Abstract
Background
Adverse childhood experiences (ACEs) are one of the greatest predictors for affective disorders for women. Periods of dynamic hormonal flux, including pregnancy, exacerbate the risk for affective disturbance and promote hypothalamic-pituitary-adrenal (HPA) axis dysregulation, a key feature of affective disorders. Little is understood as to how stress experienced in late childhood, defined as preadolescence, alters the programming unique to this period of brain maturation and its interaction with the hormonal changes of pregnancy and postpartum.
Methods
Preadolescent female mice were exposed to chronic stress and examined for changes in their HPA axis during pregnancy and postpartum, including assessment of maternal-specific stress responsiveness and transcriptomics of the paraventricular nucleus of the hypothalamus (PVN). Translationally, pregnant women with low or high ACEs were examined for their maternal stress responsiveness.
Results
As predicted, preadolescent stress in mice resulted in a significant blunting of the corticosterone response during pregnancy. Transcriptomic analysis of the PVN revealed widespread changes in expression of immediate early genes and their targets, supporting the likely involvement of an upstream epigenetic mechanism. Critically, in our human studies the high ACE women showed a significant blunting of the HPA response.
Conclusions
This unique mouse model recapitulates a clinical outcome of a hyporesponsive HPA stress axis, an important feature of affective disorders, during a dynamic hormonal period, and suggests involvement of transcriptional regulation in the hypothalamus. These studies identify a novel mouse model of female ACEs that can be used to examine how additional life adversity may provoke disease risk or resilience.
Key terms: stress, adolescence, pregnancy, paraventricular nucleus, HPA axis, postpartum
Introduction
Risk for affective disturbance in the lifetime of females is multi-factorial, although how these factors interact is not well understood. One key factor is exposure to adverse childhood experiences (ACEs), which are known to increase affective disorder risk across the lifespan for women (1–5). Furthermore, periods of dynamic hormonal flux, such as pregnancy and postpartum, can exacerbate the risk for affective disturbances and stress dysregulation (6–8). Peripartum depression and anxiety, occurring during pregnancy or shortly following birth, are associated with significant adverse and long-term effects for both mother and baby (9–11). Preclinical animal studies focused on stressors proximal to birth, including prenatal or postpartum social stress or stress hormone exposure, demonstrate significant changes in maternal behavior and offspring outcomes (12–15). However, little is known as to how adversity experienced in late childhood, or preadolescence, may reprogram the female brain to increase risk for such outcomes during and after pregnancy.
A central endophenotype of affective disorders is disruption of the hypothalamic-pituitary-adrenal (HPA) axis, which is responsible for initiating the neuroendocrine response to stressors (16; 17). Importantly, the responsiveness of the HPA axis has yet to fully mature in preadolescent animals. In response to a variety of stressors, preadolescent rodents have an HPA axis characterized by a protracted hormonal response compared to neonatal and adult animals, and an insensitivity to factors, such as gonadal hormones, that normally modulate the adult response (18–20). Thus, the preadolescent individual may have an increased risk for adversity to program long-term dysfunction of the HPA axis (21). Indeed, clinical studies show that childhood adversity is associated with HPA axis dysregulation in adult women (22–25). During pregnancy and postpartum, there are dramatic changes in hormone levels. This has important implications for regulation of stress circuitry, as these gonadal hormones and their metabolites have been shown to be potent regulators of the HPA axis (26–29). Critically, as the HPA axis response is highly conserved among vertebrates, it represents a readily translatable outcome for animal models.
We have developed a mouse paradigm to examine the hypothesis that stress experienced during the preadolescent window of brain development would program long-term changes in stress pathways that, when interacted with dynamic hormonal changes during pregnancy and postpartum, would produce dysregulation of the stress response. Female mice were exposed to chronic stress during preadolescence and were then examined for changes in HPA stress axis responsiveness during pregnancy. To examine potential mechanisms for programming changes that may have occurred following preadolescent stress, pregnancy hormones and gene expression changes related to stress circuitry, including transcriptomics of the paraventricular nucleus (PVN), were also measured. To evaluate the translational potential of this model, HPA responsiveness to a maternally-relevant stressor was assessed in preadolescent stressed mice and a cohort of women with varying levels of ACEs.
Methods and Materials
Full details of experimental procedures and analyses are provided in the Supplement.
Animals
All mice bred were virgin, in-house mixed C57BL/6:129 mice (30–33). All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preadolescent stress
Administration of preadolescent stress (PAS) was performed as described previously (33). Female mice underwent 14 days of chronic variable stress starting on postnatal day (PN) 21, during which one stressor was administered per day. Animals in the PAS group were weaned into singly-housed cages at the beginning of stress, and were pair-housed with a same-sex, same-stress cage mate at the end of stress. Control individuals remained with the dam until they were weaned at PN28 into pair-housed cages.
Breeding scheme
At 8–10 weeks old, females were bred with naïve males for 1–3 nights. Upon confirmation of a copulation plug, females were established as pregnant and were immediately removed to their own cage. Females were left undisturbed until testing.
