.74 | 0.013 | PIP3(+), AKT1(+), PDPK1(+), RAS(+), MAPK/ERK(+), PI3K(+) signaling |
| Pik3r2 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit |
−4.25 | 0.020 | PIP3(+), AKT1(+), PDPK1(+), p110 signaling |
| Pla2g2f | Phospholipase A2, group IIF | −3.80 | 0.009 | hydrolyzes phosphadylglycerol |
| Pla2g4b | Phospholipase A2, group IVB | −2.27 | 0.015 | hydrolyzes glycerophospholipids |
| Pla2g6 | Phospholipase A2, group VI (cytosolic, calcium- independent) |
−12.40 | 0.008 | arachidonic acid release, apoptosis |
| Plcg1 | Phospholipase C, gamma 1 | −5.11 | 0.013 | IP3(+), DAG(+) signaling |
| Ppp3ca | Calcineurin subunit B type 2 (CANB2) | 3.88 | 0.022 | Calcium, DNM1L, HSPB1, SSH1 signaling |
| Prkca | Protein kinase C alpha (PKCα) | −6.34 | 0.003 | RAF1, BCL2, CSPG4, TNNT2/CTNT, MAPK/ERK(+) signaling |
| Prkcg | Protein kinase C gamma (PKCγ) | −7.12 | 0.025 | calcium, DAG, p53/TP53 signaling |
Note: All genes indicated significant fold change in gene expression (p≤0.05); biologic processes include but are not limited to those above (N=3).
Indicates 100 nM Ang-(1-7) stimulated versus unstimulated endothelial cells.
Bioinformatics Signaling Pathway Analysis of Ang-(1-7)-Mediated Mas1 Signaling in the Endothelium
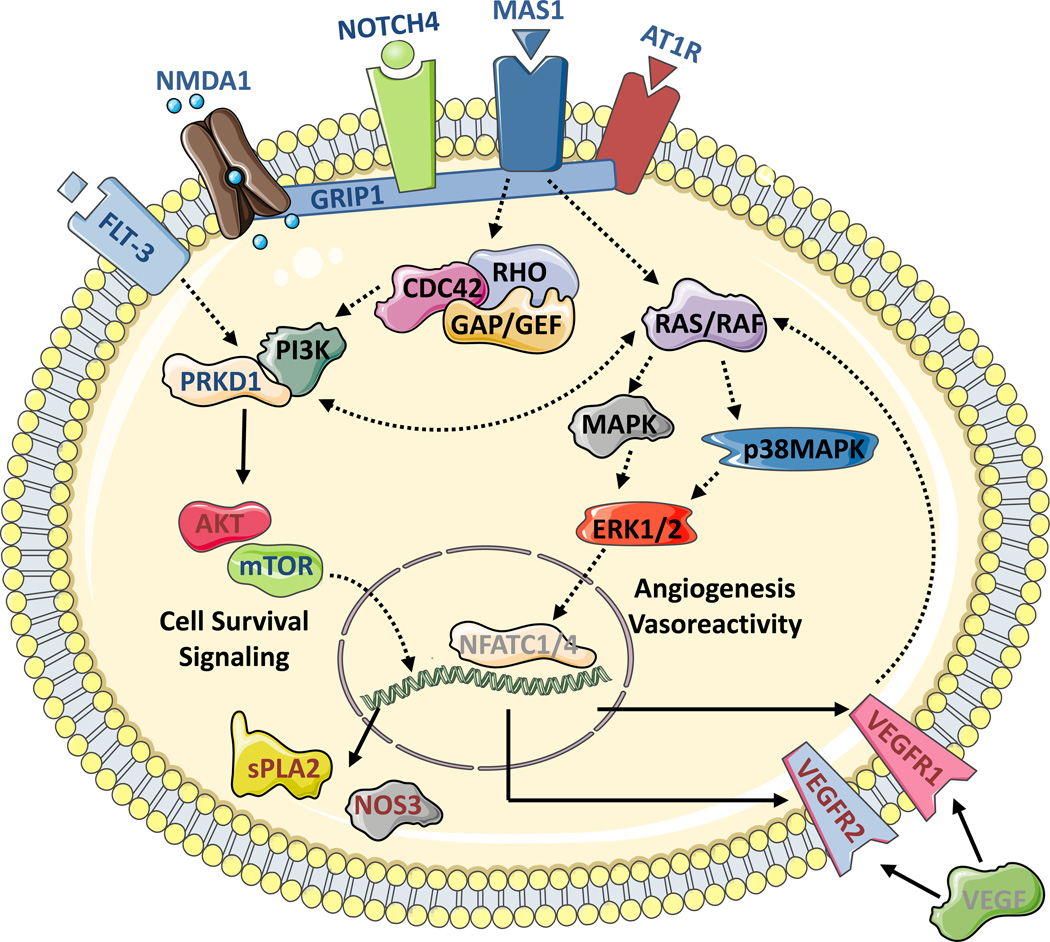
The results of a comprehensive bioinformatic analysis of the proteomic and RT-PCR dataset for pathway mapping of Ang-(1-7) stimulated Mas1 receptor signaling in RMVECs is summarized in Figure 3. Based on the proteomic data, a cell surface complex consisting of extracellular matrix proteins, the Mas1 receptor, and AT1R appear to form and signal through the Rho Family of GTPases (RHO, RAC, RAS). Further bioinformatics pathway analysis of the data indicated that G-protein signaling acted upstream of the PI3K/PRKD1/AKT and MAPK/ERK signaling pathways important for endothelial function, angiogenesis, and vasodilation. General trends observed implicated p38MAPK and ERK1/2 signaling as the important mediators of angiogenesis and vasodilation processes. This analysis suggests a possible convergence of the Ang-(1-7)/Mas1 receptor signaling in this study with previously known AngII/AT1R signaling, especially on the p38MAPK and ERK1/2 signal cascades, leading to upregulation of VEGFR signaling as indicated by downstream gene expression analysis (Figure 3). The Ang-(1-7)/Mas1 receptor signaling complex pathway data presented here is supported by previous literature showing that early stage changes in Ang-(1-7) induced phosphorylation and protein expression related to global changes in ERK1/2, AKT1, NFkB, and VEGF signaling19, 20 and further confirm the molecular basis of the cell survival and anti-inflammatory role of Mas1 receptor activation.
