Abstract
Background
The acute hospital is a challenging place for a person with dementia. Behavioural and psychological symptoms of dementia (BPSD) are common and may be exacerbated by the hospital environment. Concerns have been raised about how BPSD are managed in this setting and about over reliance on neuroleptic medication. This study aimed to investigate how BPSD are managed in UK acute hospitals.
Method(s)
A longitudinal cohort of 230 patients with dementia admitted to two acute NHS hospitals. BPSD were measured every four days (Behave‐AD scale), as well as documentation of pharmacological prescriptions and non‐pharmacological management.
Results
The overall prevalence of BPSD was 75%, with aggression and activity disturbance being the most common. Antipsychotics were prescribed for 28 (12%) patients; 70% of these prescriptions were new on admission. Benzodiazepines were prescribed for 27 (12%) patients, antidepressants were prescribed for 37 (16%) patients, and sedatives were prescribed for 14 (3%) patients. Patients who were prescribed antipsychotics, after adjusting for end of life medication, age and dementia severity, were significantly more likely to die (adjusted hazard ratio 5.78, 95% CI 1.57, 21.26, p = 0.008). Non‐pharmacological management was used in 55% of participants, most commonly psychosocial interventions (36%) with little evidence of monitoring their effectiveness. A form of restraint was used during 50 (22%) patients' admissions.
Conclusions
Antipsychotic medications and psychosocial interventions were the main methods used to manage BPSD; however, these were not implemented or monitored in a systematic fashion.
Keywords: dementia, behaviour, BPSD, antipsychotics, non‐pharmacological management, acute hospital
Introduction
People with dementia are frequently admitted to acute general hospitals (Russ et al., 2012; Mukadam and Sampson, 2011), and this is a challenging environment for them. In addition to being physically unwell, the person is in strange surroundings, with unfamiliar and changing staff. Behavioural and psychological symptoms of dementia (BPSD) include symptoms such as agitation, aggression, affective disorder, and sleep disturbance. These symptoms can be difficult to manage, cause significant distress to family and professional caregivers, and are often exacerbated during an acute hospital admission (Kales et al., 2015; Freeman and Joska, 2012; Banerjee, 2010; Lyketsos et al., 2014). Up to 75% of people with dementia admitted to the acute hospital will experience BSPD (Sampson et al., 2014). A UK national audit, the ‘National Audit of Dementia Care in General Hospitals’ found only 5% of acute hospital staff receive mandatory training in dementia, and one third felt that there were insufficient staff to meet the need of patients (2011). Many ward staff feel that they lack the skills and confidence to work with ‘confused’ patients (Griffiths et al., 2014). A randomised controlled trial (Goldberg et al., 2013) found that a specialist inpatient ward for people with dementia did not significantly reduce BPSD nor reduce admission, but it did improve the experience of the patient and their family members.
In care homes, non‐pharmacological interventions have been shown to decrease BPSD for people with dementia and improve the general well‐being of the carer (Kales et al., 2015; Deudon et al., 2009; Livingston et al., 2005). However, it has been found that pharmacological interventions, specifically antipsychotics, are often used before non‐pharmacological alternatives (Cohen‐Mansfield, 2013) despite the increased risk of mortality and adverse events such as seizures, weight gain, or extrapyramidal symptoms (Langballe et al., 2014; Gauthier et al., 2010; Ballard and Corbett, 2010). We have little detailed data describing how BPSD are managed in acute hospitals. Given the recent focus on decreasing neuroleptic use in people with dementia (Banerjee, 2010) this information could inform better care for these patients.
Aim
The aim of this study was to describe the pharmacological and non‐pharmacological management of BPSD in people with dementia over the age of 70 years who had undergone unplanned acute hospital admission.
Specific questions were as follows:
How commonly are pharmacological interventions for BPSD prescribed in this setting?
Which patient characteristics and BPSD symptoms are associated with pharmacological management?
Is there an association between antipsychotic medication and mortality in this setting?
What type of non‐pharmacological management techniques are being used to manage BPSD, and how frequently?
Methods
Study design
Longitudinal cohort data were collected as part of a larger study investigating BPSD and pain in people with dementia in the acute hospital (Scott et al., 2011; Sampson et al., 2015). This study received ethical approval by Central London REC 3 on 01/12/2010 (ref: 10/HO716/79).
Setting
Two NHS acute hospitals within the Greater London area serve over two million people encompassing socioeconomic and ethnic diversity. Between them, the hospitals cover six primary care trusts and four mental health trusts. They were chosen because they were at different stages of implementing the English National Dementia Strategy with varying provision of liaison psychiatry and service quality rating scores. Researchers spent 5 months at each site (between 4 April 2011 and 6 March 2012). In both settings, patients were admitted via a medical acute admissions unit before transfer to an elderly care ward or a general medical ward (total of 20 wards).