HPA axis responsiveness
Females that were nulliparous, in the early stage of pregnancy (7.5 days post conception (dpc)), in the late stage of pregnancy (17.5dpc), or postpartum (PN4) were tested for HPA axis responsiveness to a 15 min restraint stress as previously (34; 35). Each group represents a different set of subjects, such that females were not tested more than once.
Activation of the HPA axis by adrenocorticotropic hormone (ACTH) in the absence of any additional stressor was examined. Plasma corticosterone was measured in late pregnant females (17.5dpc) as previously, except that animals were injected with 50 μg/kg ACTH (Sigma-Aldrich, St. Louis, MO) at time 0 min and were not restrained.
Light-dark box
To assess anxiety-like behavior, late pregnant females (17.5dpc) were tested in light-dark box and analysis was performed as previously (35; 36).
Postpartum pup separation
Females (PN7) were tested for behavioral and HPA axis responsiveness to a 15 min separation from pups. Four pups (2/sex) were placed in a novel cage 5 min prior to the start of testing. The arena was outfitted with a plastic mesh gate that hemisected the cage. This allowed the dam to see, smell, and hear, but not come into physical contact with, the pups. Dams were placed in the side of the arena opposite of the pups and behavior was recorded from above. Distance travelled was quantified using ANY-maze v4.75 software (Stoelting, Kiel, WI). Following the separation, the dam was removed and tail blood was collected. The dam and pups were returned to the home cage, after which tail blood was collected from the dam at 30 and 120 min following the start of the separation test. Blood was not collected at the start of test so as to not interfere with maternal behavior during the separation.
Pup retrieval
To assess maternal care, females (PN2) underwent a pup retrieval test. The dam was removed from the cage, and two pups (1/sex) were placed each in the two corners of the cage opposite to that of the nest. The dam was placed back in the cage, and latency to retrieve each of the four pups was recorded.
Human infant separation test
Subjects
Pregnant women (age range 19–35) were recruited to an ongoing study focusing on the role of maternal life stress on pregnancy and infant outcomes conducted at the Penn Center for Women’s Behavioral Wellness. See Supplement for a full description of the cohort. The study was approved by the Perelman School of Medicine at the University of Pennsylvania Institutional Review Board and all adult participants provided written informed consent.
Assessment of preadolescent adverse experience
Upon enrollment, women were given the Adverse Childhood Experiences (ACE) questionnaire, a 10-item self-report that assesses exposure to abuse, neglect, and household adversity from birth to 18 years of age (Supplemental Table S2) (37). An item was considered to be a preadolescent ACE if the experience was reported to have first occurred at least 2 years prior to reported age of menarche. Participants were separated into low (0 ACE) and high (2+ ACEs) preadolescent ACE categories.
Infant separation test
Mothers underwent a maternal-specific laboratory stressor that consisted of exposure to infant separation, during which the infant (6 months old) experienced 3 bursts of 90 dB sound (30 sec intervals), followed by a 2 min restraint stress (38). Mothers were asked to sit quietly in a nearby room while the infant was undergoing the stressor. Salivary samples were obtained from the mother using salivettes (Salimetrics, LLC Inc., College Station, PA) after 5 minutes of rest (Baseline 1, B1), immediately after the infant was removed (B2), immediately after the infant stressor (Time 1, T1), and 15 (T2), 30 (T3), and 60 (T4) min after completion of the stressor. The infant and mother were reunited after the T1 saliva collection. Mothers were screened for depression using the Edinburgh Postnatal Depression Scale (EPDS). For an hour prior to and during the protocol, the mother and infant were not allowed to eat or drink.
Cortisol assay
Saliva was collected utilizing standard methods (39; 40). Samples were sent either to University of Pennsylvania Translational Core laboratory or Dr. C. Kirschbaum Biopsychology laboratory (Dreseden Germany) for cortisol measurement.
Mouse tissue collection and analysis
Plasma from the trunk blood of 18.5dpc females was analyzed for 17β-estradiol and progesterone levels by 125I radioimmunoassay kits (MP Biomedicals, Santa Ana, CA) (33).
Total RNA was isolated and quantitative real-time PCR (qRT-PCR) was performed and analyzed as previously to assess gene expression (Supplemental Table S1) in the adrenal gland, pituitary gland, and placenta (34; 41).
Whole brains from 18.5dpc dams were cryosectioned at −20 °C. The paraventricular nucleus (PVN) was micropunched (42) and prepared for RNA-Sequencing (Illumina, San Diego, CA). Single-end 75-bp sequencing was performed on libraries on the Illumina NextSeq 500 sequencer using the NextSeq High Output v2 kit.
Statistical analysis
An investigator blind to group conducted all data collection and analysis. Behavioral, hormonal, and gene expression measures were analyzed by t-test, Pearson correlations, or one-way analysis of variance (ANOVA) with corrections and post-hoc testing, as appropriate. The significance level was P < 0.05. All data for these measures are reported as mean ± SEM.