Figure 3.
Utilizing a combination of immunoprecipitation (IP) and tandem mass spectrometry (MS) protein identification (ID), complemented by gene expression analysis, a comprehensive Ang-(1-7) stimulated Mas1 receptor signaling pathway was generated. Signaling proteins annotated with blue lettering indicate those identified in the tandem MS analysis of the immunoprecipitation, annotated in red lettering were significantly increased during gene expression analysis, and annotated in black lettering were identified through both methods. Gray lettering indicates inferred signaling molecules.
Ang-(1-7)-Dependent Activation of RMVEC Signaling
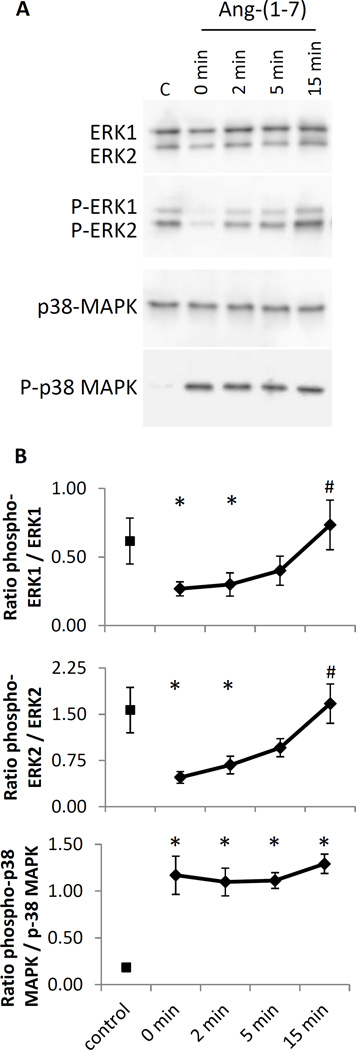
The bioinformatics signaling pathway analysis of Ang-(1-7)-mediated signaling in RMVECs indicated pathways crucial for angiogenesis and vasodilation. ERK1/2 and p38MAPK were two important protein regulators indicated by the activated Mas1 receptor immunoprecipitation proteomic MS/MS data and the Ang-(1-7) stimulated RMVEC gene expression arrays. Therefore, the activation of ERK1/2 and p38MAPK was tested by monitoring Ang-(1-7) induced phosphorylation in the RMVECs. Immunoblotting of ERK1/2 and p38MAPK, along with the phosphorylation of these molecules, in serum starved (1% FBS) RMVECs not treated and treated with Ang-(1-7) samples was used to monitor pathway activation (Figure 4). RMVECs in complete media exhibited high ERK1/2 phosphorylation but little p38MAPK phosphorylation. However, the serum starved RMVEC samples showed little ERK1/2 phosphorylation and high p38MAPK phosphorylation, suggesting that serum starvation suppressed ERK1/2 in our system but enhanced p38MAPK. Treatment of serum starved RMVECs with 100 nM Ang-(1-7) was able to significantly recover ERK1/2 phosphorylation back to levels of complete media after 15 minutes, whereas p38MAPK remained steady in activation. This data suggests ERK1/2 is activated in an Ang-(1-7)/Mas1 receptor dependent manner, while p38 MAPK is activated during serum starvation and maintained following Ang-(1-7) treatment.
Figure 4.
Phosphorylation of ERK1/2 and p38MAPK in RMVECs treated with Ang-(1-7). (A) Immunoblot of RMVECs serum starved in 1% FBS and treated with 100 nM Ang-(1-7) (C = positive control, cells in complete media). Membranes were blotted against ERK1/2, phospho-ERK1/2, p38 MAPK and phospho-p38 MAPK. (B) Quantitation of immunoblots indicated there was an Ang-(1-7)-dependent activation of ERK1/2 and that serum starvation activated p38MAPK, which remained active following Ang-(1-7) treatment. Values are expressed as ratio of phospho-protein / protein mean area (*P<0.05 vs. control cells. #P<0.05 vs. zero minute timepoint).
