Abstract
Background
Energy homeostasis is mediated by the hypothalamus, whose inflammation‐induced functional derangements contribute to the onset of anorexia in cancer. By using functional magnetic resonance imaging (fMRI), we determined the patterns of hypothalamic activation after oral intake in anorexic (A), non‐anorexic (NA) cancer patients, and in controls (C).
Methods
Lung cancer patients were considered. Hypothalamic activation was recorded in A and NA patients and in C by fMRI, before (T0), immediately after (T1) the administration of an oral nutritional supplement, and after 15 min (T2). The grey of the hypothalamus and Blood Oxygen Level Dependent (BOLD) intensity were calculated and normalized for basal conditions. Interleukin (IL)‐1, IL‐6, tumour necrosis factor (TNF)‐α, ghrelin, and leptin plasma levels were measured. A statistical parametric mapping was used.
Results
Thirteen lung cancer patients (7 M, 6 F; 9A, 4NA) and 2 C (1 M, 1 F) were enrolled. Controls had the lowest BOLD intensity. At all‐time points, anorexic patients showed lower hypothalamic activity compared with NA (P < 0.001) (T0: 585.57 ± 55.69 vs. 667.92 ± 33.18, respectively; T1: 536.50 ± 61.70 vs. 624.49 ± 55.51, respectively; T2: 556.44 ± 58.51 vs. 615.43 ± 71.50, respectively). Anorexic patients showed greater BOLD signal reduction during T0–T1 than NA (−8.5% vs. −6.80%, P < 0.001). Independently from the presence of anorexia, BOLD signals modification before and after oral challenge correlated with basal values of IL‐1 and ghrelin (P < 0.001).
Conclusions
Hypothalamic activity in A cancer patients is reduced respect to NA and responds differently to oral challenges. This suggests a central control of appetite dysregulation during cancer anorexia, before, and after oral intake.
Keywords: Anorexia, Cancer, fMRI, Inflammation, Ghrelin, Leptin
Introduction
Anorexia, i.e. the loss of the desire to eat, is frequently found among cancer patients, and its presence negatively impacts on patients' morbidity, mortality, and quality of life.1, 2 Anorexia represents a defensive mechanism against external insults, in particular infections.3 Nevertheless, during chronic diseases including cancer, it contributes to the onset of protein‐energy malnutrition and eventually cachexia.4, 5, 6 Recent evidences indicate that energy homeostasis, i.e. the control of energy intake and expenditure, is largely mediated within the hypothalamus, and centrally produced cytokines are involved in triggering the molecular changes associated with the development of cancer‐associated anorexia and cachexia.1, 7, 8 Also, experimental models of wasting indicate that muscle proteolysis during disease is influenced by hypothalamic activity. In particular, centrally produced pro‐inflammatory cytokines appear to facilitate the activity of the hypothalamic melanocortin system toward the promotion of catabolic stimuli.7
During tumour growth, the interaction with the host immune system triggers a systemic inflammatory response, whose pathogenesis is primarily mediated by pro‐inflammatory cytokines (i.e. tumour necrosis factor (TNF)‐α, interleukin (IL)‐1, IL‐6, etc.). At the central level, it has been postulated that the inflammatory response profoundly alters the activity of the hypothalamic nuclei, which are involved in the regulation of energy homeostasis. In particular, pro‐inflammatory cytokines appear to inhibit prophagic neurons activity, while enhancing the activation of the anorexigenic neurons.7 Although supported by compelling experimental evidence, it should be acknowledged that this pathogenic hypothesis has not been confirmed yet by human studies.9 Indeed, the lack of non‐invasive tools to assess human hypothalamic activity in vivo prevented the possibility to confirm its role in human cancer anorexia. Indirect evidence, such as the close association between perturbations of sympathovagal balance and cancer patients' outcome,9 appears to confirm the involvement of the hypothalamus in the metabolic disorders of cancer. Nevertheless, the direct involvement of the central nervous system during cancer anorexia in humans remains to be clearly demonstrated. Indeed, hormonal alterations might play a relevant role in the dysregulation of food intake during cancer. In fact, several experimental and clinical data highlighted the possible role of at least two specific hormones, such as ghrelin (with prophagic effects)10 and leptin (with anorexigenic effects).11
In the past few years, a sophisticated neuroimaging technique to study in vivo neurophysiology has become available, i.e. functional magnetic resonance imaging (fMRI). By means of this technique, whose features but also limitations have been largely described,12, 13 it is possible to measure, in a non‐invasive and predictable way, the spatial and temporal patterns of brain activation/inhibition after specific stimuli. So far, this imaging modality has been used to explore the relationships between eating behaviour and neural activity. As an example, fMRI has been used to correlate gustative stimuli and cerebral activity,12 but no report exists in the literature regarding its use in cancer patients before and after food intake. Considering the influence of changes in appetite on patients' outcome,2 fMRI should be implemented in patients to obtain clinically relevant information and possibly devise therapeutic strategies.