Participants
All patients admitted to the hospital with an unplanned acute medical admission, over the age of 70, with English language sufficient to complete cognitive testing, and an Abbreviated Mental Test (AMT), completed as part of the admission (Hodkinson, 1972) score of less than 7 were eligible for screening.
Screening
At the time of this study, dementia was commonly under‐detected in the UK. We therefore needed to try and include those patients who had dementia but had no pre‐existing documented diagnosis, whilst excluding those who were cognitively impaired because of delirium. Therefore, all patients who met the study criteria were screened for delirium using the Confusion Assessment Method (CAM) (Inouye et al., 1990) by a research assistant. The CAM tool has a sensitivity of 94% and specificity of over 90% for detecting delirium and distinguishing accurately between delirium and dementia (Inouye et al., 1990).
Patients with a pre‐existing diagnosis of dementia, documented in the medical notes, were approached to participate regardless of the CAM score.
Patients with no pre‐existing diagnosis of dementia who screened negatively for delirium were approached to participate.
Patients with no pre‐existing diagnosis of dementia who screened positive for delirium were re‐screened 48 hours later. At this time point, if the delirium had cleared, they were approached to participate. If the delirium was unresolved, they were excluded.
We used the consent guidelines for adults who lacked capacity within the Mental Capacity Act (2005). All patients underwent a capacity assessment with a research assistant. Those with capacity were asked to complete a consent form. When patients lacked capacity, agreement was requested from a personal consultee. In the absence of a personal consultee, a professional consultee was employed (Scott et al., 2011).
All consented participants completed a Mini Mental State Examination (MMSE). Patients with no history of dementia were excluded if they scored over 24 and did not meet the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for dementia.
Assessments
Demographic characteristics such as age, gender, ethnicity, dementia severity using the Functional Assessment Staging Tool (FAST) (Reisberg, 1988), and marital status were gathered on each patient from hospital notes.
The behaviour pathology in Alzheimer's disease rating scale
The Behaviour Pathology in Alzheimer's disease Rating Scale (BEHAVE‐AD) (Sclan et al., 1996) was used to assess BPSD. It consists of 25 items which are divided in to seven subgroups; paranoid and delusional ideation, hallucinations, activity disturbances, aggression, diurnal disturbances, affective disturbances, anxieties, and phobias. Each subgroup is scored on a 4‐point scale from 0 to 3, 3 being the most severe score. The BEHAVE‐AD has good internal consistency and reliability and sensitive to longitudinal changes (Sclan et al., 1996).
Pharmacological management
Prescriptions of antipsychotics, antidepressant, cholinesterase inhibitors, benzodiazepines, and sedatives were recorded from drug charts at each assessment. We documented whether antipsychotics were newly prescribed during this admission or whether the patient had been on these medications when admitted.
Non‐pharmacological management
Non‐pharmacological management was defined as any intervention put in place by the ward team that did not involve medication and was used with the intention to manage BPSD. Interventions were identified by speaking to the clinical team, often the nurse, responsible for the patient's care and asking how they were managing any BPSD, examining medical notes for any documentation of this since the patient's admission, and by observations on the wards whilst gathering information on the patient.
A list of overall types of non‐pharmacological interventions was developed from the types of interventions employed by the staff specifically to manage BPSD. This list was verified by two independent raters. The categories were ‘Equipment’ incorporated the use of hospital beds, hoists (where used in the context of managing behaviours), a safety mat, or a falls alarm to aid the ward staff in managing the BPSD. ‘Family assistance’ were instances when the family had been called in specifically to help manage the BPSD. ‘Psycho‐social techniques’ were techniques used by the healthcare professionals, consisting usually verbal prompts such as reorientation to time and place, allowing the patient to wander on the ward, individual care plans for confusion, or behaviour charts. ‘Increased staffing’ were instances of patients being moved to a high visibility bay, requiring close supervision, or one to one staffing. ‘Specialist referral’ were instances when the patient was referred to a Psychiatry or Psychology team. ‘Other therapy’ incorporated any complementary therapy that the patient was referred to, for example massage, spiritual support, and music therapy.
In addition to the non‐pharmacological interventions, we documented periods where the team had to restrain the patient. ‘Restraint’ incorporated any barrier to limit the patient's activities, for example mittens, nasogastric tubes with bridles, raised bed rails, and physical restraint.