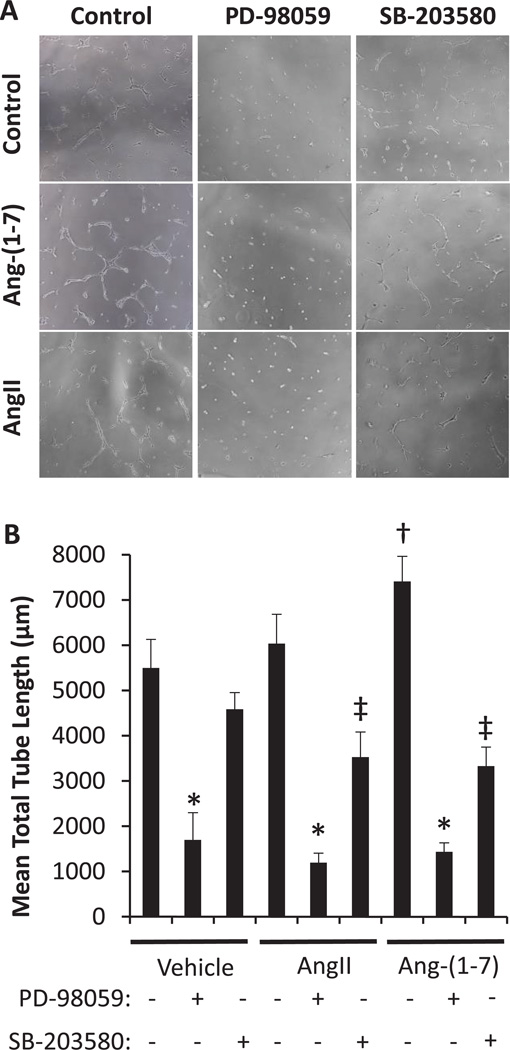
Effects of ERK1/2 and p38MAPK Inhibition on Ang-(1-7)-Stimulated Mas1 Receptor-Mediated Angiogenesis
In order to validate specific signaling pathways shown to be of importance in the Ang-(1-7) stimulation of the Mas1 receptor by proteomic and RT-PCR analyses we tested the functional effects of key modulators ERK1/2 and p38MAPK. RMVEC tube formation was performed for 24 hours in the presence or absence of 100 nM Ang-(1-7) or AngII plus or minus ERK1/2 or p38MAPK antagonists according to the methods (Figure 5). Ang-(1-7) and AngII stimulated an upward trend in RMVEC tube formation; however, only Ang-(1-7) resulted in a significant increase (p<0.001) versus RMVECs alone. The AngII data, coupled with the whole animal data in Figure 1, suggests other factors are required in vivo to aid in the AngII promotion of endothelial angiogenesis. Further experiments indicated that ERK1/2 inhibition resulted in complete loss of tube formation ability under all conditions. p38MAPK inhibition also resulted in significantly decreased tube formation versus control and stimulated conditions, but to a lesser extent than ERK1/2 inhibition. This data suggests that ERK1/2 is essential for angiogenesis processes in general and Ang-(1-7) signals directly through it, whereas p38MAPK may be one of many MAPK upstream regulators of ERK1/2 making it a less essential molecule.
Figure 5.
Endothelium-dependent tube formation. (A) Matrigel tube formation with 20,000 RMVECs treated with 100 nM AngII, 100 nM Ang-(1-7) or vehicle and co-treated with ERK pathway inhibitory PD-98059 (PD; 50 µM) or p38MAPK pathway inhibitor SB-203580 (SB; 10 µM) are shown (n=12). (B) Ang-(1-7) significantly increased (†p<0.05) tube formation compared to vehicle treatment, whereas AngII treatment produced no significant change. ERK pathway inhibition completely eliminated tube formation in all treatments (*p<0.05), while p38MAPK only exhibited significant inhibition in the AngII and Ang-(1-7) treatments (‡p<0.05).
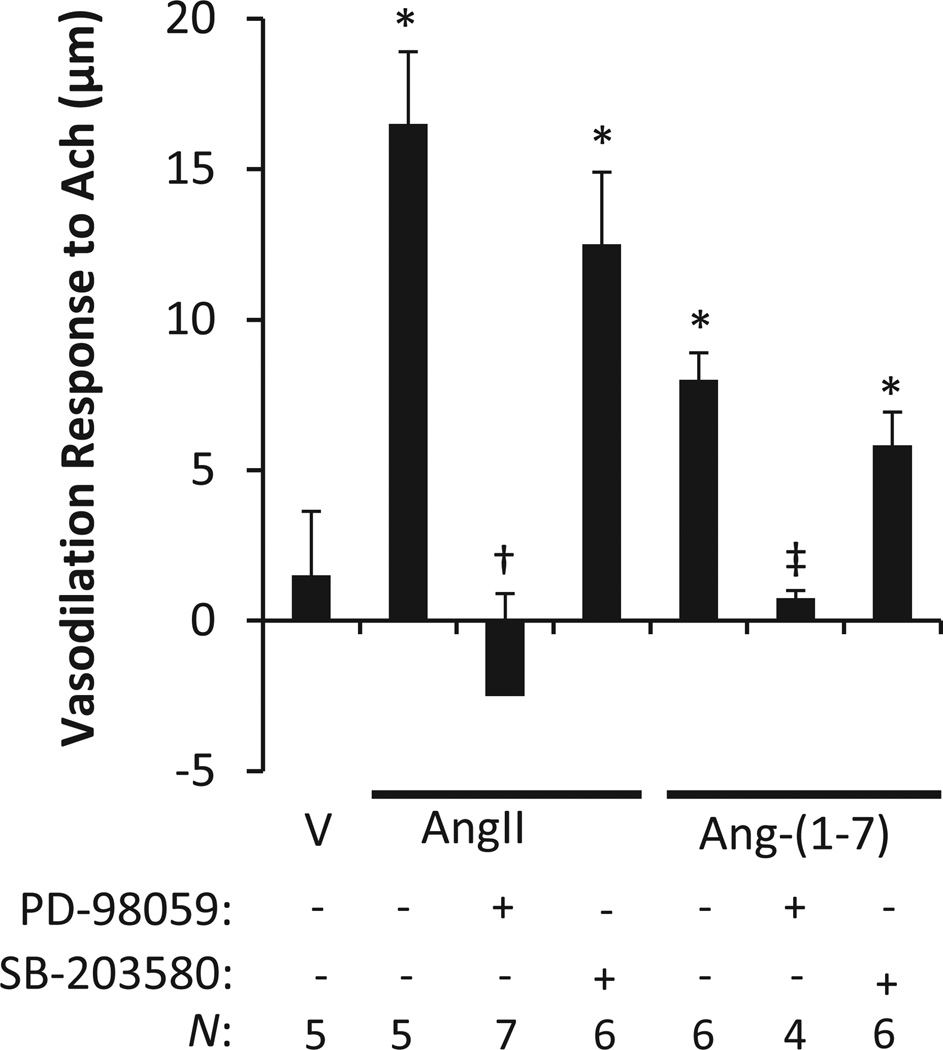
Effects of ERK1/2 and p38MAPK Inhibition on Low-Dose Ang-(1-7)-Stimulated MAS1 Receptor-Mediated Vasodilation
The influence of ERK1/2 and p38MAPK on ex vivo MCA endothelium-dependent Ach (10−5 M)-induced vasodilation was tested. SD rats on a high salt diet to suppress the renin-angiotensin system were treated in vivo with AngII (5 ng·kg−1·min−1), Ang-(1-7) (4 ng·kg−1·min−1) or DMSO vehicle (20 µL/h) and co-treated with ERK pathway inhibitor PD-98059 (PD; 10µg/hr) or p38MAPK pathway inhibitor SB-203580 (SB; 10µg/hr) for 3–5 days as described in the methods. MCAs were then isolated and tested for vasoreactivity ex vivo. The ex vivo studies revealed that a chronic infusion of Ang-(1-7) or AngII restored MCA vasodilation responses to ACh in comparison to the vehicle treatment; this phenotype was blocked by ERK1/2 inhibition (Figure 6). Conversely, p38MAPK inhibition exhibited only slight ablation of ACh induced vasodilation in the MCA from rats receiving chronic AngII or Ang-(1-7) treatments. This data suggests that ERK1/2 is essential for the vasodilation response in conjunction with upstream MAPKs, not just those in the p38MAPK family.
Figure 6.
