Summary
The establishment of spinal motor neuron subclass diversity is achieved through developmental programs that are aligned with the organization of muscle targets in the limb. The evolutionary emergence of digits represents a specialized adaptation of limb morphology, yet it remains unclear how the specification of digit-innervating motor neuron subtypes parallels the elaboration of digits. We show that digit-innervating motor neurons can be defined by selective gene markers and distinguished from other LMC neurons by the expression of a variant Hox gene repertoire and by the failure to express a key enzyme involved in retinoic acid synthesis. This divergent developmental program is sufficient to induce the specification of digit-innervating motor neurons, emphasizing the specialized status of digit control in the evolution of skilled motor behaviors. Our findings suggest that the emergence of digits in the limb is matched by distinct mechanisms for specifying motor neurons that innervate digit muscles.
Introduction
During vertebrate evolution, the creation of limbed appendages provided a critical step in the refinement of skilled motor behaviors (Shubin et al., 1997). Within the limb, the appearance of digits has been argued to represent an essential morphological adaptation that meets the need for dexterous manipulative skills (Sustaita et al., 2013). The emergence of distal limb elements appears to have been a specialized step in limb patterning, both evolutionarily and molecularly (Nakamura et al., 2016; Schneider and Shubin, 2013; Woltering and Duboule, 2010). The formation of digits may therefore have demanded the creation of an expanded diversity of motor neuron subtypes able to innervate and coordinate the activity of new digit muscles. Considerable information is available on the logic of innervation of more proximal limb muscles (Dasen and Jessell, 2009), but how motor neurons destined for digit innervation assume their distinct identities remains unclear.
The molecular logic that imposes discrete limb innervating motor neuron identities involves interactions between conserved signaling molecules and Hox transcriptional codes, and is likely to have occurred in a manner aligned with the evolutionary organization of limb pattern. Indeed, the coordination of limb and motor development is achieved in part through the use of common patterning mechanisms. The proximo-distal axis of the limb is specified by a proximal source of retinoic acid and a distal source of fibroblast growth factors (FGFs), with signals coordinated through the sequential expression of Hox genes that define the pattern of skeletal elements (Cooper et al., 2011; Mercader et al., 2000; Roselló-Díez et al., 2011; Zakany and Duboule, 2007). In parallel fashion, rostral retinoic acid and caudal FGFs determine the patterned expression of Hox genes within the neural tube and specify the spatial segregation of motor neuron subtypes that innervate different limb muscle domains (Dasen and Jessell, 2009; Vanderhorst and Holstege, 1997). At brachial and lumbar levels of the spinal cord, Hox6-8 and Hox10-11 proteins, respectively, initiate the programs that specify the lateral motor column (LMC), which innervates limb muscles (Dasen et al., 2003; Wu et al., 2008). In contrast, Hox9 proteins expressed at thoracic levels mediate the specification of motor neurons projecting to sympathetic chain ganglia and hypaxial muscles (Jung et al., 2010).
The generation of diverse motor pool subtypes involves the activities of nearly two dozen individual Hox genes, whose expression appears to be defined through cross-repressive interactions (Dasen et al., 2005). Within the LMC, motor neurons acquire finer divisional and pool transcriptional identities through a coordinated program in which Hox proteins and their conserved cofactor Foxp1 direct the synthesis of retinoic acid in limb-innervating motor neurons (Dasen et al., 2008; Sockanathan and Jessell, 1998; Sockanathan et al., 2003). Consequently, LMC motor neurons have traditionally been defined by co-expression of Foxp1 and the retinoic acid synthesis enzyme Raldh2 (Dasen et al., 2008). Variation in the expression and function of Hox genes presumably facilitated the adaptation of ancestral motor circuits to evolutionary modifications in limb morphology (Jung and Dasen, 2015; Jung et al., 2014).
We reasoned that if the emergence of limbs necessitated the recruitment of new genetic strategies to pattern limb-innervating motor neurons, then the elaboration of digits over evolution may have required the allocation of divergent molecular programs that specify digit-innervating motor neurons. We have used genetic methods in mouse and chick to explore the specification of digit-innervating motor neurons, initially identifying genes that exhibit selective expression within, or exclusion from, digit-innervating motor neurons. Using these gene markers we show that digit-innervating motor neurons can be distinguished from other LMC neurons by the expression of a distinct Hox gene repertoire and by their failure to synthesize or respond to retinoic acid. This unusual program of Hox coding and retinoid evasion is sufficient to specify digit-innervating motor neurons, potentially a reflection of the specialized status of digit control in the emergence of skilled limb motor behaviors.
Results
Genetic profiling of motor pools targeting distal limb muscles
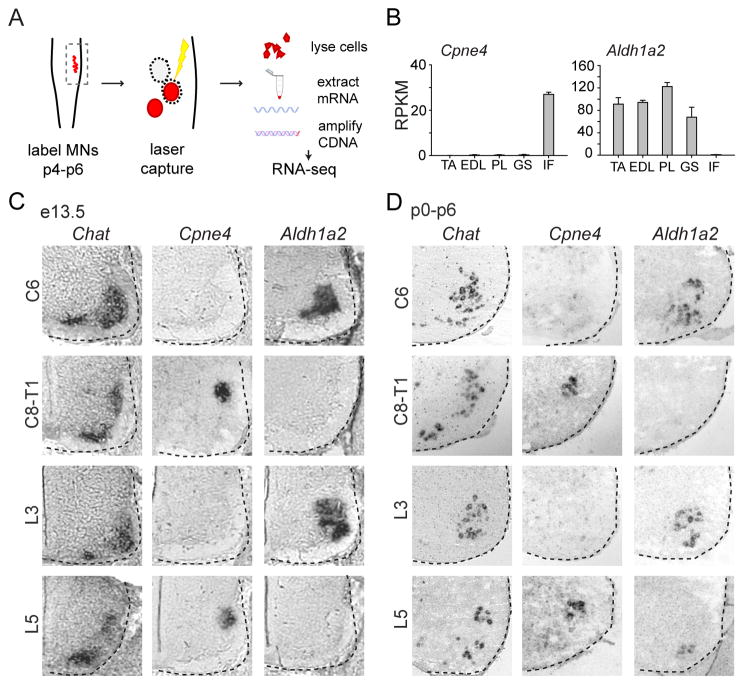
To probe molecular distinctions in the specification of motor neurons innervating digit muscles we performed a screen for genetic markers that distinguish digit innervating motor neurons from other motor pools. We focused on hindlimb motor pools, comparing motor neurons innervating the intrinsic digit muscles in the foot (IF) with three ankle synergist dorsiflexor muscles, the tibialis anterior (TA), extensor digitorum longus (EDL) and peroneus longus (PL), and one antagonist ankle extensor muscle, the gastrocnemius (GS). We assessed gene expression by performing RNA-seq. profiling on cholera toxin B subunit (CTB) labeled motor neurons isolated by laser capture from spinal cord sections of p6 wild-type mice, the earliest age at which selective motor pool labeling could reliably be performed (Figure 1A). Amongst many genes, comparative pairwise sampling between the five motor pools revealed two general categories of genes, those expressed selectively in intrinsic foot motor neurons and those excluded from them.
Figure 1. Identification of motor pool specific gene expression.
(A) Schema for motor pool genetic screen experiment.
(B) Pool-specific levels of gene expression in reads per kilobase per million (RPKM). Pools profiled were tibialis anterior (TA), extensor digitorum longus (EDL), peroneus longus (PL), gastrocnemius (GS) and intrinsic foot (IF). Error bars represent SEM from 3 biological replicates.
(C–D) Validation of gene expression by in situ hybridization at brachial and lumbar spinal levels at e13.5 (C) and p0–p6 (D). The intrinsic hand (IH) pool occupies a dorsal position at C8-T1, and the intrinsic foot (IF) pool occupies a dorsal position at L5. Intrinsic hand and intrinsic foot motor pools were identified by their settling position, with motor pool organization determined by serial sections labeled with Chat.
For more detailed screen methodology, see Experimental Procedures. For related data, see also Figure S1.
Although few genes were identified that were expressed selectively in any of the proximal motor pools that were profiled, we identified ten genes that were robustly expressed in intrinsic foot motor neurons, but not other motor pools, with an average difference in gene expression of >25-fold (Figures 1B and S1A). We examined the expression of these genes using in situ hybridization between e13.5 and p6 in serial spinal cord sections hybridized with a probe against ChAT to reveal the position of motor neurons. Digit muscles are divisible into two groups. Extrinsic digit muscles are located in the forearm or lower leg, control the digits through tendinous attachments and may also control the wrist or ankle joints. In contrast, intrinsic digit muscles are located within the hand or foot and control solely the digits. We identified intrinsic hand and foot motor neurons based on their dorsal positions at spinal levels C8-T1 and L5, respectively, as determined by retrograde tracing (Figure 2; see also Bácskai et al., 2013; McHanwell and Biscoe, 1981; Vanderhorst and Holstege, 1997).
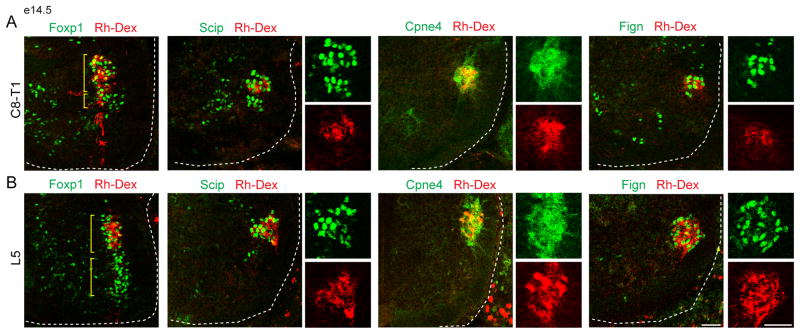
Figure 2. Validation of digit motor neuron marker specificity.