Follow‐up assessments
Data on the BEHAVE‐AD, pharmacological prescribing, and non‐pharmacological management were collected every four days until death or discharge, or until patients were deemed ‘medically fit for discharge/awaiting placement’ by their medical team. At each follow‐up assessment, any monitoring or measures of effectiveness of the intervention being used, as well as any changes since the previous assessment, were documented.
Data analysis
Patient demographics were described. The prevalence of BPSD, antipsychotic use, and non‐pharmacological management were calculated by dichotomising each variable in to present/absent at any point during the admission. Presence of a BPSD was defined as reaching a BEHAVE‐AD score above 0 for at least one of the assessments. The mean BPSD score was calculated from the patients' BEHAVE‐AD ratings over the admission.
We used chi‐squared tests and logistic regression to investigate the association of patient characteristics with specific BPSD (as indicated by the BEHAVE‐AD) with the use of antipsychotic medication during the admission. We explored the association between the use of antipsychotics and mortality with a cox regression survival analysis. Because the number of mortality events was small, a multivariable model with too many adjustment covariates can be unstable; we therefore restricted to adjust for two main confounders (age and dementia severity) in the main analysis. We completed a sensitivity analysis to exclude those patients who were on an end of life care plan and may have been receiving these medications as part of that treatment plan. To explore how results could change with further adjustment we also performed a sensitivity analysis fully adjusted for age, dementia severity, presence of delirium, and psychiatric history.
When BPSD were present during admission we tabulated the frequency and 95% confidence interval (CI) of use of each type of non‐pharmacological management. Analyses were two‐sided and considered significant when significance level was below 0.05. Analyses were conducted using STATA v13.0.
Results
Of the 292 participants approached, 230 had dementia, were consented and entered the study (refer to Study flow chart Appendix 1). For cohort characteristics, refer to Table 1.
Table 1.
Cohort characteristics
| (n = 230) | Total (%) | |
|---|---|---|
| Gender | ||
| Female | 151 | 65.7 |
| Male | 79 | 34.3 |
| Ethnicity | ||
| White British | 175 | 76.1 |
| Black Caribbean | 15 | 6.5 |
| Other | 40 | 17.4 |
| FAST | ||
| 3–5 | 86 | 37.4 |
| 6a–6c | 39 | 16.9 |
| 6d–6e | 74 | 32.2 |
| 7a–7f | 31 | 13.5 |
| Age | ||
| 75–84 | 85 | 37.0 |
| 85–94 | 118 | 51.3 |
| 95+ | 27 | 11.7 |
| Place of residence (/219) | ||
| Home/sheltered | 145 | 66.2 |
| Residential home | 26 | 11.9 |
| Nursing home | 39 | 17.8 |
| Other | 9 | 4.1 |
| Reason for admission (/229) | ||
| Infection–lungs/skin/viral | 79 | 34.5 |
| Infection–UTI/blocked catheter | 36 | 15.7 |
| Fall/fracture/pain | 31 | 13.5 |
| Cardiac | 22 | 9.6 |
| Other | 61 | 26.7 |
| Delirium on baseline (/227) | ||
| Yes | 26 | 11.4 |
| No | 201 | 88.6 |
| Died during admission | ||
| Yes | 30 | 13.0 |
| No | 200 | 87.0 |
| Previous psychiatric history | ||
| Yes | 46 | 20.0 |
| No | 184 | 80.0 |
| BPSD experienced during admission: | ||
| Any BPSD | 172 | 74.8 |
| Paranoia | 26 | 11.3 |
| Hallucination | 34 | 14.8 |
| Activity disturbance | 101 | 43.9 |
| Aggression | 130 | 56.5 |
| Sleep disturbance | 97 | 42.2 |
| Affective | 77 | 33.5 |
| Anxiety | 81 | 35.2 |
Prevalence of BPSD
Three quarters (74.8%) of participants experienced at least one BPSD during their admission (Table 1). Aggression was the most frequent type of BPSD with over half of the participants experiencing this at least once during the admission 56.5%, (95% CI 50–63). Activity disturbance occurred in 43.9% (95% CI 37–50) and sleep disturbance in 42.2% (95% CI 36–49). The admission and the course of the BPSD over admission are discussed as part of the larger study (Sampson et al., 2014; Sampson et al., 2015).
Pharmacological management of BPSD
An antipsychotic was prescribed on at least one occasion during admission to 28 participants (12.2%) (Table 2). Of these, 19/28 (67.9%) of participants were new prescriptions during this admission. None of those prescribed antipsychotics had cognitive capacity to give their consent for participation in the study and 7/28 (25%) had delirium on admission. Ten (36%) had a pre‐existing mental health diagnosis, nine (32%) had severe dementia and were at stage 7 of the FAST score, and 15/28 (54%) of participants were in stage 6 of the FAST. Over a third of those who were prescribed an antipsychotic died during their admission.