Mediation of ex vivo endothelium-dependent acetylcholine (Ach; 10−5 M) induced vasodilation. Rats on a high salt diet leading to renin-angiotensin system suppression were treated in vivo with AngII (5 ng·kg−1·min−1), Ang-(1-7) (4 ng·kg−1·min−1) or DMSO vehicle (20 µL/h) and co-treated with ERK pathway inhibitory PD-98059 (PD; 10µg/hr) or p38MAPK pathway inhibitor SB-203580 (SB; 10µg/hr) for 3–5 days. Following treatments, the middle cerebral arteries were tested ex vivo for response to acetylcholine (Ach; 10−5 M) induced vasodilation. ERK pathway inhibition eliminated AngII and Ang-(1-7) mediated vasodiation, while p38MAPK inhibition only exhibits slight ablation on angiotensin peptide restored vasodilation (AngII data replotted from McEwen et al 2009 with permissions).
Discussion
This study examined the impact of the administration of low dose Ang-(1-7) in comparison to a low, equimolar dose of AngII on vascular dysfunction in an animal model with low renin-angiotensin system activity. The data here significantly expands the Ang-(1-7)/Mas1 receptor signaling pathway and adds to the growing body of work demonstrating an important distinction between the pathological effects of elevated or suppressed AngII and the beneficial vascular effects of AngII normalization. It is well established that abnormally elevated levels of AngII stimulate superoxide production and endothelial dysfunction2, 5, 28–31; however, increasing evidence indicates pathologically suppressed AngII levels also increase oxidant stress and endothelial dysfunction relative to physiologically normal plasma AngII levels6, 7, 32, 33. Numerous studies have now shown suppression of AngII via high salt diet disrupts vascular function and low-dose AngII infusion restores function via AT1R6, 7, 27. This challenges the belief that reduction in AngII is universally beneficial to vascular health, but it does not contradict the finding that normalizing pathologically elevated AngII levels is an effective intervention. Our data supports this concept of an “ideal” range of plasma angiotensin peptides to promote effective endothelial function and suggests that an equimolar, low dose of Ang-(1-7) recapitulates the effects of this subpressor dose of AngII.
In previous work, we have consistently observed altered phenotypes of microvessel angiogenesis in response to electrical stimulation by directly, genetically or environmentally manipulating AngII levels and through pharmacological interventions, including angiotensin converting enzyme inhibition and AT1R inhibition7, 24, 33–38. Here for the first time, we demonstrate that Ang-(1-7), acting through the Mas1 receptor and not AT1R, is able to restore microvessel angiogenesis in response to electrical stimulation (Figure 1A). Many studies have shown continuous infusion of Ang-(1-7) at levels up to 160× higher than used here generate Ang-(1-7)/Mas1 receptor dependent effects that counteract the deleterious actions of high levels of AngII4, 13, 16. Our data suggest that at equimolar low doses, Ang-(1-7) and AngII have some complementary rather than antagonistic functions as seen with elevated AngII12–14. High levels of Ang-(1-7) inhibit vessel growth in tumor xenografts39; however, a recent study has shown Ang-(1-7) stimulating a significant increase in sinusoidal endothelial cells sprouting and forming tubule structures out of cavernosal strips in culture40. We show enhancement of in vitro endothelial cell tube formation, in vivo skeletal muscle angiogenesis, and ex vivo MCA vasodilation by treatment with low-dose physiological concentrations of both Ang-(1-7) and AngII.
