Abstract
We identified primary human monocyte-derived macrophages (MDM) as vulnerable target cells for Zika virus (ZIKV) infection. We demonstrate dramatic effects of hemin, the natural inducer of the heme catabolic enzyme heme oxygenase-1 (HO-1), in the reduction of ZIKV replication in vitro. Both LLC-MK2 monkey kidney cells and primary MDM exhibited hemin-induced HO-1 expression with major reductions of > 90% in ZIKV replication, with little toxicity to infected cells. Silencing expression of HO-1 or its upstream regulatory gene, nuclear factor erythroid-related factor 2 (Nrf2), attenuated hemin-induced suppression of ZIKV infection, suggesting an important role for induction of these intracellular mediators in retarding ZIKV replication. The inverse correlation between hemin-induced HO-1 levels and ZIKV replication provides a potentially useful therapeutic modality based on stimulation of an innate cellular response against Zika virus infection.
Keywords: Zika, Monocytes, Macrophages, Heme oxygenase-1, Nuclear factor erythroid-related factor 2
1. Introduction
Zika virus (ZIKV) is one of several emerging arboviruses (Fauci and Morens, 2016). First identified in 1947 in the Zika Forest of Uganda, ZIKV is a mosquito-borne flavivirus that is related to yellow fever, dengue, West Nile, and Japanese encephalitis viruses (Musso and Gubler, 2016; Petersen et al., 2016). Like other members of the genus Flavivirus, ZIKV consists of a positive single-stranded genomic RNA. Epidemiological studies indicate a wide-spread distribution of ZIKV in the northern half of the African continent, as well as in many countries in Southeast Asia, South America, and elsewhere (Rasmussen and Katze, 2016; Vorou, 2016).
ZIKV is transmitted by infected female Aedes mosquito bites. Although the cellular reservoirs of ZIKV are not yet fully defined, body fluids contain the virus; therefore, it can be transmitted by sexual contacts and potentially also by blood transfusion (Zika and Blood Transfusion). ZIKV is neuropathogenic. Among many adverse health consequences related to ZIKV infection are neurological disorders and developmental defects in the fetuses of ZIKV-infected mothers, constituting an enormous global public health concern (Waddell and Greig, 2016; Panchaud et al., 2016).
Currently, no vaccines or antiviral drugs are available to prevent or treat ZIKV infection. Therefore, development of medical countermeasures, such as new antiviral drugs and other preventive or therapeutic strategies are of the highest priority. Because both innate and subsequent adaptive immune functions are pivotal for protection against viral infections, we hypothesized that activation of a safe and effective host innate response, with an understanding of its mechanisms, may present a logical approach for protection against ZIKV infection.
We and others have previously demonstrated that stimulation of HO-1, an endogenous cytoprotective enzyme, promotes effective cellular resistance against numerous pathogens (Devadas and Dhawan, 2006; Pamplona et al., 2007; Protzer et al., 2007; Seixas et al., 2009; Hou et al., 2009; Devadas et al., 2010; Schmidt et al., 2012; Zhou et al., 2013a, 2013b; Dhawan et al., 2013; Siegert and Holt, 2008; Hill-Batorski et al., 2013). The present study supports the notion of a pivotal role for inducible HO-1 by its natural physiological substrate hemin as a new cellular protection mechanism against ZIKV infection. Our study, to our knowledge, is the first demonstration and strong rationale for use of HO-1 induction as a novel modality for developing new therapeutic strategies for the treatment of ZIKV infection. Reducing Zika virus replication by inducing HO-1 early in pathogenesis could allow patients time to develop adaptive immunity for long-term protection.
