Abstract
Tartrazine is a synthetic organic azo dye widely used in food and pharmaceutical products. The current study aimed to evaluate the possible adverse effect of this coloring food additive on renal and hepatic structures and functions. Also, the genotoxic potential of tartrazine on white blood cells was investigated using comet assay. Twenty adult male Wistar rats were grouped into two groups of 10 each, control- and tartrazine-treated groups. The control group was administered orally with water alone. The experimental group was administered orally with tartrazine (7.5 mg/kg, b.wt.). Our results showed a marked increase in the levels of ALT, AST, ALP, urea, uric acid, creatinine, MDA and NO, and a decreased level of total antioxidants in the serum of rats dosed with tartrazine compared to controls. On the other hand, administration of tartrazine was associated with severe histopathological and cellular alterations of rat liver and kidney tissues and induced DNA damage in leucocytes as detected by comet assay. Taken together, the results showed that tartrazine intake may lead to adverse health effects.
Keywords: Tartrazine, Serum biochemistry, Histopathology, Ultrastructure, Genotoxicity
Introduction
Tartrazine (E number E102) a synthetic azo dye with lemon yellow color, is a commonly used food colorant for food products that we eat almost every day (Mittal, Kurup & Mittal, 2007). Among foods containing tatrazine are soft drinks and sport drinks, flavored chips, sauces, ice creams, jams, jellies and chewing gums (Walton et al., 1999). Tartrazine is found in many non-food consumables such as soaps, cosmetics, shampoos, vitamins and certain prescription medications (Amin, Abdel Hameid & AbdElsttar, 2010). Moreover, it is used in many developing countries as a low cost alternative for saffron in cooking (Mehedi et al., 2009).
Tartrazine toxicity results directly or indirectly from the metabolic reductive biotransformation of the azo linkage (Chequer, Dorta & De Oliveira, 2011). For example, tartrazine can undergo metabolic reduction in the intestine of the animal by the intestinal microflora, thus resulting in formation of two metabolites, sulfanilic acid and aminopyrazolone (Chung, Stevens & Cerniglia, 1992). These metabolites of tartrazine can generate reactive oxygen species (ROS), generating oxidative stress, and affect hepatic and renal architectures and biochemical profiles (Himri et al., 2011).
Several in vivo studies have been administered tartrazine in different doses and revealed neither cytotoxic changes in tissues and organs nor development of neoplastic alteration in experimental animals (Rus et al., 2010).
Since literature data regarding the toxicity of tartrazine are contradictory, the present work aims to evaluate the toxic potential of tartrazine on livers and kidneys of male albino rats. In addition, this study was extended to evaluate the effect of this food colorant on the biomarkers of oxidative stress and to examine its genotoxic effect on white blood cells using comet assay.
Materials and Methods
Twenty adult male Wistar albino rats of 146–153 g body weight were used in this study. They were kept under observation for about one week before the beginning of the experiment to exclude any underlying infection and to be allowed to acclimatize.
The animals were housed in cages and were maintained under controlled conditions of temperature (24 ± 2 °C) and light (12:12 h light:dark cycle). They received a standard basal diet (fat 5%, carbohydrates 65%, protein 20.3%, fiber 5%, salt mixture 3.7%, vitamins mixture 1%) and water ad libitum. The procedures of this experiment are compatible with the guide for care and use of laboratory animal approved by IACUC of Menoufia University, Egypt, Approval No:MNSP155.
Tartrazine (CAS 1934-21-0, Purity 86.7%) was purchased from Sigma Aldrich (Germany).
Experimental design
The animals were randomly divided into two groups of 10 rats each.
Group 1: Animals were orally given distilled water 1 ml/kg b.wt. for 30 days and served as controls.
Group 2: Animals were orally given tartrazine 7.5 mg/kg b.wt.(dissolved in 1 ml of distilled water) daily for 30 days (Himri et al., 2011).
After 30 days of treatment, animals from control and treated groups were fasted overnight, and sacrificed under anesthesia. Blood samples were drawn from dorsal aorta into dry glass centrifuge tubes and left to clot, then centrifuged at 3,500 rpm for 15 min in a Beckman Model T-6 refrigerated centrifuge. Serum was separated and used for biochemical analysis.
Other blood samples were taken to isolate leucocytes for comet assay.
For histological and ultrastructure studies, small pieces of liver and kidney were quickly removed and fixed in an appropriate fixative.
Biochemical analysis
For biochemical analysis, serum was rapidly separated by centrifuging the clotted blood at 3,000 g for 10 min in a Beckman Model T-6 refrigerated centrifuge and collected into new clean and dry tubes. Sera were stored at −20 °C until assayed for the biochemical parameters. Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) were measured colorimetrically according to Steven (1996), while Alkaline phosphatase (ALP) activity was determined according to the method described by Mathieu et al. (1977).
Creatinine, urea and uric acid were estimated using the methods of Henry (1974), Patton & Crouch (1977) and Caraway (1955), respectively.
Malondialdehyde (MDA), Nitric oxide (NO) and total antioxidant capacity (TAC) were estimated by the methods of Ohkawa, Ohishi & Yagi (1979), Cortas & Wakid (1990) and Koracevic et al. (2001), respectively. They were determined using Bio-diagnostic assay kits according to the manufacturer’s instructions (Giza, Egypt).