The primary aim of the present study was to determine the baseline hypothalamic signals in anorexic and non‐anorexic cancer patients, and to measure the intensity of the hypothalamic response to a standard meal in the same population. We also aimed at revealing potential differences among patterns and at correlating them with the levels of concurrently measured circulating pro‐inflammatory cytokines.
Secondary aims were to relate the possible altered circulating levels of ghrelin, leptin, and proinflammatory cytokines to the presence of anorexia, and to the pattern of activation/inhibition of specific hypothalamic areas analysed via the fMRI.
Materials and methods
After approval of the study protocol by the Ethical Committee at our Institution (Azienda Policlinico Umberto I, Sapienza University of Rome, Italy), the study was registered on Clinicaltrials.gov (NCT01564693). Anorexic lung cancer patients, non‐anorexic lung cancer patients, and healthy individuals (control group) were studied. The sample size was determined based on previous studies of neuroimaging available in the literature12, 13 and on our preliminary observations (not published) documenting, during fMRI scansion, a hypothalamic signals variation of −9% in anorexic lung cancer patients and −7% in non‐anorexic lung cancer patients (standardized difference of 2% with a standard deviation of 1%). Therefore, a total of 13 lung cancer patients guaranteed a power of 90%, at a significance level of 5%, to detect the difference in hypothalamic activity between anorexic and non‐anorexic patients.
Patients' selection
Patients with confirmed diagnosis of non‐small cell lung cancer (NSCLC) were considered for the enrollment before the initiation of any anti‐cancer treatment, including surgery and/or chemo/radiotherapy (treatments naïve), in order to investigate the effect of cancer‐related anorexia.
The inclusion criteria were: age ≥18 years, stage of the disease IIIB or IV NSCLC, and ability to provide informed consent.
Exclusion criteria were: patients with concomitant wasting disease, such as end‐stage renal disease, liver cirrhosis or psychiatric disorders or cognitive impairment, and dysphagia or mechanical obstruction of the gastrointestinal tract. We also excluded patients with head motion (i.e. tremor disorders) and claustrophobia. All study procedures were performed in accordance with the ethical standards of the responsible institutional committee on human experimentation.
Anorexia tools
Approximately 30 min before recording hypothalamic activity, the presence/absence of anorexia was investigated using the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire, which has been recently endorsed by the European Society for Clinical Nutrition and Metabolism (ESPEN) as a reliable tool to assess disease‐associated anorexia.14 As recently shown, a FAACT score ≤ 30 is indicative of the presence of anorexia.15, 16
We also used a specific anorexia questionnaire17 investigating the presence of major symptoms, like meat aversion, taste and smell alterations, nausea and/or vomiting, and early satiety. All these symptoms interfere with eating and are likely related to deranged central nervous system regulation of feeding behaviour. The patients reporting one or more of these symptoms were considered as anorexic.17
A visual analog scale (VAS) consisting of a line of 100 mm was also utilized.18 The extremities of this line are anchored to ‘no hunger’ (0 mm) and ‘hunger’ (100 mm). Patients were asked to place a line on the VAS that corresponded to their current appetite.18
The time frame of reference for the three appetite tools was represented by the moment they were completed (current appetite). We additionally administered the self‐assessment of appetite changes, which investigates present appetite vs. appetite over the last month (increased, decreased, or unchanged).16
The percentage of food eaten by each patient at the most recent meal (ranging from 0% to 100%) was also recorded.
Functional magnetic resonance imaging and oral nutritional supplementation
On the same day of anorexia assessment, hypothalamic activation patterns were evaluated in patients and in control subjects by fMRI. Appetite intensity has been shown to fluctuate during the day.19 To minimize the influence of appetite diurnal variation, patients and controls were studied at the same time of the morning, after an overnight fast (at least 10 h). Participants kept their eyes closed during fMRI scansion, with head movement and motion‐related artifacts minimized by using foam pads, as previously described.20 After basal evaluation, all the groups received a standard oral meal, i.e. a 200 mL hypercaloric oral nutritional supplement (ONS) providing 300 Kcal (Ensure Plus, Abbott, Lake Forest, IL, USA). Immediately after the ONS intake, which took approximately 15 min, a second fMRI scan was performed for 15 consecutive minutes. During the entire fMRI scansion the Blood Oxygen Level Dependent (BOLD) intensity signals were recorded. Images were obtained using a 3.0 T MRI system (Verio, Siemens, Erlangen, Germany) equipped with magnetic field gradients of 45 mTm−1. Patients were scanned in supine position with their head placed in an eight‐channel head coil (Head matrix coil, Siemens, Erlangen, Germany). After a localizer sequence, a high resolution T1 spin echo image (TR/TE 400/8.4 ms, FOV 220 mm2, matrix 512 × 512, slice thickness 4 mm, no GAP, 15 slices, 2′50″) was acquired in sagittal plane. This sequence was performed to identify the best midsagittal slice where the hypothalamus was well assessed and used to centre the following functional scan. The functional scan consisted of a single‐slice gradient Echo‐Planar T2* weighted sequence on sagittal plane (TR/TE 707/41 ms, average: 12 FOV 215 mm2, matrix 192 × 256, a slice thickness of 10 mm, 8 s). This acquisition was repeated in the same slice for 50 times in the baseline period and 212 times after the ingestion of the oral nutritional intake, for a total scan duration time of 37 min. On the functional sequence, a circular region of interest (ROI) of 0.5 cm2 was manually drawn by one of the author (MCC) centred on the hypothalamus (Figures 1A and 1B).