2. Materials and methods
2.1. Reagents
The Zika MR766 strain was kindly provided by Dr. Barbara Johnson, Centers for Disease Control and Prevention, Fort Collins, CO. LLC-MK2 cells and C6/36 cells were purchased from the American Type Culture Collection, Manassas, VA. SYBR Green RT-PCR MasterMix was obtained from Qiagen (Valencia, CA). The FDA-approved drug Panhematin®, containing hemin as the active component, was purchased from Lundbeck, Deerfield, IL (manufactured by APP Pharmaceuticals, Raleigh, NC). Mosquito cells (C6/36) were used for propagation of the MR766 ZIKV strain; virus stocks were titered on LLC-MK2 cells by neutral red staining. Small interfering RNA (siRNA) targeting human Nrf2 was purchased from Santa Cruz Biotechnology (Catalog number sc-37030, Santa Cruz, CA). siRNAs Hs_HMOX1_1 (Catalog number SI00033089) and Hs_HMOX1_10 (Catalog number SI04435354) targeting human HO-1-coding sequences (NM_002133) and AllStars Negative Control siRNA (Catalog number 1027280) were from Qiagen. All other reagents were of reagent grade.
2.2. Isolation, culture, and infection of cultured monocytes
Human monocytes were isolated from peripheral blood mononuclear cells of donors seronegative for HIV-1 and hepatitis B after leukopheresis and were purified by countercurrent centrifugal elutriation (Wahl et al., 1984). Cell suspensions contained > 95% monocytes based on cell morphology in Wright-stained cytosmears, granular peroxidase, and nonspecific esterase. The cells were cultured for 5 days in DMEM supplemented with 10% FBS, 20 μg/ml gentamicin, and 1000 U/ml M-CSF. After 5 days, the cells were then inoculated with the Zika virus MR766 strain diluted in culture medium to the desired MOI for 2 h at 37 °C with gentle agitation every 15–20 min. After this incubation, the virus solution was removed, and the cells were washed twice with medium. Culture medium was added to each well, and the cells were incubated at 37 °C in a 95% air/5% CO2 incubator.
2.3. RNA extraction from cells
Total RNA was extracted from MDM or LLC-MK2 cells using a Qiagen RNA isolation kit (RNeasy, Qiagen, Valencia, CA) according to the manufacturer's protocol. The RNA pellet was resuspended in 30 μl of RNAse-free distilled water and stored at 80 °C. The quality of RNA was assessed by Agilent Bioanalyzer (Santa Clara, CA).
2.4. Real-time reverse-transcription polymerase chain (RT-PCR) reaction
Real-time RT-PCR amplification of viral RNA was performed by quantitative PCR. Each reaction of 25 μl contained 2.5 μl of total RNA as a template, 0.5 μM of forward primer (TGAAGTTCTCACAGCCGTTG), 0.5 μM of reverse primer (CCGAGACCACATAGCTGACA), and SYBR Green RT-PCR Master Mix (Qiagen). Amplification was performed in an Applied Biosystems 7500 real-time PCR or Applied Biosystems QuantStudio 6 Flex system with reverse transcription at 50 °C for 30 min, 95 °C for 15 min, followed by 40 amplification cycles of 95 °C for 15 s, 53 °C for 30 s, and 72 °C for 30 s. Viral copies were quantified by comparing the amplification cycle threshold (Ct) values with standard curves generated from the live Zika MR766 virus.
2.5. siRNA transfections
LLC-MK2 cells were seeded in 6-well culture plates for transfection, and incubated with 50 nM control or Nrf2 siRNA for 6 h in serum-free OPTI-MEM media, following the manufacturer's transfection instructions using Lipofectamine® RNAiMAX (Invitrogen, Carlsbad, CA; reference (Hill-Batorski et al., 2013)). Cells were then treated with 100 μM hemin 24 h prior to infection with Zika virus at MOI 0.01. Efficiency of HO-1 knockdown was assessed by Western blot analysis using specific HO-1 monoclonal antibody (Enzo Life Sciences, Farmingdale, NY) with rabbit anti-actin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) as the control. Virus infection was quantified by real-time RT-PCR.
2.6. Statistics
The statistical values were calculated by one-way ANOVA with Dunnett's multiple comparison post-test.
3. Results and discussion
We identified primary human macrophages as vulnerable targets of ZIKV infection, and examined how modulating HO-1 can disrupt pathogenesis through host defense against Zika virus infection of macrophages and other cells. Our discovery of an inducible Nrf2-dependent HO-1 regulatory pathway inhibiting ZIKV suggests a mechanism for enhancing innate host defense against Zika virus infection.