Histological and ultrastructural study
After fixation of liver and kidney samples in Bouin’s solution, they were dehydrated, cleared, embedded in paraffin wax and then prepared for histological examination using hematoxylin and eosin stain (Bancroft & Gamble, 2002).
For ultrastructural examination, small pieces of liver and kidney were immediately fixed in 4F1G in a phosphate buffer (pH 7.2) for 3 h at 4 °C, then post-fixed in 2% OsO4 in the same buffer at 4 °C for 1–2 h. The specimens were dehydrated through a graded series of ethanol, embedded in epon-araldite mixture and polymerized at 60 °C. Ultrathin sections (50 nm) from selected areas were cut with glass knives on an LKB ultra microtome, double stained with uranyl acetate and lead citrate and examined using a JEOL 100CX electron microscope.
Genotoxicity study
Isolation of leucocytes for comet assay
In order to isolate leucocytes, 4 ml of fresh blood was collected in tubes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. Blood samples were centrifuged at 10,000 rpm for 5 min. The white layer of leukocytes (buffy coat) was transferred to a new 2.0 ml microcentrifuge tube containing 2.0 ml of cold freezing mixtures (RPMI 1640 with 10% DMSO). Aliquots of 250 µl in 1.5 ml microcentrifuge tubes were stored in a −80 °C freezer until comet assay could be completed.
Comet assay
Alkaline Comet assay was performed according to Collins (2004), with some modifications. The comets were visualized by a fluorescence microscope (Zeiss Axioplan 2) equipped with an Olympus C5050 camera. About 30 comets were scored and three different samples for each group were examined. Percentage of DNA in the tail was determined by the Open Comet software (Gyori et al., 2014).
Statistical Analysis
The data of biochemical analysis and comet assay was expressed as mean ± SD of 3–5 replicates and were analyzed by one way ANOVA followed by student’s t-test. The results are expressed as mean ± SD for three independent replicates. The difference was considered statistically significant when p < 0.05.
Results
Biochemical results
Table 1 shows that treatment with tartrazine resulted in a significant (p < 0.05) increase in the activity of plasma aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (AlP) compared to control. Also, our results showed a significant (p < 0.05) increase in plasma uric acid, urea and creatinine levels in tartrazine-treated animals. Additionally, rats exhibited a significant (p < 0.05) increase in plasma lipid peroxidation and nitric oxide (NO), and a significant decrease in the total of antioxidants after treatment with tartrazine.
Table 1. Effect of tartrazine on serum biochemistry and oxidative biomarkers in male Wistar rats.
| Group parameter | Control group | Tartrazine-treated group | p value |
|---|---|---|---|
| Aspartate transaminase (U/ml) | 48.40 ± 4.26 | 128.40 ± 18.97 | 0.003* |
| Alanine transaminase (U/ml) | 29.40 ± 4.07 | 71.20 ± 7.75 | 0.001* |
| Alkaline phosphatase (U/l) | 47.0 ± 5.18 | 89.40 ± 6.66 | 0.001* |
| Creatinine (mg/dl) | 0.36 ± 0.07 | 0.72 ± 0.06 | 0.004* |
| Urea (mg/dl) | 38.20 ± 1.77 | 46.0 ± 2.12 | 0.02* |
| Uric acid (mg/dl) | 0.59 ± 0.07 | 0.92 ± 0.07 | 0.009* |
| Malondialdehyde(nM/ml) | 17.33 ± 2.03 | 46.67 ± 5.61 | 0.008* |
| Nitric oxide (µM/l) | 16.67 ± 2.85 | 41.0 ± 3.79 | 0.007* |
| Total antioxidants (mM/l) | 66.67 ± 4.63 | 28.33 ± 4.06 | 0.003* |
Notes.
Data represented as mean ± SD.
p: p value for F test (ANOVA) and significant between control and treated groups using Post Hoc Test (LSD).
Light and electron microscopic results
Light microscopic observations showed distortion of hepatic architecture in liver sections of tartrazine-treated rats as compared to controls (Figs. 1A–1C). Most of hepatocytes appeared with necrotic nuclei and cytoplasmic vacuolization. Some cells had irregular-shaped nuclei, while others were devoid of nuclei (Fig. 1C). Moreover, the blood sinusoids revealed dilatation and congestion as well as white blood cell infiltration and Kupffer cells activation (Figs. 1B and 1C).
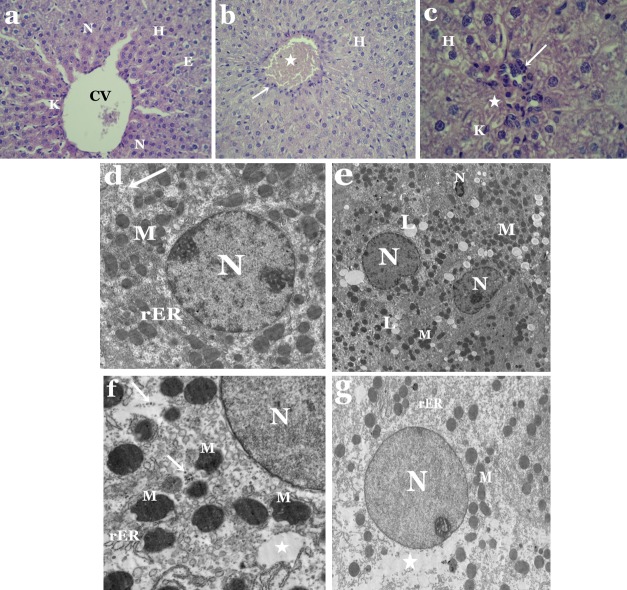
Figure 1.