Figure 1.
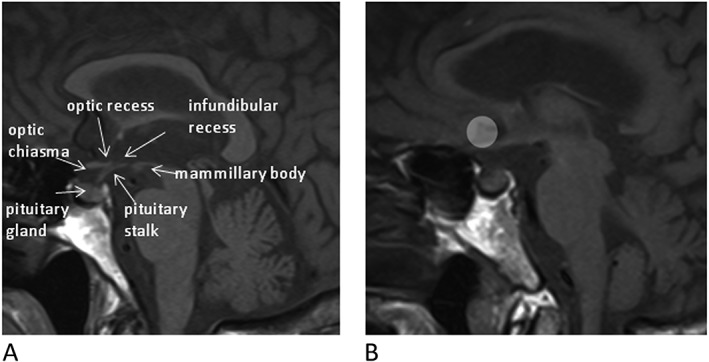
(A) Midsagittal T1 sequence. Anatomical scan of the hypothalamus‐pituitary region. (B) Midsagittal T1 sequence showing region of interest (ROI) centred on the hypothalamus.
The hypothalamic area was delineated according to anatomic landmarks, such as the optic chiasma, the mammillary bodies, and the floor of the third ventricle, as previously described.21, 22, 23 A second circular ROI of the same size was delineated in the frontal cortex, anterior of the genu of the corpus callous, as a control reference area. We considered this method as the most appropriate to observe the modifications overtime after oral intake in the whole hypothalamic area, as demonstrated in similar studies evaluating the effects of calorie intake on the hypothalamic activity.12, 22, 23 At every time point, the mean gray value in the hypothalamus was calculated, as previously shown.22, 23 Functional scans with any BOLD volume displacement >0.9 mm, or a fraction of outliers >0.19 (3dToutcount, AFNI), were rejected from analysis to prevent the influence of gross peak and mean head motion.24 This approach allows to significantly minimize signal loss because of magnetic field distortions. The imaging processing software (mean curve) used for analyses was available in the Leonardo® workstation (Siemens Medical Systems, Erlangen, Germany).
Mean values of signal intensity in the ROI were then exported on an Excel sheet (Microsoft) and used for statistical evaluation.
We analysed the BOLD signal intensity at baseline and before ONS assumption (T0, i.e. time frame 0–50), after ONS intake (T1, i.e. time frame 51–261), and at the end of the fMRI scansion (T2, i.e. time 262).
Inflammation and hormonal profile
On the day of anorexia assessment and before fMRI recording, blood samples were collected from overnight fasted cancer patients and control subjects. IL‐1, IL‐6, and TNF‐α levels were measured in duplicate by commercially available ELISA kits (Abcam, Cambridge, U.K.), as well as plasma leptin and total ghrelin levels (RayBiotech, Norcross, GA, U.S.A.)
Statistical analyses
BOLD data were first preprocessed. Images were motion‐corrected, then normalized with affine registration to an echoplanar imaging template and spatially smoothed. To better understand changes in hypothalamic activation, a ROI was identified and signal modelled only within this region. Our model included a non‐linear time effect to remove signal drift and high‐frequency variation over time. We also included an additive effect of the stimulus, a group‐specific (anorexic, non‐anorexic, and control) intercept, and, when significant, an interaction between a polynomial function of time, stimulus and/or the group indicators was included. Standard errors were obtained through a sandwich estimator to take into account dependence arising from repeated measurements on the same subjects. We then included subject‐specific serum biomarkers as covariates of the Generalized Additive Model previously defined.
Significance was evaluated through opportune Wald tests, and P values were Bonferroni adjusted for multiplicity.
Data are shown as mean ± standard deviation or median (interquartile range) when variables are not normally distributed. Percentage (%) of signals change was used to describe the time course of BOLD intensity and raw data for the analyses performed at single time point (T0, T1, T2). A P value ≤0.05 was considered statistically significant.
Results
Participants' characteristics and prevalence of anorexia
Thirteen patients and two healthy subjects, serving as controls, were studied. As previously shown, fMRI allows for obtaining robust data even if involving a limited number of subjects.12, 19, 20 Patients' anthropometric, clinical, and nutritional variables are reported in Table 1. Control group included one male and one female whose age and BMI (kg/m2) (65 ± 7.1 years; BMI: 23.95 ± 1.34, respectively) were comparable to anorexic and non‐anorexic patients.