(A–B) Identification of motor pools by retrograde Rh-Dex labeling after tracer injection into intrinsic hand (A) and intrinsic foot muscles (B) at e14.5. Intrinsic hand and intrinsic foot pools represent a dorsal domain of Foxp1+ LMC motor neurons. Fign+ and Cpne4+ motor neurons are labeled selectively after Rh-Dex injection into the intrinsic hand and intrinsic foot muscles, whereas Scip+ motor neurons can also be found in forearm flexor (FF) motor neurons unlabeled by tracer injection. Sacral (S) motor neurons represent a ventral domain at lumbar levels.
Scale bars represent 50 μm. For related data, see also Figure S2.
The ten foot-enriched genes were divisible into four expression classes based on specificity and temporal onset of expression in motor neurons (Figure S1B). One class, represented by Osmr and Col8a1, began expression postnatally, selectively at the position of intrinsic foot motor neurons. A second class, represented by Ecrg4, Reg3b and Serpinf1, also began expression postnatally, but at the position of both intrinsic hand and foot motor neurons. A third class, represented by S100a11 and Crabp2, was expressed by all LMC motor neurons at embryonic stages, but became restricted to the position of intrinsic hand and foot motor neurons from p0 onwards. A fourth class, represented by Cpne4, Fign and Pirt, was selectively expressed from e13.5 onwards at the position of intrinsic hand and foot innervating motor neurons (Figures 1C, 1D and S1B). There was little variation in the number of hand or foot motor neurons labeled by the genes identified from this screen, and each gene appeared to be expressed uniformly throughout the intrinsic hand or foot motor pools.
We used immunohistochemistry to examine the selectivity of expression of Cpne4 and Fign, two of the embryonically expressed genes specific to the intrinsic hand and foot motor neurons. Cpne4, a member of the Copine calcium-dependent phospholipid binding protein family, and Fign, a nuclear ATPase, are both associated with diverse intracellular activities, but their role in neurons is unclear. Antibodies selective for mouse Cpne4 and Fign were generated, showed similar patterns of expression to the corresponding in situ hybridization pattern, and were used to examine their motor neuron specificity, in conjunction with retrograde Rh-Dex and CTB tracing in e14.5 and p6 mice (Figures 1C, S1B, 2, S2A and S2B). As a molecular comparison we also examined the expression of Scip (Pou3F1), a transcription factor that labels motor neurons which innervate intrinsic muscles in the hand and foot, as well as extrinsic muscles of digit control in the forearm (Dasen et al., 2005; Kevin Kanning, personal communication). After injection of Rh-Dex into intrinsic hand or foot muscles we found that all retrogradely labeled motor neurons expressed Scip, Cpne4 and Fign, indicating that these markers label the entire cohort of intrinsic digit-innervating motor neurons (Figure 2). At brachial levels a more ventral population of Scip+ motor neurons could be labeled by injections into forearm digit-flexor, but not forearm digit-extensor muscles (Figures 2A and S2B). By contrast, Rh-Dex injections into forearm muscles failed to label Cpne4+ or Fign+ motor neurons (Figure S2B). Thus, Cpne4 and Fign expression are selective to intrinsic hand and foot motor neurons, whereas Scip is more broadly expressed - in both extrinsic and intrinsic digit motor pools.
The same RNAseq screen also revealed that the gene Aldh1a2, which encodes the retinoid synthetic enzyme retinaldehyde dehydrogenase-2 (Raldh2), was excluded from intrinsic foot motor neurons, but expressed by other hindlimb pools (Figure 1B). In situ hybridization and retrograde labeling, reported below, showed that Aldh1a2 was excluded from motor neurons at caudal brachial and lumbar levels, the locations at which intrinsic hand and intrinsic foot motor neurons are located, but was expressed by essentially all other LMC motor neurons (Figures 1C and 1D). Taken together, this molecular screen has identified numerous genes expressed in, and one excluded from, digit-innervating motor neurons.
Digit-innervating motor neurons lack Raldh2 expression
Given the relative scarcity of selective markers for motor pools that innervate proximal muscles, the identification of many genes that distinguish digit-innervating motor neurons argues for a fundamental distinction in their specification. Since Aldh1a2 is not expressed by digit-innervating motor neurons, it is possible that the evasion of retinoid signaling is involved in establishing this fundamental motor neuron distinction.
We first evaluated the absence of Raldh2 protein expression in intrinsic digit-innervating motor neurons using retrograde tracing. After Rh-Dex and CTB-555 injection into intrinsic hand and intrinsic foot muscles at e14.5 or p0, respectively, none of the retrogradely-labeled motor neurons expressed Raldh2 (Figures 3A and S3A). In addition, we found that intrinsic hand and foot motor neurons, as defined by Cpne4 expression, did not express Raldh2 at e11.5, prior to the axonal innervation of distal limb muscles (Figure 3B). In contrast, Raldh2 was detected in all other Foxp1+ LMC motor neurons at rostral brachial and lumbar levels (Figures 3C and 3D). Despite their failure to express Raldh2, intrinsic hand and foot motor neurons expressed Islet1 and Foxp1 at levels comparable to that of other LMC motor neurons (Figures S3B and S3E).
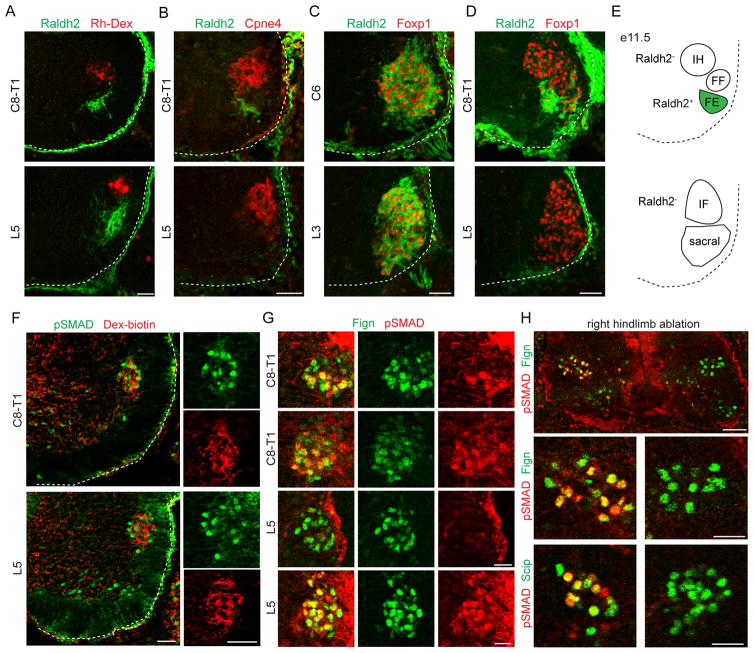
Figure 3. Digit-innervating motor neurons lack Raldh2 expression.
(A) Raldh2+ motor neurons are not labeled after Rh-Dex injection into the intrinsic hand and intrinsic foot muscles at e14.5.
(B) Cpne4+ intrinsic hand and intrinsic foot motor neurons do not express Raldh2 at e12.5.
(C) Rostral LMC motor neurons express Raldh2 at e11.5.
(D) Caudal LMC motor neurons do not express Raldh2 at e11.5.
(E) Summary of Raldh2 expression by spinal motor neurons. Forearm extensor (FE) but not forearm flexor (FF) motor neurons express Raldh2.
(F) pSMAD+ motor neurons are labeled by Dex-biotin injection into intrinsic hand and intrinsic foot muscles at e14.5.
(G) Fign+ intrinsic hand motor neurons express pSMAD by e12.5. Fign+ intrinsic foot motor neurons express pSMAD by e13.5. The timing of pSMAD expression onset in intrinsic hand and foot motor neurons correlates with the timing of axon outgrowth in the limb, as axons first invade the developing hand at e12.5, whereas the initial innervation of the developing foot is delayed by approximately 12–24 hours (Petrinovic et al., 2010; Shu et al., 2009).
(H) Right hindlimb ablation at stage 18 disrupts expression of pSMAD in right intrinsic foot motor neurons at stage 28.
Scale bars represent 50 μm in (A–F), 25 μm in (G), 50 μm in the large panel of (H) and 25 μm in the small panel of (H). For related data, see also Figure S3.
We also sought to determine whether motor neurons that innervate extrinsic digit muscles fail to express Raldh2. To resolve this issue we examined the pattern of Raldh2 expression in Scip+ motor neurons at forelimb levels, which includes forearm digit-flexor and intrinsic digit-innervating motor neurons. All Scip+ motor neurons at e12.5 failed to express Raldh2 (Figure S3D). This result demonstrates that all intrinsic and certain extrinsic digit-innervating motor neurons can be distinguished from other LMC motor neurons throughout embryonic development by the absence of Raldh2 expression.