(A–C) Light micrographs of liver sections from control and tartrazine-treated rats stained with H&E. (A) Section from control rat showing hepatocytes (H) with central spherical nucleus (N), central vein (CV), endothelial cells (E) and Kupffer cells (K). X400. (B) Section from rat treated with tartrazine showing necrosis of most hepatocytes (H), congestion of blood vessel (star) and leucocytic infiltration (arrow). X400. (C) Section from rat treated with tartrazine showing vacuolated hepatocytes (H), others devoid of nuclei (star), leucocytic infiltration (arrow) and Kupffer cells (K). X1000. (D–G) Electron micrographs of liver sections from control and tartrazine-treated rats. (D) Section from control rat, showing part of the hepatocyte with nucleus (N), rough endoplasmic reticulum (rER), numerous mitochondria (M) and glycogen particles (arrows). X2500. (E) Section from rat treated with tartrazine, showing irregular and pyknotic nuclei (N), numerous lipid droplets (L) and electron dense mitochondria (M). X1500. (F) Section from rat treated with tartrazine, showing abnormal shaped mitochondria (M), dilated rough endoplasmic reticulum cisternae (rER), scattered ribosomes (arrows) and clear area of cytoplasm (star). X4000. (G) Section from rat treated with tartrazine, showing less electron dense nucleus (N), clear area of cytoplasm (star), electron dense mitochondria (M) and fragmented endoplasmic reticulum cisternae (rER). X2000.
Electron microscope investigation revealed significant structural aberations in liver and kidney of tartrazine-treated rats. Nuclei of hepatocytes appeared irregular or pyknotic and others were with less electron dense chromatin as compared to control (Figs. 1D and 1E). Alterations in the lipid contents of cells were observed by the presence of numerous lipid droplets that vary in size and shape (Fig. 1E). Abnormal shaped mitochondria were observed with condensed opaque matrices and lack internal organization. The cisternae of rough endoplasmic reticulum appeared dilated and fragmented (Fig. 1F). Rarefied areas in the cytoplasm could be resulting from dissociation of cellular organelles as well as scattered ribosomes that were also observed (Fig. 1G).
Moreover, obvious histological changes were observed in the structure of the kidney in the animal group treated with tartrazine when compared to controls (Figs. 2A–2C). These changes includes degeneration in glomerular structure, loss of renal tubules integrity and the presence of areas of huge vacuoles (Fig. 2B). Membrane injury in apical surfaces of tubular epithelial cells and degeneration in the basal membrane of cells were also noticed (Fig. 2C).
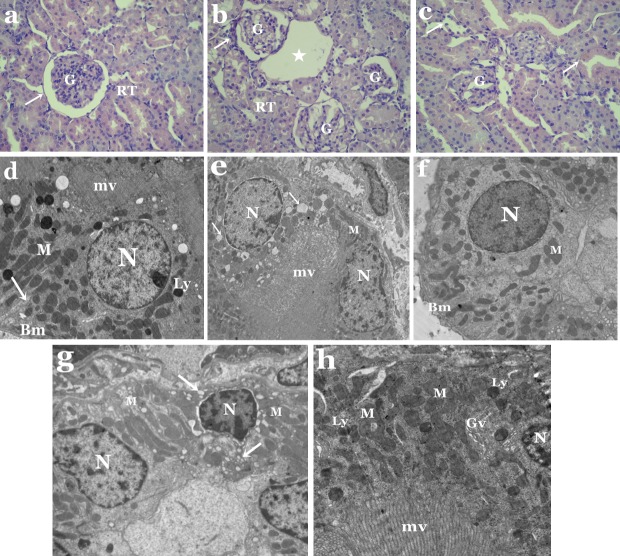
Figure 2.
(A–C) Light micrographs of kidney sections from control and tartrazine-treated rats stained with H&E. (A) Section from control rat showing Malpighian corpuscles with its glomerulus (G), Bowman’s capsule (arrow) and renal tubules (RT). X400. (B) Kidney section from rat treated with tartrazine showing degenerated glomeruli (G), loss of renal tubules integrity (RT), huge cavity with fragmented areas (star) and inflammation (arrow). X400. (C) Kidney section from rat treated with tartrazine showing damage in renal tubules membrane (arrows) and degenerated glomeruli (G). X400. (D–H) Electron micrographs of kidney sections from control and tartrazine-treated rats. (D) Kidney section from control rat showing proximal tubular cells with apical microvilli (mv), basement membrane (Bm), basal infoldings (arrow), nucleus (N), numerous mitochondria (M) and lysosomes (Ly). X2000. (E) Kidney section from rat treated with tartrazine showing disrupted proximal tubular cells with irregular nucleus (N), vacuolated cytoplasm (arrows) and disordered mitochondria (M). X2000. (F) Kidney section from control rat showing distal tubular cell with nucleus (N), mitochondria (M) and basement membrane (Bm). X2000. (G) Kidney section from rat treated with tartrazine showing disrupted distal tubular cells with nucleus (N), mitochondria (M) and vacuoles (arrows). X 2500. (H) Enlarged part of proximal tubular cell from rat treated with tartrazine showing part of pyknotic nucleus (N), destructed mitochondria (M), numerous lysosomes (Ly), Gologi vesicles (Gv) and microvilli (mv). X4000.
