Abstract
BACKGROUND
Genomic analyses have suggested that the LPA gene and its associated plasma biomarker, lipoprotein(a) (Lp[a]), represent a causal risk factor for coronary heart disease (CHD). As such, lowering Lp(a) has emerged as a therapeutic strategy. Beyond target identification, human genetics may contribute to the development of new therapies by defining the full spectrum of beneficial and adverse consequences and by developing a dose-response curve of target perturbation.
OBJECTIVES
We attempted to establish the full phenotypic impact of LPA gene variation and to estimate a dose-response curve between genetically altered plasma Lp(a) and risk for CHD.
METHODS
We leveraged genetic variants at the LPA gene from 3 data sources: individual-level data from 112,338 participants in the UK Biobank; summary association results from large-scale genome-wide association studies; and LPA gene sequencing results from cases with and controls free of CHD.
RESULTS
One standard deviation genetically lowered Lp(a) level was associated with 29% lower risk of CHD (odds ratio [OR]: 0.71; 95% confidence interval [CI]: 0.69 to 0.73), 31% lower risk of peripheral vascular disease (OR: 0.69; 95% CI: 0.59 to 0.80), 13% lower risk of stroke (OR: 0.87; 95% CI: 0.79 to 0.96), 17% lower risk of heart failure (OR: 0.83; 95% CI: 0.73 to 0.94), and 37% lower risk of aortic stenosis (OR: 0.63; 95% CI: 0.47 to 0.83). We observed no association with 31 other disorders including type 2 diabetes and cancer. Variants that led to gain of LPA gene function increased risk for CHD whereas those that led to loss of gene function reduced CHD risk.
CONCLUSIONS
Beyond CHD, genetically lowered Lp(a) is associated with a lower risk of peripheral vascular disease, stroke, heart failure, and aortic stenosis. As such, pharmacological lowering of plasma Lp(a) may impact a range of atherosclerosis-related diseases.
Keywords: coronary heart disease, genetics, phenome-wide association study, single nucleotide polymorphism
Lipoprotein(a) (Lp[a]) is a circulating lipoprotein where the constituent apolipoprotein B on a low-density lipoprotein (LDL) particle is modified by the covalent addition of another protein, namely apolipoprotein(a) (1, 2). Higher plasma Lp(a) levels are correlated with increased risk for coronary heart disease (CHD) (3), heritable, and largely determined by variation in the LPA gene, which encodes apolipoprotein(a) (2). Genetic variants in LPA that increase Lp(a) levels also increase CHD risk, suggesting that Lp(a) is a causal risk factor for development of CHD (4–6). Consequently, lowering Lp(a) levels has emerged as a therapeutic strategy to reduce CHD risk (2, 7).
Beyond identifying a therapeutic target gene, human genetics may help estimate the probable efficacy and safety of pharmacological modulation (8). Although LPA variants have been consistently reported to be associated with CHD (5, 6) and aortic valve stenosis (9, 10), there is uncertainty around the full spectrum of phenotypic consequences. Previous studies have reported conflicting evidence on whether LPA variants are associated with other cardiovascular diseases, such as stroke (11, 12). In addition, observational epidemiology has associated lower plasma levels of Lp(a) with increased risks of cancer (13) and diabetes (14).
Deoxyribonucleic acid (DNA) sequence variants might also provide a mechanism to estimate a dose-response curve. In particular, the simultaneous identification of gain-of-function as well as loss-of-function variants and an analysis of phenotypic effects can reveal a dose-response curve even before a clinical trial is initiated.
Here, we leveraged genetic variants across the allele frequency spectrum and 3 large data sources to evaluate the phenotypic consequences of genetically lowered Lp(a) levels. We estimate the effect of a genetically mediated 1 standard deviation decrease in Lp(a) levels on cardiometabolic disease and range of other disorders.
METHODS
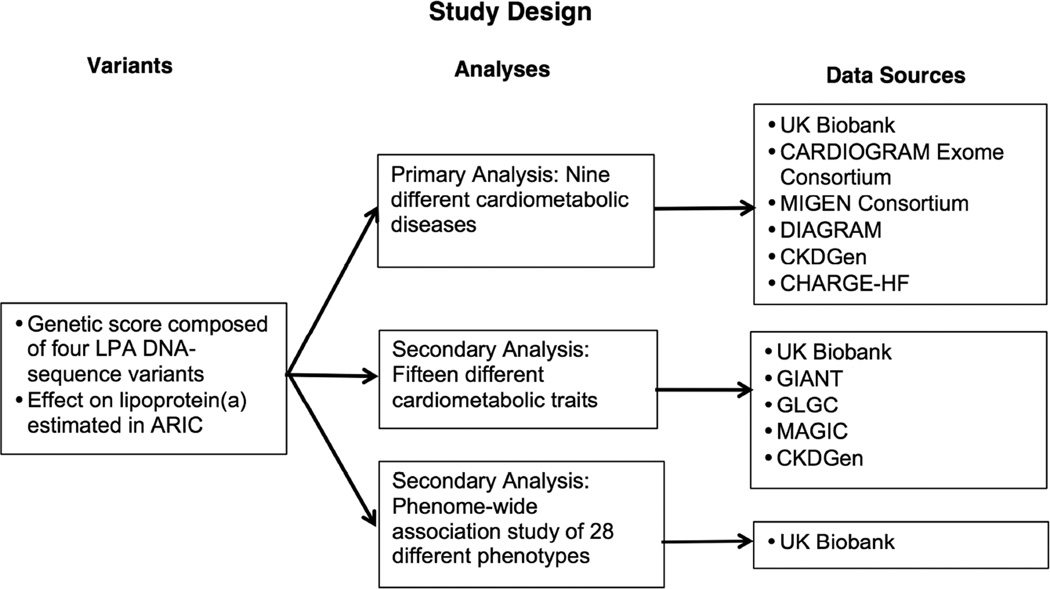
Overall study design is shown in Figure 1. We leveraged several data sources to provide greater power for estimating the effect of genetically lowered Lp(a) on cardiometabolic traits and outcomes, to conduct a phenome-wide association study, and to examine the effect of rare loss-of-function variants in the LPA gene on risk of CHD.