A repeated measures mixed-model ANOVA was performed to evaluate ACE category and time as predictors of cortisol change-from-baseline, controlling for race as a confounder. A Wilcoxon rank sum test was used to analyze EPDS data.
RNA-Seq data were analyzed in the R environment for Mac with the packages RSubread and DESeq (43–45). To identify differentially expressed genes, the Benjamini Hochbert FDR correction was applied and an adjusted P < 0.05 was used.
Results
Preadolescent stress disrupted HPA axis responsiveness to acute restraint only during pregnancy
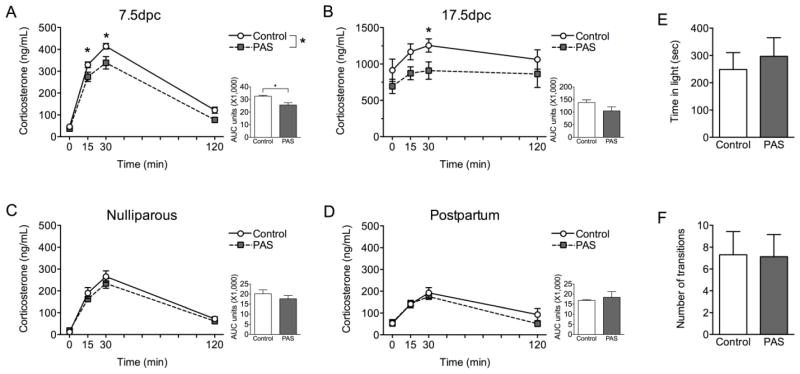
The corticosterone response to acute restraint was disrupted by PAS only during pregnancy (Figure 1). At 7.5dpc, PAS females had decreased corticosterone compared to Control females (P < 0.05, Figure 1A). The effect of PAS was also observed for total corticosterone (P < 0.05), where PAS females had decreased area under the curve (AUC). Based on an a priori hypothesis that PAS would reprogram the hypothalamus, we conducted a specific examination of the rise (15 min) and the peak (30 min) of the corticosterone response, as these time points are most indicative of hypothalamic initiation of the corticosterone response. This analysis revealed that PAS females had lower corticosterone at both 15 min (P < 0.05) and 30 min (P < 0.05). At 17.5dpc, while there was only a trend for PAS to disrupt HPA axis responsiveness (P = 0.09, Figure 1B), there was an effect of PAS on corticosterone at 30 min (P < 0.05).
Figure 1.
Preadolescent stress disrupted HPA axis responsiveness to acute restraint stress only during pregnancy. (A) The corticosterone response to restraint was blunted in PAS females compared to Controls at 7.5dpc (n = 6–8/group), as indicated by both a main effect on the corticosterone curve (Fstress(1,12) = 14.038, P = 0.0028; Ftime(2.3,28.0) = 280.35, P < 0.0001; Fstress*time(2.3,28.0) = 2.17, P = 0.13) as well as total area under the curve (AUC) measurement (t(12) = 3.70, P = 0.003). PAS females had lower corticosterone at both 15 min (t(12) = 2.32, P = 0.039) and 30 min time points (t(12) =2.67, P = 0.020). (B) In late pregnancy (17.5dpc, n = 8/group), preadolescent stress significantly altered the peak in corticosterone, as indicated by PAS females having blunted corticosterone at the 30 min time point compared to Controls (t(14) = 2.30, P = 0.038). There was no effect of PAS on corticosterone over time (Ftime(1.7,23.9) = 4.042, P = 0.036; Fstress(1,14) = 3.30, P = 0.090; Fstress*time(1.7,23.9) = 0.32, P = 0.70), AUC measurement (t(14) = 1.74, P = 0.10), or corticosterone at the 15 min time point (t(14) = 2.096, P = 0.055). Disruption of the corticosterone response to acute restraint stress is specific to pregnancy. (C) There was no effect of PAS in nulliparous females (n = 7–8/group), as indicated by an effect on the corticosterone curve (Fstress(1,13) = 0.82, P = 0.38; Ftime(2.4,31.7) = 120.43, P < 0.0001; Fstress*time(2.4,31.7) = 0.81, P = 0.47), AUC measurement (t(13) = 0.96, P = 0.35), or corticosterone at 15 min (t(13) = 0.92, P = 0.37) or at 30 min (t(13) = 0.90, P = 0.38). (D) Similarly, there was no effect of PAS on the postpartum corticosterone response (n = 3–4/group, Fstress(1,5) = 2.66, P = 0.16; Ftime(3,3) = 14.32, P = 0.028; Fstress*time(3,3) = 0.87, P = 0.55), AUC measurement (t(5) = 0.47, P = 0.65), or corticosterone at 15 min (t(5) = 0.08, P = 0.94) or at 30 min (t(5) = 0.68, P = 0.53). PAS effects on pregnancy stress responsiveness were specific to the HPA axis, as performance on the light-dark box test of anxiety-like behavior at 17.5dpc (n = 8–10/group) showed no effect of PAS on (E) total time in the light (t(16) = 0.52, P = 0.61) or (F) number of transitions between the light and dark chambers (t(16) = 0.058, P = 0.95). *P < 0.05.