Table 2.
Antipsychotics use and non‐pharmacological management at any time during admission (n = 230)
| Pharmacological management | N | % | 95% CI |
|---|---|---|---|
| Antipsychotic | 28 | 12.2 | 8–17 |
| Antidepressant | 37 | 16.1 | 12–21 |
| Benzodiazepine | 27 | 11.7 | 8–17 |
| Sedative | 14 | 6.1 | 03–10 |
| Cholinesterase Inhibitors | 35 | 15 | 11–202 |
| Non‐pharmacological management | |||
| Any | 126 | 54.8 | 48–61 |
| Equipment | 17 | 7.4 | 4–12 |
| Family assistance | 14 | 6.1 | 3–10 |
| Psychosocial intervention | 83 | 36.1 | 30–43 |
| Staffing | 38 | 16.5 | 12–22 |
| Restraint | 50 | 21.7 | 17–28 |
| Specialist referral | 23 | 10.0 | 6–15 |
| Other therapy | 3 | 1.3 | 0–4 |
Out of the 230 participants recruited, the prevalence of other medications prescribed during an admission were benzodiazepines 27/230 (12%), antidepressants 37/230 (16%) cholinesterase inhibitors 35/230 (15%), and sedative‐hypnotics 14/230 (6%).
Factors associated with pharmacological management at any time during admission.
Gender and age were not significantly associated with antipsychotic, anti‐depressant, benzodiazepine, or sedative prescribing (Table 3). For every category increase on the FAST tool, patients were 26% more likely to have an antipsychotic prescribed during their admission (OR 1.26, 95% CI 1.09, 1.46, p = 0.002).
Table 3.
Factors associated with pharmacological use (n = 230)
| Antipsychotic use | Antidepression | Cholinesterase inhibitors | Benzodiazepine | Sedative | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p‐value | OR | (95% CI) | p‐value | OR | (95% CI) | p‐value | OR | (95% CI) | p‐value | OR | (95% CI) | p‐value | |
| Gender | |||||||||||||||
| Female | 0.66 | (0.29, 1.47) | 0.314 | 1.11 | (0.52, 2.34) | 0.789 | 1.00 | (0.47, 2.14) | 0.993 | 1.05 | (0.45, 2.46) | 0.906 | 0.94 | (0.30, 2.90) | 0.912 |
| Ageǂ * | 0.98 | (0.91, 1.05) | 0.529 | 0.99 | (0.94, 1.06) | 0.915 | 0.97 | (0.91, 1.03) | 0.305 | 1.04 | (0.97, 1.12) | 0.230 | 1.05 | (0.96, 1.15) | 0.290 |
| FASTǂ, * | 1.26 | (1.09, 1.46) | 0.002 | 1.03 | (0.92, 1.16) | 0.594 | 0.97 | (0.86, 1.10) | 0.649 | 1.05 | (0.92, 1.21) | 0.445 | 0.97 | (0.81, 1.17) | 0.755 |
| BPSDǂ mean score* | 1.27 | (1.15, 1.40) | <.001 | 1.05 | (0.97, 1.15) | 0.218 | 1.02 | (0.94, 1.12) | 0.571 | 1.14 | (1.04, 1.25) | 0.003 | 1.11 | (0.99, 1.24) | 0.060 |
| BPSD experienced | |||||||||||||||
| Any BPSD | 4.99 | (1.15, 21.70) | 0.032 | 1.27 | (0.54, 2.95) | 0.583 | 2.24 | (0.83, 6.08) | 0.113 | 2.97 | (0.86, 10.27) | 0.085 | 4.66 | (0.60, 36.43) | 0.142 |
| Paranoia | 2.48 | (0.90, 6.84) | 0.079 | 1.28 | (0.45, 3.64) | 0.644 | 0.43 | (0.10, 1.92) | 0.269 | 1.97 | (0.67, 5.75) | 0.215 | 1.33 | (0.28, 6.32) | 0.717 |
| Hallucination | 5.04 | (2.10, 12.06) | <.001 | 1.43 | (0.57, 3.59) | 0.441 | 0.71 | (0.23, 2.16) | 0.545 | 2.28 | (0.88, 5.91) | 0.089 | 1.63 | (0.43, 6.17) | 0.474 |
| Activity disturbance | 5.71 | (2.22, 14.70) | <.001 | 1.85 | (0.91, 3.77) | 0.089 | 1.88 | (0.91, 3.88) | 0.090 | 1.44 | (0.64, 3.21) | 0.378 | 1.76 | (0.59, 5.26) | 0.309 |
| Aggression | 7.70 | (2.25, 26.31) | 0.001 | 0.89 | (0.44, 1.80) | 0.741 | 1.83 | (0.85, 3.95) | 0.122 | 1.63 | (0.70, 3.79) | 0.261 | 1.41 | (0.46, 4.36) | 0.547 |
| Sleep disturbance | 3.35 | (1.45, 7.79) | 0.005 | 1.37 | (0.68, 2.77) | 0.385 | 0.78 | (0.37, 1.64) | 0.513 | 1.31 | (0.59, 2.94) | 0.504 | 1.90 | (0.64, 5.67) | 0.249 |
| Affective | 1.33 | (0.59, 3.00) | 0.488 | 1.44 | (0.70, 2.97) | 0.322 | 1.39 | (0.67, 2.93) | 0.376 | 2.02 | (0.90, 4.54) | 0.090 | 1.11 | (0.36, 3.44) | 0.855 |
| Anxiety | 1.71 | (0.77, 3.79) | 0.189 | 1.31 | (0.64, 2.70) | 0.460 | 0.95 | (0.45, 2.03) | 0.900 | 2.59 | (1.15, 5.86) | 0.022 | 1.92 | (0.65, 5.68) | 0.239 |
Functional Assessment Staging Tool (FAST), Confusion Assessment Method (CAM), Behavioural and Psychological Symptoms of Dementia (BPSD).