While AT1R signaling has been well characterized2, Mas1 receptor signaling mechanisms are only beginning to be elucidated. To further investigate the actions of low-dose Ang-(1-7) for comparison, we examined cellular signaling processes activated by Ang-(1-7) stimulation of the Mas1 receptor signaling complex. We then compared the results against low- and high-dose AngII RMVEC gene expression data (Table 3) and to known pathways influenced by AngII stimulation of AT1R2. We found substantial overlap in Ang-(1-7) stimulated Mas1 receptor signaling and AngII stimulated AT1R signaling. Gene expression analysis showed that equimolar, low doses of Ang-(1-7)/Mas1 and AngII/AT1 receptor-ligand interactions lead to the activation of similar pathways, whereas high-dose AngII exhibited down regulation of the same pathways (Tables 2–3). Initial functional tests showed Ang-(1-7) significantly increased the ability of RMVECs to form tube like structures in vitro (data not shown). Importantly, this verified that Ang-(1-7) stimulation had a functional effect on the specific endothelial cell population utilized for subsequent proteomic and gene expression signaling pathway analysis. Proteomic MS/MS analysis of Ang-(1-7) stimulated Mas1 receptor signaling complexes implicated proteins involved in the regulation of G-protein, ERK/MAPK, PI3K/AKT/mTOR, CARD10/NFkB, and phosphoinositol signaling (Table 1).
Gene expression analysis indicated there were significant increases in in expression of the VEGFR family, G-protein signaling including CDC42, ERK1/2, and MAPK signaling, phosphoinositol signaling, and phopholipase signaling (Table 2). The significant increase in expression of the VEGFR1 and VEGFR2 are significant because these are important downstream effectors resulting from RAS/MAPK/ERK signaling and have been shown to mediate angiogenesis through VEGF signaling in other models27. Together these data suggest the Mas1 receptor signals through the RHO Family of GTPases (RHO, RAS, RAC), ERK/MAPK, PI3K/PRKD1/AKT/mTOR-mediated cell survival signaling to promote normal endothelial function, cell survival, angiogenesis, and vasodilation (Figure 3). Additionally, the decreased detection of CARD10 following Ang-(1-7) stimulation suggests a downregulation of NFkB signaling and an anti-inflammatory response. This Ang-(1-7)/Mas1 receptor signaling complex pathway data is supported by previous literature showing that Ang-(1-7) induced global changes in phosphorylation and protein expression related to ERK1/2, AKT1, NFkB, and VEGF signaling19, 20. The direct signaling complex and pathways implicated by this dataset correlate with early stage, but not later stage, global alterations of phosphorylation detected in the previous phosphoproteomic study on Ang-(1-7) signaling19. Interestingly, the gene expression analysis presented here for low dose AngII stimulation demonstrated a similar upregulation in CDC42, VEGFR1, p38MAPK, and RAC signaling to that of Ang-(1-7) suggesting signaling overlap, while high dose AngII exhibited a marked decrease in these signaling pathways supporting previously observed pathophysiologic phenotypes (Table 3).
Modulation of AKT-dependent pathways by Ang-(1-7) stimulation of the Mas1 receptor, including increased mTOR and decreased NFkB activity, is supported by previous literature demonstrating that Ang-(1-7) regulates eNOS activation through PIK3/PKB/AKT-dependent pathways10, 19, 20. Additionally, it is known that AngII increases p38MAPK and ERK1/2 activities41. Pathway data presented here suggest p38MAPK and ERK1/2 involvement in Ang-(1-7) mediated Mas1 signaling in the endothelium (Figure 3). Previous studies have shown that Ang-(1-7) enhances AngII activation of ERK1/2 signaling in bone marrow-derived dendritic cells; a result that is blocked with the Mas1 antagonist A77942. These studies all point to a convergence between the AngII/AT1R and Ang-(1-7)/Mas1 receptor signaling networks through ERK1/2 and p38MAPK signaling, as well as NFkB-mediated inflammatory responses3, 19, 43. Further, our data implicates ERK1/2 and p38MAPK as candidate points of convergence for AngII/AT1R and Ang-(1-7)/Mas1 receptor signaling in the endothelium important for vasodilation and angiogenesis (Figures 5,6). However, our pathway activation data suggests that p38MAPK is activated in our system but not dependent only on Ang-(1-7), while ERK1/2 is an essential molecule for the Ang-(1-7)/Mas1 receptor vasodilation and angiogenic processes shown here (Figure 4). Further analysis of these targets using in vitro RMVEC tube formation following no stimulation, AngII stimulation, or Ang-(1-7) stimulation plus or minus ERK1/2 or p38MAPK inhibition indicated that AngII/AT1R and Ang-(1-7)/Mas1 receptor signaling converge on these molecules for the promotion of in vitro angiogenesis (Figure 5). This data also suggests that ERK1/2 is a common point of convergence for angiogenesis pathways in general as it also inhibited in the vehicle control, while p38MAPK appears more specific for these renin-angiotensin system pathways. Similar conditions applied to rat MCAs indicated that AngII/AT1R and Ang-(1-7)/Mas1 receptor signaling through ERK1/2 was essential for vasodilation, while p38MAPK contributed but was not essential for this process, suggesting there may be other MAPKs involved in signaling to ERK1/2 in this instance (Figure 6). These results suggest that ERK1/2 is a point of essential convergence for ACh induced vasodilation in these pathways and angiogenesis signaling processes, including Ang-(1-7)/Mas1 and AngII/AT1R signaling.
