Abstract
Background
Post-stroke mortality is higher among residents of disadvantaged neighborhoods, but it is not known whether neighborhood inequalities are specific to stroke survival or similar to mortality patterns in the general population. We hypothesized that neighborhood disadvantage would predict higher post-stroke mortality and neighborhood effects would be relatively larger for stroke patients than for individuals with no history of stroke.
Methods and Results
Health and Retirement Study participants aged 50+ without stroke at baseline (n=15,560) were followed up to 12 years for incident stroke (1,715 events over 159,286 person-years) and mortality (5,325 deaths). Baseline neighborhood characteristics included objective measures based on census tracts (family income, poverty, deprivation, residential stability, and percent white, black or foreign-born) and self-reported neighborhood social ties. Using Cox proportional hazard models, we compared neighborhood mortality effects for people with versus without a history of stroke. Most neighborhood variables predicted mortality for both stroke patients and the general population in demographic-adjusted models. Neighborhood percent white predicted lower mortality for stroke survivors (HR=0.75 for neighborhoods in highest 25th percentile vs. below, 95 % CI: 0.62, 0.91) more strongly than for stroke-free adults (HR=0.92 (0.83, 1.02); p=0.04 for stroke-by-neighborhood interaction). No other neighborhood characteristic had different effects for people with versus without stroke. Neighborhood-mortality associations emerged within three months after stroke, when associations were often stronger than among stroke-free individuals.
Conclusions
Neighborhood characteristics predict post-stroke mortality, but most effects are similar for individuals without stroke. Eliminating disparities in stroke survival may require addressing pathways that are not specific to traditional post-stroke care.
Keywords: stroke, socioeconomic position, mortality, community, psychology and behavior, neighborhood, social ties, social support, social networks, stroke incidence
Journal Subject Terms: Cerebrovascular Disease/Stroke, Epidemiology, Lifestyle, Primary Prevention, Secondary Prevention
Introduction
Despite declines in overall stroke mortality and case-fatality rates1, improving long-term survival of stroke patients and eliminating racial, socioeconomic, and geographic disparities in stroke outcomes remains a major public health priority2. A growing body of evidence suggests that neighborhood context is associated with stroke incidence and mortality after stroke;3–15 for example, not only does a patient’s own socioeconomic position (SEP) predict higher mortality following stroke,16 but so does the average SEP of his or her neighbors.
The association between neighborhood SEP and post-stroke mortality is not surprising because neighborhood SEP predicts mortality in the general population17. It is not known, however, whether the association between neighborhood SEP and mortality among stroke survivors is stronger, weaker, or similar to neighborhood-mortality associations that prevail in the general population. If the magnitudes of the neighborhood-mortality associations are comparable for stroke survivors and stroke-free adults, this may suggest shared pathways and intervention points. Neighborhood effects on post-stroke survival may be even stronger than neighborhood effects in the general population, however, due to disparities in access and quality of acute care, rehabilitation services, and post-stroke care pathways.
We hypothesized that lower neighborhood SEP, lower residential stability, higher concentrations of minorities, and weaker social ties would predict worse post-stroke survival, and that this mortality disadvantage would be stronger (worse) in relative terms for individuals who had survived a stroke compared to individuals with no history of stroke.
Materials and Methods
We used prospective cohort data from Health and Retirement Study (HRS)18, 19 participants born 1900 to 1947 included in the 1998 assessment. Biennial interviews by telephone or in person, were conducted through 2010 (retention rates >80%). HRS was approved by the University of Michigan Health Sciences Human Subjects Committee and these analyses were approved by Harvard School of Public Health Office of Human Research Administration.
From the age-eligible sample (n=19,991), we excluded those with history of stroke at baseline (n=1,211) and with missing or implausible stroke date information (n=64). An additional 2,889 participants were excluded due to missing covariates for neighborhood social ties (n=1,666), friend information (n=8), race (n=1), marital status (n=22), education (n=54), physical activity (n=9), functional impairment (n=27), body mass index (BMI) (n=229), alcohol intake (n=3), smoking (n=120), blood pressure (BP) (n=293), diabetes (n=85), self-reported health (n=5), Census tract (n=12), and residential stability (n=355). Additionally, 267 individuals were excluded due to loss to follow-up prior to first exposure wave, leaving a final analytic sample of 15,560. In supplemental analyses, we used multiple imputation to compare models retaining individuals with partial covariate missingness, resulting in an analytic sample of 17,960.
Outcome: All-cause mortality
Mortality was obtained via linkage to the National Death Index (NDI) through 2008. If NDI information was missing we used exit interview information from proxies.
Effect Modifier: Incident Stroke
We evaluated stroke as a modifier of the association between neighborhood and mortality. First stroke was based on time-updated self- or proxy-report of a doctor’s diagnosis (“Has a doctor ever told you that you had a stroke?”). No information on transient ischemic attacks, stroke subtypes, or stroke severity was available. Interviews were conducted with proxy informants (<15%), predominantly spouses, for participants not available for direct interviews (e.g., due to death). Proxy interviews were included by design in HRS to avoid bias due to excluding respondents with low cognitive function or declining health; previous evaluations of HRS indicate that inclusion of proxy reports reduces bias due to attrition, and raises response rates.20–22 This outcome was validated using respondents with data that could be linked to records from the Centers for Medicare and Medicaid Services (CMS) (n=6,223 aged 65+ not enrolled in Medicare Health Maintenance Organizations); the self/proxy reported stroke outcome had a sensitivity of 74% and specificity of 93% to detect strokes as recorded in CMS (or 79% sensitivity and 91% specificity for identifying strokes recorded as the primary diagnosis on CMS records). Sensitivity and specificity was similar across sociodemographic and health factors (see Appendix Table 1). Respondents reported month and year of stroke diagnosis, used to calculate time since stroke in secondary analyses. We classified mortality of stroke patients based on time since stroke: less than 3 months, 3–12 months, and more than 12 months (compared to those who did not experience stroke).
Exposure: Neighborhood Environment
We considered three domains of neighborhood measures: social ties to neighbors, neighborhood SEP, and neighborhood demographic composition.
Social ties to neighbors were assessed based on presence of friends and (separately) relatives via this item “Do you have any close friends (relatives) in the neighborhood?” Neighborhood-based social interactions were assessed by two items: “Do you get together with any of your neighbors for social reasons?” and “How often do you get together with neighbors per month?” These two items were combined and dichotomized at one or more times/month (versus zero). We then created an index by averaging these three dichotomous variables (each coded 0, 1), for an index ranging 0–1, with higher values denoting better social integration.