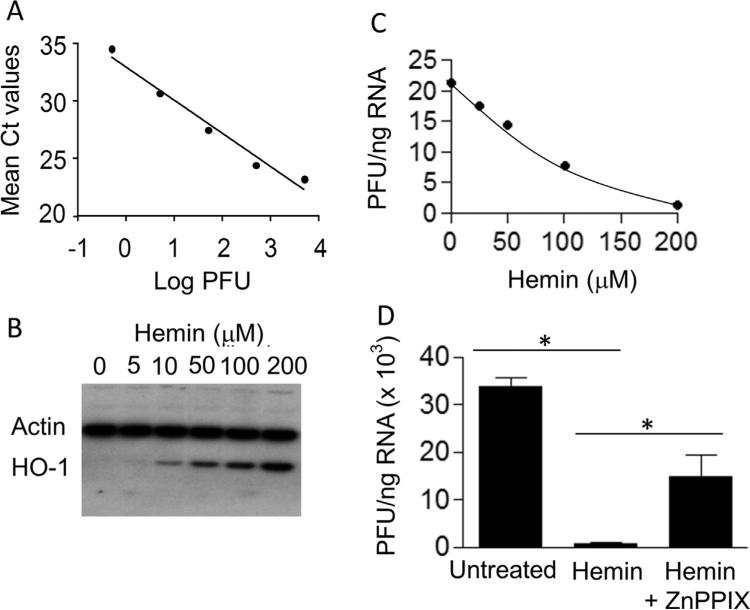
To quantify ZIKV by real-time RT-PCR, we first generated a standard curve by amplifying serial ten-fold dilutions of a Zika virus stock with a known infectious titer of 2×107 plaque-forming units (pfu)/ml without pre-extracting the RNA. Fig. 1A shows excellent linearity (R2 > 0.99) between the amplification threshold values (Ct) and varying pfu over a broad dynamic range. We used this standard curve to convert the Ct values of untitered samples into pfu equivalent units (PFU).
Fig. 1.
(A) Real-time RT-PCR amplification of infectious Zika virus. Ct values were determined by direct RT-PCR amplification of live Zika virus with a known titer of 2×107 plaque-forming units (pfu)/ml without RNA pre-extraction. The data are representative of two independent experiments demonstrating direct correlation between the Ct values observed at the indicated viral pfu. (B) Western blot analysis of hemin-treated MDM. Cells were incubated with the indicated concentrations of hemin, cellular proteins were separated on an SDS-polyacrylamide gel, transferred to PVDF nitrocellulose membrane, and probed simultaneously with HO-1 and actin antibodies. (C) Hemin treatment inhibits Zika virus infection of MDM. Cells were infected in the absence or presence of the indicated concentrations of hemin as described in Materials and Methods. These representative data demonstrate suppression of Zika virus replication in MDM treated with various concentrations of hemin. (D) HO-1 inhibitor ZnPPIX attenuates hemin-induced suppression of Zika virus replication in MDM. Cells were incubated with 10 μM ZnPPIX, a competitive inhibitor of HO-1 enzymatic activity, for 2 h before treatment with 25 μM hemin, and subsequently infected with Zika virus. Total RNA was isolated and quantified for virus replication by real-time RT-PCR 24 h after infection. *p < 0.05.
We used an FDA-approved pharmaceutical formulation of hemin to induce the endogenous host protective factor, HO-1. To establish that hemin increases HO-1 expression, MDM were treated for 24 h in the presence of various concentrations of hemin and examined for HO-1 induction by Western blot analysis. Blots were simultaneously probed with human anti-mouse HO-1 mAb and goat-anti-human actin polyclonal antibody. As expected from its established mode of action, hemin treatment induced HO-1 protein expression in a dose-dependent manner without altering the housekeeping protein actin (Fig. 1, panel B).