It is important to note that AT1R was consistently identified as part of the Ang-(1-7)/MAS1 receptor signaling complex. It is well known that Ang-(1-7) does not signal through AT1R. Santos et al. (2003) showed that Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas independent of AT1R8. Our data in Figure 1 also demonstrates the specificity of Ang-(1-7) for the Mas1 receptor independent of AT1R stimulation. However, it is important to note that there have been previous reports of a functional interaction between the Mas1 receptor, AT1R, and AT2R that may be of importance to the receptor signaling44, 45. It has also been shown that EGF receptor transactivation is essential for ERK1/2 signaling mediated by AT1R27, 46. We cannot rule out a similar paradigm here given the presence of the AT1R in the Ang-(1-7)/MAS1 receptor signaling complex and the convergence of AT1R and Mas1 signaling. While our vasodilation and angiogenesis data using AT1R and Mas1 receptor inhibitors show that Ang-(1-7) acts specifically through the Mas1 receptor (Figure 1), we cannot rule out that there could be formation of a complex between the receptors at the cell surface required for signaling. This transactivation and the potential for tissue/cell specific differences in the balance between AT1R and Mas1 receptor signaling warrant examination in future studies.
The results of the current study suggest that AngII/AT1R and Ang-(1-7)/Mas1 receptor signaling converge on an essential common pathway of importance for both angiogenesis and vasodilation in the renin-angiotensin system involving ERK1/2 and p38MAPK signaling. The study also indicated ERK1/2 signaling related specifically to phenotypes directed by AT1R and Mas1 receptor; it appears to be essential for global angiogenesis signaling processes as a point of convergence and demonstrated an Ang-(1-7) activation dependence in our system (Figure 4). This data, along with inhibition studies in Figures 5 and 6, suggested that ERK1/2 was essential for Ang-(1-7)/Mas1 receptor mediated vasodilation and angiogenesis. Overall, the innovative approach utilized for signal pathway analysis in combination with in vitro, ex vivo, and in vivo functional assays allowed for an increased understanding of Ang-(1-7) stimulated Mas1 receptor signaling in relation to low, subpressor dose AngII (normal physiological levels) signaling through AT1R. Our data in endothelial cells and data from other labs in other cell types have shown that dosage of the peptide is a significant factor in how a cell responds to AngII or Ang-(1-7)47, 48. There is also growing evidence that AngII/AT1and Ang-(1-7)/Mas1 signaling are co-occurring, dose-dependent, and more complex than once thought13. The complimentary action of equimolar, low dose AngII and Ang-(1-7) suggests a delicate balance in the regulation of these two peptides at both the receptor and intracellular signaling level.
Supplementary Material
HIGHLIGHTS.
This study demonstrates the importance of the synergystic effects of “ideal” plasma concentations of Ang-(1-7)and AngII in normal physiological homeostasis.
Low, subpressor doses of Ang-(1-7) on the Mas1 receptor and AngII on AT1R exhibit similar signaling effects contributing to angiogenesis and endothelial-dependent vasodilation.
At low-doses the effects of the Ang-(1-7) on the Mas1 receptor and AngII on the AT1R exhibit similar pathway points of covergence that are opposed to high-dose AngII.
ERK1/2 was essential for vasodilation and angiogenesis following Ang-(1-7) signaling through the Mas1 receptor, whereas p38MAPK can contribute but was not essential.
Acknowledgments
All mass spectrometry was performed in the Medical College of Wisconsin Dedicated Mass Spectrometry Facility and the Biomedical Engineering Department.
SOURCES OF FUNDING: This work was funded by the National Institutes of Health grants P01-HL082798 to A.G., T32-HL094273 and K01-DK105043 to B. H., R01-HL65289 and R56-HL06529 to J.L., and by a generous donation from Drs. Robert D. and Patricia E. Kern. Losartan used in this study was a generous donation by Merck.
Abbreviations
- AKT
serine/threonine protein kinase AKT
- AngII
angiotensin II
- AT1R
angiotensin II receptor type 1
- Ang-(1-7)
angiotensin 1-7
- ACh
acetylcholine
- CARD10
caspase recruitment domain family member 10
- CDC42
cell division control protein 42
- ERK
extracellular signal-related kinases
- MAPK
mitogen-activated protein kinase
- MCA
middle cerebral artery
- MS/MS
tandem mass spectrometry
- mTOR
mechanistic target of rapamycin
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NOS
nitric oxide synthase
- PI3K
phosphatidylinositol 3-kinase
- PRKD1
serine/threonine protein kinase D1
- RMVEC
rat microvascular endothelial cell
- SD
Sprague Dawley
- VEGF
vascular endothelial growth factor
- VEGFR1
vascular endothelial growth factor receptor 1
- VEGFR2
vascular endothelial growth factor receptor 2
Footnotes
DISCLOSURES: Authors of this study have no conflicts of interest to indicate.