In nulliparous females (Figure 1C), PAS had no impact on the corticosterone response or on total corticosterone as measured by AUC. Similarly, nulliparous PAS and Control females did not differ in corticosterone at 15 min or at 30 min. There was no effect of PAS on the postpartum corticosterone response (Figure 1D), as indicated by corticosterone over time, AUC measurement, or corticosterone at 15 min or at 30 min.
To examine the effect of PAS on anxiety-like behavior and locomotion during late pregnancy, females were tested in the light-dark box (Figure 1E,F). PAS did not impact total time in the light chamber of the box or the number of transitions between the light and dark chambers.
Preadolescent stress altered maternal stress responding in mice and humans
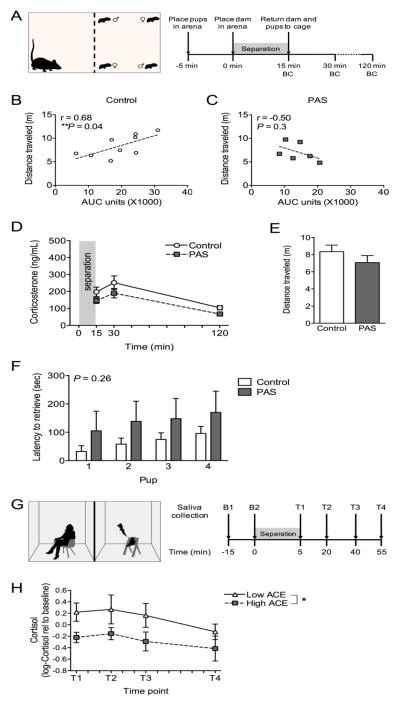
To examine several aspects of maternal responsiveness to pups, females underwent either separation testing or pup retrieval testing (Figure 2). When females were separated from pups by a plastic mesh barrier (Figure 2A), we observed a disorganization of maternal responsiveness in PAS females. When the correlation between total corticosterone released and total distance travelled was examined, PAS disrupted the expected positive relationship. In Control subjects, there was a positive correlation between AUC and distance travelled (P < 0.05, Figure 2B). In contrast, there was no longer a relationship between AUC and distance travelled in PAS females (P < 0.05, Figure 2C), indicating a disorganization of maternal responsiveness. There was no effect of PAS on corticosterone measured over time or on total distance travelled during the separation (Figure 2D,E). In pup retrieval testing (Figure 2F), there was no effect of PAS on the latency to retrieve pups.
Figure 2.
Separation from offspring resulted in disrupted postpartum stress responsiveness in both mice and humans with adverse preadolescent experience. (A) During a postpartum separation test in mice, females (n = 7–10/group) were separated from pups (2 male, 2 female, PN7) by a mesh screen for 15 min, during which distance travelled was measured, and following which there was blood collection (BC) to assess corticosterone response to the test. (B) A predicted positive correlation between total distance travelled and total corticosterone (area under the curve units, AUC) was observed in Control females (r = 0.68, P = 0.042). (C) However, this relationship was disrupted in PAS females (r = −0.50, P = 0.31). There was no effect of PAS alone on (D) corticosterone measurement over time (Fstress(1,15) = 1.23, P = 0.28; Ftime(1.6,24.0) = 30.089, P < 0.0001; Fstress*time(1.6,24.0) = 0.072, P = 0.89) or (E) distance travelled during the 15 min separation (t(13) = 1.10, P = 0.29). (F) Preadolescent stress did not alter maternal behavior, as assessed on a pup retrieval task (PN2, n = 4–6/group), as indicated by latency to retrieve pups (Fstress(1,8) = 1.48, P = 0.26; Fpup(1.5,12.2) = 9.34, P = 0.0054; Fstress*pup(1.5,12.2) = 0.041, P = 0.93). (G) Women were assessed in a similar task at 6 months postpartum (n = 9–11/group). (H) High ACE women have a significant blunting of salivary cortisol (P = 0.013). There was also a predicted significant effect of time on salivary cortisol (P = 0.011). *P < 0.05.
For the mothers who underwent infant separation testing (Figure 2G), ACE category showed significance in predicting log-transformed cortisol change-from-baseline (P < 0.05), with a beta estimate of −0.553 for high ACE women (Figure 2H). After exponentiating, this indicates that high ACE women had, on average, a 42% lower cortisol response to the infant separation paradigm than did low ACE women. The time point variable also showed statistical significance (P < 0.05). Preadolescent ACE exposure did lead to an increased incidence of depression symptoms (P < 0.05, Figure S1).