We then tested our hypothesis whether or not induced HO-1 could elicit innate cellular protection against Zika virus infection. Using Zika-specific primers, we performed real-time RT-PCR amplification of the total RNA from ZIKV-infected MDM inoculated at a multiplicity of infection (MOI) of 0.1 in the presence of the various concentrations of hemin for 2 h and then cultured for 24 h in the presence of hemin. The HO-1 induction was accompanied by dramatically reduced Zika infection, with > 90% inhibition of the Zika virus at the highest dose of hemin (Fig. 1C). To evaluate whether inhibition of HO-1 activity would attenuate suppression of Zika virus replication, MDM were incubated with 10 μM ZnPPIX, a competitive inhibitor of HO-1 enzymatic activity, for 2 h before treatment with 25 μM hemin, and subsequently infected with Zika virus. The results shown in Fig. 1D reveal that pretreatment with ZnPPIX significantly attenuated the effect of hemin-induced inhibition of Zika virus replication, indicating that functional activity of HO-1 was involved in the inhibitory effect of hemin on viral replication. Although the exact mechanism of action for inhibition of Zika virus infection is not known, the induction of HO-1, by virtue of its anti-inflammatory and anti-apoptotic properties, together with the production of biliverdin, bilirubin and CO, could likely function as a potent inhibitor of virus replication.
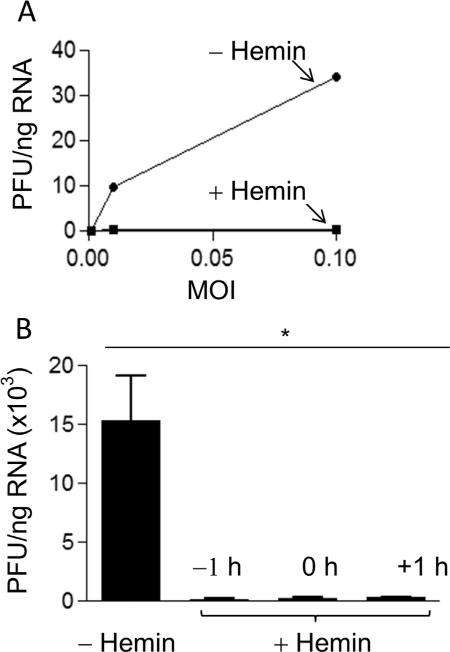
The ability of hemin to suppress virus replication in MDM infected with ZIKV at various MOI in the absence or presence of 100 μM hemin was examined. Total RNA was isolated and the level of infection was quantified after 48 h as described above. As shown in Fig. 2A, hemin treatment markedly inhibited Zika virus replication as compared with untreated cells. To address a key question whether the observed hemin-induced inhibition of viral replication was a result of blocking virus entry or a post-viral entry event, we treated MDM with hemin 1 h before infection, at the time of infection, and 1 h after infection with Zika virus. Total cellular RNA was extracted 48 h post-infection, and the level of Zika RNA was quantified by real-time RT-PCR. The results shown in Fig. 2B show that 1 h pretreatment of MDM with hemin substantially decreased the level of viral replication. In addition, hemin treatment inhibited Zika virus replication in MDM when added at the time of infection or even 1 h post-inoculation, providing evidence for the involvement of intracellular host factors even after initial infection.
Fig. 2.
Inhibition of Zika virus infection of MDM by hemin at various MOI. (A) MDM were infected with Zika virus at MOI 0.01 or MOI 0.1 in the absence or presence of 100 μM hemin, and total RNA was isolated and quantified for virus replication by real-time RT-PCR 48 h after infection. (B) MDM were treated with 100 μM hemin either 1 h before infection, at the time of infection, or 1 h after infection with Zika. Virus replication was determined by RT real-time PCR 48 h after infection. Data are presented as mean ±SEM; *p < 0.05.