Electron microscope investigation showed remarkable ultrastructural alterations in the kidney of rats treated with tartrazine. These changes includes degenerated proximal and distal tubular cells with nuclei containing clumps of marginated heterochromatin (Figs. 2D and 2F). Some of these nuclei appeared with irregular outline,while others were pyknotic. Vacuolated cytoplasm was a prominent feature in both proximal and distal tubular cells (Figs. 2E and 2G). Also, pleomorphic and disorganized mitochondria as well as increased number of lysosomes and Golgi vesicles were observed in proximal tubular cells (Fig. 2H). In distal tubular cells, some mitochondria appeared normal while others were disrupted (Fig. 2G).
Genotoxicity results
Comet assay resulted that tartrazine possesses a genotoxic effect in the white blood cells of treated rats (Figs. 3A and 3B). This genotoxic effect was observed as a significant increase (p < 0.05) in the percentage of DNA in the comet tail in the nuclei of leucocytes of tartrazine-treated animals as compared to controls.
Figure 3. The genotoxic effect of tartrazine on leucocytes of rats.
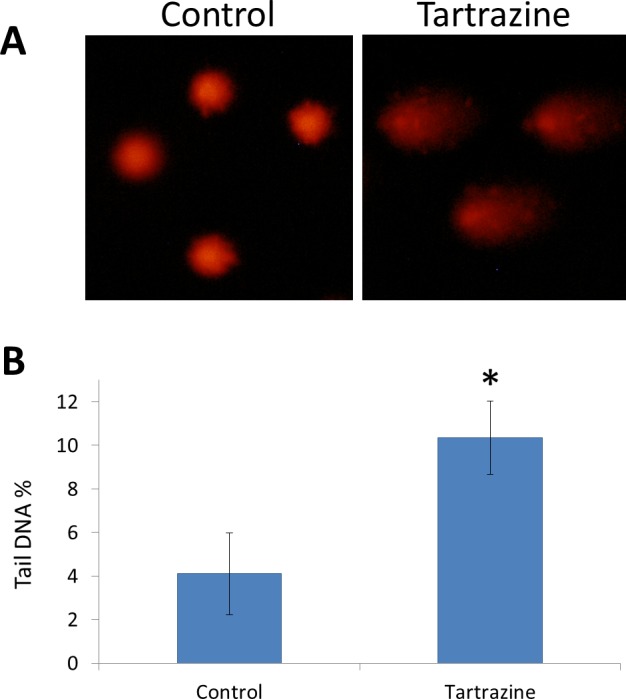
(A) Fluorescence micrograph representing nuclei of leucocytes after the comet assay. Control nuclei of untreated animals appear intact with no detectable DNA damage. Nuclei of tartrazine-treated animals appear damaged. (B) Bar graph showing the tail DNA damage in percentage in nuclei of leucocytes of control and tartrazine-treated animals subjected to comet assay. Data are Mean ± SD (n = 3, t-test, ∗p < 0.05).
Discussion
In the present work, administration of tartrazine significantly elevated the activity of serum ALT, AST and ALP. The increased levels of liver enzyme activities in blood is an indication of possible tissue damage. These results are in accordance with data reported by other investigators who attributed similar changes in liver function to hepatocellular impairment. This liver damage would releases greater than normal levels of intracellular enzymes into the blood (Amin, Abdel Hameid & AbdElsttar, 2010; Himri et al., 2011; El-Wahab & Moram, 2015; Saxena & Sharma, 2015).
Serum levels of creatinine and urea are major factors in determination of both, the glomerular filtration efficacy and proximal tubular secretion rate (Azu et al., 2010). Such damage in the the filtering compartments of kidney, blood levels of creatinine and urea increase. Our results show a significantly elevated serum levels of creatinine, urea and uric acid in tartrazine-treated group as compared to controls. These results are in agreement with Tawfek et al. (2015) who found a significant increase in serum creatinine and urea in rats following the consumption of different types of food additives including tartrazine, sunset yellow and sodium benzoate. Similar finding was observed by Ashour & Abdelaziz (2009) in rats dosed with organic azo dye (fast green) orally for 35 days. In addition, these results are in agreement with data reported by Amin, Abdel Hameid & AbdElsttar (2010) who revealed a significant increase in serum creatinine and urea levels in rats after consumption of tartrazine. Recently, Nabila et al. (2013) mentioned that tartrazine application induced significant elevations in urea and creatinine levels that is associated with impaired renal function and the inability of the kidney to filter body fluids.