REFERENCES
- 1.Moon JY. Recent update of renin-angiotensin-aldosterone system in the pathogenesis of hypertension. Electrolyte Blood Press. 2013;11:41–45. doi: 10.5049/EBP.2013.11.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balakumar P, Jagadeesh G. A century old renin-angiotensin system still grows with endless possibilities: At1 receptor signaling cascades in cardiovascular physiopathology. Cell Signal. 2014;26:2147–2160. doi: 10.1016/j.cellsig.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Xu P, Sriramula S, Lazartigues E. Ace2/ang-(1-7)/mas pathway in the brain: The axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Bodiga S, Das SK, Lo J, Patel V, Oudit GY. Role of ace2 in diastolic and systolic heart failure. Heart Fail Rev. 2012;17:683–691. doi: 10.1007/s10741-011-9259-x. [DOI] [PubMed] [Google Scholar]
- 5.Kamo T, Akazawa H, Komuro I. Pleiotropic effects of angiotensin ii receptor signaling in cardiovascular homeostasis and aging. Int Heart J. 2015 doi: 10.1536/ihj.14-429. [DOI] [PubMed] [Google Scholar]
- 6.Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin-(1-7) and low-dose angiotensin ii infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2010;299:H1024–H1033. doi: 10.1152/ajpheart.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen MC, Munzenmaier DH, Greene AS. Angiotensin ii infusion restores angiogenesis in the skeletal muscle of rats on a high salt diet. Am J Physiol Heart Circ Physiol. 2006;291:H114–H120. doi: 10.1152/ajpheart.01116.2005. [DOI] [PubMed] [Google Scholar]
- 8.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, et al. Angiotensin-(1-7) is an endogenous ligand for the g protein-coupled receptor mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin-(1-7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with l-name. Am J Physiol Heart Circ Physiol. 2006;290:H684–H691. doi: 10.1152/ajpheart.00632.2005. [DOI] [PubMed] [Google Scholar]
- 10.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor mas mediates endothelial nitric oxide synthase activation via akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Qian C, Roks AJ, Westermann D, Schumacher SM, Escher F, et al. Circulating rather than cardiac angiotensin-(1-7) stimulates cardioprotection after myocardial infarction. Circ Heart Fail. 2010;3:286–293. doi: 10.1161/CIRCHEARTFAILURE.109.905968. [DOI] [PubMed] [Google Scholar]
- 12.Raffai G, Durand MJ, Lombard JH. Acute and chronic angiotensin-(1-7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am J Physiol Heart Circ Physiol. 2011;301:H1341–H1352. doi: 10.1152/ajpheart.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue H, Zhou L, Yuan P, Wang Z, Ni J, Yao T, et al. Counteraction between angiotensin ii and angiotensin-(1-7) via activating angiotensin type i and mas receptor on rat renal mesangial cells. Regul Pept. 2012;177:12–20. doi: 10.1016/j.regpep.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Souza AP, Sobrinho DB, Almeida JF, Alves GM, Macedo LM, Porto JE, et al. Angiotensin ii type 1 receptor blockade restores angiotensin-(1-7)-induced coronary vasodilation in hypertrophic rat hearts. Clin Sci (Lond) 2013;125:449–459. doi: 10.1042/CS20120519. [DOI] [PubMed] [Google Scholar]
- 15.Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, et al. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 16.Guimaraes PS, Santiago NM, Xavier CH, Velloso EP, Fontes MA, Santos RA, et al. Chronic infusion of angiotensin-(1-7) into the lateral ventricle of the brain attenuates hypertension in doca-salt rats. Am J Physiol Heart Circ Physiol. 2012;303:H393–H400. doi: 10.1152/ajpheart.00075.2012. [DOI] [PubMed] [Google Scholar]
- 17.Fraga-Silva RA, Da Silva DG, Montecucco F, Mach F, Stergiopulos N, da Silva RF, et al. The angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor axis: A potential target for treating thrombotic diseases. Thromb Haemost. 2012;108:1089–1096. doi: 10.1160/TH12-06-0396. [DOI] [PubMed] [Google Scholar]
- 18.Liu GC, Oudit GY, Fang F, Zhou J, Scholey JW. Angiotensin-(1-7)-induced activation of erk1/2 is camp/protein kinase a-dependent in glomerular mesangial cells. Am J Physiol Renal Physiol. 2012;302:F784–F790. doi: 10.1152/ajprenal.00455.2011. [DOI] [PubMed] [Google Scholar]
- 19.Verano-Braga T, Schwammle V, Sylvester M, Passos-Silva DG, Peluso AA, Etelvino GM, et al. Time-resolved quantitative phosphoproteomics: New insights into angiotensin-(1-7) signaling networks in human endothelial cells. J Proteome Res. 2012;11:3370–3381. doi: 10.1021/pr3001755. [DOI] [PubMed] [Google Scholar]
- 20.Meinert C, Gembardt F, Bohme I, Tetzner A, Wieland T, Greenberg B, et al. Identification of intracellular proteins and signaling pathways in human endothelial cells regulated by angiotensin-(1-7) J Proteomics. 2016;130:129–139. doi: 10.1016/j.jprot.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karcher JR, Greene AS. Bone marrow mononuclear cell angiogenic competency is suppressed by a high-salt diet. Am J Physiol Cell Physiol. 2014;306:C123–C131. doi: 10.1152/ajpcell.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman MJ, Flister MJ, Nunez L, Xiao B, Greene AS, Jacob HJ, et al. Female-specific hypertension loci on rat chromosome 13. Hypertension. 2013;62:557–563. doi: 10.1161/HYPERTENSIONAHA.113.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freed JK, Greene AS. Proteomic analysis of shear stress-mediated protection from tnf-alpha in endothelial cells. Microcirculation. 2010;17:259–270. doi: 10.1111/j.1549-8719.2010.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, et al. Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the dahl s hypertensive rat. Physiol Genomics. 2007;31:228–235. doi: 10.1152/physiolgenomics.00280.2006. [DOI] [PubMed] [Google Scholar]