FIGURE 1. Study Design.
This study included 1 primary and 2 secondary analyses to estimate the effect of a lipoprotein(a) (Lp[a]) on a range of outcomes. ARIC = Atherosclerosis Risk in Communities; CARDIoGRAM = Coronary ARtery DIsease Genome wide Replication and Meta-analysis; CHARGE-HF = Cohorts for Heart and Aging Research in Genomic Epidemiology – Heart Failure Consortium; CKDGen = Chronic Kidney Disease Genetics Consortium; DIAGRAM = DIAbetes Genetics Replication And Meta-analysis; DNA = deoxyribonucleic acid; GIANT = Genetic Investigation of ANthropometric Traits; GLGC = Global Lipids Genetics Consortium; MAGIC = Meta-Analyses of Glucose and Insulin-related traits Consortium; MIGen = Myocardial Infarction Genetics.
We used individual-level data from 112,338 individuals of European ancestry from the UK Biobank, a large population-based cohort (Online Supplement) (15). Characteristics of individuals are provided in Online Table 1. We supplemented this individual-level data with summary results from 7 genome-wide association study (GWAS) consortia examining blood lipids, anthropometric traits, glycemic traits, diabetes, CHD, heart failure (HF), and renal dysfunction, all predominantly containing individuals of European descent (Online Supplement and Table 1) (16–23). Our estimates for CHD were derived from the CARDIoGRAM Exome Consortium analysis of up to 42,335 CHD cases and 78,240 controls (Online Supplement). Finally, we used LPA gene sequences from 15,251 participants of European ancestry from the Myocardial Infarction Genetics (MIGen) Consortium.
Table 1.
Characteristics of Genome-Wide Association Studies Utilized
| Consortium | Outcome/Trait | Sample Size | Genotyping |
|---|---|---|---|
| GLGC (17) | LDL cholesterol HDL cholesterol Total cholesterol Triglycerides |
Up to 188,587 individuals |
37 studies using Metabochip, 23 studies using various arrays |
| MAGIC (18) | Fasting glucose Fasting insulin 2-h glucose HbA1c |
Up to 133,010 individuals |
Various arrays, imputation to 2.5 million SNPs using HapMap reference panel |
| GIANT (37,38) | Waist-to-hip ratio Waist circumference Hip circumference Body mass index |
Up to 322,154 individuals |
Various arrays, imputation to 2.5 million SNPs using HapMap reference panel |
| CKDGen (39) | Serum estimated glomerular filtration rate Chronic kidney disease |
Up to 133,413 individuals |
Various arrays, imputation to 2.5 million SNPs using HapMap reference panel |
| CARDIoGRAM Exome Consortium (22) |
Coronary heart disease |
Up to 42,335 cases/ 78,240 controls |
Illumina HumanExome BeadChip array or the Illumina OmniExome array |
| DIAGRAM (20) | Diabetes | Up to 34,840 cases/ 114,981 controls |
37 studies using Metabochip, 23 studies various arrays, imputation to 2.5 million SNPs using HapMap reference panel |
| CHARGE-HF (23) | Heart failure | Up to 2,526 cases / 18,400 controls |
Various arrays, imputation to 2.5 million SNPs using HapMap reference panel |
CARDIoGRAM = Coronary ARtery DIsease Genome wide Replication and Meta-analysis; CHARGE-HF = Cohorts for Heart and Aging Research in Genomic Epidemiology-Heart Failure; CKDGen = Chronic Kidney Disease Genetics Consortium; DIAGRAM = DIAbetes Genetics Replication And Meta-analysis; GIANT = Genetic Investigation of ANthropometric Traits; GLGC = Global Lipids Genetics Consortium; GWAS = genome-wide association study; HbA1c = glycosylated hemoglobin; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MAGIC = Meta-Analyses of Glucose and Insulin-related traits Consortium; SNP = single nucleotide polymorphism.
In our primary analysis, we examined the effect of genetically lowered Lp(a) on 9 different cardiometabolic diseases: CHD; stroke; HF; atrial fibrillation; aortic stenosis; peripheral vascular disease (PVD); venous thromboembolism; diabetes; and chronic kidney disease (CKD) (Online Table 2). We additionally examined the effect of genetically lowered Lp(a) on 15 cardiometabolic quantitative traits (Online Supplement): waist-to-hip ratio; waist circumference; hip circumference; body mass index; systolic blood pressure; diastolic blood pressure; total cholesterol; LDL cholesterol; high-density lipoprotein (HDL) cholesterol; triglyceride levels; fasting glucose; fasting insulin; 2-hour glucose; glycosylated hemoglobin; and serum creatinine-derived estimated glomerular filtration rate (eGFR). All traits were standardized (that is, reported in units of SDs) to allow for comparisons among traits (Online Supplement). Using the UK Biobank cohort, we also conducted a phenome-wide association study for 28 additional diseases, including endocrine, renal, urological, gastrointestinal, neurological, musculoskeletal, respiratory and neoplastic disorders (Online Table 2).