Peripheral factors of HPA axis responsiveness were not disrupted by preadolescent stress
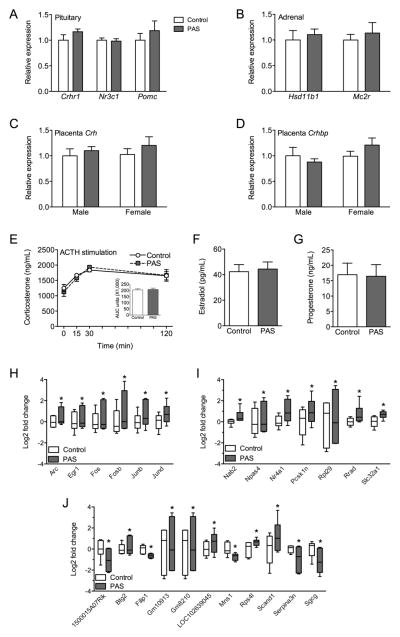
To understand the mechanism by which PAS disrupted HPA axis responsiveness to acute restraint, we examined gene expression in several nodes of the peripheral HPA axis that contribute to stress responsiveness during late pregnancy. Analysis of gene expression in the pituitary and adrenal suggests there was no reprogramming by PAS on these tissues during late pregnancy (Figure 3A,B). There was no effect of PAS on Crhr1, Nr3c1, or Pomc expression in the pituitary, or on Hsd11b1 or Mc2r expression in the adrenal.
Figure 3.
Preadolescent stress resulted in long-term reprogramming of gene expression in the PVN but did not alter peripheral regulators of HPA axis responsiveness. At 17.5dpc (n = 5–6/group), there was no effect of PAS on (A) pituitary expression (relative to Control) of Crhr1 (t(9) = 1.45, P = 0.18), Nr3c1 (t(9) = 0.27, P = 0.80), or Pomc (t(9) = 0.78, P = 0.45), nor was there an effect on gene expression in (B) the adrenal gland, including Hsd11b1 (t(9) = 0.51, P = 0.62) and Mc2r (t(9) = 0.55, P = 0.59). As the placenta can contribute CRF to maternal circulation, we also measured gene expression in the late pregnancy placenta, but there was no effect of PAS on either (C) Crh (Fstress(1,20) = 1.066, P = 0.31; Fsex(1,20) = 0.23, P = 0.64; Fstress*sex(1,20) = 0.080, P = 0.78) or (D) Crhbp expression (relative to Control male; Fstress(1,20) = 0.15, P = 0.70; Fsex(1,20) = 1.76, P = 0.20; Fstress*sex(1,20) = 1.94, P = 0.18). (E) Responsiveness of the adrenal gland to ACTH stimulation was assessed, and preadolescent stress had no effect on corticosterone response (n = 6–10/group), as indicated by corticosterone over time (Fstress(1,12) = 0.057, P = 0.81; Ftime(3,10) = 12.76, P = 0.0009; Fstress*time(3,12) = 0.82, P = 0.51) or AUC measurement (t(12) = 0.24, P = 0.82). The effect of PAS on the HPA axis is unlikely to be related to alterations in circulating hormone levels, as there was no effect of PAS on (F) plasma estradiol (t(13) = 0.25, P = 0.80) or (G) plasma progesterone (t(13) = 0.095, P = 0.93) in 18.5dpc females (n = 7–8/group). Differential gene expression analysis of RNA-Seq data revealed that 24 genes in the late pregnancy PVN differed between PAS and Control females (n = 6/group). These genes fell into three categories that suggest a broad change in gene expression as a result of preadolescent stress. (H) Of the differentially expressed genes, 6 were immediate early genes (IEGs), all of which were increased in the PVN of PAS females. Log2 fold change is relative to Control value. (I) Of the remaining genes, 7 were identified as having at least one of the six IEGs as a transcription factor. Interestingly, many of these genes (Nab2, Npas4, Nr4a1, Rpl29, and Rrad) are involved in transcriptional or translational regulation. Other genes that were identified as being regulated by the IEGs include Pcsk1, which regulates the cleavage of neuroendocrine peptide precursors, and Slc32a1, the vesicular GABA transporter. (J) Other differentially expressed genes were not directly regulated by the any of the IEGs and have a variety of biological functions, including regulation of transcription (Btg2, Scand1) and response to hormone stimulus (Serpina3n). *significantly different between Control and PAS.
During late pregnancy, each placenta can contribute CRF to the maternal circulation, which may alter HPA axis responsiveness during pregnancy (Figure 3C,D). At 18.5dpc, there was no effect of PAS on Crh or Crhbp expression in the placenta.
To assess adrenal responsiveness, an ACTH stimulation test was administered at 17.5dpc (Figure 3E). There was no effect of PAS on corticosterone production following ACTH stimulation. Similarly, there was no effect of PAS on corticosterone AUC.
As circulating hormone levels are both increased during late pregnancy and can impact HPA axis responsiveness, 17β-estradiol and progesterone levels in plasma were examined at 18.5dpc (Figure 3F,G). There was no effect of PAS on the amount of circulating 17β-estradiol or progesterone.