- 25.Widlansky ME, Gokce N, Keaney JFJ, Vita J. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann BR, Wagner JR, Prisco AR, Janiak A, Greene AS. Vascular endothelial growth factor-a signaling in bone marrow-derived endothelial progenitor cells exposed to hypoxic stress. Physiol Genomics. 2013;45:1021–1034. doi: 10.1152/physiolgenomics.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen ST, Schmidt JR, Somberg L, Cruz Ldl, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin ii infusion in rats fed a high-salt diet. Microcirculation. 2009;16:220–234. doi: 10.1080/10739680802544177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghiadoni L, Versari D, Magagna A, Kardasz I, Plantinga Y, Giannarelli C, et al. Ramipril dose-dependently increases nitric oxide availability in the radial artery of essential hypertension patients. Journal of hypertension. 2007;25:361–366. doi: 10.1097/HJH.0b013e3280115901. [DOI] [PubMed] [Google Scholar]
- 29.Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin ii and oxidative stress. Current opinion in cardiology. 2007;22:311–315. doi: 10.1097/HCO.0b013e3281532b53. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa S, Mori T, Nako K, Kato T, Takeuchi K, Ito S. Angiotensin ii type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension. 2006;47:699–705. doi: 10.1161/01.HYP.0000203826.15076.4b. [DOI] [PubMed] [Google Scholar]
- 31.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin ii in smooth muscle cells from resistance arteries of hypertensive patients: Role of phospholipase d-dependent nad(p)h oxidase-sensitive pathways. Journal of hypertension. 2001;19:1245–1254. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, et al. Role of superoxide and angiotensin ii suppression in salt-induced changes in endothelial ca2+ signaling and no production in rat aorta. Am J Physiol Heart Circ Physiol. 2006;291:H929–H938. doi: 10.1152/ajpheart.00692.2005. [DOI] [PubMed] [Google Scholar]
- 33.de Resende MM, Amaral SL, Moreno C, Greene AS. Congenic strains reveal the effect of the renin gene on skeletal muscle angiogenesis induced by electrical stimulation. Physiol Genomics. 2008;33:33–40. doi: 10.1152/physiolgenomics.00150.2007. [DOI] [PubMed] [Google Scholar]
- 34.Kaczorowski CC, Stodola TJ, Hoffmann BR, Prisco AR, Liu PY, Didier DN, et al. Targeting the endothelial progenitor cell surface proteome to identify novel mechanisms that mediate angiogenic efficacy in a rodent model of vascular disease. Physiol Genomics. 2013;45:999–1011. doi: 10.1152/physiolgenomics.00097.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stodola TJ, Resende MMd, Sarkis AB, Didier DN, Jacob HJ, Huebner N, et al. Characterization of the genomic structure and function of regions influencing renin and angiogenesis in the ss rat. Physiol Genomics. 2011;43:808–817. doi: 10.1152/physiolgenomics.00171.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Resende MM, Greene AS. Effect of ang ii on endothelial cell apoptosis and survival and its impact on skeletal muscle angiogenesis after electrical stimulation. Am J Physiol Heart Circ Physiol. 2008;294:H2814–H2821. doi: 10.1152/ajpheart.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amaral SL, Linderman JR, Morse MM, Greene AS. Angiogenesis induced by electrical stimulation is mediated by angiotensin ii and vegf. Microcirculation. 2001;8:57–67. [PubMed] [Google Scholar]
- 38.Linderman JR, Kloehn MR, Greene AS. Development of an inmplantable muscle stimulator: Measurement of stimulated angiogenesis and poststimulus vessel regression. Microcirculation. 2000;7:119–128. [PubMed] [Google Scholar]
- 39.Soto-Pantoja DR, Menon J, Gallagher PE, Tallant EA. Angiotensin-(1-7) inhibits tumor angiogenesis in human lung cancer xenografts with a reduction in vascular endothelial growth factor. Molecular cancer therapeutics. 2009;8:1676–1683. doi: 10.1158/1535-7163.MCT-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh N, Vasam G, Pawar R, Jarajapu Y. Angiotensin-(1-7) reverses angiogenic dysfunction in corpus cavernosum by acting on the microvascularture and bone marrow-derived cells in diabetes. J Sex Med. 2104;11:2153–2163. doi: 10.1111/jsm.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamarat R, Silvestre JS, Durie M, Levy BI. Angiotensin ii angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab Invest. 2002;82:747–756. doi: 10.1097/01.lab.0000017372.76297.eb. [DOI] [PubMed] [Google Scholar]
- 42.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 43.Nie W, Yan H, Li S, Zhang Y, Yu F, Zhu W, et al. Angiotensin-(1-7) enhances angiotensin ii induced phosphorylation of erk1/2 in mouse bone marrow-derived dendritic cells. Mol Immunol. 2009;46:355–361. doi: 10.1016/j.molimm.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Von Bohlen und Halbach O, Walther T, Bader M, Albrecht D. Interaction between mas and the angiotensin at1 receptor in the amygdala. J Neurophysiol. 2000;83:2012–2021. doi: 10.1152/jn.2000.83.4.2012. [DOI] [PubMed] [Google Scholar]
- 45.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor mas with at1 and at2 receptors in the mouse heart. Hypertension. 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- 46.McEwen ST, Balus SF, Durand MJ, Lombard JH. Angiotensin ii maintains cerebral vascular relaxation via egf receptor transactivation and erk1/2. Am J Physiol Heart Circ Physiol. 2009;297:H1296–H1303. doi: 10.1152/ajpheart.01325.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimpelmann J, Burns KD. Angiotensin-(1-7) activates growth-stimulatory pathways in human mesangial cells. Am J Physiol Renal Physiol. 2009;296:F337–F346. doi: 10.1152/ajprenal.90437.2008. [DOI] [PubMed] [Google Scholar]
- 48.Zhou LM, Shi Z, Gao J, Han Y, Yuan N, Gao XY, et al. Angiotensin-(1-7) and angiotension ii in the rostral ventrolateral medulla modulate the cardiac sympathetic afferent reflex and sympathetic activity in rats. Pflugers Arch. 2010;459:681–688. doi: 10.1007/s00424-010-0793-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.