Per each unit increase of age, FAST score, and BPSD.
Patients with any form of BPSD during their admission were five times more likely to have an antipsychotic prescribed during the admission (OR 4.99, 95% CI 1.15, 21.70, p = 0.032). Antipsychotic prescription was five times more likely in people who experienced hallucinations (OR 5.04, 95% CI 2.10, 12.06, p ≤ 0.001) or activity disturbances (OR 5.71, 95% CI 2.22, 14.70, p ≤ 0.001) and seven times more likely with aggressive behaviours (OR 7.70, 95% CI 2.25, 26.31, p = 0.001). Patients were three times more likely to have an antipsychotic prescribed when they experienced sleep disturbance (OR 3.35, 95% CI 1.45, 7.79, p = 0.005).
Prescription of benzodiazepine was associated with anxiety (OR 2.59, 95% CI 1.15, 5.86, p = 0.022). The prescription of antidepressants and sedatives was not significantly associated with BPSD overall or by subgroups.
Patients prescribed antipsychotics had a higher risk of mortality during admission ( hazard ratio (HR) 3.19, 95% CI 1.49, 6.83, p = 0.003). This association remained significant after adjustment for age and dementia severity (HR 2.78, 95% CI 1.27, 6.07; p = 0.010). In a sensitivity analysis, where participants on an end of life care regime were excluded, the association between mortality and antipsychotics remained significant, whilst still adjusting for age and dementia severity (HR 5.78, 95% CI 1.57, 21.26, p = 0.008). In an exploratory analysis, the association adjusted for age, previous psychiatric history, dementia severity, and delirium on admission, the association became non‐significant (HR 1.45, 95% CI 0.39, 5.32, p = 0.577).
Non‐pharmacological management of BPSD
In total, 55% of participants received non‐pharmacological management during their admission. The most commonly used techniques were psychosocial interventions (36%) and staffing (17%) (Table 2). We found no evidence in the nursing or medical notes of ongoing monitoring or review of the effectiveness of these non‐pharmacological interventions, or of a systematic way of using these techniques.
Restraint was used during 50 (22%) patients' admissions. In 119 of the 128 individual incidents this consisted of using bed rails (93%). Other forms of restraint were restricting the patients' ability to remove a naso‐gastric tube, that is by the use of mittens, or preventing the patient from removing the oxygen mask by holding it in place. Referrals to specialist services, such as the hospital liaison psychiatry team, occurred for 23 participants during their admission (10%).
Discussion
Overall, three quarters of the participants experienced BPSD during their admission. Almost 40% (88/230) of patients had a pharmacological intervention and 55% (126/230) had a non‐pharmacological intervention at some point during their admission.
Antipsychotics were prescribed to 12% of patients during their admission. Over two thirds (70%) of prescriptions for antipsychotic drugs were new during this admission. Hallucinations, activity disturbance, aggression, and sleep disturbance were the subgroups of BPSD that were more strongly with associated antipsychotic prescription. This echoes findings from carer surveys in Alzheimer's Society ‘Counting the Cost’ report (2009). These are more problematic for healthcare professionals to manage in a busy hospital and more noticeable, so are more likely to lead to the prescription of an antipsychotic; patients who are withdrawn and have ‘less troublesome’ symptoms such as depression, may not demand such attention. We found that delirium and dementia severity were significantly associated to the prescription of antipsychotics. With delirium, and as dementia severity increases, people not only lose capacity to make informed decisions, but also lose the ability to communicate their needs effectively. Whilst the prescription of antipsychotic medication may be appropriate in some cases of delirium or severe BPSD, the risk of stroke or death may outweigh the benefits for people who are also acutely physically unwell (Azermai et al., 2012). Our findings suggested that mortality was greater in patients prescribed an antipsychotic; however, after adjusting for age, previous psychiatric history, dementia severity, and delirium this relationship was not significant. The confounding influence of delirium when investigating mortality in patients with dementia is consistent with previous research (Fick et al., 2002).