DNA SEQUENCE VARIANTS
To estimate the effect of genetically lowered Lp(a) on a wide range of phenotypes, we combined individual-level data from UK Biobank with summary-level data from large-scale GWAS. We used 4 single nucleotide polymorphisms (SNP) in the LPA gene that have been previously associated with plasma Lp(a) levels: rs10455872; rs3798220; rs41272114; and rs143431368 (Online Table 3). Together, rs10455872 and rs3798220 explain about 36% of variation in plasma Lp(a) levels (5); the other 2, rs41272114 and rs143431368, are loss-of-function variants associated with lower Lp(a) levels (Online Table 3).
To standardize our estimates to 1 SD decrease in Lp(a) levels, we used estimates of the effect of each variant on Lp(a) levels from the ARIC (Atherosclerosis Risk in Communities) study (Online Table 3 and Online Supplement). ARIC is a community-based study of 15,792 white and black participants, ages 45 to 64 years, who were first enrolled in 1987 (24). We restricted our analysis to 2,758 individuals of European ancestry in the ARIC cohort who had Lp(a) levels measured at the baseline visit and measured using a double-antibody enzyme-linked immunosorbent assay (25). Participants fasted for 12 to 24 h before blood collection. Plasma was separated from cells with centrifugation within 1 h of collection and stored at −70°C. Analyses were performed within 2 weeks. The assay was shown to have high internal reliability in a validation study in ARIC (r = 0.9) (25) and in a separate comparison to a newer assay calibrated using International Federation of Clinical Chemistry reference material (r = 0.88) (26). We used linear regression, adjusting for age, sex, and 5 principal components of ancestry to estimate the association between each variant and Lp(a) level in an additive model. As Lp(a) levels were non-normally distributed, we used log-transformed Lp(a) levels, as previously described (5).
STATISTICAL ANALYSIS
For analyses of both UK Biobank and summary-level data, we created a gene variant score out of the 4 SNPs. For each variant, we modeled the Lp(a)-lowering allele and weighted by the effect of each SNP on log-transformed Lp(a) levels in SD units (Online Table 4). We then examined the effect of this gene variant score on each trait and outcome, standardized per SD decrease in log-transformed Lp(a) levels.
For UK Biobank, we generated an LPA gene variant score in units of SD Lp(a) by multiplying each variant by its effect on Lp(a) levels. We then included this gene variant score in a logistic regression analysis adjusting for age, sex, 10 principal components of ancestry, and a dummy variable for array type. For the summary-level data, this approach was equivalent to an inverse-variance-weighted fixed-effects meta-analysis of the effect of each variant on a trait or outcome of interest, divided by the effect of each variant on Lp(a) levels (27).
For our primary outcomes (the 9 cardiometabolic diseases), we set a Bonferroni-adjusted level of significance of p = 0.05/9 = 0.0056. For our secondary analysis of cardiometabolic traits, which included 15 traits, we set a level of significance of p = 0.05/15 = 0.003. For our phenome-wide association study of 28 phenotypes, we set a level of significance of p = 0.05/28 = 0.0018.
LOSS OF FUNCTION VARIANT ANALYSIS
To examine whether loss-of-function variants in the LPA gene influence CHD risk, we used whole exome sequencing data from the MIGen Consortium (Online Supplement). This consortium is composed of 10 coronary artery disease case-control studies (28, 29). Loss-of-function variants were defined as: 1) nonsense mutations that resulted in early termination of the apolipoprotein(a) protein; 2) frameshift mutations due to insertions or deletions of DNA; or 3) splice-site mutations that resulted in an incorrectly spliced protein. We combined these loss-of-function variants in the MIGen consortium with loss-of-function variants that were genotyped (either directly or imputed) in the UK Biobank. Variants are provided in Online Tables 5 and 6. We analyzed rare variants (<1%) separately to a common loss-of-function variant in the LPA gene (rs41272114, allele frequency of 3.8% in UK Biobank) (30, 31).
We tested for the association of CHD with presence of a loss-of-function variant using logistic regression. In MIGen, we adjusted for sex, 5 principal components of ancestry, and a dummy variable for each cohort. We did not adjust for age in MIGen as cases in some cohorts were selected for early-onset myocardial infarction, resulting in age being significantly and inversely associated with the presence of CHD. In the UK Biobank, we adjusted for age, sex, 10 principal components of ancestry, and array type.
All analyses were performed using R version 3.2.3 software (The R Project for Statistical Computing, Vienna, Austria).
RESULTS
We first estimated the effect of LPA gene variant score on plasma Lp(a) levels in ARIC participants. Variants rs3798220 and rs10455872 altered Lp(a) levels by 0.98 and 0.91 SD, respectively, while rs41272114 and rs143431368 altered Lp(a) levels by 0.62 SD and 0.92 SD respectively (Online Table 4). In this study, 1 SD in Lp(a) levels equaled 0.332 μmol/l. The distribution of LPA gene variant score in the UK Biobank is provided (Online Table 6).