Table 1.
Patient's characteristics according to the presence of anorexia evaluated using the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire (FAACT score ≤30)
| Anorexic (n = 9) Mean ± SD | Non‐anorexic (n = 4) Mean ± SD | |
|---|---|---|
| Gender | ||
| Male | 6 | 1 |
| Female | 3 | 3 |
| Age (years) | 71 ± 9.4 | 76 ± 6.9 |
| Height (m) | 1.69 ± 0.08 | 1.58 ± 0.14 |
| Habitual weight (kg) | 73 ± 8.2 | 61 ± 16.3 |
| % Weight loss in the previous 6 months | 11.7 ± 9.5 | 9.1 ± 8.8 |
| Body mass index (kg/m2) | 22.9 ± 2.0 | 24.2 ± 0.3 |
| Visual analog scale | 3 ± 2.2 | 9 ± 1.2* |
| % eaten | 50 ± 27.9 | 81.3 ± 37.5 |
| Appetite | ||
| Increased | – | – |
| Usual | 3 | 3 |
| Reduced | 6 | 1 |
| Anorexia questionnaire | ||
| Anorexic | 8 | 1 |
| Non‐anorexic | 1 | 3 |
| Stage | ||
| IIIB | 3 | 4 |
| IV | 6 | – |
P < 0.0001.
In particular, no significant differences were observed between anorexic and non‐anorexic patients regarding gender distribution, age, anthropometric variables, and tumour stage.
Based on the results obtained with our criterion standard for the presence of anorexia (FAACT score ≤ 30), nine patients (6 M, 3 F) were considered as anorexic and four patients (1 M, 3 F) as non‐anorexic. Moreover, anorexic patients showed significantly lower VAS values respect to non‐anorexic (3 ± 2.2 vs. 9 ± 1.2, respectively; P < 0.0001) (Table 1).
Functional magnetic resonance imaging: Blood Oxygen Level Dependent signals and their association with oral challenge
Differences between anorexic, non‐anorexic, and control at the time points (inter‐groups)
BOLD intensity at T0, T1, and T2 is shown in Table 2. The time course of BOLD intensity in the 3 groups during the entire acquisition is shown in Figure 2.
Table 2.
Blood Oxygen Level Dependent (BOLD) signal intensity (mean ± SD) in the three groups studied at different time points
| Time 0 | Time 1 | Time 2 | |
|---|---|---|---|
| Anorexic (A) | 586 ± 56 | 536 ± 62 | 556 ± 59 |
| Non‐anorexic (NA) | 668 ± 33 | 624 ± 56 | 615 ± 71 |
| Control (C) | 511 ± 79 | 542 ± 41 | 530 ± 58 |
T0: A vs. NA P < 0.001; A vs. C P < 0.001; NA vs. C P < 0.001.
T1: A vs. NA P < 0.001; A vs. C P < 0.001; NA vs. C P < 0.001.
T2: A vs. NA P < 0.001; A vs. C P < 0.001; NA vs. C P = 0.01.
Figure 2.
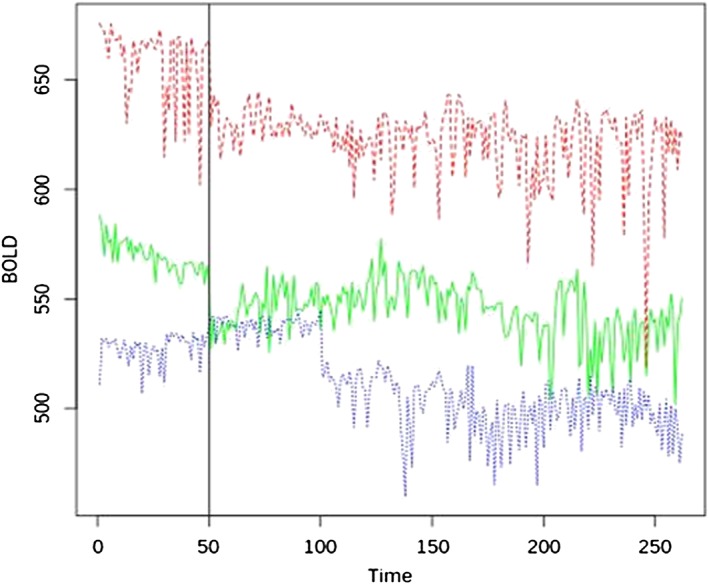
The three different lines indicate the raw BOLD in each group during functional magnetic resonance imaging scansion at baseline (time frame 0–50) and after the standard meal assumption (time frame 51–262). Red line indicates non‐anorexic cancer patients, green line indicates anorexic cancer patients, and blue line indicates control group.