The expression of ETS and other pool-specific transcriptional programs depends on extrinsic signals from the limb mesenchyme (Dasen and Jessell, 2009). To assess the role of limb-derived cues in digit-motor neuron patterning, we performed unilateral hindlimb ablations in chick embryos. We used Scip and Fign to mark digit-innervating motor neurons in chick, since chick Cpne4 does not display the pool specificity evident in mouse (Figures S3J and S3K). We performed unilateral hindlimb ablation in chick embryos at stage 18, prior to the onset of hind-limb axon innervation. Embryos were permitted to develop until stage 28, prior to the peak period of motor neuron cell death (Calderó et al., 1998; Tosney and Landmesser, 1985). Hindlimb ablation did not eliminate Raldh2 expression at spinal level L3, nor was Raldh2 expression induced ectopically at caudal lumbar levels (Figure S3L). Moreover, hindlimb ablation did not disrupt the generation of Scip+ and Fign+ intrinsic foot motor neurons (Figure 3H). Thus, the generation of digit-innervating motor neurons and their failure to express Raldh2 occurs independently of limb-derived cues.
Intrinsic digit-innervating motor neurons engage in pSMAD signaling
We also sought to address the potential contribution of other signaling pathways to digit-motor neuron specification. BMPs regulate limb and spinal patterning, through phosphorylation of SMAD1, SMAD5 and SMAD8, and BMP2, BMP4 and BMP7 are expressed in the apical ectodermal ridge of the developing autopod (Liu and Niswander, 2005; Robert, 2007). We therefore considered the possibility that the BMP-pSMAD pathway contributes to the development of digit-innervating motor neurons. To assess whether digit-innervating motor neurons engage in pSMAD signaling, we injected Dex-biotin into intrinsic hand or intrinsic foot muscles at e14.5 and observed that nearly all labeled digit-innervating motor neurons expressed pSMAD (Figure 3F). Intrinsic hand and foot motor neurons first expressed pSMAD at e12.5 and e13.5, respectively, with the number of pSMAD-labeled motor neurons increasing from e12.5–e14.5 (Figures 3G and S3I). In contrast, Scip+ forearm digit-flexor motor neurons did not express pSMAD, indicating that pSMAD is expressed selectively by intrinsic digit-innervating motor neurons (Figure S3I). The timing of pSMAD expression is consistent with the timing of limb axon innervation, suggesting selective induction in intrinsic digit-innervating motor neurons upon the arrival of their axons in the developing autopod.
Intrinsic digit-innervating motor neurons also express pSMAD in chick (Figure S3K). To determine whether the induction of pSMAD in intrinsic digit-innervating motor neurons depends on signals from the limb mesenchyme, we examined chick embryos in which the right hindlimb had been ablated. We found that hindlimb ablation disrupted the expression of pSMAD in Scip+ and Fign+ intrinsic foot motor neurons (Figure 3H). Taken together, these finding argue that pSMAD signaling is induced in intrinsic digit motor neurons by limb-derived cues, occurs after the initial specification of these motor neurons, but is not involved in the exclusion of Aldh1a2.
Retinoid signaling disrupts digit-innervating motor neurons
We next sought to address whether retinoid signaling influences the generation of digit-innervating motor neurons. If the specification of digit-innervating motor neurons requires the absence of retinoid signaling, constitutive retinoid induction might be expected to disrupt their development. We activated retinoic acid receptor signaling in digit-innervating and other motor neurons by chick neural tube electroporation of a constitutively active retinoic acid receptor, VP16RAR-GFP, in which the retinoic acid receptor RARα is fused to the transcriptional activator VP16 (Novitch et al., 2003; Sockanathan et al., 2003). This constitutively activated RAR receptor mediates transcription at retinoic acid response elements (RAREs) (Figure S4A), through heterodimer formation with RXR receptors, which are widely expressed in the spinal cord (data not shown). Electroporations were performed using a pCAGGS driver at brachial levels at stages 14–16, prior to the acquisition of motor neuron divisional or pool identity. Although chickens have undergone evolutionary digit loss at forelimb levels, with wings containing three rather than five digits, wing digit muscles are still innervated by motor neurons (Hollyday and Jacobson, 1990; Straznicky and Tay, 1983).
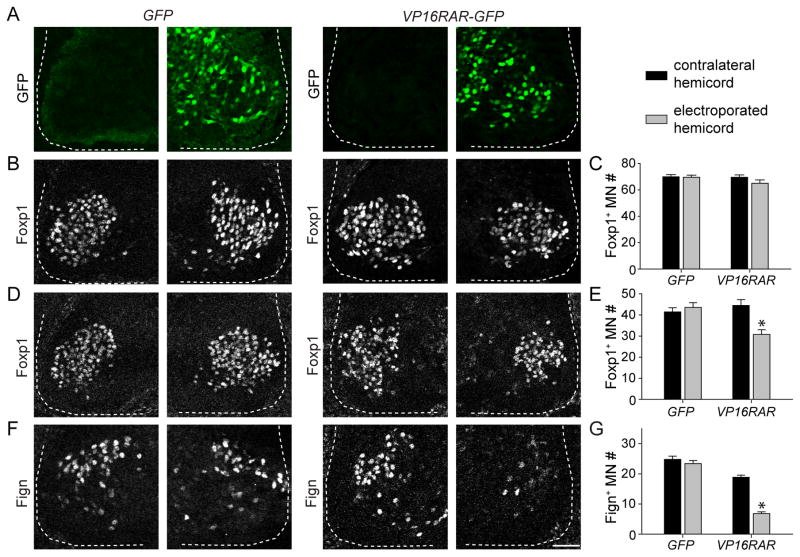
We first examined whether expression of a constitutively active retinoid receptor perturbs the development of LMC neurons. Electroporation of VP16RAR-GFP or a control GFP construct did not change the number of Foxp1+ motor neurons at spinal level C6–C7 (Figures 4B and 4C), an indication that constitutive retinoid induction does not have a general impact on motor neuron number. In contrast, electroporation of a dominant negative retinoic acid receptor, RAR403, was lethal to developing motor neuron progenitors (data not shown). In addition, motor neurons electroporated with VP16RAR-GFP or a control GFP construct maintained consistent levels of Foxp1 expression, indicating that retinoid induction does not disrupt induction of Foxp1 expression (Figure S4J). In contrast, electroporation of VP16RAR-GFP selectively reduced the average number of Foxp1+ motor neurons at C8-T1 by 30%, despite a similar fraction of Foxp1+ motor neurons expressing either VP16RAR-GFP or control GFP construct (Figures 4D and 4E; p < 0.001, Figure S4I). The moderate loss of Foxp1+ neurons is consistent with the observation that only ~50% of motor neurons at C8-T1 innervate digit muscles (Figures 4E, 4G and S4H). These findings reveal that retinoid induction maintains Foxp1 expression, but results in a selective loss of Foxp1+ motor neurons at segmental levels at which digit-innervating motor neurons are located.
Figure 4. Constitutive retinoic acid receptor expression disrupts digit motor neuron development.
(A) Electroporation of VP16RAR-GFP or control GFP plasmids in stage 14–17 chick embryos.
(B and C) Electroporation of VP16RAR-GFP or control GFP does not affect the number of Foxp1+ motor neurons at rostral brachial levels (n = 5 embryos). Quantification in (C) represents motor neuron counts per spinal cord hemisection.
(D and E) Electroporation of VP16RAR-GFP, but not control GFP, reduces the number of Foxp1+ motor neurons at caudal brachial levels by 30% (p < 0.001, Mann-Whitney Rank Sum Test, n = 8 embryos).
(F and G) Electroporation of VP16RAR-GFP, but not control GFP, reduces the number Fign+ motor neurons at brachial levels by 69% (p < 0.001, Mann-Whitney Rank Sum Test, n = 8 embryos for GFP and 15 embryos for VP16RAR).
All data reported as mean ± SEM. Scale bar represents 50 μm. For related data, see also Figure S4.
Retinoic acid plays an early role in directing divisional identity within the LMC (Ji et al., 2006; Sockanathan et al., 2003), prompting us to examine the effect of retinoid induction on medial-lateral divisional specification within the LMC. We used the medial LMC marker Isl1, since the lateral LMC marker Lim1 is not expressed at late embryonic stages. We found that electroporation of VP16RAR-GFP at brachial levels reduced the average number of Isl1+ motor neurons by 22% (Figures S4C and S4D; p < 0.001). Similarly, VP16RAR-GFP electroporation reduced the average number of medial Pea3+ motor neurons by 28% (Figures S4E and S4F; p < 0.001). The maintenance of the overall number of Foxp1+ motor neurons at C6–C7 argues that retinoid induction induces only a modest medial-to-lateral shift in LMC divisional specification.
We next examined the effect of retinoid induction on the expression of digit-innervating motor neuron markers at brachial levels. We found that electroporation of VP16RAR-GFP at brachial levels reduced the average number of Scip+ and Fign+ motor neurons by 64% and 69%, respectively (Figures 4F, 4G, S4G and S4H; p < 0.001). Given the proportion of motor neurons that innervate digits at C8-T1, the observed reduction of digit-motor neuron marker expression is consistent with the observed 30% reduction in Foxp1+ neurons at these segmental levels. Thus, it is unlikely that the digit-innervating motor neurons were redirected to other LMC pool identities. Moreover, of the Scip+ and Fign+ motor neurons that remained after electroporation, the percentage expressing VP16RAR-GFP was reduced by 36% and 39%, respectively, as compared to those expressing GFP control (Figures S4I and S4K). Taken together with the reduction of Foxp1+ at caudal brachial levels, these results provide evidence, indicated by selective marker expression, that retinoid induction results in a selective loss of motor neurons fated to innervate digits.