Oxidative stress is referred to a reactive oxygen species (ROS)/antioxidant imbalance. It occurs when the overall level of ROS exceeds the potential of the antioxidants. Thus, oxidative stress may occur because of accelerated ROS production, a drop of the antioxidant mechanisms, or both (France’s et al., 2013). In the present study, elevated levels of malondialdehyde (MDA, end product of lipid peroxidation) and nitric oxide (NO) clearly indicates oxidative stress occurrence in the tartrazine-treated rats. These results are in hand with data reported by Omca, Zhang & Ercal (2012), who studied the oxidative effects of tartrazine and other azo dyes on Chinese hamsters. The increased lipid peroxidation may be attributed to the generation of ROS that result from tartrazine administration. Because tartrazine belongs to the group of azo dye food colorants,it is metabolized inside the body into aromatic amines by intestinal microflora. These formed amines are able to generate ROS as part of their metabolism by the interaction of the active amino groups with nitrite or nitrate containing foods (Moutinho, Bertges & Assis, 2007). NO is considered as another important source of free radicals that might contribute to alterations in energy metabolism. Peresleni et al. (1996) demonstrated that oxidative stress to epithelial cells increases NO syntheses which results in elevated NO release, nitrite production and decreased cell viability.
The increased total tissue antioxidant capacity (TAC) provides a gross estimation of how the body can react against oxidative stress (Costantini & Verhulst, 2009).
In the current work, the decrease in the level of TAC in the serum of tartrazine-treated animals may be related to increased free radical generation due to tartrazine administration and/or impaired antioxidant machinery leading to increased oxidative stress.
Histological and ultrastructural results clearly showed that administration of tartrazine had led to distortion of hepatic architecture as well as degeneration in kidney structure of rats. Light microscopic figures showed tartrazine-induced necrosis of most hepatocytes, congestion of blood sinusoids, infiltration of white blood cell, activated Kupffer cells, damaged glomerular and renal tubule membranes. These findings are in agreement with Himri et al. (2011), Mehedi et al. (2013) and Saxena & Sharma (2015) who indicated that tartrazine administration alters the histological structure of livers and kidneys in experimental animals. Rus et al. (2010) found that tartrazine and carmoisine causes congestion, stasis and edema in liver and kidney of Guinea pigs leading to apoptosis in hepatocytes and atrophy of renal structures.
Several ultrastructural alterations were recorded in the livers and kidneys of rats treated with tartrazine. The hepatocytes and renal tubule epithelium appeared with irregular or pyknotic nuclei. Large numerous lipid droplets, disorganization of mitochondria, rough endoplasmic reticuli (rER) and degenerated cytoplasmic areas were observed in the cytoplasm of hepatocytes. Meanwhile, proximal and distal tubular cells possess vacuolated cytoplasm and defective mitochondria. These results are similar to those of previous studies conducted with food additives, sodium benzoate and citric acid (Bakar & Aktac, 2014; Chen et al., 2014; Aktac et al., 2008; Kaboglu & Aktac, 2002). The presence of pyknotic nuclei and necrosis of hepatocytes and tubular cells in the current work clearly indicates toxicosis as previously described by Deveci et al. (2011) and Sarkar & Ghosh (2012). Structural changes in nuclei and ER, the critical structures for biosynthesis of glycoconjugates, could probably impairs glycosylation mechanisms and hence affect cell surface sialylation (Bakar & Aktac, 2014).
The increased amount of hepatocellular lipids was previously reported by Sinha & D’Souza (2010) and Khidr, Makhlouf & Ahmed (2012) after sodium benzoate administration in experimental animals. Cheville (1988) reported that toxins may affect ribosomes and their ability to produce peptide chains and decreases the amount of proteins involved in the transport of triglycerides, that are produced at their normal rates, causing accumulation of lipid globules. Deformation of mitochondria and rER in hepatocytes and renal tubular cells cytoplasm after tartrazine administration could be a response to chemical stressors (Khidr, Makhlouf & Ahmed, 2012). Reyes, Valim & Vercesi (1996) found that synthetic food coloring agents such as tartrazine, inhibited mitochondrial respiration in liver and kidney of rats. It also affected the integrity of mitochondrial membranes, which is critical for maintaining vital mitochondrial functions and determination of apoptosis in cells. Bakar & Aktac (2014) reported that degeneration of mitochondrial membranes and cristae negatively affect oxidative metabolism of cells.
Our electron microscopic results showed a clear destruction of hepatocytic cytoplasm. Sinha & D’Souza (2010) reported that damage from toxic insults may cause swallowing of hepatocytes with large clear spaces. The integrity of cytoplasm is important for regular intracellular trafficking which in turn would be damaged due to cytoplasmic vacuolization (Bakar & Aktac, 2014). The distortion of renal structures and tubular vacuolization found in the present work was previously described by other workers in kidneys of animals treated with the food additive, monosodium glutamate (Eweka, 2007; Abass & Abd El-Haleem, 2011; Afeefy, Mahmoud & Arafa, 2012).
Concerning the genotoxicity of synthetic colors, the obtained results revealed that tartrazine caused DNA damage in leucocytes as detected by comet assay. This genotoxic effect is probably due to the direct contact of tartrazine with nuclear DNA (Himri et al., 2012). Data pertaining to the genotoxic effect of tartrazine with positive results are available. This finding agrees with Mpountoukas et al. (2010) who investigated the toxic effect of tartrazine at 0.02–8 mM in human peripheral blood cells in vitro. In addition, tartrazine has been shown to induce chromosomal aberrations in fibroblast cells of Muntiacus muntjac (Chung, Fulk & Andrews, 1981). Hassan (2010) also revealed that administration of a daily dose of tartrazine (7.5 and 15 mg/kg b.wt.) for seven weeks leads to liver and kidney DNA damage.