We found significant differences in BOLD signal intensity between anorexic, non‐anorexic patients, and controls, which were independent from the gender. In particular, anorexic patients had significant lower BOLD signal intensity, at the three different time points, respect to non‐anorexic patients. At T0 and T2, anorexic patients had increased hypothalamic signals vs. controls (P < 0.001), while at T1 anorexic patients had reduced activity respect to control group (Table 2). Also, we observed a significantly greater BOLD signal reduction during T0–T1 period in anorexic patients respect to non‐anorexic (−8.5% vs. −6.80%, respectively; P < 0.001). Conversely, during T1–T2 period we did not find a significant difference between the increase of the BOLD signal between anorexic and non‐anorexic patients (+0.06% vs. +0.04%, respectively; P = n.s.). No significant BOLD signal changes were detected in the control area in all groups at each time point and overtime.
Differences in anorexic, non‐anorexic, and control at the time points (intra‐group)
We observed significant differences in anorexic, non‐anorexic, and control at different time‐point. Specifically, anorexic patients had increased BOLD signal intensity at T0 vs. T1, and at T0 vs. T2 (P < 0.001), while hypothalamic BOLD signal intensity was significantly reduced at T1 vs. T2 (P < 0.001) (Table 2). Moreover, during T0–T1 period, we observed a significant reduction (−8.51%, CI 8.03–9.19; P < 0.001) of BOLD signal intensity and a progressive significant signal increase during T1–T2 period (+0.06%, CI 0.05–0.07; P < 0.001).
Non‐anorexic patients had increased BOLD signal intensity at T0 vs. T1, at T0 vs. T2 (P < 0.001), and T1 vs. T2 (P < 0.001) (Table 2). Moreover, during T0–T1 period we observed a significant reduction (−6.8%, CI 5.51–7.06; P < 0.001) of BOLD signal intensity and a subsequent significant signal increase during T1–T2 period (+0.04%, CI 0.03–0.04; P < 0.001).
In control group, hypothalamic activity at T0 was significantly reduced vs. T1 (P = 0.001) and T2 (P = 0.012), whereas it was reduced at T2 vs. T1 (P = 0.006) (Table 2). Interestingly, during T0–T1 period, we also observed a significant reduction of BOLD signal intensity (−0.46%, CI 0.29–0.67; P < 0.001), as well as a significant BOLD intensity increase during T1–T2 period (+0.04%, CI 0.03–0.04; P < 0.001) (Figure 2).
Inflammation, leptin, and ghrelin
No significant differences in circulating levels of cytokines, leptin, and ghrelin were observed between the three groups. However, cytokines, leptin, and ghrelin had a significant impact on BOLD intensity both pre‐ and post‐stimulus (pre‐ and post‐ONS assumption) (Table 3). In particular, serum levels of IL‐1, IL‐6, TNF‐α, leptin, and ghrelin were significantly associated with normalized BOLD signal intensity independently from the presence/absence of anorexia and independently from the presence NSCLC (Table 3). Moreover, we observed a significant difference regarding the pre‐ and post‐stimulus effect only for IL‐1 and ghrelin levels (P < 0.001).
Table 3.
Association between biomarkers and normalized BOLD signal intensity in anorexic, non anorexic patients, and controls
| Biomarker | Values° | Pre‐stimulus effect† | Post‐stimulus effect† |
|---|---|---|---|
| IL‐1* (pg/mL) | 8.31 (0.90, 21.2) | 0.0003 (0.0001; 0.0004) | 0.0155 (0.0111; 0.0219) |
| IL‐6 * (pg/mL) | 8.25 (2.06, 11.6) | −0.0004 (−0.0004; −0.0003) | −0.0004 (−0.0004; −0.0003) |
| TNF‐α # (pg/mL) | 99.2 (3.3, 707.8) | 0.000007 (0.000006; 0.000009) | 0.000007 (0.000006; 0.000009) |
| Leptin * (pg/mL) | 542.5 (318.9, 620.1) | 0.005 (0.003; 0.006) | 0.005 (0.003; 0.006) |
| Ghrelin* (ng/mL) | 323.6 (115.0, 2524.9) | 0.00001 (0.00000; 0.00002) | −0.000025 (−0.000022; −0.000029) |
Abbreviations: interleukin‐1 (IL‐1), interleukin‐6 (IL‐6), tumour necrosis factor (TNF)‐α.
Values: Median (25th, 75th interquartile).
Blood Oxygen Level Dependent (BOLD) signal intensity (absolute value).
P‐value refers to the overall association between the biomarker and normalized BOLD; 95% CI in parentheses.
Differences between pre‐ and post‐stimulus.
P < 0.001.
P = 0.01.