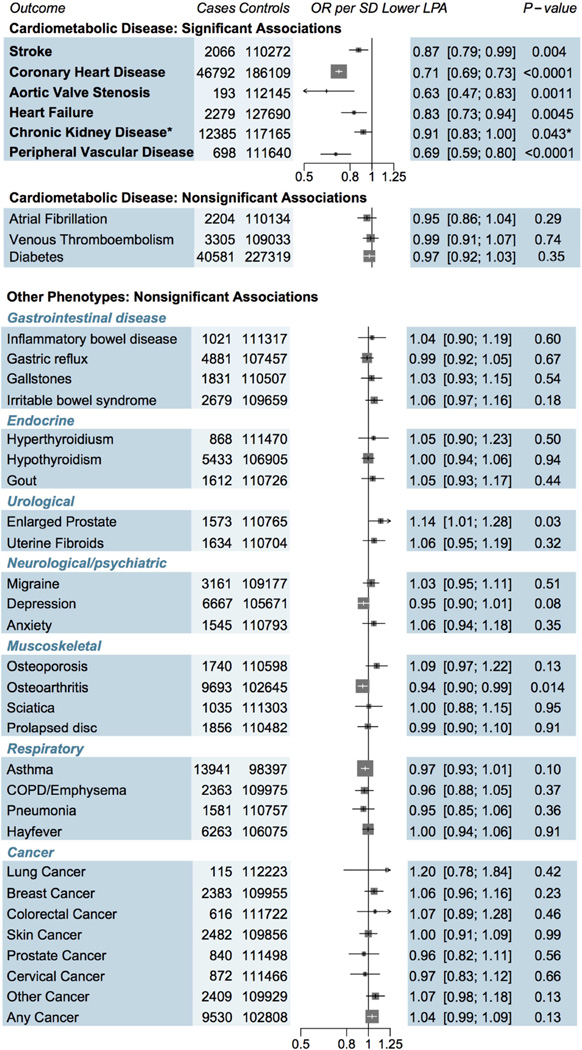
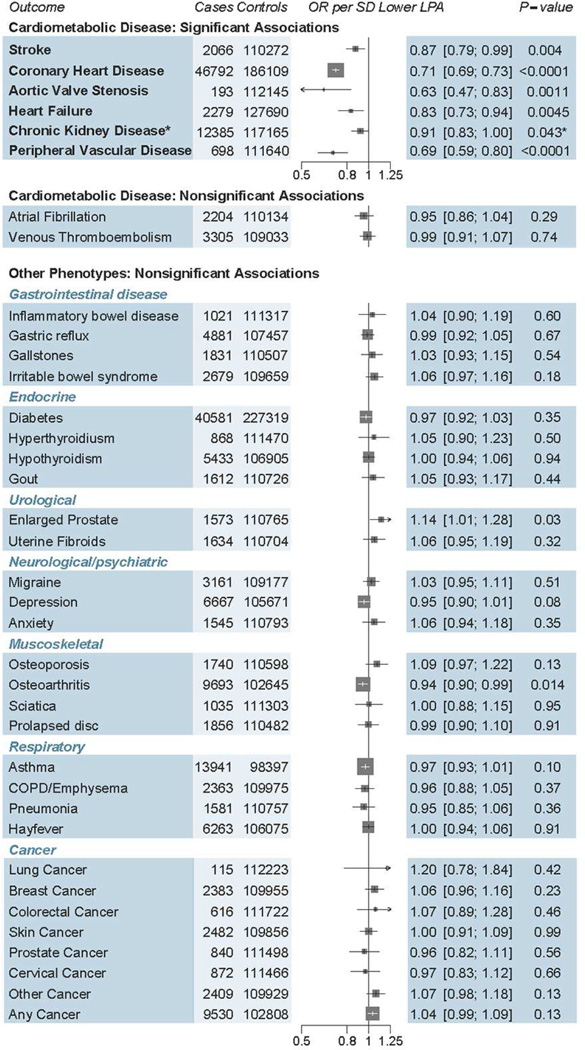
We examined the effect of genetically lowered Lp(a) on 9 different cardiometabolic diseases (Central Illustration). Genetically lowered lipoprotein(a), a 1 SD genetic decrease, was associated with 29% lower risk of CHD (odds ratio [OR]: 0.71; 95% confidence interval [CI]: 0.69 to 0.73; p = 3.2*10−90). Genetically lowered Lp(a) had similar strengths of association with CHD across subpopulations (Online Figure 1). Beyond CHD, genetically lowered Lp(a) was associated with 31% lower risk of PVD (OR: 0.69; 95% CI: 0.59 to 0.80; p = 1.9 × 10−6), 13% lower risk of stroke (OR: 0.87; 95% CI: 0.79 to 0.96; p = 0.004), 37% lower risk of aortic stenosis (OR: 0.63; 95% CI: 0.47 to 0.83; p = 0.0011), and 17% lower risk of HF (OR: 0.83; 95% CI: 0.73 to 0.94; p = 0.0045; Central Illustration). While genetically lowered Lp(a) levels were only nominally associated with a 9% lower risk of CKD (OR: 0.91; 95% CI: 0.81 to 1.00; p = 0.043), it was highly significantly associated with the underlying quantitative trait (eGFR) as described later. Genetically lowered Lp(a) was not associated with diabetes, venous thromboembolism, or atrial fibrillation. To examine if the association of genetically lowered Lp(a) with HF and aortic stenosis was mediated by CHD, we excluded participants with CHD in the UK Biobank (n = 4,461). After exclusion, a 1 SD genetic decrease in Lp(a) levels had similar strengths of association with HF (OR: 0.84; 95% CI: 0.66 to 1.07; n = 107,877) and aortic stenosis (OR: 0.70; 95% CI: 0.49 to 0.99; n = 107,877). A sensitivity analysis excluding those with prevalent aortic stenosis (n = 193) yielded a similar strength of association for the association between a 1 SD decrease in Lp(a) levels and HF (OR: 0.85; 95% CI: 0.72 to 1.02; n = 112,145).