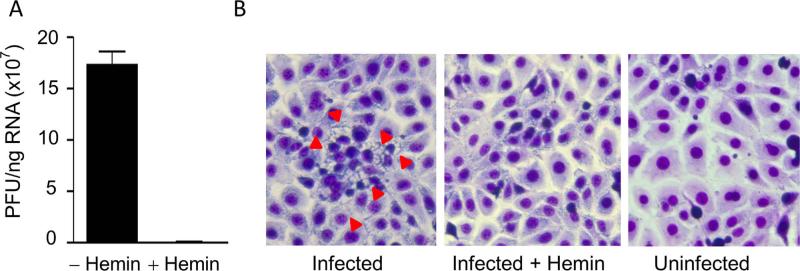
We next asked if the virus produced by Zika-infected macrophages was infectious. We inoculated LLC-MK2 cells with the culture medium from infected MDM in the absence or presence of 100 μM hemin for 2 h and then replaced the medium without hemin. On day 2, total RNA was isolated and viral RNA was quantified by real-time RT-PCR. The results shown in Fig. 3A show that: (a) LLC-MK2 cells were susceptible to Zika virus infection and confirm that the virus produced by Zika-infected macrophages was infectious; and (b) hemin treatment inhibited Zika infection of LLC-MK2 cells. Consistent with these results, LLC-MK2 cells exhibited cytopathic effects as evidenced by vacuoles in the cell cytoplasm shown by the red arrowheads in Fig. 3B when examined on day 7 after infection (left panel). The number of vacuoles was dramatically reduced in cells cultured in the presence of hemin (Fig. 3B, middle panel). The morphology of uninfected LLC-MK2 cells is shown in Fig. 3B (right panel).
Fig. 3.
(A) Hemin treatment inhibits Zika virus replication in LLC-MK2 cells. (A) LLC-MK2 cells were incubated with culture supernatant from Zika-infected macrophages in the absence or presence of 100 μM hemin for 2 days. Virus infectivity was determined by real-time RT-PCR amplification of total cellular RNA using Zika-specific primers. (B) Hemin treatment reduces cytopathic effects in Zika-infected LLC-MK2 cells. LLC-MK2 cells incubated in the absence or presence of 100 μM hemin with culture supernatant from Zika-infected macrophages. Cells were Wright-stained and microscopically examined for Zika-induced cytopathic effects 7 days after infection. Zika-induced cytopathic effects are shown by red arrowheads.
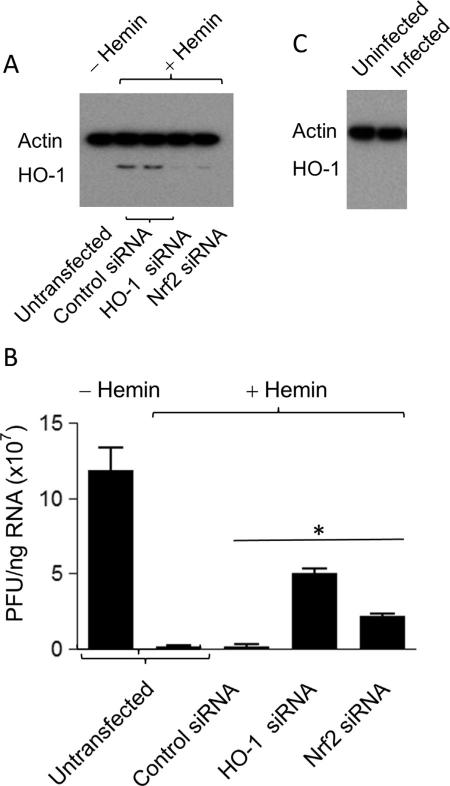
To explore the mechanism of hemin induction of HO-1 in protection against Zika virus infection, we tested whether the putative HO-1-mediated protective mechanism involved Nrf2, which is one of several important nuclear factors that transcriptionally activate HO-1 expression (Balogun et al., 2003; Lee et al., 2012; Chen et al., 2013; Kang et al., 2014; Joo Choi et al., 2014). We transfected LLC-MK2 cells with siRNAs specific for Nrf2 or HO-1 and examined HO-1 protein expression by Western blot analysis. Consistent with previously published reports (references (Balogun et al., 2003; Lee et al., 2012; Chen et al., 2013; Kang et al., 2014; Joo Choi et al., 2014)), silencing of either HO-1 or its upstream regulator Nrf2 using siRNAs revealed substantially reduced HO-1 induction, confirming that hemin-mediated upregulation of HO-1 requires Nrf2 (Fig. 4A). Furthermore, transfection with HO-1 and Nrf2 siRNAs strongly diminished the protective effect of hemin against Zika infection (Fig. 4B). These results establish that: (a) the expression of HO-1 protein is important for the inhibitory effect on Zika virus replication; and (b) reducing HO-1 expression correlates with increased Zika replication in Nrf2 siRNA-transfected cells, suggesting Nrf2 as an upstream mediator of hemin-induced HO-1 host protective effects. Hemin induction of HO-1 through its upstream regulatory gene, Nrf2, may provide an unconventional therapeutic approach for stimulating an innate cellular response against ZIKV infection. Although HO-1 induction can be expected in response to various environmental and cellular oxidative stresses, we did not observe increased HO-1 expression in Zika-infected LLC-MK2 cells (Fig. 4C).