Discussion
During the last decades, a number of specific physiological mechanisms have been described in different experimental settings as determinants of anorexia.25 Nevertheless, the neurocognitive mechanisms regulating food intake in cancer patients remain unclear. Recent evidence showed that anorexic cancer patients display a reduced activation in the brain regions linked to food stimuli processing.26 In particular, BOLD activation of brain areas of non‐anorexic patients, including frontal areas in the premotor and prefrontal cortices, was observed only during stimulation by unpleasant food. In contrast, anorexic cancer patients showed no brain activation during stimulation by either pleasant or unpleasant food images.26
Our study extends these previous observations by assessing for the first time in anorexic and non‐anorexic cancer patients and control, the effects on hypothalamic function of a standard meal during a prolonged observational time. Our dynamic observation included a baseline recording, to specifically assess possible neurophysiological modifications after food intake.
The specifically designed protocol of our study allowed revealing significant different hypothalamic BOLD intensities in the three groups. Anorexic cancer patients showed a significant reduction of the hypothalamic signals from baseline to immediately after meal intake when compared with non‐anorexic patients. Interestingly, anorexic patients presented increased BOLD activity at baseline and at the end of the observation period when compared with the control group, while non‐anorexic patients showed the highest BOLD activity at baseline. Also, anorexic patients had significantly reduced BOLD signals at T1 respect to non‐anorexic and control. This evidence appears clinically relevant and provides neurophysiological background to the impaired appetite of anorexic cancer patients at baseline and to the frequently observed worsening of appetite after meal.
The results of our study demonstrate that anorexic, non‐anorexic cancer patients, and healthy controls react differently to the same food challenge. In anorexic cancer patients, hypothalamic activity at baseline was significantly higher than those after food intake and at the end of fMRI scansion. Also, BOLD activity after the meal was significantly lower than that at the end of fMRI recording. Hypothalamic activity of anorexic cancer patients behaved similarly to non‐anorexic patients during the periods T0–T1 and T0–T2. However, and in contrast with non‐anorexic cancer patients, BOLD signals of anorexic cancer patients in the period T1–T2 increased significantly, which suggests enhanced anorexigenic stimuli in response to food intake. During the period T0–T1, BOLD activity in anorexic and non‐anorexic cancer patients was significantly reduced, but the reduction observed in anorexic patients was more robust than that observed in non‐anorexic patients. The increase of BOLD signal during the period T1–T2 was not statistically different between the anorexic and non‐anorexic patients.
Interestingly, although at baseline healthy subjects presented with the lowest BOLD signal activity respect to cancer patients (independently from the presence/absence of anorexia), they responded to food intake similarly to non‐anorexic patients, reducing hypothalamic activity from the period immediately after meal intake to the end of the fMRI scansion. Also, hypothalamic signals in control subjects decreased during the period T0–T1, and increased, as percent of variation, during the entire period T1–T2. This evidence might suggest a different response of cancer patients to food challenge respect to healthy subjects.
We acknowledge that cancer anorexia results from the complex interaction between activated hypothalamic nuclei and inhibited areas.7, 9 The imaging modality we used does not allow to capture such a complexity. In this respect, our data do not allow to identify cancer anorexia as a perturbation of eating behaviour related either to activation or inhibition of hypothalamic activity. In addition, studies reported that it is possible to observe different connectivity profiles in the hypothalamus region when using two seeds in this area corresponding to a medial and lateral sector of the hypothalamus.27 This method was able to assess neural response to emotional stimuli.27 In our study, we did not aim to evaluate differences in term of connectivity in different sectors of the hypothalamus. In this light, we analysed the whole hypothalamic area which was considered a reliable method to evaluate differences in BOLD activity before and after oral calorie intake.12, 22, 23
Our results strongly point at a functional association between the presence of anorexia and differences in BOLD intensity. In particular, cancer patients showed that reduced appetite is associated with impaired hypothalamic activity. It is understood that, by using a standard meal, we could not assess the relative influences of personal food preferences on BOLD signals.
We acknowledge that food preferences and food aversion may contribute to the severity of cancer‐associated anorexia. However, they may also confound the interpretation of the results when the aim of the study is to clarify the relation between being anorexic and impaired hypothalamic activity during cancer.
As previously demonstrated, both in humans28 and in experimental models,29, 30, 31 proinflammatory cytokines, including IL‐1, IL‐6, and TNF‐α are involved in the pathophysiology of cancer anorexia. Particularly, IL‐1 levels were significantly increased in the cerebrospinal fluid of anorexic tumour‐bearing rats and inversely correlated with energy intake.32 Inhibition of TNF‐α activity, by the administration of the recombinant human soluble TNF receptor, resulted in amelioration of food intake in experimental cancer anorexia.33 In our study, we could not find significant differences in the levels of IL‐1, IL‐6, and TNF‐α between anorexic, non‐anorexic patients, and healthy subjects. Conversely, the circulating levels of cytokines were significantly associated with normalized BOLD signals before and after oral challenge in all individuals enrolled for this study, independently from the presence/absence of anorexia and from the presence of cancer. The significant association with BOLD signals was also found for leptin and ghrelin levels. Leptin reduces appetite and increases energy expenditure via its effects on the central nervous system, and in particular via its functional interplay with hypothalamic neuropeptides downstream of leptin signalling regulating food intake and energy homeostasis.34 Similarly, ghrelin levels have been reported to be significantly increased during secondary anorexia, which in turn may reduce food intake by mechanisms of action involving the hypothalamus and the stomach.35
We acknowledge the limitations of our study. We included a small number of patients, and particularly of healthy individuals, possibly limiting the interpretation of our results, although similar experiences using fMRI to investigate the function of the hypothalamus included comparable number of participants.12, 22, 23, 26 Moreover, we have not prospectively assessed the performance of anorexia instruments to determine whether they are sensitive to changes in appetite or food intake.