We geocoded participants’ 1998 addresses and linked to 1990 Census tract data for the remainder of neighborhood variables described below. Using census tracts to proxy for neighborhood definitions is common and valid, since tracts correspond roughly to a spatial unit of a neighborhood.23–25 We chose the functional form of the variables (i.e., quartiles vs. binary breaks) based on preliminary bivariate associations. Neighborhood SEP was measured as average tract family income (in quartiles, modeled ordinally), % of residents below the poverty line (dichotomized at the sample’s 75th percentile, above 17.7% poor), and an index of deprivation (in quartiles, modeled ordinally). We derived a deprivation score from a principal components analysis of five census-based deprivation variables including % households in poverty, % unemployed civilians aged 16+, % households receiving public assistance, % female-headed households with children, and % persons aged 25+ with less than a high school education26, 27.
Finally, we examined Census-tract measures of neighborhood demographics: % residents who identified as non-Hispanic (NH) black (dichotomized at sample’s 75th percentile: 12.7%), % NH white (dichotomized at sample’s 25th percentile: 61.3%), % foreign born (dichotomized at sample’s 95th percentile, 23.0%). Neighborhood residential stability was defined as % of residents living at the same address 5 years ago (dichotomized at sample’s 25th percentile: 44.7%). To avoid bias if stroke caused individuals to move to different types of neighborhoods, we did not time-update neighborhood characteristics; all are based on 1998 residence, when everyone was stroke-free.
Covariates
All covariates are measured at the individual level, and reported prospectively in the 1998 (our baseline) survey. Demographic variables included race/ethnicity (non-Hispanic white, non-Hispanic black, Latino/Hispanic, or non-Hispanic other), baseline age, gender, birth in a southern state, marital status, and nativity. Individual-level SEP was measured by self-reported own years of completed education, parental education, self-reported household income and (separately) household wealth in 1998. Income and wealth were equivalized for household size. Behavioral risk factors included smoking status; vigorous physical activity; and weekly alcohol use. Health conditions included BMI and self-rated health. Comorbidities/chronic health problems included self-reported diagnoses of diabetes and (separately) of hypertension; elevated depressive symptoms (measured by a modified 8-item Center for Epidemiological Studies Depression (CES-D) Scale, modeled as binary, <3 vs. ≥3)28; limitations in activities of daily living (ADLs: needing help to get across a room, dress, bathe, eat, get in and out of bed, or use the toilet) and, separately, instrumental activities of daily living (IADLs : needing help to prepare meals, make telephone calls, shop for groceries, or take medications), each recoded as any vs. none. See Table 1 for additional coding detail.
Table 1.
Baseline Sample Characteristics and Incident Disease: HRS 1998 (Unweighted).
| n/mean/median | %/SD/Q | |
|---|---|---|
| Total (n, %) | 15,560 | 100% |
| Incident stroke by 2010 (n,%) | 1,715 | 11.0% |
| Mortality by 2010 (n, %) | 5,325 | 34.2% |
| Years of follow-up 1998–2010 (mean) | 10.2 | |
| Total person-years of follow-up 1998–2010 (sum) | 159,286 | |
| Demographic Variables | ||
| Age in 1998 (mean, SD) | 66.2 | 10.0 |
| Male (n, %) | 6,700 | 43.1% |
| Race/Ethnicity (n, %) | ||
| Non-Hispanic White (n, %) | 12,002 | 77.1% |
| Non-Hispanic Black (n, %) | 2,160 | 13.9% |
| Hispanic (n, %) | 1,097 | 7.1% |
| Non-Hispanic Other * (n, %) | 301 | 1.9% |
| Married (vs. widowed/divorced/never married) (n, %) | 10,584 | 68.0% |
| Foreign-born (n, %) | 1,355 | 8.7% |
| Southern birth state (n, %) | 5,534 | 35.6% |
| Socioeconomic Variables | ||
| Parental education † ≥ 8 yrs (n, %) | 10,905 | 70.1% |
| Years of education attained (mean, SD) | 12.1 | 3.2 |
| Equivalized household income ‡ (mean, SD) | $34,813 | $54,429 |
| Equivalized household wealth ‡ (mean, SD) | $232,184 | $890,808 |
| Behavioral Risk Factors | ||
| Vigorous Physical Activity (≥3 times/wk) (n, %) | 6,973 | 44.8% |
| Body Mass Index (BMI) | ||
| Normal weight (BMI<25) (n, %) | 5,897 | 37.9% |
| Overweight (25 <=BMI< 30) (n, %) | 6,106 | 39.2% |
| Obese (BMI >= 30) (n, %) | 3,557 | 22.9% |
| Alcohol use (past week) | ||
| No alcohol use (n, %) | 10,687 | 68.7% |
| Moderate alcohol use (1–2 days drinking) (n, %) | 2,322 | 14.9% |
| Heavy alcohol use (≥3 days drinking) (n, %) | 2,551 | 16.4% |
| Tobacco Use | ||
| Never smoker (n, %) | 6,373 | 41.0% |
| Current Smoker (n, %) | 2,582 | 16.6% |
| Former Smoker (n, %) | 6,605 | 42.5% |
| Activities of Daily Living (≥1 limitation) (n, %) | 2,177 | 14.0% |
| Instrumental Activities of Daily Living (≥1 limitation) (n, %) | 1,801 | 11.6% |
| Chronic Conditions | ||
| CES-D Depressive Symptom Score (mean, SD) | 1.5 | 1.8 |
| Fair/Poor (v.s. excellent/very good/good) Self-Assessed Health (n, %) | 4,453 | 28.6% |
| Hypertension (n, %) | 7,231 | 46.5% |
| Diabetes (n, %) | 2,135 | 13.7% |
| Neighborhood Variables | ||
| 1990 Census Variables | ||
| neighborhood deprivation score (1990) | −0.013 | 0.91 |
| neighborhood family income (1990) (mean, SD) | $42,476 | $20,999 |
| neighborhood % poverty (mean, SD) | 13.4% | 11.8 |
| neighborhood % black (mean, SD) | 13.9% | 25.4 |
| neighborhood % non-hispanic white (mean, SD) | 74.1% | 30.6 |
| neighborhood % foreign-born (mean, SD) | 6.3% | 9.3 |
| neighborhood % residential stability (5+ years) (mean, SD) | 52.3% | 12.1 |
| 75th percentile neighborhood % poverty (>17.7%) (n) | 3897 | |
| 75th percentile neighborhood % black (> 12.7%) (n) | 3883 | |
| 75th percentile neighborhood % white (> 61.3%) (n) | 3890 | |
| 95th percentile neighborhood % foreign born (> 23.0%) (n) | 778 | |
| 25th percentile neighborhood % residential stability (> 44.7%) (n) | 11670 | |
| Survey Based Variables | ||
| Any Relatives in the neighborhood (n, %) | 4,654 | 29.9% |
| Any Close friends in the neighborhood (n, %) | 11,061 | 71.1% |
| Any Monthly contact with neighbors (n, %) | 11,759 | 75.6% |
| 3-Item Neighborhood Social Ties Index (mean, SD) | 0.59 | 0.30 |
Notes: Baseline defined in 1998 for this analytic sample. All variables except stroke were defined in 1998. Sample members had never experienced stroke at baseline (1998). SD = standard deviation; Q1 = first quartile, Q3 = 3rd quartile, CES-D=Center for Epidemiologic Studies Depression Scale.