Hoxc8 and Hoxc9 are needed for specification of brachial digit-innervating motor neurons
LMC neurons acquire distinct pool subtype identities through the actions of Hox transcription factors, which also regulate the expression of Raldh2 (Dasen et al., 2005, 2008). We examined the role of Hox genes in the specification of digit-innervating motor neurons, focusing on their role at forelimb levels, where the contributions of individual Hox genes is well defined (Figure 5A; Dasen et al., 2005). We focused on the expression of Hoxc8 and Hoxc9, which are found at caudal brachial and thoracic levels and known to regulate motor neuron differentiation (Dasen et al., 2005; Jung et al., 2010).
Figure 5. Brachial Hox expression in digit-innervating motor neurons.
(A) Organization of Hox proteins, motor columns and pools. At brachial levels, Pea3+ motor neurons project to the cutaneous maximus (CM) muscle and latissimus dorsi (LD) muscle. Scip+ motor neurons project to forearm flexor (FF) muscles and intrinsic hand (IH) muscles. Cpne4+ and Fign+ motor neurons project to the intrinsic hand (IH) muscles.
(B) Rostrocaudal distribution of Pea3+, Scip+ and Cpne4/Fign+ motor neurons at brachial levels. Error bars represent SEM from >3 animals.
(C) Expression of Hoxc8 and Hoxc9 in Pea3+ and Scip+ motor pools at defined rostrocaudal levels.
(D) Pea3+ and Scip+ motor neurons express similar levels of Hoxc8 protein. Scip motor neurons express higher levels of Hoxc9 than Pea3+ motor neurons (p < 0.001, Mann-Whitney Rank Sum Test). Scip+ motor neurons express Hoxc9 at lower levels than thoracic PGC motor neurons (p < 0.001, Mann-Whitney Rank Sum Test) (all panels, n = 3 animals, 25–50 motor neurons).
Scale bar in (C) represents 50 μm. For related data, see also Figure S5.
Analysis of the rostro-caudal expression domains of Hoxc8 and Hoxc9 using selective antibodies revealed that Hoxc8 is expressed in caudal brachial and rostral thoracic segments, whereas Hoxc9 is expressed in the extreme caudal LMC and in all thoracic segments (Figures 5B and 5C). Pea3+ and Scip+ motor neurons thus express high levels of Hoxc8 at spinal level C8 (Figure 5C). In contrast, dorsally positioned Scip+ motor neurons express Hoxc9 at spinal level C8, whereas more ventrally positioned Pea3+ motor neurons lack Hoxc9 expression (Figure 5C). The level of Hoxc9 in Scip+ motor neurons does not vary from C8 to T1, but is nevertheless approximately three times lower than the level of Hoxc9 found in PGC motor neurons at thoracic levels (Figures 5D and S5B). Thus, digit-innervating motor neurons at forelimb levels express high levels of Hoxc8 and low levels of Hoxc9.
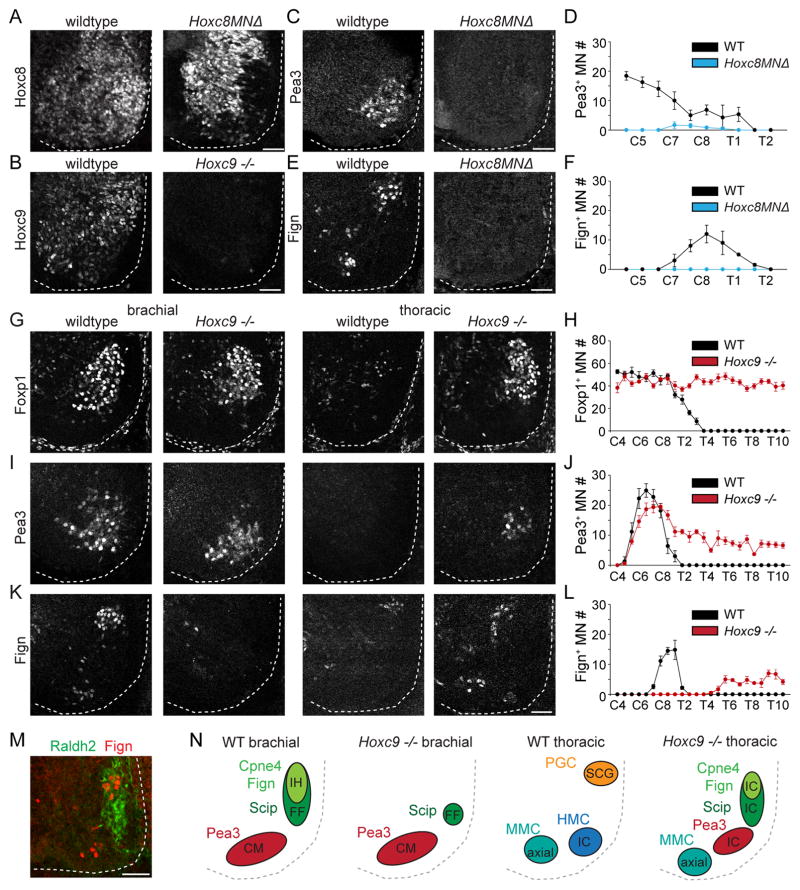
We used Hox mutants to determine whether Hoxc8 and Hoxc9 expression at brachial levels is required for the specification of digit-innervating motor neurons. In conditional Hoxc8MNΔ mutants, in which Hoxc8 is deleted selectively in motor neurons using the driver Olig2::Cre, we found that expression of Pea3, Scip, Cpne4 or Fign was no longer detected in motor neurons at brachial levels, suggesting that Hoxc8 is required for caudal LMC specification (Figures 6A, 6C–6F and S6A–S6C). Previous work has indicated that loss of Hoxc9 leads to a selective reduction in Scip+ motor neurons at caudal brachial levels and a loss of motor innervation of intrinsic hand muscles (Jung et al., 2010). We found that expression of Cpne4+ and Fign+ was completely absent at these segmental levels in Hoxc9 mutants, whereas Pea3+ motor neurons are maintained at brachial levels (Figure 6I–6L and S6F and S6G). A small number Scip+ motor neurons remain at C8-T1 in Hoxc9 mutants, presumably those that normally project to forearm flexor muscles (Figure S6D and S6E). These results suggest that both Hoxc8 and Hoxc9 contribute to the specification of digit-innervating motor neurons.
Figure 6. Hoxc8 and Hoxc9 are necessary for brachial digit-innervating motor neuron specification.
(A) Loss of Hoxc8 protein in motor neurons in Hoxc8MNΔ mutants at e12.5.
(B) Loss of Hoxc9 protein at thoracic levels in Hoxc9 mutants at e12.5.
(C and E) Expression of Pea3 (C) and Fign (E) at e12.5 in wildtype and Hoxc8MNΔ mutants.
(D and F) Quantification of Pea3+ (D) and Fign+ (F) motor neurons per spinal cord hemisection along the rostrocaudal axis at e12.5 in wildtype and Hoxc8MNΔ mutants (n = 3 embryos).
(G, I and K) Expression of Foxp1 (G), Pea3 (I), and Fign (K) at e12.5 in wildtype and Hoxc9 mutants.
(H, J and L) Quantification of Foxp1+ (H), Pea3+ (J), and Fign+ (L) motor neurons per spinal cord hemisection along the rostrocaudal axis at e12.5 in wildtype and Hoxc9 mutants (n = 3 embryos).
(M) Ectopic Fign+ motor neurons generated at thoracic levels in Hoxc9 mutants express Raldh2.
(N) Summary of pool specific reorganization in Hoxc9 mutants shown in cross section. Pools are labeled in black according to axonal projection based on connectivity changes reported in (Jung et al., 2010). In Hoxc9 mutants, Pea3+ motor neurons are maintained at brachial levels and continue to project to cutaneous maximus (CM). Projections to the intrinsic hand (IH) muscles are lost. Remaining Scip+ motor neurons at caudal brachial levels may continue to project to forearm flexor (FF) muscles. Ectopically produced motor neurons at thoracic levels project to intercostal muscles (IC).
All data reported as mean ± SEM. Scale bars represent 50 μm. For related data, see also Figure S6.
We next examined the pattern of motor neuron generation at thoracic levels in Hoxc9 mutants. We observed that Foxp1+ motor neurons extend into thoracic segments, a consequence of ectopic expression of forelimb level Hox activity in Hoxc9 mutant embryos (Jung et al., 2010; Figure 6G and 6H). Moreover, Pea3+, Scip+, Cpne4+ and Fign+ motor neurons are each ectopically induced at thoracic levels in Hoxc9 mutants (Figures 6I–6L and Figure S6D–S6G). Previous work indicates that the axons of these neurons project to intercostal muscles (Jung et al., 2010), the normal target of thoracic hypaxial motor column (HMC) neurons. The generation of Cpne4+ and Fign+ motor neurons at thoracic levels in the absence of Hoxc9 suggested that other thoracic Hox9 genes may compensate for the absence of Hoxc9 in specifying digit-innervating motor neurons. The ectopic Scip+, Cpne4+ and Fign+ motor neurons generated at thoracic levels express Raldh2 (Figure 6M), consistent with the finding that only Hoxa9 and Hoxc9 paralogs are capable of repressing Raldh2 expression (Jung et al., 2014). Thus, it is possible that other Hox9 paralogs can facilitate the specification of a small number of digit-innervating motor neurons, but are less effective than Hoxc9 at silencing Raldh2 expression.