Functional magnetic resonance imaging might not represent the most accurate methodology to evaluate changes in hypothalamic activity, particularly after calorie intake,36 when compared with brain perfusion measures, such as hypothalamic regional cerebral blood flow,37 or other methods for quantification of the hypothalamic intrinsic oscillations.38
Finally, we did not find significant differences between anorexic and non‐anorexic cancer patients regarding baseline values of BMI and body weight change. This might indicate low accuracy of our instruments in selecting the two groups of patients. However, we specifically aimed at investigating the neural implication of the presence/absence of cancer anorexia, independently from the presence of involuntary body weight loss.
In conclusion, the results obtained indicate the crucial role of the central nervous system and, in particular of the hypothalamus, in the pathogenesis of cancer anorexia.
Data obtained may be extremely useful to provide neurophysiological basis for the use of novel therapeutic strategies against cancer anorexia, particularly, for the use of specific molecules, such as ghrelin and ghrelin mimetics, able to improve food intake via the direct effects on the central nervous system.39
This project was supported by the Italian Society of Internal Medicine (SIMI) (Research Grant to Dr. Alessio Molfino).
Dr. Alessio Molfino received a research fellowship (Young Investigator Programme 2012–2013) by Fondazione Umberto Veronesi, Italy.
Conflict of interest
None declared.
Acknowledgements
We thank Dr. Patrizia Seminara, M.D., for her important support in enrolling cancer patients.
We thank Angelo Pittalis, radiology technician, for his valuable help in collecting fMRI data.
We thank Cesarina Ramaccini, lab technician, for her valuable help in measuring serum biomarkers.
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.40
Molfino, A. , Iannace, A. , Colaiacomo, M. C. , Farcomeni, A. , Emiliani, A. , Gualdi, G. , Laviano, A. , and Rossi Fanelli, F. (2017) Cancer anorexia: hypothalamic activity and its association with inflammation and appetite‐regulating peptides in lung cancer. Journal of Cachexia, Sarcopenia and Muscle, 8: 40–47. doi: 10.1002/jcsm.12156.
References
- 1. Laviano A, Meguid MM, Inui A, Muscaritoli M. Rossi Fanelli F. Therapy insight: cancer anorexia–cachexia syndrome—when all you can eat is yourself. Nat Clin Pract Oncol 2005;2:158–65. [DOI] [PubMed] [Google Scholar]
- 2. Molfino A, Laviano A, Rossi FF. Contribution of anorexia to tissue wasting in cachexia. Curr Opin Support Palliat Care 2010;4:249–53. [DOI] [PubMed] [Google Scholar]
- 3. Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol 2009;7:e1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95. [DOI] [PubMed] [Google Scholar]
- 5. Braun TP, Marks DL. Pathophysiology and treatment of inflammatory anorexia in chronic disease. J Cachexia Sarcopenia Muscle 2010;1:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle 2015;6:28–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Molfino A, Rossi Fanelli F, Laviano A. The interaction between pro‐inflammatory cytokines and the nervous system. Nat Rev Cancer 2009;9:224. [DOI] [PubMed] [Google Scholar]
- 8. Cooper C, Burden ST, Cheng H, Molassiotis A. Understanding and managing cancer‐related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle 2015;6:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laviano A, Inui A, Marks DL, Meguid MM, Pichard C, Rossi Fanelli F, et al. Neural control of the anorexia–cachexia syndrome. Am J Physiol Endocrinol Meta 2008;295:E1000–8. [DOI] [PubMed] [Google Scholar]
- 10. DeBoer MD. Ghrelin and cachexia: will treatment with GHSR‐1a agonists make a difference for patients suffering from chronic wasting syndromes? Mol Cell Endocrinol 2011;340:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engineer DR, Garcia JM. Leptin in anorexia and cachexia syndrome. Int J Pept 2012;2012:287457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr 2006;83:1297–305. [DOI] [PubMed] [Google Scholar]
- 13. Li W, Lai TM, Bohon C, Loo SK, McCurdy D, Strober M, et al. Anorexia nervosa and body dysmorphic disorder are associated with abnormalities in processing visual information. Psychol Med 2015;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia–anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–9. [DOI] [PubMed] [Google Scholar]