Half the patients in the study (55%) received some form of non‐pharmacological intervention. Simple interventions such as allowing the patient to wander, using simplified instructions, and re‐orientating the patient to time and place were the most common techniques used. Other therapies such as aromatherapy, music, massage, or spiritual support were used much less frequently (1.3% in this study). Previous research from long‐term settings suggests that these therapies can be effective in reducing BPSD. However, these are challenging to implement in a hospital where staff are managing the acute illness, lack knowledge or time, and frequently changeover.
Almost a quarter of patients were restrained during their admission. The most commonly used restraints were bed rails. The use of restraints may exacerbate BPSD and can also lead to numerous physical problems. For example, reducing mobility through the use of bed rails can lead to an increase risk of deep vein thrombosis, pressure sores, constipation, delirium, or chest infections (Evans et al., 2003; Inouye and Charpentier, 1996). Of the study population, 201 (87.4%) lacked the capacity to provide consent to participate in the study. Restraints with individuals who do not have capacity should only be used as a last resort with the deprivation being proportionate to the perceived risk, as outlined by the Deprivation of Liberty Safeguards within the Mental Capacity Act (Cairns, 2009).
For patients where a non‐pharmacological intervention was implemented, there was little evidence of review or monitoring of effectiveness. Behavioural charts were completed with several patients; however, these became a replication of the nursing notes and did not appear to change the care plan. This concurs with community studies which have found that clinicians feel that they do not have the resources, time, or knowledge required to implement non‐pharmacological intervention (Ballard et al., 2009; Kales et al., 2015; Bishara et al., 2009). Recent reports such as ‘Meeting needs and reducing distress’(2014) and a randomised controlled trial looking at the effectiveness of specialist units (Goldberg et al., 2013) are beginning to detail practical guidance on managing challenging behaviours within the acute setting; however, more evidence‐based research is needed.
Strengths and limitations
To our knowledge, this is the first study which examines pharmacological and non‐pharmacological management of BPSD within the acute hospital setting. We were able to recruit a representative population of people with dementia, including those with severe dementia.
We acknowledge that during the screening phase we may have missed patients with dementia. Those patients who scored more than 7 on the AMT with no documented diagnosis of dementia were excluded. Although a standardised tool, it has been shown that the AMT has a sensitivity rate of 81%.
There are several limitations including potential reporting bias; when researchers asked hospital staff about BPSD they were asked to recall what had occurred over the last four days. It is possible that more ‘troubling’ and overt BPSD were more likely to be reported. We collected information on the prescription of antipsychotic but did not discuss the rationale for the medication with the prescriber, or their decision‐making processes. Prescription of antipsychotics may have been appropriate in some cases. In addition, we did not continuously observe ward staff and their interactions with the person with dementia. They may have been using non‐pharmacological interventions such as reassurance or diversion whilst caring for the person but these may have not been documented. Whilst we have adjusted for factors routinely collected as part of the larger dataset, we also acknowledge that there could be residual confounders which influenced the prescription of pharmacological or non‐pharmacological interventions.
The method of collecting data on the non‐pharmacological management of BPSD was simple and naturalistic, with no standardised method of assessing the effectiveness. The subsequent categories described in this study were derived from what the medical team were doing as part of their care.
Research implications
A randomised controlled trial (Goldberg et al., 2013) found that a specialist inpatient ward for people with dementia did not significantly reduce BPSD but it did improve the overall experience for patients and their carers; however, this study did not document the specific non‐pharmacological interventions that were implemented in the unit. Our research presents data on non‐pharmacological methods employed by ward staff to reduce BPSD. We found little documented evidence that the effectiveness of interventions was reviewed. Future research can build upon these findings by developing and testing complex interventions, taking into account the need for stepped implementation of pharmacological and behavioural management for BPSD.
Clinical implications
The National Audit of Dementia Care in General Hospitals (2011) found that only 5% of staff had received training on dementia care and wards are often understaffed. The management of BPSD in the acute hospital setting should be supported by evidence‐based research and systematic guidelines for healthcare professionals of all levels to use, with built‐in monitoring and an escalation to antipsychotic use when necessary and appropriate. It may be possible to adapt holistic models of care which have been developed for the management of BPSD in care homes, such as the Serial Trial Intervention to the acute hospital (Kovach et al., 2006).