Joint Hoxc8 and Hoxc9 expression drives specification of digit-innervating motor neurons
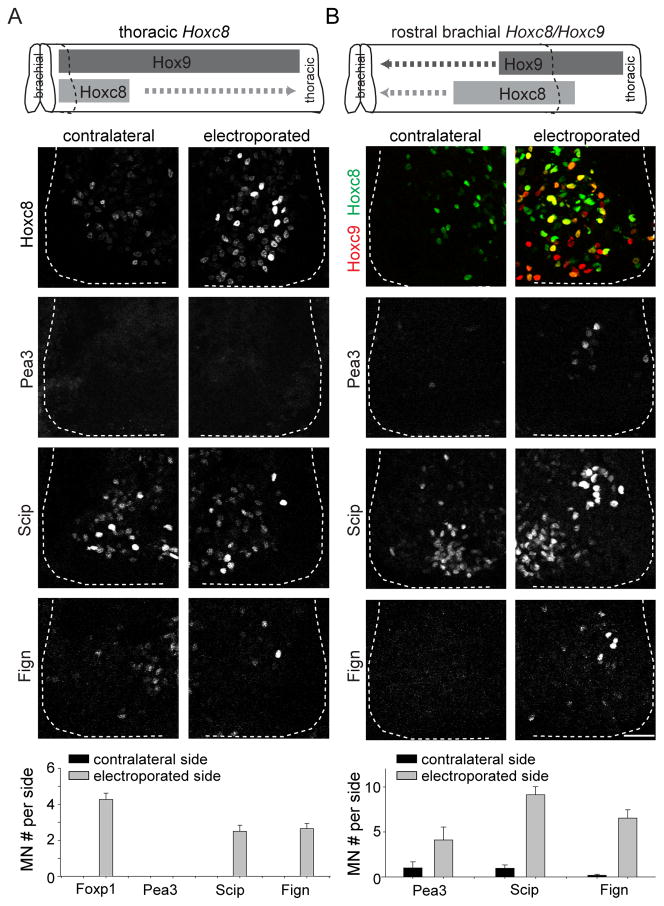
To determine the sufficiency of Hoxc8 and Hoxc9 in inducing digit-innervating motor neurons, individually or together, we performed Hoxc8 and Hoxc9 electroporation in chick neural tube. Expression of Hoxc8 at rostral brachial levels, where Hoxc9 is not expressed, induced ectopic Pea3+ neurons but not Scip+ or Fign+ motor neurons (Figure S7A), a finding consistent with previous reports (Dasen et al., 2005). Expression of Hoxc9 at rostral brachial levels reduced the number of Foxp1+ motor neurons and did not ectopically induce Pea3+, Scip+ or Fign+ motor neurons, consistent with previous findings (Figure S7B; Dasen et al., 2008; Jung et al., 2010). Thus, neither Hoxc8 nor Hoxc9 alone are sufficient to induce digit-innervating motor neurons. But expression of Hoxc8 at thoracic levels, a domain of Hoxc9 expression, resulted in induction of Scip+ and Fign+, but not Pea3+ motor neurons (Figure 7A). Thus, induction of Scip+ and Fign+ motor neurons is achieved in the context of both Hoxc8 and Hoxc9.
Figure 7. Hoxc8 and Hoxc9 coexpression are sufficient for brachial digit-innervating motor neuron specification.
(A) Expression of Hoxc8 at thoracic levels, where Hoxc9 is highly expressed, ectopically induces Scip+ and Fign+, but not Pea3+ motor neurons (n = 5 embryos). Quantification panel at bottom represents motor neuron counts per spinal cord hemisection.
(B) Co-eletroporation of Hoxc8 and Hoxc9 at rostral brachial levels in chick neural tube induces Scip+ and Fign+ motor neurons. A smaller number of Pea3+ motor neurons are also induced (n = 5 embryos).
All data reported as mean ± SEM. Scale bar represents 50 μm. For related data, see also Figure S7.
To determine whether joint Hoxc8 and Hoxc9 expression is sufficient for the specification of forelimb digit-innervating motor neurons, we performed co-electroporation of Hoxc8 and Hoxc9. With joint co-electroporation, 76% of the electroporated motor neurons expressed both genes, with 14% expressing Hoxc8 alone and 10% expressing Hoxc9 alone (Figure S7D). Co-electroporation of Hoxc8 and Hoxc9 at rostral brachial levels induced an average of 3 ectopic Pea3+ motor neurons per hemisection, compared to an average of 30 Pea3+ neurons present at C8 (Figures 7B and S4F). These ectopic Pea3+ motor neurons are presumably due to an influence of Hoxc8 expression in the absence of Hoxc9, as co-electroporation of Hoxc8 and Hoxc9 at caudal brachial levels, where Hoxc8 is broadly expressed, reduced Pea3 expression (Figure S7C). A more dramatic increase in the number of ectopic Scip+ and Fign+ motor neurons was obtained at rostral brachial levels, with an average of 8 and 6 ectopic motor neurons per hemisection, respectively, compared to an average of 25 Scip+ and Fign+ neurons present at C8-T1 (Figures 7B, 4G and S4H). These ectopic neurons express Foxp1 at high levels, indicating that they are LMC motor neurons (Figure S7E). Thus, co-expression of Hoxc8 and Hoxc9 is able to induce digit-innervating motor neurons at forelimb levels.
Discussion
The emergence of digits represents a specialized adaptation of limb morphology for use in fine motor control. The formation of limbed appendages necessitated the recruitment of novel genetic mechanisms for specifying limb-innervating motor neurons, suggesting a parallel allocation of molecular programs that specify digit-innervating motor neurons. Our results indicate that digit-innervating motor neurons can be distinguished from other LMC motor neurons through the expression of specific genetic markers and by their reliance on a distinct Hox gene specification code that ensures that digit innervating motor neurons fail to synthesize retinoic acid. This divergent program of novel Hox coding and retinoid evasion is sufficient to induce the specification of digit-innervating motor neurons in vivo. Taken together, our findings suggest that the late evolutionary emergence of specialized genetic programs for digit elaboration in the limb is matched by a parallel adaptation in motor neurons that innervate digit muscles.
A Hox and retinoid model of digit motor neuron specification
Our Hox and retinoid manipulation studies suggest a model for the specification of digit-innervating motor neurons which implicates Hox genes in regulating the expression of Raldh2 (Figure 8). The status of motor neuron Raldh2 expression is known to depend on ongoing Hox activity (Dasen et al., 2008). Foxp1 contributes to the activation of Raldh2, but is not sufficient to act as a Hox intermediary that directly induces Raldh2 expression (Dasen et al., 2008). Thus, the absence of retinoic signaling in Foxp1 expressing digit-innervating motor neurons is likely related to their Hox status. The caudal position of digit-innervating motor neurons within the LMC allows for overlapping expression of Hoxc8 and Hoxc9 at brachial levels of the spinal cord, which in turn is necessary and sufficient for the specification of forelimb level digit-innervating motor neurons. This variant Hoxc8/Hoxc9 profile is likely responsible for the failure of digit-innervating motor neurons to synthesize retinoic acid.
Figure 8. A model of digit-innervating motor neuron specification.
Digit-innervating motor neurons can be distinguished from motor pools that innervate more proximal muscles in the forearm by their low levels of Hoxc9 expression. The specification of digit-innervating motor neurons at brachial levels requires both Hoxc8 and Hoxc9, in likely combination with FGF and Shh signaling. At high levels, Hoxc9 blocks expression of Foxp1 and Raldh2. Low levels of Hoxc9 in digit-innervating motor neurons may thus be sufficient to block expression of Raldh2 while maintaining Foxp1. The absence of retinoid production in digit-innervating motor neurons consequently contributes to their proliferation and maintenance.
The most salient feature of the Hox specification of digit-innervating motor neurons at brachial levels is their expression of low levels of Hoxc9, a feature which distinguishes them from motor pools that innervate more proximal muscles. High levels of Hoxc9 block expression of brachial Hox genes and of Foxp1, which both contribute to the loss of Raldh2 expression (Dasen et al., 2003, 2008; Jung et al., 2010). We surmise that low levels of Hoxc9 expression block expression of Raldh2 in digit-innervating motor neurons while permitting expression of Foxp1. The finding that Hoxc9 is involved in conferring the identity of digit-innervating motor neurons provides a plausible explanation for the absence of Raldh2 in these neurons. It is likely that a similar logic applies for different Hox genes at lumbar levels of the spinal cord. Nevertheless, the reciprocal relationship between retinoid signaling and Hox activity requires more detailed interrogation. It is possible that just as early retinoid signaling establishes the boundaries of Hox expression that mediate columnar identity, later retinoid signaling may dynamically regulate the expression of more refined Hox programs to mediate divisional and pool subtype specification. However, it remains unclear how ongoing retinoid signaling might be expected to shape Hox expression in developing motor neurons, as previous genetic experiments that reduced retinoid signaling yielded conflicting results regarding whether Hoxc8 expression was increased or decreased (Ji et al., 2006; Vermot et al., 2005).
As with other brachial and lumbar LMC neurons, intrinsic hand and foot motor neurons are generated at different levels of the spinal cord and engage distinct Hox programs. Yet both digit-innervating motor neuron sets express many of the same genetic markers. Our findings argue that the absence of retinoid signaling serves as a common element in the specification of intrinsic hand and foot motor neurons, permitting expression of conserved downstream genes in each pool. Retinoic acid plays an early role in blocking PGC and HMC columnar specification and inducing lateral character within the LMC (Ji et al., 2006; Novitch et al., 2003; Sockanathan et al., 2003; Vermot et al., 2005). Our results implicate an additional role for retinoids in motor pool specification, suppressing the acquisition of digit-innervating pool identities, as determined by selective marker expression. Although it is unclear whether the observed loss of digit motor neuron marker expression following retinoid induction implies the absence of axonal digit-innervation in the limb, previous work together with our findings indicate that in Hox mutants, the absence of selective marker expression is accompanied by the absence of corresponding peripheral innervation (Jung et al., 2010).