Non-Hispanic other race/ethnicity was combined with the Non-Hispanic White group in regression models, due to small sample size.
Parental education modeled as the highest education of the two parents.
Income and wealth were equivalized by dividing by the square root of the number of household members.
Analyses
We applied Cox proportional hazard survival regression models for the outcome of death, measured by continuous failure time as date of death, or right censoring as the last contact date before loss to follow-up, or the 2010 survey. We estimated several sets of models; each neighborhood variable was always modeled one at a time. The first set of models tested the main effects of neighborhood context on mortality, first adjusted for stroke and demographic covariates (Model 1); in Model 2 we then added CVD risk factors including health behaviors (physical activity, alcohol use, tobacco use), health conditions (obesity, self-rated health), and comorbidities/chronic health problems (depressive symptoms, hypertension, diabetes, functional impairment (ADL, IADL)), in addition to demographics and stroke, but not individual-level SEP. Model 3 built on Model 1 to add individual-level SEP, in addition to stroke and demographics, but not CVD risk factors. Model 4 included all covariates simultaneously (stroke, demographic, health behaviors, health conditions, and comorbidities/chronic health problems, individual SEP). Extensive evidence suggests that neighborhood disadvantage influences health behaviors and comorbid conditions, so we consider models adjusted for these covariates to underestimate the total effects of neighborhood on mortality.
A second set of models estimated a covariate-adjusted association between incident stroke and mortality excluding neighborhood variables, using the same model-building strategy above (Models 1 and 4) (reported in the text).
The third set of models tested our primary hypothesis of equivalent effects for stroke patients and stroke-free individuals by specifying a stroke-neighborhood interaction predicting mortality, adjusted for demographic covariates (Model 1) and for all covariates (Model 4). We present the p-value from those interaction tests and effect estimates (and 95 % CI) from pooled interaction models of neighborhood associations with mortality for people with and people without history of stroke.
To test whether associations between neighborhood environment and mortality depended on time since stroke, we interacted neighborhood with time since stroke indicator variables, and report associations of neighborhood on mortality within each time since stroke stratum.
We confirmed the proportional hazards assumptions held for the main effects models and directly evaluated heterogeneity in effects for time since stroke models. We used SAS 9.3 (Cary, NC) PROC PHREG and accounted for clustering of individuals in tracts using robust sandwich estimators29. We applied HRS sampling weights to render the sample representative of the 1998 US population aged 50+ years. We estimated a subset of our models using multiple imputation to retain individuals with partially missing data and found substantively identical results.
Results
In our sample (N=15,560), 1,715 participants (11.0%) experienced stroke, and 5,325 participants died (34.2%), from 1998–2010. Mean follow-up time was 10.2 years, and the cohort accrued 159,286 person years of follow-up (Table 1).
Main Effects of Stroke on Mortality
After adjustment for baseline demographic covariates, respondents who had ever experienced a stroke had 2-fold higher mortality risk, hazard ratio (HR)=2.17 (95 % CI: 2.00, 2.36). This association declined to 1.90 (95 % CI: 1.74, 2.08) after adjustment for all covariates. These associations were consistent regardless of the neighborhood variable modeled.
Main Effects of Neighborhood Context on Mortality
Figure 1 and Appendix Table 2 present the mortality HRs associated with neighborhood characteristics, adjusted for stroke and covariates, for the entire follow-up, pooled across stroke status. We found no statistically-significant evidence of proportional hazards violations in any model; p>0.15 for all tests. After demographic adjustment (Model 1), neighborhood social ties, higher neighborhood family income, and high neighborhood % white all predicted lower mortality. For example, those living in the highest (best) neighborhood family income quartile (Quartile 4) experienced 24% lower mortality (HR=0.76, 95 % CI: 0.70, 0.83), than those living in the lowest neighborhood family income quartile (Quartile 1)(p for trend <0.0001). As hypothesized, participants living in higher neighborhood deprivation and higher neighborhood poverty had significantly higher mortality risk.
Figure 1. Main Effects of Neighborhood Context on Hazard Ratio of Mortality.
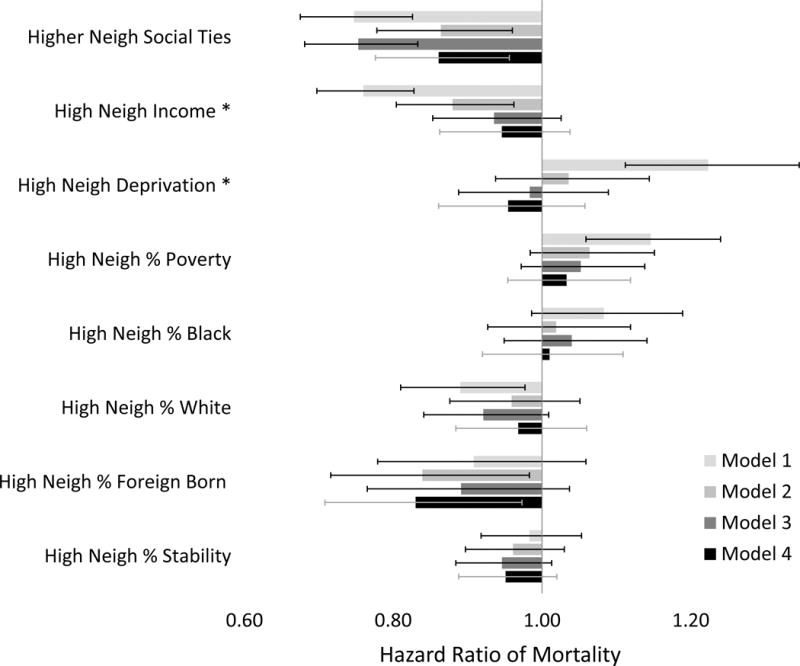
Estimates, confidence intervals, and p-values reported in Appendix Table 2. All models adjusted for stroke status. Model 1 adjusted for demographic variables. Model 2 adjusted for demographics plus CVD risk factors. Model 3 adjusted for demographics plus SEP variables. Model 4 adjusted for demographic, CVD, and SEP variables. * Neighborhood family income and neighborhood deprivation are modeled in quartiles modeled ordinally; hazard ratio models a change from 4th vs. 1st quartiles.
