Abstract
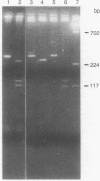
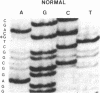
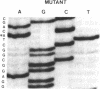
Identical single-base changes in the C1 inhibitor gene that may result in dysfunctional inhibitor proteins are described in two different families with type II hereditary angioneurotic edema. Initially, a restriction fragment length polymorphism was defined that resulted from loss of a Pst I site within exon VIII, which encodes the region containing the reactive center. Exon VIII from the normal and abnormal allelles was amplified by the polymerase chain reaction. Amplified DNA product was cloned into plasmid pUC18; clones representing normal and mutant allelles were distinguished by the presence and absence, respectively, of the Pst I restriction site. DNA sequence analysis revealed a G----A mutation in the codon for alanine-436, which would result in replacement with a threonine residue. This position is nine amino acid residues amino-terminal to the reactive-center arginylthreonine peptide bond. In contrast, previously defined mutations in type II hereditary angioneurotic edema result in replacement of the reactive-center arginine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulak K. S., Pemberton P. A., Rosen F. S., Carrell R. W., Lachmann P. J., Harrison R. A. Dysfunctional C1-inhibitor(At), isolated from a type II hereditary-angio-oedema plasma, contains a P1 'reactive centre' (Arg444----His) mutation. Biochem J. 1988 Jul 15;253(2):615–618. doi: 10.1042/bj2530615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock S. C., Marrinan J. A., Radziejewska E. Antithrombin III Utah: proline-407 to leucine mutation in a highly conserved region near the inhibitor reactive site. Biochemistry. 1988 Aug 9;27(16):6171–6178. doi: 10.1021/bi00416a052. [DOI] [PubMed] [Google Scholar]
- Bock S. C., Skriver K., Nielsen E., Thøgersen H. C., Wiman B., Donaldson V. H., Eddy R. L., Marrinan J., Radziejewska E., Huber R. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry. 1986 Jul 29;25(15):4292–4301. doi: 10.1021/bi00363a018. [DOI] [PubMed] [Google Scholar]
- Bruch M., Weiss V., Engel J. Plasma serine proteinase inhibitors (serpins) exhibit major conformational changes and a large increase in conformational stability upon cleavage at their reactive sites. J Biol Chem. 1988 Nov 15;263(32):16626–16630. [PubMed] [Google Scholar]
- Carrell R. W., Owen M. C. Plakalbumin, alpha 1-antitrypsin, antithrombin and the mechanism of inflammatory thrombosis. Nature. 1985 Oct 24;317(6039):730–732. doi: 10.1038/317730a0. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Pemberton P. A., Boswell D. R. The serpins: evolution and adaptation in a family of protease inhibitors. Cold Spring Harb Symp Quant Biol. 1987;52:527–535. doi: 10.1101/sqb.1987.052.01.060. [DOI] [PubMed] [Google Scholar]
- Carter P. E., Dunbar B., Fothergill J. E. Genomic and cDNA cloning of the human C1 inhibitor. Intron-exon junctions and comparison with other serpins. Eur J Biochem. 1988 Apr 5;173(1):163–169. doi: 10.1111/j.1432-1033.1988.tb13980.x. [DOI] [PubMed] [Google Scholar]
- Cicardi M., Igarashi T., Kim M. S., Frangi D., Agostoni A., Davis A. E., 3rd Restriction fragment length polymorphism of the C1 inhibitor gene in hereditary angioneurotic edema. J Clin Invest. 1987 Dec;80(6):1640–1643. doi: 10.1172/JCI113252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- DONALDSON V. H., EVANS R. R. A BIOCHEMICAL ABNORMALITY IN HEREDIATRY ANGIONEUROTIC EDEMA: ABSENCE OF SERUM INHIBITOR OF C' 1-ESTERASE. Am J Med. 1963 Jul;35:37–44. doi: 10.1016/0002-9343(63)90162-1. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol. 1988;6:595–628. doi: 10.1146/annurev.iy.06.040188.003115. [DOI] [PubMed] [Google Scholar]
- Devraj-Kizuk R., Chui D. H., Prochownik E. V., Carter C. J., Ofosu F. A., Blajchman M. A. Antithrombin-III-Hamilton: a gene with a point mutation (guanine to adenine) in codon 382 causing impaired serine protease reactivity. Blood. 1988 Nov;72(5):1518–1523. [PubMed] [Google Scholar]