It remains unclear mechanistically how differential retinoid signaling regulates the acquisition of motor neuron subtype identity. Preliminary work suggests that retinoids are likely to suppress the initial specification of these neurons, rather than promote a process of programmed cell death after their generation (Figure S4L). Nevertheless, future efforts will be required to determine the downstream targets of retinoid signaling that coordinate the expression of selective digit motor neuron markers, and to characterize the functional role of the gene markers expressed in digit-innervating motor neurons.
Taken together, our findings suggest a model in which the specification of digit innervating motor neuron identity emerges through a cell intrinsic network of Hox expression and evasion of retinoid signaling. Nevertheless, limb-derived cues also play a role in later aspects of motor neuron differentiation, and the expression of certain motor pool specific transcription factors depends on GDNF and other signals from limb mesenchyme (Haase et al., 2002; Lin et al., 1998). It is possible that pSMAD signaling may regulate later aspects of intrinsic digit-motor neuron development, coordinating the expression of late-onset genes involved in motor neuron survival, maintenance or differentiation.
Linking the spinal motor system to limb evolution
Is there a broader significance of these studies on digit innervating motor neurons? The cellular and molecular properties of neurons with long-distance axonal projections are typically organized in alignment with the position and identity of their target cells. Such matching occurs despite the fact that the differentiation of neurons and their distant targets occurs in spatially and developmentally distinct regions of the nervous system. These features raise the issue of how the coordinate programming of neurons and their distant targets has been achieved during evolution. Our findings highlight that one strategy for linking the development of the spinal motor system and limb is the use of shared patterning mechanisms. We identify variant Hox coding and retinoid evasion as a feature common to both the distal limb bud and the motor neurons that innervate distal digit muscles. Moreover, the observation that precise levels of retinoids are required for digit muscle and tendon development (Rodriguez-Guzman et al., 2007) raises the possibility that the absence of Raldh2 in digit-innervating motor neurons may be required for proper autopod development
Several recent studies have addressed the degree to which the evolutionary emergence of limb elements involved the adaptation of preexisting genetic programs from the fins of bony fish (Nakamura et al., 2016; Woltering et al., 2014). In vertebrates, appendage innervating motor neurons are thought to have emerged in fish as populations of motor neurons that innervate the pectoral and pelvic fins (Murakami and Tanaka, 2011). However, it is still unclear to what extent the molecular programs involved in specifying limb-innervating motor neurons were adapted from programs already present in fish, as motor neurons in zebrafish share only the most basic molecular features with mammalian LMC motor neurons (Murata et al., 2010; Uemura et al., 2005). It is likely that Hox-dependent limb innervation programs first emerged in transitional organisms in which bony fins first began to develop limb-like structure. However, clarifying when and how mechanisms that specify digit-innervating motor neurons first emerged is challenging, given that digit-like structures first appeared in now extinct tetrapodomorphs (Johanson and Joss, 2007; Schneider and Shubin, 2013; Woltering and Duboule, 2010). It is possible that Raldh2+ LMC motor neurons emerged first, with additional Hox programs recruited later to extinguish Raldh2 expression in motor neurons fated to innervate the digits.
Regardless of precise evolutionary order, it seems that rearrangements in Hox activity were responsible for the emergence of limb- and digit- innervating motor neurons, since species-specific patterns of Hox expression control motor neuron columnar organization in a manner that accommodates different vertebrate body plans (Jung and Dasen, 2015; Jung et al., 2014). Moreover, analysis of Hox activity from different vertebrate species suggests that N-terminus motifs within the Hoxc9 gene responsible for promoting thoracic identity and repressing LMC specification emerged at the time that vertebrates acquired paired appendages (Jung et al., 2014). This conclusion suggests that the expression of a small number of essential regulatory genes can reorganize the structure of preexisting motor circuits to adapt to evolutionary modifications in limb morphology and motor behavior. Our results argue that the specification of digit-motor neurons involved the adaptation of preexisting Hox programs in such a way as to mediate all-or-none distinctions in retinoid signaling, leading to more dramatic downstream differences in gene expression.
Motor neurons for digit control
The finding that digit motor neurons express distinct genetic programs has several implications for the coordination of digits. The use of distinct Hox codes and the absence of retinoic acid production implies the expression of different surface molecules involved in target recognition, as both Hox and retinoid programs are known to contribute to directing motor neurons to the appropriate muscle target (Dasen et al., 2005; Sockanathan et al., 2003). The particular mechanisms by which digit-innervating motor neurons project to distal muscles have yet to be defined. Previous work has identified programs involved in mediating the choice to innervate the ventral versus dorsal limb, but not proximal versus distal, mesenchyme (Kania and Jessell, 2003). The position at which motor neurons are generated also contributes to their axonal trajectories, as preliminary work suggests the coexpression of Hoxc8 and Hoxc9 may not be strictly deterministic of distal innervation patterns (Figure S7E).
The observation that digit motor neurons fail to express Raldh2 complicates our understanding of LMC divisional identity. Traditionally, LMC motor neurons have been defined by the joint expression of Foxp1 and Raldh2, and LMC divisional identity has been defined by the differential expression of Isl1 and Lim1. Digit motor neurons thus represent a distinct category within the LMC in that they express Foxp1 and Isl1, but not Raldh2. Thus, it is unclear whether digit-innervating motor neurons represent a separate division within the LMC.
Our studies have also identified genes expressed in all intrinsic digit motor neurons, posing the question of whether smaller subdivisions within the intrinsic hand and foot motor cohort can be distinguished genetically. Intrinsic hand and foot pools may consist of many smaller pools corresponding to each individual muscle within the autopod. However, it is unclear whether the motor neurons that innervate distinct intrinsic muscles are topographically segregated, as with motor pools that innervate more proximal muscles (Jenny and Inukai, 1983). Moreover, whereas proximal limb motor neurons that innervate the dorsal or ventral limb differentially express the LMC divisional markers Isl1 and Lim1, intrinsic digit innervating motor neurons all express Isl1.
The divergent patterns of gene expression in digit innervating motor neurons also has implications for the coordinate control of digit motor neurons by premotor interneurons. Intrinsic foot motor neurons, unlike more proximal hindlimb motor pools, lack recurrent inhibition from Renshaw interneurons, raising the possibility that they are innervated by a distinct subset of premotor interneurons (Bikoff et al., 2016). If locally produced retinoids are involved in interneuron specification, the finding that digit-innervating motor neurons do not make retinoids could have broader consequences for the establishment of spinal circuitry for fine motor control.
STAR Methods
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to the Lead Contact, Thomas Jessell, tmj1@columbia.edu.
Experimental model and subject details
Scip-LacZ (Bermingham et al., 1996), Hoxc8MNΔ−/− (Olig2::Cre; Hoxc8flox, Catela et al., 2016, Blackburn et al., 2009, Dessaud et al., 2007) and Hoxc9 −/− (Jung et al., 2014, McIntyre et al., 2007) mouse strains have been described. C57BL/6J mice (The Jackson Labs) were used as the wildtype strain in this study. Male and female mice were both used in this study depending on availability, and were maintained using standard husbandry and housing conditions. All experiments were performed in accordance with the National Institutes of Health Guidelines on the Care and Use of Animals and approved by the Columbia University animal care and use committee. Pathogen free fertilized chicken eggs were obtained from Charles River laboratories.
Method details
Motor pool genetic screen
Individual motor pools were labeled by injecting 0.1% CTB-Alexa 555 into individual muscles of p4 mice. At p6, the animals were anaesthetized on ice, then decapitated and eviscerated. In all instances possible, bench spaces and tools were subsequently cleaned with RNAzap (Life Technologies). Ventral laminectomies were quickly performed in cold PBS and the unfixed spinal cords were embedded longitudinally, ventral side down, in OCT and frozen at −80°. The muscles were then examined under epifluorescence for specificity, and spinal cords from non-specific injections were discarded.
Prior to cryosectioning, RNAse free 1.0 PEN MembraneSlides (Zeiss) were UV crosslinked for 30 minutes at optimal power. The cryostat was cleaned with 95% EtOH. Frozen blocks were cryosectioned at 12 μm, with the angle of the block adjusted to section the spinal cord at a perpendicular cutting surface. The MembraneSlides were then placed in a slide box and frozen at −80° for laser capture microdissection. Laser capture microdissection was performed on the PALM Microbeam System (Zeiss). A solution of 300 μl lysis buffer with 2.1 μl BME was prepared for cell lysis. Cells from a set of slides corresponding to a single animal were preserved in 40 μl of the lysis buffer/BME mixture. The surface area captured in um2 was recorded for each set of slides.
Each biological replicate required approximately 1 ng of RNA, corresponding to approximately 170000 um2 of laser captured surface area. For RNA purification, samples from individual animals were pooled to provide at least 250000 um2 of laser captured surface area per biological replicate. Depending on the size of the motor pool, each biological replicate thus contained 30–40 cells from 2–5 animals.
RNA was purified with the Absolutely RNA Nanoprep Kit (Agilent). cDNA was subsequently amplified with the Ovation RNA-Seq System V2 (NuGEN). cDNA libraries were constructed using the Nextera DNA Sample Prep Kit (Illumina) and sequenced on an Illumina HiSeq 2000 to a depth of 25–30 million single-end reads per sample.