CENTRAL ILLUSTRATION. Impact of Genetically Mediated Lp(a) Reduction (1 SD) on Disease Risk.
This study to establish the full phenotypic impact of LPA gene variation and to estimate a dose-response curve between genetically altered plasma lipoprotein (Lp) (a) and risk for coronary heart disease. Estimates were derived in UK Biobank using logistic regression, adjusted for age, sex, 10 principal components and array type, with the exception of chronic kidney disease (CKD), which was derived using summary statistics from CKDGen. One SD genetically lowered Lp(a) level was associated with reduced risk of 5 cardiometabolic diseases. Although the estimate for CKD did not reach Bonferroni adjusted significance, it was included as a significant outcome as the underlying trait (estimated glomerular filtration rate) was significantly associated with Lp(a) (p = 2 × 10−5). OR = odds ratio.
In contrast to the effects of Lp(a) on cardiometabolic disorders, we found no association of genetically lowered Lp(a) with any of 28 different disorders, including 4 gastrointestinal disorders, 3 endocrine disorders, 2 renal/urological disorders, 3 psychiatric disorders, 4 musculoskeletal disorders, 4 respiratory disorders, and 8 different cancers (all p > 0.01) (Figure 2).
FIGURE 2. Associations of Genetically Lowered Lp(a) With a Range of Diseases.
While 1 SD genetically lowered Lp(a) level was significantly associated with reduced risk of coronary heart disease, stroke, aortic stenosis, heart failure, chronic kidney disease, and peripheral vascular disease, there was no significant association seen for 3 other cardiometabolic disorders as well as 28 other diseases. COPD = chronic obstructive pulmonary disease; OR = odds ratio; other abbreviations as in Figure 1.
We next estimated the effect of LPA gene variant score on 15 quantitative traits (Figure 3). We observed a significant association of genetically lowered Lp(a) with improved kidney function: a 0.04 SD (95% CI: 0.02 to 0.05) increase in eGFR per SD genetically lowered Lp(a) (p = 1.4 × 10−5). This corresponds to an approximate 2.0 ml/min increase in eGFR per SD lower Lp(a). As expected, a 1 SD genetically lowered Lp(a) was associated with total cholesterol and LDL cholesterol (0.14 SD decrease in total cholesterol [95% CI: 0.11 to 0.16; p = 3.5 × 10−27) and a 0.14 SD decrease in LDL cholesterol (95% CI: 0.11 to 0.16; p = 4.7 × 10−27) (Figure 3). These estimates correspond, approximately, to a 0.14 mmol/l decrease in total cholesterol and a 0.13 mmol/l decrease in LDL cholesterol. We saw no significant association of LPA genetic risk score with waist-to-hip ratio, waist circumference, hip circumference, body mass index, systolic blood pressure, diastolic blood pressure, HDL cholesterol, triglycerides, fasting glucose, fasting insulin, 2-h glucose, or glycosylated hemoglobin (p > 0.05 for each). LPA gene variant risk score remained unassociated with systolic and diastolic blood pressure when use of antihypertensive therapy was not accounted for (0 SD, 95% CI: −0.02 to 0.01 and 0 SD, 95% CI: −0.01 to 0.02 per SD lower Lp[a], respectively).
FIGURE 3. Association of Genetically Lowered Lp(a) (1 SD Decrease) with Cardiometabolic Quantitative Traits.
Genetically lowered Lp(a) was associated with reductions in total and low-density lipoprotein (LDL) cholesterol as well as improved kidney function. There were no other significant associations seen between 1 SD decrease in Lp(a) and other traits measured. BMI = body mass index; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HbA1c = glycosylated hemoglobin; HDL = high-density lipoprotein; SBP = systolic blood pressure; SNP = single nucleotide polymorphism; other abbreviations as in Figures 1 and 2.