Conclusions
People with dementia and BPSD present with complex symptoms exacerbated by an unfamiliar and distressing environment, acute medical illness, and in some cases delirium. Rather than two parallel models for antipsychotic prescribing and non‐pharmacological management, our findings support calls for a cohesive model that incorporates the two paradigms (Livingston et al., 2014).
Conflict of interest
None declared.
Key points.
People with dementia may present with complex behavioural and psychological symptoms exacerbated by a difficult environment, medical illness, and sometimes delirium.
In this cohort aggression and activity disturbance were the most common behavioural disturbances.
Antipsychotic medications and simple “psychosocial” interventions were the main methods used to manage BPSD; however, these were not implemented or monitored in a systematic fashion.
Patients who were prescribed antipsychotics were significantly more likely to die.
A form of restraint was used during a fifth of patients’ admissions.
Author contribution
ELS, SS, and LJ conceived, designed and obtained funding for the study. NW, KL, BL, SS, and ELS were involved in the analysis and interpretation of data. All authors were involved in drafting the article, revising it critically, and giving final approval of the version to be published.
Acknowledgements
This project is funded jointly by Alzheimer's Society and the BUPA Foundation (Grant reference number: 131). We would like to thank our Alzheimer's Society Quality Research Monitors Barbara Di Vita, Sylvia Wallach, and Lynn Whittaker; the staff from Health Services for Older People at the Royal Free NHS Trust and the North Middlesex University Trust (Dr Sophie Edwards and Dr Dan Lee, Consultants in Care of the Elderly); and particularly our professional consultees Jo James, Jenny Kenward, Pippa Street, Bridget Cooney, Jane Dunne, and Dr. Ada Chime.
Appendix 1. Recruitment flow chart
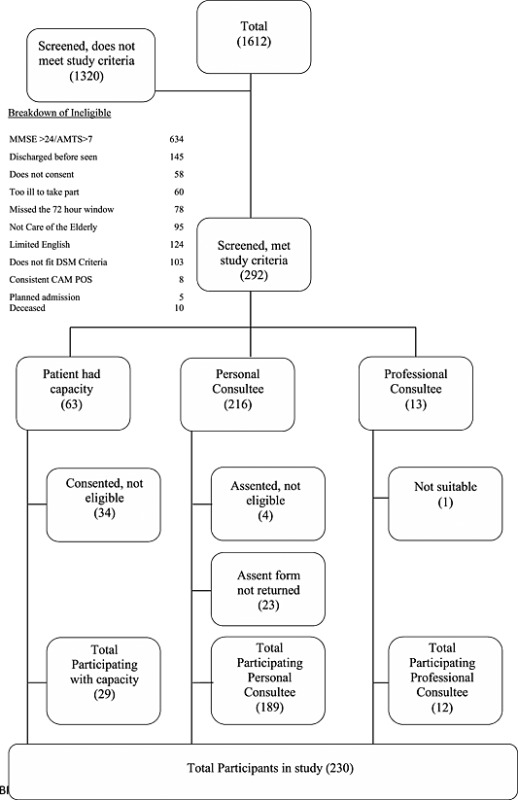
White, N. , Leurent, B. , Lord, K. , Scott, S. , Jones, L. , and Sampson, E. L. (2017) The management of behavioural and psychological symptoms of dementia in the acute general medical hospital: a longitudinal cohort study. Int J Geriatr Psychiatry, 32: 297–305. doi: 10.1002/gps.4463.
References
- 2005. The Mental Capacity Act [Online]. Available: http://www.legislation.gov.uk/ukpga/2005/9/contents.
- 2009. Alzheimer's Society Report ‘Counting the Cost’.
- 2011. Report of the National Audit of Dementia Care in General Hospitals.
- 2014. Meeting Needs and Reducing Distress. accessed from: http://www.reducingdistress.co.uk/reducingdistress/wp‐content/uploads/2014/02/Meeting_needs_and_reducing_distress.pdf.