Higher neighborhood family income predicted lower mortality even after adjusting for CVD risk factors (Model 2, HR for highest vs. lowest quartile neighborhood income=0.88, 95 %CI: 0.80, 0.96; p for linear trend=0.005). Other neighborhood SEP variables no longer significantly predicted mortality in Model 2, and mortality associations with all three neighborhood SEP variables were also attenuated after adjusting for individual-level SEP (Models 3, 4). However, neighborhood social ties (HR=0.86, 95 % CI 0.78, 0.96) and neighborhoods with high proportions of immigrants (HR=0.83, 95 % CI: 0.71, 0.97) significantly predicted lower mortality after adjustment for stroke, demographics, CVD risk factors, and individual SEP (Figure 1, Model 4).
Effect Modification of Neighborhood Context-Mortality Association by Stroke
Neighborhood social ties predicted significantly lower mortality for stroke patients (HR=0.76, 95 % CI: 0.59, 0.99) as well as for individuals with no history of stroke (HR=0.74, 95 % CI: 0.67, 0.83) after demographic adjustment; effect estimates were statistically comparable (stroke interaction with neighborhood social ties p=0.87) (Table 2, Model 1). Likewise, there were significant protective effects of neighborhood family income on mortality after demographic adjustment (Model 1, comparing 4th to 1st quartile), among both stroke-free (Model 1 HR=0.70, 95 % CI: 0.58, 0.85) and stroke populations (Model 1 HR=0.77, 95 % CI: 0.70, 0.85); effects were homogeneous by stroke (interaction p=0.36). Significantly harmful patterns were observed for other measures of neighborhood SEP (neighborhood poverty; deprivation) in Model 1, again with similar patterns by stroke. However, these mortality-neighborhood SEP associations were attenuated after adjustment for individual-level SEP (Table 2, Model 4).
Table 2.
Stratum-Specific Estimates of Neighborhood Context on Survival (Hazard Ratios of Mortality) within Strata of Ever-Stroke Status.
| Model 1 | Model 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ever Stroke | Never Stroke | Inter-action | Ever Stroke | Never Stroke | Inter-action | |||||||||
| HR | 95 % CI | HR | 95 % CI | p | HR | 95 % CI | HR | 95 % CI | p | |||||
| Higher Neighborhood Social Ties | 0.76 | (0.59 | ,0.99) | 0.74 | (0.67 | ,0.83) | 0.87 | 0.93 | (0.71 | ,1.21) | 0.85 | (0.76 | ,0.95) | 0.53 |
| Higher Neighborhood Family Income (ordinal, 4th vs. 1st Q) | 0.70 | (0.58 | ,0.85) | 0.77 | (0.70 | ,0.85) | 0.36 | 0.89 | (0.72 | ,1.10) | 0.96 | (0.87 | ,1.06) | 0.54 |
| Higher Neighborhood Deprivation (ordinal, 4th vs. 1st Q) | 1.22 | (0.99 | ,1.50) | 1.22 | (1.11 | ,1.36) | 0.96 | 0.98 | (0.79 | ,1.23) | 0.95 | (0.85 | ,1.06) | 0.76 |
| High Neighborhood % Poverty | 1.19 | (1.01 | ,1.41) | 1.14 | (1.04 | ,1.24) | 0.60 | 1.09 | (0.92 | ,1.29) | 1.02 | (0.94 | ,1.11) | 0.50 |
| High Neighborhood % Black | 1.17 | (0.97 | ,1.40) | 1.07 | (0.96 | ,1.18) | 0.38 | 1.10 | (0.91 | ,1.32) | 0.99 | (0.90 | ,1.10) | 0.29 |
| High Neighborhood % White | 0.75 | (0.62 | ,0.91) | 0.92 | (0.83 | ,1.02) | 0.04 | 0.80 | (0.66 | ,0.96) | 1.01 | (0.91 | ,1.11) | 0.02 |
| High Neighborhood % Foreign Born | 1.13 | (0.78 | ,1.65) | 0.87 | (0.74 | ,1.03) | 0.21 | 1.11 | (0.76 | ,1.64) | 0.79 | (0.66 | ,0.94) | 0.12 |
| High Neighborhood % Residentially Stable | 0.94 | (0.81 | ,1.10) | 0.99 | (0.92 | ,1.07) | 0.57 | 0.97 | (0.82 | ,1.16) | 0.95 | (0.88 | ,1.02) | 0.79 |
Stratum-specific neighborhood-mortality estimates within strata of ever-stroke status derived from interaction models (interacting neighborhood context variable with stroke status). Model 1 adjusted for demographic variables (stroke status, age, gender, race, ethnicity, southern birth, nativity, and marital status). Model 4 additionally adjusted for SES variables (parental education, education, income, and wealth), and CVD risk factors (physical activity, ADL, IADL, obesity, alcohol use, smoking status, depressive symptoms, hypertension, diabetes, self-rated health). Q=Quartile. Neighborhood social ties modeled with a 3-item index; hazard ratio models a change from 0 to 3 social ties. Neighborhood family income and neighborhood deprivation are modeled in quartiles modeled ordinally; hazard ratio models a change from 4th vs. 1st quartiles.