- Donaldson V. H., Harrison R. A., Rosen F. S., Bing D. H., Kindness G., Canar J., Wagner C. J., Awad S. Variability in purified dysfunctional C1(-)-inhibitor proteins from patients with hereditary angioneurotic edema. Functional and analytical gel studies. J Clin Invest. 1985 Jan;75(1):124–132. doi: 10.1172/JCI111664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes C. D., Pensky J., Ratnoff O. D. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J Lab Clin Med. 1970 Nov;76(5):809–815. [PubMed] [Google Scholar]
- Gadek J. E., Hosea S. W., Gelfand J. A., Frank M. M. Response of variant hereditary angioedema phenotypes to danazol therapy. Genetic implications. J Clin Invest. 1979 Jul;64(1):280–286. doi: 10.1172/JCI109449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Mason J. W., Colman R. W., Austen K. F. Interaction of plasma kallikrein with the C1 inhibitor. J Immunol. 1970 Mar;104(3):574–581. [PubMed] [Google Scholar]
- Harpel P. C., Cooper N. R. Studies on human plasma C1 inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. 1975 Mar;55(3):593–604. doi: 10.1172/JCI107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. E., Lijnen H. R., Nelles L., Kluft C., Nieuwenhuis H. K., Rijken D. C., Collen D. Alpha 2-antiplasmin Enschede: alanine insertion and abolition of plasmin inhibitory activity. Science. 1987 Oct 9;238(4824):209–211. doi: 10.1126/science.2958938. [DOI] [PubMed] [Google Scholar]
- LANDERMAN N. S., WEBSTER M. E., BECKER E. L., RATCLIFFE H. E. Hereditary angioneurotic edema. II. Deficiency of inhibitor for serum globulin permeability factor and/or plasma kallikrein. J Allergy. 1962 Jul-Aug;33:330–341. doi: 10.1016/0021-8707(62)90032-1. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Pemberton P. A., Harrison R. A., Lachmann P. J., Carrell R. W. The structural basis for neutrophil inactivation of C1 inhibitor. Biochem J. 1989 Feb 15;258(1):193–198. doi: 10.1042/bj2580193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H. Some properties of an esterase derived from preparations of the first component of complement. J Exp Med. 1957 Aug 1;106(2):327–343. doi: 10.1084/jem.106.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragg H. A new member of the plasma protease inhibitor gene family. Nucleic Acids Res. 1986 Jan 24;14(2):1073–1088. doi: 10.1093/nar/14.2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnoff O. D., Pensky J., Ogston D., Naff G. B. The inhibition of plasmin, plasma kallikrein, plasma permeability factor, and the C'1r subcomponent of the first component of complement by serum C'1 esterase inhibitor. J Exp Med. 1969 Feb 1;129(2):315–331. doi: 10.1084/jem.129.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen F. S., Alper C. A., Pensky J., Klemperer M. R., Donaldson V. H. Genetically determined heterogeneity of the C1 esterase inhibitor in patients with hereditary angioneurotic edema. J Clin Invest. 1971 Oct;50(10):2143–2149. doi: 10.1172/JCI106708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Radziejewska E., Silbermann J. A., Donaldson V. H., Bock S. C. CpG mutations in the reactive site of human C1 inhibitor. J Biol Chem. 1989 Feb 25;264(6):3066–3071. [PubMed] [Google Scholar]
- Stephens A. W., Thalley B. S., Hirs C. H. Antithrombin-III Denver, a reactive site variant. J Biol Chem. 1987 Jan 25;262(3):1044–1048. [PubMed] [Google Scholar]
- Stoppa-Lyonnet D., Tosi M., Laurent J., Sobel A., Lagrue G., Meo T. Altered C1 inhibitor genes in type I hereditary angioedema. N Engl J Med. 1987 Jul 2;317(1):1–6. doi: 10.1056/NEJM198707023170101. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Y., Kuo K. C., Gehrke C. W., Huang L. H., Ehrlich M. Heat- and alkali-induced deamination of 5-methylcytosine and cytosine residues in DNA. Biochim Biophys Acta. 1982 Jun 30;697(3):371–377. doi: 10.1016/0167-4781(82)90101-4. [DOI] [PubMed] [Google Scholar]
- Whitehead A. S., Woods D. E., Fleischnick E., Chin J. E., Yunis E. J., Katz A. J., Gerald P. S., Alper C. A., Colten H. R. DNA polymorphism of the C4 genes. A new marker for analysis of the major histocompatibility complex. N Engl J Med. 1984 Jan 12;310(2):88–91. doi: 10.1056/NEJM198401123100204. [DOI] [PubMed] [Google Scholar]