Fig. 4.
Nrf2-mediated regulation of HO-1 expression and Zika virus infection. (A) LLC-MK2 cells were transfected with control siRNA, Nrf2-specific siRNA or HO-1-specific siRNA, and hemin-induced HO-1 expression was examined by Western blot analysis using actin as the housekeeping gene control. (B) Zika virus replication in LLC-MK2 cells transfected with control siRNA, HO-1-specific siRNA, or Nrf2-specific siRNA, respectively, as described in Materials and Methods. untransfected and transfected cells were infected in the absence or presence of 100 μM hemin for 48 h, and examined for Zika RNA by real-time RT-PCR. Representative data are presented as mean±SEM from two experiments. P < 0.05. (C) Western blot analysis in total protein isolated from uninfected and Zika-infected LLC-MK2 cells using HO-1 and actin antibodies.
Our findings are the first, to our knowledge, demonstrating a pivotal role for induction of HO-1 by its natural physiological substrate hemin as a mediator of cellular protection against ZIKV infection. We propose that induction of nascent cellular defense responses may provide a novel alternative or concurrent therapeutic strategy against Zika virus infection, especially since the inducer – hemin – is already FDA-approved for another medical condition. Our study presents a rationale for exploring HO-1 induction as a novel modality for developing new therapeutic strategies for the treatment of ZIKV infection.
Acknowledgments
We thank Dr. Rana Nagarkatti and Dr. Sreenivas Gannavaram for critical review of the manuscript. The findings and conclusions in this paper have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy. This work was supported by FDA and NIDCR Intramural Research Programs.
Footnotes
Authorship
S.D., conceptualized, designed, performed, analyzed experiments and wrote the paper; H.H., B.F., and K.T. performed and analyzed experiments; K.M.Y., analyzed experiments. All authors declare no conflict of interest.