We found few significant associations of neighborhood characteristics on mortality that were different by stroke status subgroups (our key hypothesis, Table 2); results were very similar when based on multiply imputed data (Appendix Table 3). Living in a predominantly white neighborhood, however, was associated with substantially better survival among stroke patients in demographically-adjusted models (HR mortality=0.75, 95 % CI: 0.62, 0.91), but not among stroke-free populations (HR=0.92, 95 % CI: 0.83, 1.02; interaction p=0.04; Table 2 Model 1). Associations changed little after comprehensive adjustment in Model 4 (HR mortality for stroke patients=0.80, 95 % CI: 0.66, 0.96; HR mortality among stroke-free populations =1.01, 95 % CI: 0.91, 1.11; interaction p=0.02).
Time Since Stroke
Estimated effects of neighborhoods on mortality were often different in the short term (stroke occurred <3 months from last contact) compared to those never experiencing stroke. Figure 2 demonstrates that neighborhood deprivation had adverse associations with mortality for recent stroke patients (Figure 2, Appendix Table 4: HR=1.35, 95 %CI: 1.03, 1.77; interaction vs. never stroke: p=0.02), with no effect for other stroke subgroups. Neighborhood % white was protective for recent stroke patients (HR=0.54, 95 %CI: 0.26, 1.11, interaction p=0.09), but the magnitude was less protective or null for other groups. Unexpectedly, recent stroke patients experienced adverse effects of more neighborhood social ties on mortality (HR=2.90, 95 %CI: 0.97, 8.61), while the association was significantly protective for the non-stroke population (HR=0.85, 95 %CI: 0.76–0.95; interaction p=.03). For follow-up periods 3 months or greater, there were few statistical differences for stroke, although estimates were imprecise. Results were similar when estimated in multiply imputed data sets (Appendix Table 5), although the unexpected adverse association between neighborhood social ties and mortality within 3 months after stroke was attenuated (HR=1.74, 95 % CI: 0.59, 5.16; interaction p=.31).
Figure 2. Stratum-Specific Estimates of Neighborhood Context on Hazard Ratios of Mortality within Strata of Time Since Stroke.
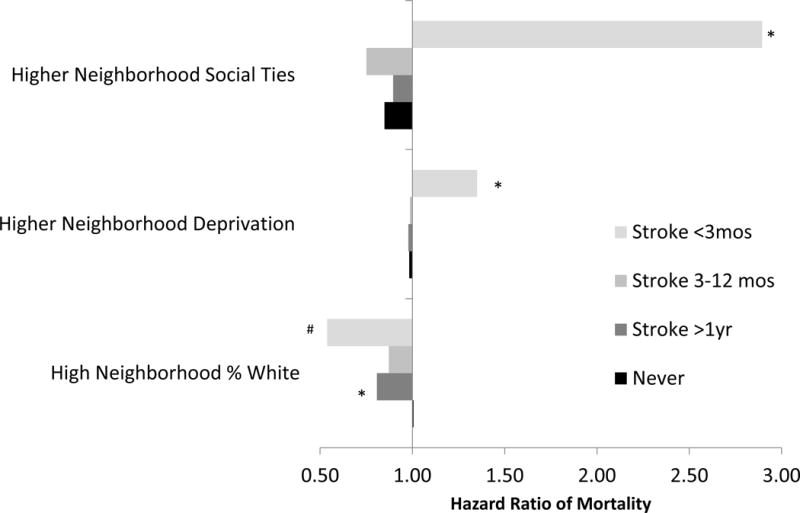
* P<.05 # P<.10, where P denotes the P-value for the interaction (compared to never stroke). All Interaction P-values, and stratum specific estimates and confidence intervals for all neighborhood variables are reported in Appendix Table 4. Model 4 adjusted for demographic, CVD risk factors, and SEP variables, in addition to modeling the effect modification of neighborhood context with stroke status.
Discussion
In this nationally representative cohort, we found that several aspects of neighborhood context predicted better survival. Higher neighborhood SEP, living in a predominantly white neighborhood, and sharing social ties with neighbors predicted better survival for stroke patients, in partial support of our hypothesis. However, these associations were also present, and similar in relative magnitude, for those who never experienced a stroke, contrary to our hypothesis that associations would be larger in stroke survivors. Only the estimated effect of neighborhood racial composition (specifically, percent white) appeared specific to stroke survivorship. Although relative effects (e.g., ratios of mortality rates, such as hazard ratios) are similar, the absolute impact of neighborhood characteristics on mortality would be larger in stroke patients because they have higher underlying mortality.
Our findings complement a growing body of literature linking neighborhood disadvantage to shorter survival after stroke3, 12–16 or after acute cardiovascular events15, 27, 30–36. Guidance on how to interpret and respond effectively to these disparities represents a major gap in previous literature. Our research attempts to address this gap by including individuals both with and without prior acute events, to evaluate whether inequalities were specific to post-event care. Our findings have three important implications for stroke care. Several neighborhood factors are strong predictors of mortality among stroke patients; addressing these inequalities will probably require looking beyond conventional stroke care. For at least one domain (low neighborhood percent white), there may be mechanisms that are specifically detrimental to stroke survivors, and these mechanisms are relevant from the first months after stroke (particularly for neighborhoods that are deprived and low percent white). These neighborhood characteristics are presumably not causal, but proxies for other underlying neighborhood risk factors, such as access to high-quality acute or long-term stroke care (e.g., residential segregation of nursing homes)37, 38. Access to high-quality care is patterned by location, and may be driven by availability of specialized services in more affluent urban neighborhoods13. Therefore, interventions deriving from these findings might focus on ensuring access to high-quality care in the immediate aftermath of stroke, particularly for those living in racially segregated or deprived neighborhoods, with close follow-up soon after stroke. Our data on time of death were not sufficiently precise to evaluate whether early mortality was due to in-hospital deaths or mortality after discharge. Other data sources, such as the “Get With the Guidelines” stroke database39 might support such analyses. However since many of the mortality associations were evident in both stroke patients and stroke-free populations, our results also point to the need to address social determinants of health in poorer-quality neighborhoods that may underlie vulnerability to mortality risk, for example community outreach to elders to prevent social isolation and provide both instrumental and emotional social support.
We documented that those with better neighborhood-based social ties exhibited lower risk of mortality, after comprehensive adjustment. This is consistent with prior findings that social isolation, social support, and social cohesion are associated with stroke outcomes.10, 40–42 Our results extend these previous findings, suggesting the possibility of the specific relevance of ties to neighbors, by examining both stroke patients and stroke-free populations.