- 15. Arezzo di Trifiletti A, Misino P, Giannantoni P, Giannantoni B, Cascino A, Fazi L, et al. Comparison of the performance of four different tools in diagnosing disease‐associated anorexia and their relationship with nutritional, functional and clinical outcome measures in hospitalized patients. Clin Nutr 2013;32:527–32. [DOI] [PubMed] [Google Scholar]
- 16. Molfino A, Kaysen GA, Chertow GM, Doyle J, Delgado C, Dwyer T, et al. Validating appetite assessment tools among patients receiving hemodialysis. J Ren Nutr 2016;26:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cangiano C, Cascino A, Ceci F, Laviano A, Mulieri M, Muscaritoli M, et al. Plasma and CSF tryptophan in cancer anorexia. J Neural Transm Gen Sect 1990;81:225–33. [DOI] [PubMed] [Google Scholar]
- 18. Iyer S, Taylor‐Stokes G, Roughley A. Symptom burden and quality of life in advanced non‐small cell lung cancer patients in France and Germany. Lung Cancer 2013;81:288–93. [DOI] [PubMed] [Google Scholar]
- 19. Kim TW, Jeong JH, Hong SC. The impact of sleep and circadian disturbance on hormones and metabolism. Int J Endocrinol 2015;2015:591729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eiler WJ 2nd, Džemidžić M, Case KR, Soeurt CM, Armstrong CL, Mattes RD, et al. The apéritif effect: alcohol's effects on the brain's response to food aromas in women. Obesity 2015;23:1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999;48:1801–6. [DOI] [PubMed] [Google Scholar]
- 22. Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage 2005;24:363–8. [DOI] [PubMed] [Google Scholar]
- 23. Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr 2005;82:1011–6. [DOI] [PubMed] [Google Scholar]
- 24. Jiang A, Kennedy DN, Baker JR, WeisKoff RM, Tootell RBH, Woods RP, et al. Motion detection and correction in functional MR imaging. Hum Brain Mapp 1995;3:224–35. [Google Scholar]
- 25. Gordon JN, Green SR, Goggin PM. Cancer cachexia. QJM 2005;98:779–88. [DOI] [PubMed] [Google Scholar]
- 26. Sánchez‐Lara K, Arrieta O, Pasaye E, Laviano A, Mercadillo RE, Sosa‐Sánchez R, et al. Brain activity correlated with food preferences: a functional study comparing advanced non‐small cell lung cancer patients with and without anorexia. Nutrition 2013;29:1013–9. [DOI] [PubMed] [Google Scholar]
- 27. Kullmann JS, Grigoleit JS, Lichte P, Kobbe P, Rosenberger C, Banner C, et al. Neural response to emotional stimuli during experimental human endotoxemia. Hum Brain Mapp 2013;34:2217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mantovani G, Macciò A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Crit Rev Oncog 1998;9:99–106. [DOI] [PubMed] [Google Scholar]
- 29. Laviano A, Molfino A, Seelaender M, Frascaria T, Bertini G, Ramaccini C, et al. Carnitine administration reduces cytokine levels, improves food intake, and ameliorates body composition in tumor‐bearing rats. Cancer Invest 2011;29:696–700. [DOI] [PubMed] [Google Scholar]
- 30. Molfino A, De Luca S, Muscaritoli M, Citro G, Fazi L, Mari A, et al. Timing of antioxidant supplementation is critical in improving anorexia in an experimental model of cancer. Int J Food Sci Nutr 2013;64:570–4. [DOI] [PubMed] [Google Scholar]
- 31. Molfino A, Logorelli F, Citro G, Bertini G, Ramaccini C, Bollea MR, et al. Stimulation of the nicotine antiinflammatory pathway improves food intake and body composition in tumor‐bearing rats. Nutr Cancer 2011;63:295–9. [DOI] [PubMed] [Google Scholar]
- 32. Opara EI, Laviano A, Meguid MM, Yang ZJ. Correlation between food intake and CSF IL‐1 alpha in anorectic tumor bearing rats. Neuroreport 1995;6:750–2. [DOI] [PubMed] [Google Scholar]
- 33. Torelli GF, Meguid MM, Moldawer LL, Edwards CK 3rd, Kim HJ, Carter JL, et al. Use of recombinant human soluble TNF receptor in anorectic tumor‐bearing rats. Am J Physiol 1999;277:R850–5. [DOI] [PubMed] [Google Scholar]
- 34. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. [DOI] [PubMed] [Google Scholar]
- 35. Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005;54:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Purnell JQ, Klopfenstein BA, Stevens AA, Havel PJ, Adams SH, Dunn TN, et al. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes Metab 2011;13:229–34. [DOI] [PubMed] [Google Scholar]
- 37. Page KA, Chan O, Arora J, Belfort‐Deaguiar R, Dzuira J, Roehmholdt B, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 2013;309:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kilpatrick LA, Coveleskie K, Connolly L, Labus JS, Ebrat B, Stains J, et al. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology 2014;146:1212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo‐controlled, double‐blind trials. Lancet Oncol 2015;16:108–16. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