PVN transcriptome during pregnancy was altered by preadolescent stress
Gene expression patterns in the late pregnancy PVN were analyzed using RNA-Seq to investigate long-term reprogramming of the HPA. Analysis of differentially expressed genes revealed a total of 24 genes that were dependent upon PAS experience. These genes fell into three categories that suggest a potential for broad changes in gene transcription that were reprogrammed by PAS. First, six of the differentially expressed genes were immediate early genes (IEGs), all of which were significantly increased in the PVN of PAS females (Figure 3H). Second, several genes were identified as being under the transcriptional regulation of at lest one of the identified IEGs (Figure 3I). As we would predict based on the relationship between IEGs and their downstream targets, these genes are also significantly increased in the PVN of PAS females. Many of these genes (Nab2, Npas4, Nr4a1, Rpl29, Rrad) are involved in transcriptional or translational regulation. Other genes that were identified as being regulated by the IEGs include Pcsk1, which regulates the cleavage of neuroendocrine peptide precursors, and Slc32a1, the vesicular GABA transporter. Finally, other differentially expressed genes were not directly regulated by any of the IEGs (Figure 3J). Here, genes have a variety of biological functions that may control the PVN response to stimuli, including regulation of transcription (Btg2, Scand1) and response to hormone stimulus (Serpina3n).
Discussion
Exposure to adverse childhood experiences (ACEs) increases the risk for depressive and anxiety disorders in women (1–5). This long-term programming may interact across the female lifespan with periods of hormonal fluctuation such as those experienced during pregnancy to precipitate affective disturbance and altered stress reactivity (6–8). Peripartum depression and anxiety that occurs during pregnancy or in the postpartum period is associated with negative outcomes for both the mother and offspring (9–15). A key underlying feature of most neuropsychiatric diseases is disruption of the HPA stress axis, which undergoes dynamic changes during preadolescence and during pregnancy (16–18). Further, the HPA axis represents an important translational measure, as the underlying neural circuitry is highly conserved among vertebrates. As the mechanisms by which preadolescent adversity impacts stress responsiveness during pregnancy and postpartum are unknown, we developed a novel preclinical mouse model to examine this connection. Translationally, pregnant women with high or low ACE experience were tested for stress responsiveness.
Consistent with our hypothesis, preadolescent stressed (PAS) females had a significantly blunted corticosterone response to stress during both early and late pregnancy. While the effect is more clear in early pregnancy, it is likely that the increased variability due to the heightened hormonal state of late pregnancy obscures the effect. To determine the specificity of this hyporesponsive HPA to the pregnancy period, we also compared mice prior to pregnancy and postpartum. At both time points, the corticosterone response was normal, suggesting that the period of pregnancy is a unique physiological state that interacts with the PAS programming to blunt the HPA axis. No differences between groups were found for stress-related behaviors or locomotion, suggesting the PAS programming may be specific for neuroendocrine regulation and the precipitation of behavioral changes would require additional insults. As the postpartum period is a unique hormonal state, we hypothesized that a more maternal-relevant stressor may be important in assessing the HPA axis, and therefore utilized a pup separation stressor postpartum (46). In this test, the dam was separated from pups by a mesh barrier, which allowed for visual, auditory, and olfactory cues to reach the dam, but barred the dam from retrieving or interacting with the pups. As the corticosterone response is largely suppressed during the postpartum window, we were concerned about a floor effect obscuring the ability to detect a group differences. An advantage of this separation test is that females were freely moving, providing more detailed information about general arousal state. Therefore, we measured both the corticosterone response to this stressor and the distance travelled during the test in order to allow a correlation of these measures, permitting more nuanced insight into the responsiveness of the females. As predicted, when a small cohort was examined, there was a positive correlation between these measures in nonstressed controls, while in PAS mice there was no longer a correlation. As both measures indicate energy mobilization which is likely instigated by sympathetic nervous system activation, the loss of a correlation in PAS mice indicates a disorganization of the typically coordinated adaptive stress response (7; 47). This may lead to vulnerability of chronic or severe stressors experienced during pregnancy or postpartum to induce affective dysfunction. Similar to behaviors during pregnancy, PAS did not alter any behavioral measures postpartum, indicated by normal maternal care during a pup retrieval task (48). This suggests potential reprogramming of the HPA axis that lasts into the postpartum window but may be stimulus-specific, and is consistent with work showing that pup separation can alter anxiety-like behavior in postpartum females (49). Together, these data suggest that PAS in female mice interacts with pregnancy and postpartum to produce a disrupted stress response.
As our goal is to provide translational insight, we recruited pregnant women with low or high ACE and examined them for maternal stress responsiveness in a manner similar to that of the mouse model. We examined the effect of preadolescent ACE experience on maternal cortisol during an infant separation test, during which time the infants experienced mild distress and crying that the mother could hear while waiting in an adjacent space. The same effect of preadolescent adversity was observed when a small cohort was examined, where women who experienced a high level of preadolescent stress (2+ ACEs) responded with a 42% decrease in the cortisol response compared to women who experienced a low level of preadolescent stress (0 ACEs). Further, these results support our hypothesis that the HPA axis dysfunction may be a risk factor for subsequent life insults to precipitate disease, as depression symptoms postpartum were higher, albeit in the non-clinical range, in high versus low ACE women, and suggests that our mouse model recapitulates clinically relevant findings of a hyporesponsive HPA axis and would be a useful tool for examination of such studies.