Lower neighborhood SEP predicted higher mortality rates in our study, although this association was substantially attenuated by careful control for individual-level SEP, which is generally in contrast with prior studies3, 11–15. This discrepancy with previous reports may be due to availability of unusually comprehensive measures of individual SEP available in our cohort. Prior reports of significant effects of neighborhood SEP on survival after stroke12–14 may have attributed some individual-level SEP effects16 to neighborhood characteristics17, 43. Our study better controls for individual SEP than any prior study on the topic. Prior studies have used medical records, which typically include no measures of individual SEP12–14. Notably, in models controlling only for demographic variables, all our indicators of neighborhood SEP were strongly associated with mortality, suggesting that studies not including individual SEP were likely picking up the strong association between individual SEP and mortality in the neighborhood SEP coefficients.
Neighborhood context may influence survival17 via mechanisms related to both neighborhood SEP and neighborhood social context, such as receipt of social support; exposure to violence; physical environments that influence health behaviors like exercise; support for chronic disease management; and access to acute care and clinical services to manage comorbid conditions or to aid rehabilitation (see conceptualization of possible mechanisms in Figure 3). Given the controversy about whether neighborhood effect models should be adjusted for individual SEP, with many arguing that individual SEP is a mediator of neighborhood effects on health,17 we view the best estimates of effects of neighborhoods as falling somewhere between demographic-adjusted and SEP-adjusted models.
Figure 3.
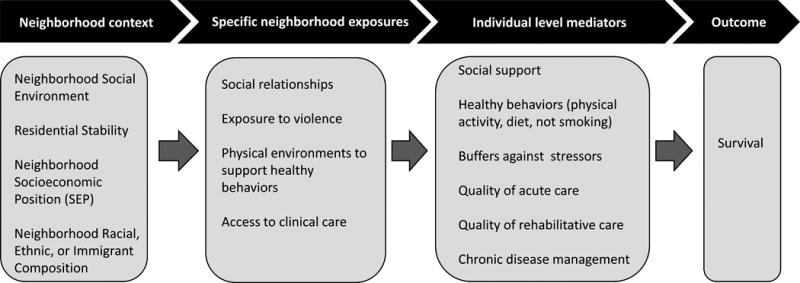
Potential mechanisms linking neighborhood context to survival.
Although models examining time since stroke had less statistical power, we documented that recent stroke patients displayed elevated mortality in deprived neighborhoods, while nonstroke populations and other stroke patients had no such mortality association. These results suggest that harmful risks present in impoverished neighborhoods, whether health care-related (e.g., proximity to high-quality treatment facilities or response time by health care professionals), or not (e.g., stress from exposure to crime and violence in high-poverty areas) may interact with the physiological vulnerability of the recent stroke patient to exacerbate mortality risk. Unexpectedly, recent stroke patients also exhibited elevated risk of death in neighborhoods with higher neighborhood social ties, while nonstroke populations exhibited protective effects. Although we can speculate on potential explanations for this pattern including potential negative consequences of social ties,44 we consider it likely to be a spurious association given that it did not hold up when evaluated in multiple imputed data sets.
Strengths and Limitations
Like many other studies,10, 11, 13 HRS did not include measures of stroke severity. Since stroke severity is a strong predictor of mortality following a stroke, particularly soon after,45 our associations of neighborhood context on mortality among recent stroke patients may reflect severity. However prior work suggests that stroke severity may not be influential in accounting for neighborhood associations with mortality.12, 13 We adjusted for numerous measures of baseline health and frailty, far more than available in prior research in this area. HRS includes only self- or proxy-reported measures of stroke, which is a good but not perfect measure of clinical stroke and inevitably misses undiagnosed ischemic cerebrovascular injury46. However, we found that these reports had 74% sensitivity and 93% specificity for stroke diagnoses reported in Medicare billing records, demonstrating that HRS measures of self or proxy reports have good validity.
Although we did not model cause-specific mortality due to misclassification on death certificates47, by calculating the attributable risk percent in the exposed, we find that the majority (54%) of the deaths among stroke patients were directly attributable to stroke.
As discussed above, causality remains uncertain in this observational study: we may have omitted important confounders or adjusted for factors on the causal chain. This causal inference challenge is unlikely to account for our finding that neighborhood-mortality associations are similar by stroke status. For example, we chose to model neighborhood context at baseline, to establish temporal order of neighborhood context prior to stroke or mortality. While neighborhood context after baseline, including after stroke, may be etiologically relevant, it is on the causal chain between baseline neighborhood and mortality and may be affected by the patient’s level of impairment after stroke. In other words, the most impaired patients may be differentially moved to disadvantaged neighborhoods or neighborhoods where they have no social contacts, creating a spurious association between neighborhood characteristics and post-stroke mortality. However, not accounting for such changes in neighborhood prior to stroke may also bias results if current neighborhood of residence is most relevant to mortality, although such bias may be minimal since residential mobility for elders is relatively low compared to younger populations.48
Since eligibility to enroll in HRS was restricted to those aged 50+, those who did not survive to age 50 or who had a stroke prior to 1998 were excluded. Strokes are rare below age 50 however,49, 50 so such a selection is unlikely to introduce substantial bias. Nonetheless, there may be differential associations of neighborhood context with mortality among younger populations and this is an important topic for future research.
This study has several unique strengths and adds substantively to prior literature in this area, and to our conceptual understanding of the determinants of post-stroke mortality. This is one of few nationally representative cohorts with sufficient power to model effects of stroke on mortality. We avoid selection bias that may be present in hospital-based studies.12 Moreover, studies based on administrative data sources would not include such detailed demographic, socioeconomic, and social variables available in HRS. These covariates, especially SEP, are important in order to evaluate whether neighborhoods per se have relevance beyond individual SEP for post-stroke outcomes. By including individuals with and without stroke, we were able to assess whether the neighborhood effects were most likely indicating mechanisms specific to stroke care.
Conclusions
Neighborhood disadvantage, racial composition, and social ties predict survival of stroke patients. Most characteristics of neighborhoods have similar estimated effects on stroke survivors and individuals never having a stroke. Many important pathways linking neighborhoods and post-stroke mortality are therefore likely not specific to conventional stroke care, but may include general mortality risk factors, including social determinants of health. These results represent an opportunity to improve long-term survival of stroke patients by identifying specific mechanisms accounting for geographic inequalities in mortality.
Supplementary Material
What is known
Post-stroke mortality is higher among residents of disadvantaged neighborhoods, but it is not known whether neighborhood inequalities are specific to stroke survival or similar to mortality patterns in the general population.
Most studies examining how neighborhood characteristics influence mortality among stroke survivors do not include a comparison group of those who never experienced stroke.
