Abstract
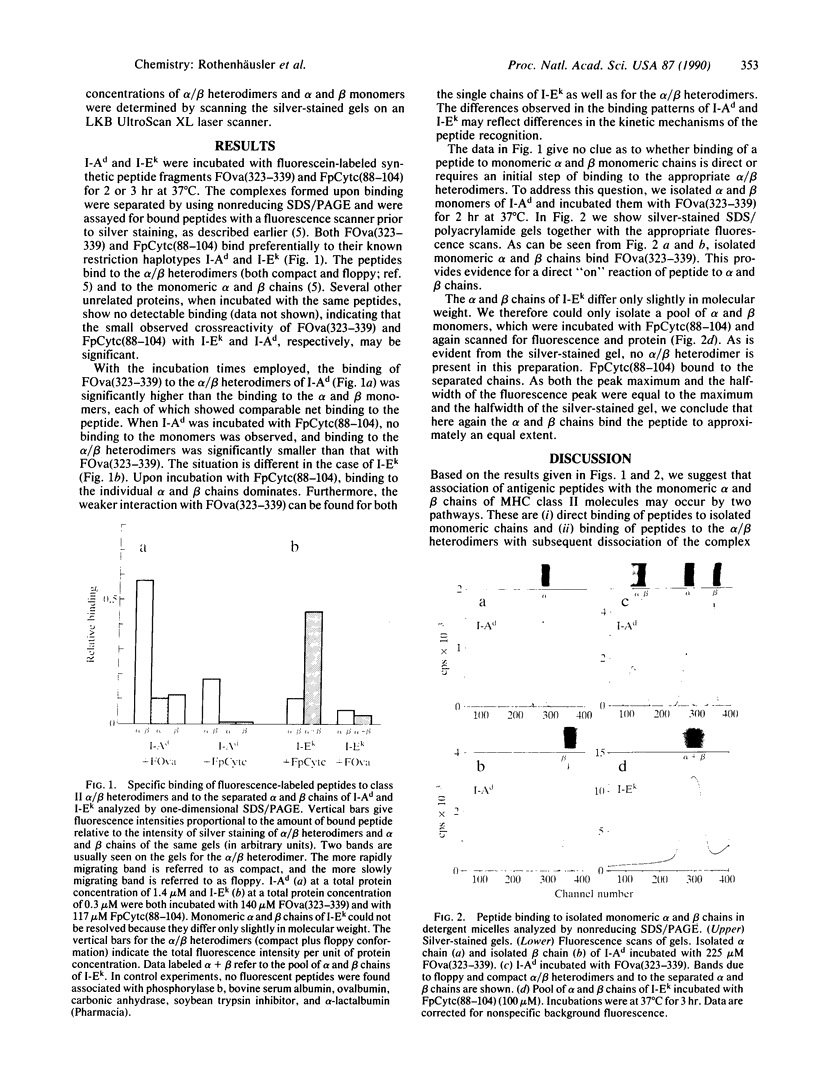
Class II molecules of the major histocompatibility complex bind antigenic peptides and present them to T-helper cells. Class II molecules are heterodimers consisting of one alpha and one beta chain. Here we report that each isolated alpha and beta chain binds antigenic peptides and that this binding is specific. The specificity of peptide binding was investigated by employing the murine major histocompatibility complex haplotypes I-Ad and I-Ek and fluorescence-labeled peptides of chicken ovalbumin and pigeon cytochrome c, respectively, which are known to be specific for these haplotypes. The major histocompatibility complex molecules were incubated with these peptides and subjected to SDS/PAGE under nondenaturing conditions. The gels were then scanned for the fluorescent peptides and, after silver staining, for proteins. We found that the fluorescence-labeled peptide fragment of ovalbumin bound preferentially to the isolated alpha and beta chains of I-Ad, whereas the fluorescence-labeled peptide fragment of pigeon cytochrome c bound preferentially to the isolated alpha and beta chains of I-Ek. The alpha and beta chains of each haplotype bound their specific peptides about equally well, suggesting comparable affinities. Our results indicate that in vivo the kinetic pathway for the formation of antigenic peptide complexes with the alpha/beta heterodimers may involve the initial formation of complexes of the alpha and/or beta chains with the specific antigenic peptides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Colon S., Smith C., Freed J. H., Miles C., Grey H. M. Interaction between a "processed" ovalbumin peptide and Ia molecules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Gorga J. C., Horejsí V., Johnson D. R., Raghupathy R., Strominger J. L. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987 Nov 25;262(33):16087–16094. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989 Jan 19;337(6204):274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., McConnell H. M. High-affinity fluorescent peptide binding to I-Ad in lipid membranes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9660–9664. doi: 10.1073/pnas.83.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]