In our mechanistic studies to determine where along the HPA stress axis PAS programming changes may have occurred, we first examined compelling peripheral gene candidates in the pituitary gland, adrenal gland, and placenta that might underlie a pregnancy-specific change in stress reactivity. No differences were found between groups in relevant gene expression in these tissues. Additionally, we utilized an ACTH stimulation test to confirm peripheral endocrine responses were similar between groups, and found that similar corticosterone levels were produced in response to exogenous application of ACTH, suggesting that PAS did not alter functioning of the adrenal. We also examined estradiol and progesterone levels to ensure the gonadal axis was not accounting for group differences. No differences for either hormone were found between groups during late pregnancy. However, we were not able to examine neurosteroids or their metabolites that may be changed and specific to pregnancy, such as allopregnanolone, which could act locally in the PVN on the inhibitory GABA neurons to dampen the HPA stress axis (50–52). Taken together, these findings suggest that the impact of PAS to alter the HPA axis is likely central.
Therefore, to examine potential central mechanisms altered by PAS, we measured programmatic gene expression changes using a broad transcriptomics approach of RNA-Seq in the paraventricular nucleus (PVN), the key hypothalamic regulator of the HPA stress axis. We found that PAS altered baseline gene expression in the PVN of pregnant females, with differentially expressed genes falling into several functional categories. PAS females had significantly increased expression of six immediate early genes (IEGs; Arc, Egr1, Fos, Fosb, Junb, Jund), which is intriguing given the extensive actions of IEGs. IEGs are the first genes activated in response to cellular stimuli, act as important transcription factors for later gene expression, and subserve a wide variety of neural processes such as brain development and plasticity (53). Indeed, many of the differentially expressed genes identified by RNA-Seq are also targets of these IEGs, suggesting that even at baseline, PAS females have heightened transcriptional regulation. Further, these IEGs are components of the activator protein-1 transcription factor, which is a critical component of estrogen receptor-mediated enhancement of gene expression at estrogen response elements and has numerous downstream targets that are relevant to PVN function, including CRF and the glutamate AMPA receptor (54–56). While there was no effect of PAS on circulating estradiol or progesterone levels during pregnancy, this does not rule out a hormonal mechanism for pregnancy-specific alterations in HPA axis responsiveness, as this is still a period in which hormone levels are high for extended periods of time. Estradiol is a potent regulator of HPA axis responsiveness, and the possibility remains that the increased IEG expression is interacting with hormonal control of the PVN to produce pregnancy-specific HPA axis changes (26; 27). Additionally, as IEGs are rapidly expressed due to their open chromatin and association with permissive histone structures, the increased presence of these genes in the PVN of PAS females suggests a tighter control of neuronal activation and ability during acute stress to quickly inactivate these neurons.
These studies demonstrate that preadolescence is a period of brain development during which stress experience can reprogram aspects of the PVN. When this altered PVN baseline then interacts with the dynamic hormonal period of pregnancy, it manifests into a hypo-responsive HPA stress axis. Future studies are necessary to precisely pinpoint the likely epigenetic mechanism by which this outcome is specific to pregnancy. While the PVN is the central regulator of HPA axis responsiveness, it is part of an extended stress circuit in the brain, including the prefrontal cortex, amygdala, hippocampus, and bed nucleus of the stria terminalis, which is subject to long-term reprogramming by life stress exposures (57–61). Nonetheless, these studies confirm clinical reports suggesting that preadolescent ACE are an important risk factor, and may be a first insult contributing to, affective disorder risk across the lifespan, especially during periods of dynamic hormonal flux.
Supplementary Material
Acknowledgments
We thank Jessica Fluharty for technical assistance, Dr. Daniel Beiting and Dr. Ana Misic for assistance with RNA-sequencing, and Dina H. Appleby for assistance with statistical analysis. This work was supported by NIH Grants MH073030 (TLB), MH091258 (TLB), MH087597 (TLB), MH099910 (TLB and CNE), MH104184 (TLB), K12 HD085848 (TLB and CNE), R01 AG048839 (CNE), K24 DA030301 (CNE) and K23 MH092399 (DRK).
Footnotes
Financial Disclosures
Dr. Epperson reports that she consults for Asarina Pharma and on behalf of Forest Laboratories. Dr. Epperson receives research funding from SAGE Therapeutics. Dr. Epperson or her family disclose investments in the following pharmaceutical companies; Johnson and Johnson, Merck, Pfizer, Abbvie and Abbott.