What the study adds
Neighborhood percent white predicted mortality for stroke survivors more strongly than for stroke-free adults, which may signal underlying risk factors, such as access to high-quality acute or long-term stroke care.
Neighborhood-mortality associations emerged within three months after stroke, when associations were often stronger than among stroke-free individuals.
This study found that characteristics of the neighborhoods where stroke patients reside predict post-stroke mortality over 12 years of follow up, but estimated effects of most neighborhood characteristics are similar for individuals without stroke; addressing these neighborhood-mortality inequalities will likely require looking beyond conventional stroke care.
Acknowledgments
We gratefully acknowledge assistance from Anna Kosheleva on validation of the self-reported stroke measures.
Funding Sources: Dr. Osypuk was supported by NIH grants R01MD006064, R03HD080848, R21HD066312. Dr. Glymour was supported by the American Heart Association 10SDG2640243 and National Institute on Aging R21AG34385. Dr. Gilsanz was supported by the National Heart, Lung, and Blood Institute F31HL112613, and the National Institute on Aging T32AG049663. Ms. Ehntholt was supported by the American Heart Association 10SDG2640243.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A. Factors influencing the decline in stroke mortality: A statement from the american heart association/american stroke association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pahigiannis K, Waddy S, Koroshetz W. Toward solutions for minimizing disparities in stroke: National institute of neurological disorders and stroke update. Stroke. 2013;44:e129–130. doi: 10.1161/STROKEAHA.113.001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thrift AG, Dewey HM, Sturm JW, Paul SL, Gilligan AK, Srikanth VK, Macdonell RAL, McNeil JJ, Macleod MR, Donnan GA. Greater incidence of both fatal and nonfatal strokes in disadvantaged areas: The northeast melbourne stroke incidence study. Stroke. 2006;37:877–882. doi: 10.1161/01.STR.0000202588.95876.a7. [DOI] [PubMed] [Google Scholar]
- 4.Menec VH, Shooshtari S, Nowicki S, Fournier S. Does the relationship between neighborhood socioeconomic status and health outcomes persist into very old age? A population-based study. J Aging Health. 2010;22:27–47. doi: 10.1177/0898264309349029. [DOI] [PubMed] [Google Scholar]
- 5.Brown P, Guy M, Broad J. Individual socio-economic status, community socio-economic status and stroke in new zealand: A case control study. Soc Sci Med. 2005;61:1174–1188. doi: 10.1016/j.socscimed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Brown AF, Liang L-J, Vassar SD, Stein-Merkin S, Longstreth WT, Ovbiagele B, Yan T, Escarce JJ. Neighborhood disadvantage and ischemic stroke: The cardiovascular health study (chs) Stroke. 2011;42:3363–3368. doi: 10.1161/STROKEAHA.111.622134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engström G, Jerntorp I, Pessah-Rasmussen H, Hedblad B, Berglund G, Janzon L. Geographic distribution of stroke incidence within an urban population: Relations to socioeconomic circumstances and prevalence of cardiovascular risk factors. Stroke. 2001;32:1098–1103. doi: 10.1161/01.str.32.5.1098. [DOI] [PubMed] [Google Scholar]
- 8.Lisabeth L, Diez Roux A, Escobar J, Smith M, Morgenstern L. Neighborhood environment and risk of ischemic stroke: The brain attack surveillance in corpus christi (basic) project. Am J Epidemiol. 2007;165:279–287. doi: 10.1093/aje/kwk005. [DOI] [PubMed] [Google Scholar]
- 9.Morgenstern LB, Escobar JD, Sánchez BN, Hughes R, Zuniga BG, Garcia N, Lisabeth LD. Fast food and neighborhood stroke risk. Ann Neurol. 2009;66:165–170. doi: 10.1002/ana.21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark CJ, Guo H, Lunos S, Aggarwal NT, Beck T, Evans DA, Mendes de Leon C, Everson-Rose SA. Neighborhood cohesion is associated with reduced risk of stroke mortality. Stroke. 2011;42:1212–1217. doi: 10.1161/STROKEAHA.110.609164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AF, Liang L-J, Vassar SD, Merkin SS, Longstreth WT, Ovbiagele B, Yan T, Escarce JJ. Neighborhood socioeconomic disadvantage and mortality after stroke. Neurology. 2013;80:520–527. doi: 10.1212/WNL.0b013e31828154ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslanyan S, Weir CJ, Lees KR, Reid JL, McInnes GT. Effect of area-based deprivation on the severity, subtype, and outcome of ischemic stroke. Stroke. 2003;34:2623–2628. doi: 10.1161/01.STR.0000097610.12803.D7. [DOI] [PubMed] [Google Scholar]
- 13.Kapral MK, Wang H, Mamdani M, Tu JV. Effect of socioeconomic status on treatment and mortality after stroke. Stroke. 2002;33:268–275. doi: 10.1161/hs0102.101169. [DOI] [PubMed] [Google Scholar]
- 14.Martinez J, Pampalon R, Hamel D. Deprivation and stroke mortality in quebec. Chronic Dis Can. 2003;24:57–64. [PubMed] [Google Scholar]
- 15.Pedigo A, Aldrich T, Odoi A. Neighborhood disparities in stroke and myocardial infarction mortality: A gis and spatial scan statistics approach. BMC Public Health. 2011;11:644. doi: 10.1186/1471-2458-11-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox AM, McKevitt C, Rudd AG, Wolfe CDA. Socioeconomic status and stroke. Lancet Neurol. 2006;5:181–188. doi: 10.1016/S1474-4422(06)70351-9. [DOI] [PubMed] [Google Scholar]
- 17.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: A critical review. J Epidemiol Community Health. 2001;55:111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juster FT, Suzman R. An overview of the health and retirement study. J Hum Resour. 1995;30:S7–S56. [Google Scholar]
- 19.Heeringa SG. Technical description of the asset and health dynamics (ahead) survey sample (vol Dr-003) Ann Arbor, MI: Institute for Social Research, University of Michigan; 1995. [Google Scholar]