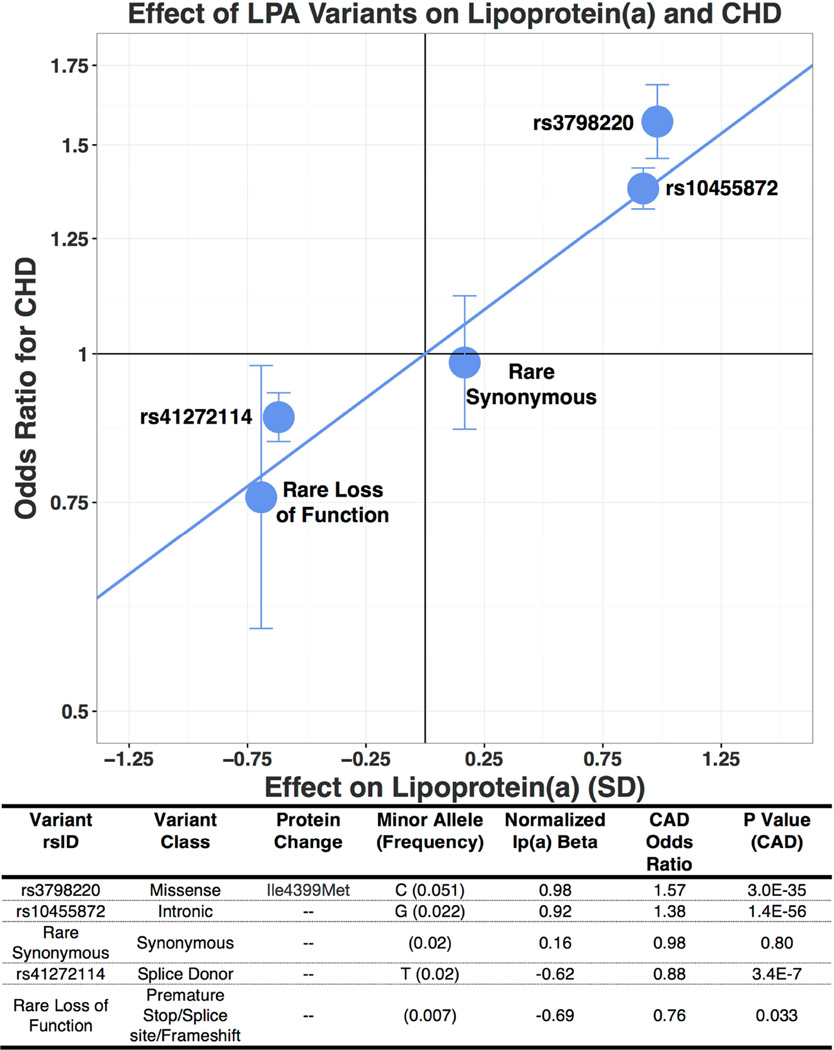
Figure 4 provides a dose-response curve for CHD derived from gain and loss-of-function variants at the LPA gene locus. The impact of LPA variation on CHD risk is directly proportional to its effect on circulating Lp(a) levels. The Lp(a)-increasing alleles of common variants rs3798220 and rs10455872, which increase Lp(a) levels by 0.98 and 0.91 SD, increased risk of CHD by 57% (OR: 1.57; 95% CI: 1.46 to 1.69) and 38% (OR: 1.38; 95% CI: 1.33 to 1.43), respectively. Rare synonymous variants, which had no significant effect on Lp(a) levels, also had no significant effect on CHD (OR: 0.98; 95% CI: 0.86 to 1.12) (Figure 4). A common loss-of-function variant rs41272114, which decreased Lp(a) levels by 0.62 SD, was associated with a 12% lower risk of CHD (OR: 0.88; 95% CI: 0.84 to 0.93; p = 3.4 × 10−7). Presence of a rare (allele frequency <1%) loss-of-function variant in the LPA gene was associated with a 24% lower risk of CHD (OR: 0.76; 95% CI: 0.59 to 0.98; p = 0.033) (Figure 4; Online Figure 2).
FIGURE 4. Effect of LPA Variants on Lp(a) and CHD.
Logistic regression was used to test the association of coronary heart disease (CHD) as an outcome and DNA sequence variant as a predictor, adjusting for sex and principal components of ancestry, with additional adjustment for array type and age in UK Biobank. The impact of LPA variation on CHD risk is directly proportional to its effect on circulating Lp(a) levels. Abbreviations as in Figure 1.
DISCUSSION
To evaluate the phenotypic consequences of genetically lowered Lp(a), we leveraged: 1) 4 DNA sequence variants that alter plasma Lp(a); 2) individual-level genotype and phenotype data from >100,000 participants in UK Biobank; 3) summary genetic association results from 7 large-scale GWAS; and 4) LPA gene sequences in >15,000 participants. We found that 1 SD genetically lowered Lp(a) is associated with a range of atherosclerosis-related diseases including CHD, PVD, stroke, HF, and aortic stenosis, but was not associated with 31 other different diseases in a phenome-wide association study.
These data allowed for several conclusions. First, using naturally occurring DNA sequence variation, we provided a dose-response relationship between perturbation of Lp(a) and risk for CHD. We examined the effects of both common and rare variants, as well as gain-of-function variants that increase Lp(a) levels and loss-of-function variants that decrease Lp(a) levels. The effects of these different variants on CHD were consistently proportional to their effect on Lp(a). Consistent with 2 recent reports (30, 32), a low-frequency loss-of-function variant (rs41272114) and a burden of rare loss-of-function variants in LPA protected against CHD. In combination, these results suggested that greater pharmacological reductions in Lp(a) levels should produce proportionally greater reductions in CHD risk, thus supporting intensive Lp(a) lowering.
Second, these results suggested that Lp(a) inhibition may be a viable therapeutic strategy to prevent a range of diseases beyond CHD. This study extends prior work demonstrating LPA variants to be associated with cardiovascular disease (5, 6, 11, 12, 33, 34). In a study of up to 12,716 individuals from 35 case-control studies, LPA variants were associated with peripheral arterial disease, ischemic stroke, and coronary artery disease (11). In contrast, in an analysis of 14,465 individuals in the Heart Protection Study, LPA variants were associated with PVD but not stroke (12). Our results suggested that LPA variants are associated with PVD, stroke, and HF. Furthermore, our report of a significant association with aortic stenosis are consistent with recent analyses demonstrating a significant effect of LPA variants on aortic valve calcification and stenosis (9, 10). Inclusion of these diseases in composite endpoints of trials of Lp(a)-reducing therapies (in addition to CHD) may increase the likelihood of a positive trial outcome, highlighting the potential benefits of genetic analyses for trial design and clinical drug development.