In situ hybridization
In situ hybridization was performed on 16 μm cryostat sections as previously described using digoxigenin (DIG)-labeled cRNA probes (Tsuchida et al., 1994). Embryonic tissue from e11.5–e13.5 animals was fixed overnight in 4% PFA/0.1M PB at 4°, washed several times with PBS and cryoprotected overnight in 30% sucrose in 0.1M PB, prior to embedding in OCT and freezing at −80°. Tissue from animals aged e16.5 and older was embedded directly in OCT without fixation. The protocol used in this study for generating and hybridizing in situ probes is available at http://sklad.cumc.columbia.edu/jessell//pdf/DIG_Labelled_In_Situ.pdf.
Anti-sense probes were generated by polymerase chain reaction (PCR) from mouse spinal cord and whole embryo cDNA libraries. cDNA libraries were synthesized using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) from RNA purified using the Absolutely RNA Miniprep Kit (Agilent). Primer design was performed with the assistance of the web based Primer3 program (http://bioinfo.ut.ee/primer3-0.4.0/) with an optimal probe length of 500–600 base pairs. Amplification of the probe was performed with specific primers (Operon), after which PCR products were purified on a gel, subcloned into Topo-TA (Life Sciences) and sequenced. DNA templates were amplified by PCR from the resulting plasmids and used to synthesize digoxegenin (DIG) or fluorescein labeled probes using the appropriate RNA labeling kit (Roche). The primer sequences used for generating probes are listed in Table S1.
Immunohistochemistry
Immunohistochemical labeling of spinal cord tissue was performed on cryostat (12–14 μm) sections as described using fluorophore-conjugated secondary antibodies (Jackson Labs and Invitrogen) (Demireva et al., 2011).
Embryonic tissue was processed for immunohistochemistry by fixing in 4% PFA/0.1M PB for various times depending on age as follows: e11.5– 1 hour, e12.5-e13.5- 1.5 hours, e14.5– 1 hour and 45 minutes, e16.5-p0- 2 hours. At e12.5 and later, ventral laminectomy was performed prior to fixation. After fixation, tissue was washed several times with PBS and cryoprotected overnight in 30% sucrose in 0.1M PB, prior to embedding in OCT and freezing at −80°.
After cryosectioning tissue onto glass slides, immunohistochemistry was performed by overnight incubation of the tissue at 4° in primary antibody diluted in phosphate buffered saline (PBS) with 0.1% trition, 0.1% BSA and 0.01% NaAz. The tissue was then washed 3x in PBS for 5 minutes each and incubated with secondary antibody diluted in PBS for 1 hour at room temperature. Sections were then washed in PBS and mounted in Vectashield (Vector Laboratories). Images were acquired on Zeiss LSM-510 Meta confocal microscopes.
Antibodies used in this study were as follows: goat anti-CTB (1:8000, List Biological Laboratories), rabbit anti-tetramethylrhodamine (1:1000, Invitrogen), rabbit anti-CRABP2 (1:500, Proteintech), guinea pig anti-Foxp1 (1:16000, Dasen et al., 2008), rabbit anti-Foxp1 (1:64000, Dasen et al., 2008), guinea pig anti-Raldh2 (1:16000, Dasen et al., 2008), rabbit anti-Raldh2 (mouse only, 1:8000, Dasen et al., 2008), guinea pig anti-mScip (mouse only, 1:8000), rabbit anti-mScip (mouse only, 1:8000), guinea pig anti-cScip (chicken only, 1:16000, Dasen et al., 2005), chicken anti-βgal (1:1000, Abcam), rabbit anti-pSMAD1/5/8 (Dasen et al., 2008), guinea pig anti-Hoxc9 (1:16000, Jung et al., 2010), mouse anti-Hoxc9 (1:100, Liu et al., 2001), mouse anti-Hoxc8 (1:100, Liu et al., 2001), Alexa 488-conjugated mouse anti-Hoxc8 (1:2000, provided by Jeremy Dasen), guinea pig anti-Isl1 (1:10000, Tanabe et al., 1998), rabbit anti-Isl1 (1:5000, Tsuchida et al., 1994), sheep anti-GFP (1:1000, AbD Serotec), rabbit anti mPea3 (mouse only, 1:4000), rabbit anti-cPea3 (chicken only, 1:6000, Lin et al., 1998), rabbit anti-Caspase (1:500, Cell Signaling) Additional antibodies were generated in guinea pig using the following peptide sequences Fign: KRKAFYMAGQGDMDSSYG (1:8000 dilution, compatible with mouse and chick tissue), Cpne4: KKMSNIYESAANTLGIFNS(1:3000 dilution, compatible with mouse only).
Secondary antibodies were generated in Donkey (Jackson Immunoresarch Laboratories) and conjugated to FITC, Cy3 or Cy5 or Alexa 488 (Invitrogen). Those conjugated to FITC, Cy3 or Alexa 488 were diluted 1:1000 and those conjugated to Cy5 were diluted 1:500. Primary antibodies or dyes coupled to biotin were detected using Streptavidin conjugated to Alexa 488 (Invitrogen), diluted 1:1000.
Retrograde labeling of motor neurons
Motor neurons supplying individual muscles were retrogradely labeled using 0.1% CTB-555, 10% 3000 MW Tetramethylrhodamine-dextran or 10% 3000 MW Dextran-biotin (Molecular probes) in vivo as described (Demireva et al., 2011; Sürmeli et al., 2011). Biotin was detected using Streptavidin-488 (Molecular probes).
P0–p4 mice were anaesthetized on ice and a small incision was performed on the skin to expose the muscle of interest. Dye was loaded into pulled glass microelectrodes by suction and injected into the muscle through application of positive pressure. Dextran injections were performed at multiple sites, whereas CTB injections were performed at a single site. For motor neuron visualization, animals were sacrificed 1–2 days following dye injection.
E14.5 embryos were eviscerated and dissected in ice-cold aCSF (127 mM NaCl, 3 mM KC1, 1.25 mM NaH2P04, 26 mM NaHC03, 10 mM D-glucose, 1 mM MgCl2 and 2 mM CaCl2). The embryos were pinned and the dorsal epidermis was removed. A pulled glass capillary was used to inject 10% Tetramethylrhodamine Dextran or Dextran Biotin into individual muscles. After injection, embryos were incubated in continuously oxygenated circulating aCSF (95/5% CO2/O2) for 4–5 hours at 27°.
Chick limb ablation
Unilateral chick hindlimb ablations were performed as described (Calderó et al., 1998). After covering the shell with packing tape, a window was cut in the shell and the right limb bud was removed using Vannas spring scissors (FST). The window was then resealed with packing tape. Limb bud ablations were performed between stages 18 and 20, and embryos were permitted to develop to stage 28. Only embryos missing the entire leg and pelvic girdle were used for subsequent analysis.
RARE reporter assay
HEK293 cells were seeded at 1 x 105 cells per well and were transfected 24 hours later with a combination of Cignal RARE Reporter luciferase kit (Qiagen) and control or pCHA-VP16RAR constructs using Opti-MEM (Thermo Fisher Scientific) according to the manufacturer’s instructions. 3 wells were used for each experimental condition. Cells were incubated for 24 hours, after which they were solubilized and assayed for firefly and Renilla luciferase activities using the Dual-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions. Firefly luciferase activity was normalized to the transfection efficiency using Renilla luciferase activity.
In ovo electroporation
In ovo electroporation was performed at brachial levels of stage 14–16 embryos as described and analyzed at stage 28 (Dasen et al., 2005). After egg windowing, embryos were visualized by injecting an india ink solution below the vitelline membrane using a pulled glass micropipette, and a DNA solution containing 1% Fast Green was subsequently injected into the neural tube. Embryos were pulsed 3 times at 30 v with an interval of 1 second between pulses.
For misexpression of Hox genes, plasmids were titrated at 100–500 ng/μl pCAGGS vector to maximize electroporation efficiency and generate levels of ectopic protein expression qualitatively similar to endogenous levels using pBluescript-SK as carrier DNA to a total level of 2 ug/μl. Hox misexpression results are representative of >5 experiments in which the electroporation efficiency in MNs was >50%. For expression of the constitutively active retinoic acid receptor, pCIG-VP16RAR was titrated at 1 ug/μl using pBSK as carrier DNA. VP16RAR misexpression results are representative of >15 experiments in which the electroporation efficiency in MNs was >50%.
Quantification and statistical analysis
Statistical analysis
All statistical details of experiments are described in the figure legends. Statistical tests were performed using Sigmaplot.
RNA-seq analysis
Analysis was carried out using ExpressionPlot software (Friedman and Maniatis, 2011). Briefly, reads were aligned to the mouse mm9 genome assembly and annotated using the Ensembl v62 database. Differential expression analysis was performed using DESeq (Anders et al., 2010).
Quantification of protein levels
Average fluorescent intensity was determined on cryostat sections at sub-saturating antibody concentrations. Images were obtained by confocal microscopy using identical laser and gain configurations. Nuclear pixel intensity was determined using Photoshop. Mean pixel intensities for > 50 MN nuclei are shown.
Data and software availability
Motor pool sequencing data and analyses were deposited into the GEO repository under accession number GSE91402.