References
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Wang SY, Chiu CC, Tseng CK, Lin CK, Wang HC, Lee JC. Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction. Antimicrob. Agents Chemother. 2013;57:1180–1191. doi: 10.1128/AAC.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J. Immunol. 2006;176:4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- Devadas K, Hewlett IK, Dhawan S. Lipopolysaccharide suppresses HIV-1 replication in human monocytes by protein kinase C-dependent heme oxygenase-1 induction. J. Leukoc. Biol. 2010;87:915–924. doi: 10.1189/jlb.0307172. [DOI] [PubMed] [Google Scholar]
- Dhawan S, Debrabant A, Yamada KM. Therapeutic potential of endogenous heme oxygenase-1 activation in pathogenic infections. Curr. Trends Immunol. 2013;14:65–70. [Google Scholar]
- Fauci AS, Morens DM. Zika virus in the Americas – yet another arbovirus threat. N. Engl. J. Med. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- Hill-Batorski L, Halfmann P, Neumann G, Kawaoka Y. The cytoprotective enzyme heme oxygenase-1 suppresses Ebola virus replication. J. Virol. 2013;87:13795–13802. doi: 10.1128/JVI.02422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou WH, Rossi L, Shan Y, Zheng JY, Lambrecht RW, Bonkovsky HL. Iron increases HMOX1 and decreases hepatitis C viral expression in HCV-expressing cellsWorld J. Gastroenterol. 2009;15:4499–4510. doi: 10.3748/wjg.15.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo Choi R, Cheng MS, Shik Kim Y. Desoxyrhapontigenin up-regulates Nrf2-mediated heme oxygenase-1 expression in macrophages and inflammatory lung injury. Redox Biol. 2014;2:504–512. doi: 10.1016/j.redox.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Han MH, Kim GY, Kim CM, Kim BW, Hwang HJ, Hyun Y. Nrf2-mediated HO-1 induction contributes to antioxidant capacity of a Schisandrae Fructus ethanol extract in C2C12 myoblasts. Nutrients. 2014;6:5667–5678. doi: 10.3390/nu6125667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Han YM, Kim EH, Kim YJ, Hahm KB. A possible involvement of Nrf2-mediated heme oxygenase-1 up-regulation in protective effect of the proton pump inhibitor pantoprazole against indomethacin-induced gastric damage in rats. BMC Gastroenterol. 2012;12:143. doi: 10.1186/1471-230X-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, Gubler DJ. Zika virus. Clin. Microbiol Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, Baud D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clin. Microbiol Rev. 2016;29:659–694. doi: 10.1128/CMR.00014-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N. Engl. J. Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- Protzer U, Seyfried S, Quasdorff M, Sass G, Svorcova M, Webb D, Bohne F, Hosel M, Schirmacher P, Tiegs G. Antiviral activity and hepatoprotection by heme oxygenase-1 in hepatitis B virus infection. Gastroenterology. 2007;133:1156–1165. doi: 10.1053/j.gastro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Rasmussen AL, Katze MG. Genomic signatures of emerging viruses: a new era of systems epidemiology. Cell Host Microbe. 2016;19:611–618. doi: 10.1016/j.chom.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WN, Mathahs MM, Zhu Z. Heme and HO-1 inhibition of HCV, HBV, and HIV. Front. Pharmcol. 2012;3:129. doi: 10.3389/fphar.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, Rebelo S, Penido C, Smith NR, Coutinho A, Soares MP. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc. Natl. Acad. Sci. USA. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert SW, Holt RJ. Physicochemical properties, pharmacokinetics, and pharmacodynamics of intravenous hematin: a literature review. Adv. Ther. 2008;25:842–857. doi: 10.1007/s12325-008-0094-y. [DOI] [PubMed] [Google Scholar]
- Vorou R. Zika virus, vectors, reservoirs, amplifying hosts, and their potential to spread worldwide: what we know and what we should investigate urgently. Int. J. Infect. Dis. 2016;48:85–90. doi: 10.1016/j.ijid.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Waddell LA, Greig JD. Scoping review of the Zika virus literature. PLoS One. 2016;11:e0156376. doi: 10.1371/journal.pone.0156376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl LM, Katona IM, Wilder RL, Winter CC, Haraoui B, Scher I, Wahl SM. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984;85:373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Zhou ZH, Kumari N, Catalano J, Nekhai S, Wise J, Yamada KM, Dhawan S. Heme oxygenase-1-mediated host cell response inhibits the susceptibility of prostate cancer cells to retroviral infection and retards their proliferation. Curr. Trends Immunol. 2013a;14:53–56. [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Kumari N, Nekhai S, Clouse KA, Wahl LM, Yamada KM, Dhawan S. Heme oxygenase-1 induction alters chemokine regulation and ameliorates human immunodeficiency virus-type-1 infection in lipopolysaccharide-stimulated macrophages. Biochem Biophys. Res Commun. 2013b;435:373–377. doi: 10.1016/j.bbrc.2013.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zika and Blood Transfusion. 〈 http://www.cdc.gov/zika/transmission/blood-transfusion.html 〉 .