The remaining authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–90. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 2.Janssen I, Krabbendam L, Bak M, Hanssen M, Vollebergh W, de Graaf R, van Os J. Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatr Scand. 2004;109:38–45. doi: 10.1046/j.0001-690x.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 3.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–25. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 4.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277:1362–8. [PubMed] [Google Scholar]
- 5.Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35:686–93. doi: 10.1016/j.psyneuen.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- 7.Dorn LD, Chrousos GP. The neurobiology of stress: understanding regulation of affect during female biological transitions. Semin Reprod Endocrinol. 1997;15:19–35. doi: 10.1055/s-2008-1067965. [DOI] [PubMed] [Google Scholar]
- 8.Babb JA, Deligiannidis KM, Murgatroyd CA, Nephew BC. Peripartum depression and anxiety as an integrative cross domain target for psychiatric preventative measures. Behav Brain Res. 2015;276:32–44. doi: 10.1016/j.bbr.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesga-López O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65:805–15. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 11.Borri C, Mauri M, Oppo A, Banti S, Rambelli C, Ramacciotti D, et al. Axis I psychopathology and functional impairment at the third month of pregnancy: Results from the Perinatal Depression-Research and Screening Unit (PND-ReScU) study. J Clin Psychiatry. 2008;69:1617–24. doi: 10.4088/jcp.v69n1012. [DOI] [PubMed] [Google Scholar]
- 12.Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14:677–84. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol. 2010;22:258–71. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 14.Brummelte S, Pawluski JL, Galea LA. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: A model of post-partum stress and possible depression. Horm Behav. 2006;50:370–82. doi: 10.1016/j.yhbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Brummelte S, Lieblich SE, Galea LA. Gestational and postpartum corticosterone exposure to the dam affects behavioral and endocrine outcome of the offspring in a sexually-dimorphic manner. Neuropharmacology. 2012;62:406–18. doi: 10.1016/j.neuropharm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. Journal of endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. Soc Endocrinology. [DOI] [PubMed] [Google Scholar]
- 17.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 18.Romeo RD, Kaplowitz ET, Ho A, Franco D. The influence of puberty on stress reactivity and forebrain glucocorticoid receptor levels in inbred and outbred strains of male and female mice. Psychoneuroendocrinology. 2013;38:592–6. doi: 10.1016/j.psyneuen.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79:125–32. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- 20.Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80:387–93. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- 21.Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry. 1997;42:680–6. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–7. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 26.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–95. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 29.Brunton PJ, Russell JA. Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat. Stress. 2011;14:6–12. doi: 10.3109/10253890.2010.482628. [DOI] [PubMed] [Google Scholar]
- 30.Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee K-FF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–9. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison KE, Narasimhan S, Fein E, Bale TL. Peripubertal Stress With Social Support Promotes Resilience in the Face of Aging. Endocrinology. 2016;157:2002–14. doi: 10.1210/en.2015-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–12. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howerton AR, Roland AV, Bale TL. Dorsal raphe neuroinflammation promotes dramatic behavioral stress dysregulation. J Neurosci. 2014;34:7113–23. doi: 10.1523/JNEUROSCI.0118-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal anti-inflammatory treatment. Endocrinology. 2014;155:2635–2646. doi: 10.1210/en.2014-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 38.Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe Z. Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. J Child Psychol Psychiatry. 2008;49:1099–107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–92. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–92. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- 41.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 4. Academic Press Inc; 2012. [Google Scholar]
- 43.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 44.Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrati D, Lonstein JS. Affective changes during the postpartum period: Influences of genetic and experiential factors. Horm Behav. 2016;77:141–52. doi: 10.1016/j.yhbeh.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 48.Murgatroyd CA, Peña CJ, Podda G, Nestler EJ, Nephew BC. Early life social stress induced changes in depression and anxiety associated neural pathways which are correlated with impaired maternal care. Neuropeptides. 2015;52:103–11. doi: 10.1016/j.npep.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragan CM, Lonstein JS. Differential postpartum sensitivity to the anxiety-modulating effects of offspring contact is associated with innate anxiety and brainstem levels of dopamine beta-hydroxylase in female laboratory rats. Neuroscience. 2014;256:433–44. doi: 10.1016/j.neuroscience.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, et al. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–9. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunton PJ. Neuroactive steroids and stress axis regulation: Pregnancy and beyond. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Brunton PJ, McKay AJ, Ochedalski T, Piastowska A, Rebas E, Lachowicz A, Russell JA. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. 2009;29:6449–60. doi: 10.1523/JNEUROSCI.0708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pérez-Cadahía B, Drobic B, Davie JR. Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol. 2011;89:61–73. doi: 10.1139/O10-138. [DOI] [PubMed] [Google Scholar]
- 54.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–7. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 55.Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, et al. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–85. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- 56.Rylski M, Kaczmarek L. Ap-1 targets in the brain. Front Biosci. 2004;9:8–23. doi: 10.2741/1207. [DOI] [PubMed] [Google Scholar]
- 57.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106:912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci USA. 2012;109:16318–23. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nisticò R, McEwen BS. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci USA. 2015;112:14960–5. doi: 10.1073/pnas.1516016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gafford GM, Guo J-DD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)α1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci USA. 2012;109:16330–5. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–84. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.