Supplementary Material
Acknowledgments
We thank the members of the Dasen lab, particularly Myungin Baek, Catarina Catela and Maggie Shin, for useful discussions, technical advice and providing Hox mutant mice. Justin Lee provided the laser-capture microdissection protocol. Sean O’Keeffe performed the RNA-seq data analysis. Kevin Kanning provided the Scip-LacZ mice. Susan Brenner-Morton assisted with antibody production. We thank Franck Polleux and Lynn Landmesser for useful discussions and Richard Axel, Richard Mann, Hynek Wichterle, David Ng, Jay Bikoff, Sara Fenstermacher and Nikolaus Balaskas for comments on the manuscript. We thank Erica Famojure for lab support and Kathy McArthur for administrative assistance. J.S.D. was supported by R01 NS062822. T.M.J. was supported by RO1 NS033245, the Brain Research Foundation, the Harold and Leila Y. Mathers Foundation, and Project A.L.S. and is an HHMI investigator.
Footnotes
Author Contributions
A.I.M. and T.M.J. devised the project, designed experiments and prepared the manuscript. A.I.M. performed all experiments and data analysis. J.S.D provided conceptual guidance throughout the project and provided the Hox mutant lines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anders S, Huber W, Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M, Mortazavi A, et al. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bácskai T, Fu Y, Sengul G, Rusznák Z, Paxinos G, Watson C. Musculotopic organization of the motor neurons supplying forelimb and shoulder girdle muscles in the mouse. Brain Struct Funct. 2013;218:221–238. doi: 10.1007/s00429-012-0396-3. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Scherer SS, O’Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bikoff JB, Gabitto MI, Rivard AF, Drobac E, MacHado TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ, et al. Spinal Inhibitory Interneuron Diversity Delineates Variant Motor Microcircuits. Cell. 2016;165:207–219. doi: 10.1016/j.cell.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn J, Rich M, Ghitani N, Liu JP. Generation of conditional Hoxc8 loss of-function and Hoxc8-->Hoxc9 replacement alleles in mice. Genesis. 2009;47:680–687. doi: 10.1002/dvg.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderó J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo. J Neurosci. 1998;18:356–370. doi: 10.1523/JNEUROSCI.18-01-00356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catela C, Shin MM, Lee DH, Liu JP, Dasen JS. Hox Proteins Coordinate Motor Neuron Differentiation and Connectivity Programs through Ret/Gfrα Genes. Cell Rep. 2016;14:1901–1915. doi: 10.1016/j.celrep.2016.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KL, Hu JK-H, ten Berge D, Fernández-Terán MA, Ros MÁ, Tabin CJ. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science (80-) 2011;332:1083–1086. doi: 10.1126/science.1199499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Elsevier Inc; 2009. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Demireva EY, Shapiro LS, Jessell TM, Zampieri N. Motor neuron position and topographic order imposed by β- and γ-catenin activities. Cell. 2011;147:641–652. doi: 10.1016/j.cell.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- Haase G, Dessaud E, Garcès A, De Bovis B, Birling MC, Filippi P, Schmalbruch H, Arber S, DeLapeyrière O. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Hollyday M, Jacobson RD. Location of motor pools innervating chick wing. J Comp Neurol. 1990;302:575–588. doi: 10.1002/cne.903020313. [DOI] [PubMed] [Google Scholar]
- Jenny AB, Inukai J. Principles of motor organization of the monkey cervical spinal cord. J Neurosci. 1983;3:567–575. doi: 10.1523/JNEUROSCI.03-03-00567.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S-J, Zhuang B, Falco C, Schneider A, Schuster-Gossler K, Gossler A, Sockanathan S. Mesodermal and neuronal retinoids regulate the induction and maintenance of limb innervating spinal motor neurons. Dev Biol. 2006;297:249–261. doi: 10.1016/j.ydbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Johanson Z, Joss J. Fish fingers: digit homologues in sarcopterygian fish fins. Zool Part B. 2007;768:757–768. doi: 10.1002/jez.b.21197. [DOI] [PubMed] [Google Scholar]
- Jung H, Dasen JS. Evolution of Patterning Systems and Circuit Elements for Locomotion. Dev Cell. 2015;32:408–422. doi: 10.1016/j.devcel.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Lacombe J, Mazzoni EO, Liem KF, Grinstein J, Mahony S, Mukhopadhyay D, Gifford DK, Young RA, Anderson KV, et al. Global control of motor neuron topography mediated by the repressive actions of a single hox gene. Neuron. 2010;67:781–796. doi: 10.1016/j.neuron.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Mazzoni EO, Soshnikova N, Hanley O, Venkatesh B, Duboule D, Dasen JS. Evolving Hox Activity Profiles Govern Diversity in Locomotor Systems. Dev Cell. 2014;29:171–187. doi: 10.1016/j.devcel.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Lin JH, Saito T, Anderson DJ, Lance-Jones C, Jessell TM, Arber S. Functionally Related Motor Neuron Pool and Muscle Sensory Afferent Subtypes Defined by Coordinate ETS Gene Expression. Cell. 1998;95:393–407. doi: 10.1016/s0092-8674(00)81770-5. [DOI] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The Localization of Motoneurons Supplying the Hindlimb Muscles of the Mouse. Philos Trans R Soc B Biol Sci. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Rakshit S, Yallowitz AR, Loken L, Jeannotte L, Capecchi MR, Wellik DM. Hox patterning of the vertebrate rib cage. Development. 2007;134:2981–2989. doi: 10.1242/dev.007567. [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Piedra ME, Martínez-A C, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Tanaka M. Evolution of motor innervation to vertebrate fins and limbs. Dev Biol. 2011;355:164–172. doi: 10.1016/j.ydbio.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Murata Y, Tamura M, Aita Y, Fujimura K, Murakami Y, Okabe M, Okada N, Tanaka M. Allometric growth of the trunk leads to the rostral shift of the pelvic fin in teleost fishes. Dev Biol. 2010;347:236–245. doi: 10.1016/j.ydbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Gehrke AR, Lemberg J, Szymaszek J, Shubin NH. Digits and fin rays share common developmental histories. Nature. 2016:1–16. doi: 10.1038/nature19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Petrinovic MM, Duncan CS, Bourikas D, Weinman O, Montani L, Schroeter A, Maerki D, Sommer L, Stoeckli ET, Schwab ME. Neuronal Nogo-A regulates neurite fasciculation, branching and extension in the developing nervous system. Development. 2010;137:2539–2550. doi: 10.1242/dev.048371. [DOI] [PubMed] [Google Scholar]
- Robert B. Bone morphogenetic protein signaling in limb outgrowth and patterning. Dev Growth Differ. 2007;49:455–468. doi: 10.1111/j.1440-169X.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Guzman M, Montero JA, Santesteban E, Gañan Y, Macias D, Hurle JM. Tendon-muscle crosstalk controls muscle bellies morphogenesis, which is mediated by cell death and retinoic acid signaling. Dev Biol. 2007;302:267–280. doi: 10.1016/j.ydbio.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Roselló-Díez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science. 2011;332:1086–1088. doi: 10.1126/science.1199489. [DOI] [PubMed] [Google Scholar]
- Schneider I, Shubin NH. The origin of the tetrapod limb: from expeditions to enhancers. Trends Genet. 2013;29:419–426. doi: 10.1016/j.tig.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Shu RZ, Zhang F, Liu XS, Li CL, Wang L, Tai YL, Wu XL, Yang X, Liao XD, Jin Y, et al. Target deletion of the cytoskeleton-associated protein palladin does not impair neurite outgrowth in mice. PLoS One. 2009:4. doi: 10.1371/journal.pone.0006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40:97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- Straznicky C, Tay D. The localization of motoneuron pools innervating wing muscles in the chick. Anat Embryol (Berl) 1983;166:209–218. doi: 10.1007/BF00305083. [DOI] [PubMed] [Google Scholar]
- Sürmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustaita D, Pouydebat E, Manzano A, Abdala V, Hertel F, Herrel A. Getting a grip on tetrapod grasping: Form, function, and evolution. Biol Rev. 2013;88:380–405. doi: 10.1111/brv.12010. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT. Development of the major pathways for neurite outgrowth in the chick hindlimb. Dev Biol. 1985;109:193–214. doi: 10.1016/0012-1606(85)90360-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Uemura O, Okada Y, Ando H, Guedj M, Higashijima SI, Shimazaki T, Chino N, Okano H, Okamoto H. Comparative functional genomics revealed conservation and diversification of three enhancers of the isl1 gene for motor and sensory neuron-specific expression. Dev Biol. 2005;278:587–606. doi: 10.1016/j.ydbio.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- Vermot J, Schuhbaur B, Le Mouellic H, McCaffery P, Garnier JM, Hentsch D, Brûlet P, Niederreither K, Chambon P, Dollé P, et al. Retinaldehyde dehydrogenase 2 and Hoxc8 are required in the murine brachial spinal cord for the specification of Lim1+ motoneurons and the correct distribution of Islet1+ motoneurons. Development. 2005;132:1611–1621. doi: 10.1242/dev.01718. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Duboule D. The origin of digits: expression patterns versus regulatory mechanisms. Dev Cell. 2010;18:526–532. doi: 10.1016/j.devcel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Noordermeer D, Leleu M, Duboule D. Conservation and Divergence of Regulatory Strategies at Hox Loci and the Origin of Tetrapod Digits. PLoS Biol. 2014;12:e1001773. doi: 10.1371/journal.pbio.1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang G, Scott SA, Capecchi MR. Hoxc10 and Hoxd10 regulate mouse columnar, divisional and motor pool identity of lumbar motoneurons. Development. 2008;135:171–182. doi: 10.1242/dev.009225. [DOI] [PubMed] [Google Scholar]
- Zakany J, Duboule D. The role of Hox genes during vertebrate limb development. Curr Opin Genet Dev. 2007;17:359–366. doi: 10.1016/j.gde.2007.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.