Third, a surprising finding of this study was that genetically lowered Lp(a) was associated with a modest but significant improvement in kidney function as assessed by 2 phenotypes, eGFR and prevalence of CKD. This lower risk of CKD may be mediated through a reduction in renal atherosclerotic burden. These findings are consistent with a recent GWAS of metabolites that revealed a strong association between LPA rs10455872 and creatinine levels (35). These results implicate Lp(a) metabolism in the development of CKD.
STUDY LIMITATIONS
This study’s major strength was the scale and variety of data sources, which improved our power to detect an effect of genetically lowered Lp(a) on a wide range of diseases and cardiometabolic traits. Our use of the largest available cohorts provided requisite power to demonstrate that genetic Lp(a) lowering was associated with a lower risk of PVD, stroke, HF, and CKD while our use of the UK Biobank allowed us to examine the association of genetic LPA variants across a wide range of noncardiovascular diseases, for which we failed to find an association.
Several study limitations deserve mention. First, our use of a 2-sample design, with exposure estimates from ARIC and outcome estimates from UK Biobank and various GWAS, prevented us from examining whether the effect of LPA variants differed by baseline levels of Lp(a). Second, our phenome-wide association study might have been underpowered to detect a significant effect of Lp(a) on many of the outcomes. As the UK Biobank develops validated phenotypes and accumulates a greater number of events, a phenome-wide association study may be better powered to detect an effect on different disorders. Third, we used prevalent events based on a verbal interview with a nurse for our phenome-wide association study of 28 different disorders. Although these events are likely to be of greater specificity than coded hospitalization data, they have not been independently validated. Finally, our population was limited to individuals of European ancestry and our results may not be generalizable to individuals of different ancestry. Indeed, both Lp(a) levels and the number of Kringle IV domains in Lp(a) have been shown to vary substantially with ancestry, suggesting that the impact of Lp(a) on cardiovascular disease may also differ by ancestry (36).
CONCLUSIONS
In conclusion, genetically decreased lipoprotein(a) is associated with a range of cardiometabolic disorders, including CHD, stroke, PVD, aortic stenosis, HF, and renal dysfunction. Pharmacological lowering of Lp(a) may reduce the risk of these disorders.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Elevated blood levels of Lp(a) have been associated with the risk of developing coronary artery disease, stroke, PVD, aortic stenosis, HF, and CKD but are not associated with the risk of developing type 2 diabetes, gastrointestinal disorders, or specific cancers.
TRANSLATIONAL OUTLOOK
Further research should be conducted to determine whether more intensive lowering of lP(a) levels results in proportionally greater reductions in cardiovascular risk.
Acknowledgments
CAE is supported by the Rhodes Trust. AVK is supported by an ACCF/Merck Cardiovascular Research Fellowship and has received consulting fees from Merck and Amarin. HHW was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning(2016R1C1B2007920). GMP is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL125751. NOS reports funding from K08HL114642 and R01HL131961, has received a research grant form AstraZeneca and consulting fees from Regeneron. DJR has received consulting fees from Aegerion Pharmaceuticals, Alnylam Pharmaceuticals, Eli Lilly and Company, Pfizer, Sanofi, and Novartis; is an inventor on a patent related to lomitapide that is owned by the University of Pennsylvania and licensed to Aegerion Pharmaceuticals; and is a cofounder of Vascular Strategies and Staten Biotechnology. DA has received speaker fees from AstraZeneca, Boehringer Ingelheim, Johnson & Johnson, Bayer, Daiichi Sankyo, GlaxoSmithKline, Eli Lilly and Company, Boston Scientific, Bristol-Myers Squibb, Menarini Group, Novartis, and Sanofi-Aventis; and research grants from GlaxoSmithKline, Eli Lilly and Company, Pfizer, and Novartis. DS has received grants from Pfizer and the National Institutes of Health. SK is supported by a research scholar award from the Massachusetts General Hospital, the Donovan Family Foundation, and R01 HL127564; he has received grants from Bayer Healthcare, Aegerion Pharmaceuticals, and Regeneron Pharmaceuticals; and consulting fees from Merck, Novartis, Sanofi, AstraZeneca, Alnylam Pharmaceuticals, Leerink Partners, Noble Insights, Quest Diagnostics, Genomics PLC, and Eli Lilly and Company; and holds equity in San Therapeutics and Catabasis Pharmaceuticals.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the NHLBI; the National Institutes of Health; or the U.S. Department of Health and Human Services
REGICOR study was supported by the Spanish Ministry of Economy and Innovation through the Carlos III Health Institute (Red Investigación Cardiovascular RD12/0042, PI09/90506), European Funds for Development (ERDF-FEDER), and by the Catalan Research and Technology Innovation Interdepartmental Commission (2014SGR240). Samples for the Leicester cohort were collected as part of projects funded by the British Heart Foundation (British Heart Foundation Family Heart Study, RG2000010; UK Aneurysm Growth Study, CS/14/2/30841) and the National Institute for Health Research (NIHR Leicester Cardiovascular Biomedical Research Unit Biomedical Research Informatics Centre for Cardiovascular Science, IS_BRU_0211_20033). The Munich MI Study is supported by the German Federal Ministry of Education and Research (BMBF) in the context of the e:Med program (e:AtheroSysMed) and the FP7 European Union project CVgenes@target (261123). Additional grants were received from the Fondation Leducq (CADgenomics: Understanding Coronary Artery Disease Genes, 12CVD02). This study was also supported through the Deutsche Forschungsgemeinschaft cluster of excellence “Inflammation at Interfaces” and SFB 1123. The Italian Atherosclerosis, Thrombosis, and Vascular Biology (ATVB) Study was supported by a grant from RFPS-2007-3-644382 and Programma di ricerca Regione-Università 2010-2012 Area 1–Strategic Programmes–Regione Emilia-Romagna. Funding for the exome-sequencing project (ESP) was provided by RC2 HL103010 (HeartGO), RC2 HL102923 (LungGO), and RC2 HL102924 (WHISP). Exome sequencing was performed through RC2 HL102925 (BroadGO) and RC2 HL102926 (SeattleGO). Exome sequencing in ATVB, PROCARDIS, Ottawa and Southern German Myocardial Infarction Study was supported by 5U54HG003067 (to Drs. Lander and Gabriel). For a full list of CHARGE-HF working group members contributing to this work and for CHARGE-HF acknowledgements, please see PMID 20445134.