- Azermai M, Petrovic M, Elseviers MM, et al. 2012. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Res Rev 11: 78–86. [DOI] [PubMed] [Google Scholar]
- Ballard C, Corbett A. 2010. Management of neuropsychiatric symptoms in people with dementia. CNS Drugs 24: 729–739. [DOI] [PubMed] [Google Scholar]
- Ballard CG, Gauthier S, Cummings JL, et al. 2009. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol 5: 245–255. [DOI] [PubMed] [Google Scholar]
- Banerjee S. 2010. Living well with dementia—development of the national dementia strategy for England. Int J Geriatr Psychiatry 25: 917–922. [DOI] [PubMed] [Google Scholar]
- Bishara D, Taylor D, Howard RJ, Abdel‐Tawab R. 2009. Expert opinion on the management of behavioural and psychological symptoms of dementia (BPSD) and investigation into prescribing practices in the UK. Int J Geriatr Psychiatry 24: 944–954. [DOI] [PubMed] [Google Scholar]
- Cairns R. 2009. Deprivation of liberty. Psychiatry 8: 487–489. [Google Scholar]
- Cohen‐Mansfield J. 2013. Nonpharmacologic treatment of behavioral disorders in dementia. Curr Treat Options Neurol 15: 765–785. [DOI] [PubMed] [Google Scholar]
- Deudon A, Maubourguet N, Gervais X, et al. 2009. Non‐pharmacological management of behavioural symptoms in nursing homes. Int J Geriatr Psychiatry 24: 1386–1395. [DOI] [PubMed] [Google Scholar]
- Evans D, Wood J, Lambert L. 2003. Patient injury and physical restraint devices: a systematic review. J Adv Nurs 41: 274–282. [DOI] [PubMed] [Google Scholar]
- Fick DM, Agostini JV, Inouye SK. 2002. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 50: 1723–1732. [DOI] [PubMed] [Google Scholar]
- Freeman CP, Joska J. 2012. Management of behavioural and psychological symptoms of dementia. CME: Your SA J CPD: Psychiatry 30: 110–113. [Google Scholar]
- Gauthier S, Cummings J, Ballard C, et al. 2010. Management of behavioral problems in Alzheimer's disease. Int Psychogeriatr 22: 346–372. [DOI] [PubMed] [Google Scholar]
- Goldberg SE, Bradshaw LE, Kearney FC, et al. 2013. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 347:f4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths A, Knight A, Harwood R, Gladman JRF. 2014. Preparation to care for confused older patients in general hospitals: a study of UK health professionals. Age Ageing 43: 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson HM. 1972. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1: 233–238. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Charpentier PA. 1996. Precipitating factors for delirium in hospitalized elderly persons: predictive model and interrelationship with baseline vulnerability. Jama 275: 852–857. [PubMed] [Google Scholar]
- Inouye SK, Van Dyck CH, Alessi CA, et al. 1990. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med 113: 941–948. [DOI] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, Lyketsos CG. 2015. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 350: h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CR, Logan BR, Noonan PE, et al. 2006. Effects of the Serial Trial Intervention on discomfort and behavior of nursing home residents with dementia. Am J Alzheimers Dis Other Demen 21: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langballe EM, Engdahl B, Nordeng H, et al. 2014. Short‐and long‐term mortality risk associated with the use of antipsychotics among 26,940 dementia outpatients: a population‐based study. Am J Geriatr Psychiatry 22: 321–331. [DOI] [PubMed] [Google Scholar]
- Livingston G, Johnston K, Katona C, et al. 2005. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. A J Psychiatry 162: 1996–2021. [DOI] [PubMed] [Google Scholar]
- Livingston G, Kelly L, Lewis‐Holmes E, et al. 2014. Non‐pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry 205: 436–442. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Sheppard JME, Rabins PV. 2014. Dementia in elderly persons in a general hospital. Am J Psychiatry 157(5), 704–707. [DOI] [PubMed] [Google Scholar]
- Mukadam N, Sampson EL. 2011. A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr 23: 344–355. [DOI] [PubMed] [Google Scholar]
- Reisberg B. 1988. Functional Assessment Staging (FAST). Psychol Bull 24: 653–659. [PubMed] [Google Scholar]
- Russ TC, Shenkin SD, Reynish E, et al. 2012. Dementia in acute hospital inpatients: the role of the geriatrician. Age Ageing 41: 282–284. [DOI] [PubMed] [Google Scholar]
- Sampson EL, White N, Leurent B, et al. 2014. Behavioural and psychiatric symptoms in people with dementia admitted to the acute hospital: prospective cohort study. Br J Psychiatry 205: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson EL, White N, Lord K, et al. 2015. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: a longitudinal cohort study. Pain 156: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclan SG, Saillon A, Franssen E, et al. 1996. The Behaviour Pathology in Alzheimer's Disease Rating Scale (BEHAVE‐AD): reliability and analysis of symptom category scores. Int J Geriatr Psychiatry 11: 819–830. [Google Scholar]
- Scott S, Jones L, Blanchard MR, Sampson EL. 2011. Study protocol: the behaviour and pain in dementia study (BePAID). BMC Geriatr 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]