On one hand, a study carried out by Poul et al. (2009) showed that acute oral administration of tartrazine did not induce genotoxic alterations in the micronucleus gut assay in mice at doses up to 2 g/kg b.wt. On the other hand, induction of tartrazine-induced DNA damage in comet assays was observed in cells from the colon of mice (Sasaki et al., 2002) at a dose that is slightly higher than the recommended human daily intake approved by the Joint FAO/WHO Expert Committee on Food Additives (MHW, 1999). Therefore, further comet assay investigations performed according to the latest recommended protocol (Hartmann et al., 2003) might be useful to clarify these discrepancies.
According to the above-discussed results, it can be concluded that tartrazine is able to generate ROS thus accelerating oxidative stress, altering the structure and biochemical profiles in hepatic and renal tissues. Therefore, controlling the consumption of tartrazine is important for the health and limiting the use of tartrazine, especially in foods used by children, is highly advisable.
Supplemental Information
Funding Statement
This work was supported by the Institute of Scientific Research and Revival of Islamic Heritage at Umm-Al Qura University, project number 43405033. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Latifa Khayyat analyzed the data, wrote the paper, reviewed drafts of the paper.
Amina Essawy conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Jehan Sorour performed the experiments, contributed reagents/materials/analysis tools, wrote the paper.
Ahmed Soffar performed the experiments, analyzed the data, prepared figures and/or tables.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The procedures of this experiment are compatible with the guide for care and use of laboratory animal approved by IACUC of Menoufia University, Egypt, Approval No:MNSP155.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplemental File.
References
- Abass & Abd El-Haleem (2011).Abass MA, Abd El-Haleem MR. Evaluation of monosodium glutamate inducedneurotoxicity and nephrotoxicity in adult male albino rats. Journal of American Science. 2011;7(8):264–276. [Google Scholar]
- Afeefy, Mahmoud & Arafa (2012).Afeefy A, Mahmoud M, Arafa M. Effect of honey on monosodium glutamate induced nephrotoxicity (histological and electron microscopic studies) Journal of American Science. 2012;8(1s):146–156. [Google Scholar]
- Aktac et al. (2008).Aktac T, Kaboglu A, Kizilay G, Bakar E. The short-term effects of single toxic citric acid doses on mouse tissues—histopathological study. Fresenius Environmental Bulletin. 2008;17:311–315. [Google Scholar]
- Amin, Abdel Hameid & AbdElsttar (2010).Amin KA, Abdel Hameid H, AbdElsttar AH. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food and Chemical Toxicology. 2010;48(10):2994–2999. doi: 10.1016/j.fct.2010.07.039. [DOI] [PubMed] [Google Scholar]
- Ashour & Abdelaziz (2009).Ashour AA, Abdelaziz I. Role of fast green on the blood of rats and the therapeutic action of vitamins C or E. International Journal of Integrative Biology. 2009;6(1):6–11. [Google Scholar]
- Azu et al. (2010).Azu O, Francis I, Abraham A, Crescie C, Stephen O, Abayomi O. Protective agent, Kigelia Africana fruit extract, against cisplatin-induced kidney oxidant injury in Sprague-Dawley rats. Asian Journal of Pharmaceutical and Clinical Research. 2010;3:84–88. [Google Scholar]
- Bakar & Aktac (2014).Bakar E, Aktac T. Effects of sodium benzoate and citric acid on serum, liver and kidney tissue total sialic acid levels: an ultrastructural study. JABS—Journal of Applied Biological Sciences. 2014;8(2):9–15. [Google Scholar]
- Bancroft & Gamble (2002).Bancroft J, Gamble M. Theory & practice of histological technique. 5th edition. Churchill Livingstone; New York: 2002. p. 796. [Google Scholar]
- Caraway (1955).Caraway W. Determination of uric acid in serum by carbonate method. American Journal of Clinical Pathology. 1955;25:840–845. doi: 10.1093/ajcp/25.7_ts.0840. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2014).Chen X, Qiongxia LV, Liu Y, Deng W. Study on injury effect of food additive citric acid on liver tissue in mice. Cytotech. 2014;66(2):275–282. doi: 10.1007/s10616-013-9567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chequer, Dorta & de Oliveira (2011).Chequer F, Dorta D, De Oliveira D. Azo dyes and their metabolites: does the discharge of the azo dye into water bodies represent human and ecological risks? In: Hauser PJ, editor. Advances in treating textile effluent. In Tech; Rijeka: 2011. pp. 28–48. [Google Scholar]
- Cheville (1988).Cheville NF. Introduction to veterinary pathology. Iowa State University Press; Ames: 1988. 537. [Google Scholar]