ABBREVIATIONS AND ACRONYMS
- CHD
coronary heart disease
- HDL
high-density lipoprotein
- HF
heart failure
- GWAS
genome-wide association study
- LDL
low-density lipoprotein
- Lp(a)
lipoprotein(a)
- PVD
peripheral vascular disease
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Utermann G. The mysteries of lipoprotein(a) Science. 1989;246:904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- 2.Tsimikas S, Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandholzer C, Saha N, Kark JD, et al. Apo(a) isoforms predict risk for coronary heart disease. A study in six populations. Arterioscler Thromb. 1992;12:1214–1226. doi: 10.1161/01.atv.12.10.1214. [DOI] [PubMed] [Google Scholar]
- 5.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 6.Kamstrup PR, Tybjærg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 7.Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 8.Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12:581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- 9.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Helgadottir A, Gretarsdottir S, Thorleifsson G, et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60:722–729. doi: 10.1016/j.jacc.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 12.Hopewell JC, Clarke R, Parish S, et al. Lipoprotein(a) genetic variants associated with coronary and peripheral vascular disease but not with stroke risk in the Heart Protection Study. Circ Cardiovasc Genet. 2011;4:68–73. doi: 10.1161/CIRCGENETICS.110.958371. [DOI] [PubMed] [Google Scholar]
- 13.Sawabe M, Tanaka N, Mieno MN, et al. Low lipoprotein(a) concentration is associated with cancer and all-cause deaths: a population-based cohort study (the JMS cohort study) PLoS ONE. 2012;7:e31954. doi: 10.1371/journal.pone.0031954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem. 2010;56:1252–1260. doi: 10.1373/clinchem.2010.146779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Consortium for Blood Pressure Genome-Wide Association Studies. Ehret GB, Munroe PB, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Lipids Genetics Consortium. Willer CJ, Schmidt EM, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott RA, Lagou V, Welch RP, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat. Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CARDIoGRAMplusC4D Consortium. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators. Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. N Engl J Med. 2016;374:1134–1144. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith NL, Felix JF, Morrison AC, et al. Association of genome-wide variation with the risk of incident heart failure in adults of European and African ancestry: a prospective meta-analysis from the cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Circ Cardiovasc Genet. 2010;3:256–266. doi: 10.1161/CIRCGENETICS.109.895763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 25.Chambless LE, McMahon RP, Brown SA, Patsch W, Heiss G, Shen YL. Short-term intraindividual variability in lipoprotein measurements: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1992;136:1069–1081. doi: 10.1093/oxfordjournals.aje.a116572. [DOI] [PubMed] [Google Scholar]
- 26.Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–249. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do R, Stitziel NO, Won H-H, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khera AV, Won H-H, Peloso GM, et al. Diagnostic Yield of Sequencing Familial Hypercholesterolemia Genes in Patients with Severe Hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–2579. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyriakou T, Seedorf U, Goel A, et al. A common LPA null allele associates with lower lipoprotein(a) levels and coronary artery disease risk. Arterioscler Thromb Vasc Biol. 2014;34:2095–2099. doi: 10.1161/ATVBAHA.114.303462. [DOI] [PubMed] [Google Scholar]
- 31.Ogorelkova M, Gruber A, Utermann G. Molecular basis of congenital lp(a) deficiency: a frequent apo(a) “null” mutation in caucasians. Hum Mol Genet. 1999;8:2087–2096. doi: 10.1093/hmg/8.11.2087. [DOI] [PubMed] [Google Scholar]
- 32.Lim ET, Würtz P, Havulinna AS, et al. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet. 2014;10:e1004494. doi: 10.1371/journal.pgen.1004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laschkolnig A, Kollerits B, Lamina C, et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res. 2014;103:28–36. doi: 10.1093/cvr/cvu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chasman DI, Shiffman D, Zee RYL, et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203:371–376. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kettunen J, Demirkan A, Würtz P, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7:11122. doi: 10.1038/ncomms11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraft HG, Lingenhel A, Pang RW, et al. Frequency distributions of apolipoprotein(a) kringle IV repeat alleles and their effects on lipoprotein(a) levels in Caucasian, Asian, and African populations: the distribution of null alleles is non-random. Eur J Hum Genet. 1996;4:74–87. doi: 10.1159/000472175. [DOI] [PubMed] [Google Scholar]
- 37.Shungin D, Winkler TW, Croteau-Chonka DC, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pattaro C, Teumer A, Gorski M, et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun. 2016;7:10023. doi: 10.1038/ncomms10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.