- 20.Wu Q, Tchetgen EJT, Osypuk TL, White K, Mujahid M, Glymour MM. Combining direct and proxy assessments to reduce attrition bias in a longitudinal study. Alzheimer Dis Assoc Disord. 2013;27:207–212. doi: 10.1097/WAD.0b013e31826cfe90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weir D, Faul J, Langa K. Proxy interviews and bias in the distribution of cognitive abilities due to non-response in longitudinal studies: A comparison of hrs and elsa. Longit Life Course Stud. 2011;2:170–184. doi: 10.14301/llcs.v2i2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnega A, Faul J, Ofstedal M, Langa K, Phillips J, Weir D. Cohort profile: The health and retirement study (hrs) Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osypuk TL, Galea S. What level macro? Choosing appropriate levels for modeling space, place, and health. In: Galea S, editor. Macrosocial determinants of population health. New York, NY: Springer Media; 2007. pp. 399–435. [Google Scholar]
- 24.Krieger N. A century of census tracts: Health & the body politic (1906–2006) Bull N Y Acad Med: J Urban Health. 2006;83:355–361. doi: 10.1007/s11524-006-9040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian S. Painting a truer picture of us socioeconomic and racial/ethnic health inequalities: The public health disparities geocoding project. Am J Public Health. 2005;95:312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osypuk TL, Kehm R, Misra DP. Where we used to live: Validating retrospective measures of childhood neighborhood context. PLoS One. 2015;10:e0124635. doi: 10.1371/journal.pone.0124635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkleby M, Sundquist K, Cubbin C. Inequities in chd incidence and case fatality by neighborhood deprivation. Am J Prev Med. 2007;32:97–106. doi: 10.1016/j.amepre.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turvey CL, Wallace RB, Herzog R. A revised ces-d measure of depressive symptoms and a dsm-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute Inc. Sas/stat® 9.2 user’s guide Chapter 64: The phreg procedure. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 30.Gerber Y, Benyamini Y, Goldbourt U, Drory Y, for the Israel Study Group on First Acute Myocardial Infarction Neighborhood socioeconomic context and long-term survival after myocardial infarction. Circulation. 2010;121:375–383. doi: 10.1161/CIRCULATIONAHA.109.882555. [DOI] [PubMed] [Google Scholar]
- 31.Chaix B, Rosvall M, Merlo J. Neighborhood socioeconomic deprivation and residential instability: Effects on incidence of ischemic heart disease and survival after myocardial infarction. Epidemiology. 2007;18:104–111. doi: 10.1097/01.ede.0000249573.22856.9a. [DOI] [PubMed] [Google Scholar]
- 32.Tonne C, Schwartz J, Mittleman M, Melly S, Suh H, Goldberg R. Long-term survival after acute myocardial infarction is lower in more deprived neighborhoods. Circulation. 2005;111:3063–3070. doi: 10.1161/CIRCULATIONAHA.104.496174. [DOI] [PubMed] [Google Scholar]
- 33.Rao SV, Schulman KA, Curtis LH, Gersh BJ, Jollis JG. Socioeconomic status and outcome following acute myocardial infarction in elderly patients. Arch Intern Med. 2004;164:1128–1133. doi: 10.1001/archinte.164.10.1128. [DOI] [PubMed] [Google Scholar]
- 34.Tydén P, Hansen O, Engström G, Hedblad B, Janzon L. Myocardial infarction in an urban population: Worse long term prognosis for patients from less affluent residential areas. J Epidemiol Community Health. 2002;56:785–790. doi: 10.1136/jech.56.10.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engström G, Göransson M, Hansen O, Hedblad B, Tydén P, Tödt T, Janzon L. Trends in long-term survival after myocardial infarction: Less favourable patterns for patients from deprived areas. J Intern Med. 2000;248:425–434. doi: 10.1046/j.1365-2796.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- 36.Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999;341:1359–1367. doi: 10.1056/NEJM199910283411806. [DOI] [PubMed] [Google Scholar]
- 37.Smith DB, Feng Z, Fennell ML, Zinn JS, Mor V. Separate and unequal: Racial segregation and disparities in quality across u.S. Nursing homes. Health Aff. 2007;26:1448–1458. doi: 10.1377/hlthaff.26.5.1448. [DOI] [PubMed] [Google Scholar]
- 38.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med. 2005;48:S42–53. [PubMed] [Google Scholar]
- 39.American Heart Association. Get with the guidelines-stroke database – overview. 2016 Accessed at: Http://www.Heart.Org/heartorg/professional/getwiththeguidelines/getwiththeguidelines-stroke/get-with-the-guidelines-stroke-overview_ucm_308021_article.Jsp#.Wcpvudurj7o.
- 40.Glass T, Matchar D, Belyea M, Feussner J. Impact of social support on outcome in first stroke. Stroke. 1993;24:64–70. doi: 10.1161/01.str.24.1.64. [DOI] [PubMed] [Google Scholar]
- 41.Colantonio A, Kasl S, Ostfeld A, Berkman L. Prestroke physical function predicts stroke outcomes in the elderly. Arch Phys Med Rehabil. 1996;77:562–566. doi: 10.1016/s0003-9993(96)90295-6. [DOI] [PubMed] [Google Scholar]
- 42.Boden-Albala B, Litwak E, Elkind M, Rundek T, Sacco R. Social isolation and outcomes post stroke. Neurology. 2005;64:1888–1892. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 43.Duncan C, Jones K, Moon G. Context, composition, and heterogeneity: Using multilevel models in health research. Soc Sci Med. 1998;46:97–117. doi: 10.1016/s0277-9536(97)00148-2. [DOI] [PubMed] [Google Scholar]
- 44.Burg MM, Seeman TE. Families and health: The negative side of social ties. Ann Behav Med. 1994;16:109–115. [Google Scholar]
- 45.Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: Stroke severity, mortality, and risk factors. Stroke. 2009;40:2068–2072. doi: 10.1161/STROKEAHA.108.540112. [DOI] [PubMed] [Google Scholar]
- 46.Reitz C, Schupf N, Luchsinger JA, Brickman AM, Manly JJ, Andrews H, Tang MX, DeCarli C, Brown TR, Mayeux R. Validity of self-reported stroke in elderly african americans, caribbean hispanics, and whites. Arch Neurol. 2009;66:834–840. doi: 10.1001/archneurol.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: Time for a reassessment? J Am Coll Cardiol. 1999;34:618–620. doi: 10.1016/s0735-1097(99)00250-8. [DOI] [PubMed] [Google Scholar]
- 48.Ihrke DK, Faber CS. Geographical mobility: 2005 to 2010. Current population reports. P20–567. Washington DC: U.S. Census Bureau; 2012. [Google Scholar]
- 49.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the framingham heart study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, Broderick JP, Kleindorfer DO. Age at stroke: Temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