- Chung, Fulk & Andrews (1981).Chung KT, Fulk EG, Andrews AW. Mutagenicity testing of some commonly used dyes. Applied and Environmental Microbiology. 1981;42:641–648. doi: 10.1128/aem.42.4.641-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, Stevens & Cerniglia (1992).Chung KT, Stevens JR, Cerniglia CE. The reduction of dyes by the intestinal microflora. Critical Reviews in Microbiology. 1992;18(3):175–190. doi: 10.3109/10408419209114557. [DOI] [PubMed] [Google Scholar]
- Collins (2004).Collins R. The comet assay for DNA damage and repair: principles, applications, and limitations. Molecular Biotechnology. 2004;26(3):249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- Cortas & Wakid (1990).Cortas N, Wakid N. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clinical Chemistry. 1990;36(8 Pt 1):1440–1443. [PubMed] [Google Scholar]
- Costantini & Verhulst (2009).Costantini D, Verhulst S. Does high antioxidant capacity indicate low oxidative stress? Functional Ecology. 2009;23:506–509. doi: 10.1111/j.1365-2435.2009.01546.x. [DOI] [Google Scholar]
- Deveci et al. (2011).Deveci E, Söker S, Baran O, Tunik S, Ayaz E, Deveci, Abayomi O. Ultrastructural changes in the kidney cortex of rats treated with lead acetate. Journal of Morphology. 2011;29(3):1058–1061. doi: 10.4067/S0717-95022011000300067. [DOI] [Google Scholar]
- El-Wahab & Moram (2015).El-Wahab HM, Moram GS. Toxic effects of some synthetic food colorants and/or flavor additives on male rats. Toxicology International. 2015;22(1):152–157. doi: 10.1177/0748233711433935. [DOI] [PubMed] [Google Scholar]
- Eweka (2007).Eweka AO. Histological studies of the effects of monosodium glutamate on the kidney of adult Wistar rats. The Internet Journal of Health. 2007;6(2):45–67. [Google Scholar]
- France’s et al. (2013).France’s DE, Ingaramo PI, Ronco MT, Carnovale CE. Diabetes, an inflammatory process: oxidative stress and TNF-alpha involved in hepatic complication. Journal of Biomedical Science and Engineering. 2013;6:645–653. doi: 10.4236/jbise.2013.66079. [DOI] [Google Scholar]
- Gyori et al. (2014).Gyori BM, Venkatachalam G, Thiagarajan PS, Hsu D, Clement MV. OpenComet: An automated tool for comet assay image analysis. Redox Biology. 2014;2:457–465. doi: 10.1016/j.redox.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan (2010).Hassan G. Effects of some synthetic coloring additives on DNA damage and chromosomal aberrations of rats. Arab Journal of Biotechnology. 2010;13(1):13–24. [Google Scholar]
- Hartmann et al. (2003).Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR. Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- Henry (1974).Henry TJ. Clinical chemical principles and techniques. 2nd edition. New York: Harper & Row Publishers; 1974. Creatinine measurements with colorimetric method. [Google Scholar]
- Himri et al. (2011).Himri I, Bellahcen S, Souna F, Belmakki F, Aziz M, Bnouham M, Zoheir J, Berkia Z, Aziz M, Saalaoui E. A 90-day oral toxicity study of tartrazine, a synthetic food dye, in wistar rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3l(3):159–169. [Google Scholar]
- Himri et al. (2012).Himri I, Souna F, Aziz M, Hakkou A, Saalaoui E. DNA Damage Induced by Tartrazine in rat whole blood using Comet Assay (Single Cell Gel Electrophoresis) Advances in Environmental Biology. 2012;6(11):2875–2881. [Google Scholar]
- Kaboglu & Aktac (2002).Kaboglu A, Aktac T. A study of the effects of sodium benzoate on the mouse liver. Biologia. 2002;57:375–382. [Google Scholar]
- Khidr, Makhlouf & Ahmed (2012).Khidr B, Makhlouf M, Ahmed S. Histological and ultrastructural study on the effect of sodiumbenzoate on the liver of adult male albino rats. Assiut University Journal of Zoology. 2012;41(1):11–39. [Google Scholar]
- Koracevic et al. (2001).Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. Journal of Clinical Pathology. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu et al. (1977).Mathieu M, Bretaudiere JP, Galteau MM, Guidollet J, Lalegerie P, Bailly M, Buret P, Dorche C, Louisot P, Schiele F. Recommandations pour la mesure de l’activité catalytique des phosphatases alcalines dans le sérum humain. Annales de Biologie Clinique. 1977;35:271–273. [PubMed] [Google Scholar]
- Mehedi et al. (2009).Mehedi N, Ainad-Tabet S, Mokrane N, Addou S, Zaoui C, Kheroua O, Saidi D. Reproductive toxicology of tartrazine (FD and C Yellow No. 5) in Swiss albino mice. American Journal of Pharmacology and Toxicology. 2009;4(4):128–133. doi: 10.3844/ajptsp.2009.130.135. [DOI] [Google Scholar]
- Mehedi et al. (2013).Mehedi N, Mokrane N, Alami O, Ainad-Tabet S, Zaoui C, Kheroua O, Saidi D. A thirteen week ad libitum administration toxicity study of tartrazine in Swiss mice. African Journal of Biotechnology. 2013;12(28):4519–4529. [Google Scholar]
- MHW (1999).Ministry of Health and Welfare (MHW) of Japan . The Japan’s specification and standards for food additives. 7th edition. Japan Food Additives Association; Tokyo: 1999. [Google Scholar]
- Mittal, Kurup & Mittal (2007).Mittal A, Kurup L, Mittal J. Freundlich and langmuir adsorption isotherms and kinetics for the removal of tartrazine from aqueous solutions using hen feathers. Journal of Hazardous Materials. 2007;146(1–2):243–248. doi: 10.1016/j.jhazmat.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Moutinho, Bertges & Assis (2007).Moutinho I, Bertges L, Assis R. Prolonged use of the food dye tartrazine (FD & C Yellown degrees 5) and its effects on the gastric mucosa of Wistar rats. Brazilian Journal of Biology. 2007;67(1):141–145. doi: 10.1590/S1519-69842007000100019. [DOI] [PubMed] [Google Scholar]
- Mpountoukas et al. (2010).Mpountoukas P, Pantazaki A, Kostareli E, Christodoulou P, Kareli D, Poliliou S, Mourelatos C, Lambropoulou V. Theodore Lialiaris cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food and Chemical Toxicology. 2010;48:2934–2944. doi: 10.1016/j.fct.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Nabila et al. (2013).Nabila M, Mokrane N, Alami O, Tabet S, Zaoui C, Kheroua O, Saidi D. A thirteen week ad libitum administration toxicity study of tartrazine in Swiss mice. African Journal of Biotechnology. 2013;12(28):4519–4529. doi: 10.5897/AJB2013.12125. [DOI] [Google Scholar]
- Ohkawa, Ohishi & Yagi (1979).Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Omca, Zhang & Ercal (2012).Omca D, Zhang X, Ercal N. Oxidative effects of Tartrazine (CAS No. 1934-21-0) and New Coccin (CAS No. 2611-82-7) azo dyes on CHO cells. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2012;7:229–236. doi: 10.1007/s00003-012-0782-z. [DOI] [Google Scholar]
- Patton & Crouch (1977).Patton C, Crouch S. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Analytical Chemistry. 1977;49:464–468. doi: 10.1021/ac50011a034. [DOI] [Google Scholar]
- Peresleni et al. (1996).Peresleni T, Noiri E, Bahou W, Goligorsky M. Anti-sense oligodeoxynucleotides to inducible NO synthase rescue epithelial cells from oxidative stress injury. American Journal of Physiology. 1996;270(6):971–977. doi: 10.1152/ajprenal.1996.270.6.F971. [DOI] [PubMed] [Google Scholar]
- Poul et al. (2009).Poul M, Jarry G, Ould Elhkim M, Poul JM. Lack of genotoxic effect of food dyes amaranth, sunset yellow and tartrazine and their metabolites in the gut micronucleus assay in mice. Food and Chemical Toxicology. 2009;47(2):443–448. doi: 10.1016/j.fct.2008.11.034. [DOI] [PubMed] [Google Scholar]
- Reyes, Valim & Vercesi (1996).Reyes FG, Valim MF, Vercesi AE. Effect of organic synthetic food colours on mitochondrial respiration. Food Additives and Contaminants. 1996;13(1):5–11. doi: 10.1080/02652039609374376. [DOI] [PubMed] [Google Scholar]
- Rus et al. (2010).Rus V, Gherman C, Miclăuş V, Mihalca A, Nadăş G. Comparative toxicity of food dyes on liver and kidney in guinea pigs: A histopathological study. Annals of RSCB. 2010;15(1):161–165. [Google Scholar]
- Sarkar & Ghosh (2012).Sarkar R, Ghosh AR. Metanil Yellow—an azo dye induced histopathological and ultrastructural changes in albino rat (Rattusnorvegicus) The Bioscan. 2012;7(3):427–432. [Google Scholar]
- Sasaki et al. (2002).Sasaki Y, Kawaguchi S, Kamaya A, Ohshima M, Kabasawa K, Iwam K, Taniguchi K, Tsuda S. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutation Research/DNA Repair. 2002;519:103–119. doi: 10.1016/s1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Saxena & Sharma (2015).Saxena B, Sharma S. Food color induced hepatotoxicity in Swiss albino rats, Rattus norvegicus. Toxicology International. 2015;22(1):152–157. doi: 10.4103/0971-6580.172286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha & D’Souza (2010).Sinha R, D’Souza D. Liver cell damage caused due to sodium benzoatetoxicity in mice. International Journal of Biochemistry and Biotechnology. 2010;6(4):549–554. [Google Scholar]
- Steven (1996).Steven CK. Alanine and Aspartat aminotransferase, principle and usage. In: James JT, Jenifer R, editors. Liver function, in clinical chemistry theory, analysis and correlation. 3rd edition M. Mosby; London: 1996. pp. 504–527. [Google Scholar]
- Tawfek et al. (2015).Tawfek N, Amin H, Abdalla A, Fargali S. Adverse effects of some food additives in adult male albino rats. Current Science International. 2015;4(4):525–537. [Google Scholar]
- Walton et al. (1999).Walton K, Walker R, Sandt J, Castell J. The application of in vitro data in the derivation of the acceptable daily intake of food additives. Food and Chemical Toxicology. 1999;37(12):1175–1197. doi: 10.1016/S0278-6915(99)00107-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplemental File.