ABSTRACT
Leishmaniasis chemotherapy remains very challenging due to high cost of the drug and its associated toxicity and drug resistance, which develops over a period of time. Combination therapies (CT) are now in use to treat many diseases, such as cancer and malaria, since it is more effective and affordable than monotherapy. CT are believed to represent a new explorable strategy for leishmaniasis, a neglected tropical disease caused by the obligate intracellular parasite Leishmania. In the present study, we investigated the effect of a combination of a traditional Indian medicine (ayurveda), a natural product curcumin and miltefosine, the only oral drug for visceral leishmaniasis (VL) using a Leishmania donovani-hamster model. We developed an oral nanoparticle-based formulation of curcumin. Nanoformulation of curcumin alone exhibited significant leishmanicidal activity both in vitro and in vivo. In combination with miltefosine, it exhibited a synergistic effect on both promastigotes and amastigotes under in vitro conditions. The combination of these two agents also demonstrated increased in vivo leishmanicidal activity accompanied by increased production of toxic reactive oxygen/nitrogen metabolites and enhanced phagocytic activity. The combination also exhibited increased lymphocyte proliferation. The present study thus establishes the possible use of nanocurcumin as an adjunct to antileishmanial chemotherapy.
KEYWORDS: Leishmania donovani, oral nanoparticle-based formulation, curcumin, miltefosine, combination therapy, in vivo efficacy, nanocurcumin
INTRODUCTION
Visceral leishmaniasis (VL), commonly known as kala-azar, is caused by Leishmania donovani, a protozoan parasite, which belongs to the class Kinetoplastida. The disease is transmitted by phlebotomine sand flies in Old World and is fatal if left untreated (1). Although the disease is endemic in more than 60 countries, with 200 million people at risk, 90% of the 500,000 cases every year happen in five countries: India, Bangladesh, Nepal, Sudan, and Brazil (2). VL, along with other neglected tropical diseases, was selected by the World Health Organization (WHO) for elimination by 2015 (3). Since antileishmanial vaccines are still under development, control of the disease depends mostly on chemotherapy (4). Treatments available for VL have limitations such as parenteral administration, a long course of treatment, toxic side effects, and high cost (5). The first-line therapy for leishmaniasis includes sodium stibogluconate; however, its efficacy is now threatened by the emergence of drug-resistant parasites (6). The drug has now been replaced with liposomized amphotericin B, but its use is limited due to very high cost (7). A major milestone in chemotherapy of VL was the discovery of miltefosine, an analogue of phosphatidylcholine, initially developed as an anticancer agent (8). It is an effective oral drug, but its use in women of child-bearing age is restricted due to teratogenicity. In addition, it has a long half-life, which might encourage the emergence of resistance. Interestingly a few relapsed cases have already been reported (9).
Drug combinations that aim to delay or prevent the emergence of resistance, increase efficacy, or shorten the course of treatment are the standard practice in the treatment of several viral, bacterial, and parasitic infections (10). In VL also, the combination of sodium stibogluconate plus paromomycin or allopurinol (11–15) has been investigated experimentally and clinically. In addition, antileishmanial efficacy of miltefosine was also found to be enhanced when given in combination with amphotericin B or paromomycin (5). Cure of leishmaniasis is also dependent upon the development of an effective immune response (16). The efficacy of miltefosine has also been enhanced in combination with immunomodulators (17, 18).
In traditional Indian medicine (ayurveda), a natural product, curcumin (diferuloylmethane), the main yellow bioactive component of turmeric, acts as an immunomodulator in regulating the host defense. It has also been shown to have a wide spectrum of biological action, including leishmanicidal activity in vitro, making it a suitable candidate as antileishmanial agent (19). The molecule is well tolerated in humans. However, curcumin has the major disadvantage that it is insoluble in water and displays poor oral bioavailability as a result of low absorption. This limits the use of this phytochemical as a potential chemotherapeutic agent. Curcumin as such does not show antileishmanial efficacy in vivo; however, either its diarylheptanoid derivatives or squalenoyl-curcumin nanoassemblies have shown good in vivo efficacy against Leishmania amazonensis (20, 21). Nanoparticle-mediated drug delivery systems have proven to be one of the finest drug delivery systems; therefore, a nanoformulation of curcumin may improve the in vivo antileishmanial potential of this promising phytochemical.
In this study, we report the synthesis, physicochemical characterization, and antileishmanial efficacy of a polymer encapsulated formulation of curcumin. We tested the antileishmanial effects of nanocurcumin alone and in combination with miltefosine under in vitro and in vivo conditions in a Leishmania donovani-hamster model. We also monitored alterations in biochemical and immunological parameters such as the production of toxic oxygen/nitrogen metabolites, lymphocyte proliferation, and phagocytosis.
RESULTS
Synthesis and characterization of CNPs.
Curcumin nanoparticles (CNPs) were synthesized according to a single-emulsion/solvent evaporation method. The nanoparticles were characterized for their hydrodynamic diameter and zeta potential using a dynamic light scattering method. The mean size and charge of curcumin nanoparticles were found to be 182.3 ± 7.404 nm and −12.7 ± 0.141 mV, respectively, and the polydispersity index (PDI) was 0.281 ± 0.015 (Table 1). Transmission electron microscopy (TEM) was used to assess the absolute diameter of the nanoparticles. The average diameter of CNP assessed by TEM was found to be 25.52 ± 5.21 nm (Fig. 1). The entrapment and loading efficiencies of curcumin in the nanoformulation were observed to be 93.275 ± 3.924% and 18.655 ± 0.785%, respectively. An in vitro drug release study revealed a biphasic release pattern; a burst release of curcumin was observed in the initial 28 to 30 h, followed by sustained release for rest of the duration of the experiment. In a 12-day experimental setup, as evident from Fig. 2 (top panel), an increase in pH of the sink buffer resulted in a corresponding enhanced drug release from the nanoformulation. Almost 60, 75, and 90% of the loaded curcumin was released from the NPs when the pH of the sink buffers was kept at 1.8, 6.8, and 7.4, respectively. The drug release experiment carried out at various pH levels (Fig. 2, bottom panel) also revealed that a low-pH (1.8) medium did not favor drug release, with an almost negligible amount of curcumin released in the initial 2 h, followed by a rapid release at pH 6.8 and 7.4 of the media. Approximately 80% of the curcumin release was observed from the particles in 24 h.
TABLE 1.
Physical characterization of curcumin PLGA nanoparticles (CNP)
| Parameter | Mean CNP nanoformulation result ± SD |
|---|---|
| Size (nm) | 182.3 ± 7.404 |
| PDI | 0.281 ± 0.015 |
| Zeta potential (mV) | −12.7 ± 0.141 |
| Entrapment efficiency (%) | 93.275 ± 3.924 |
| Loading efficiency (%) | 18.655 ± 0.785 |
FIG 1.
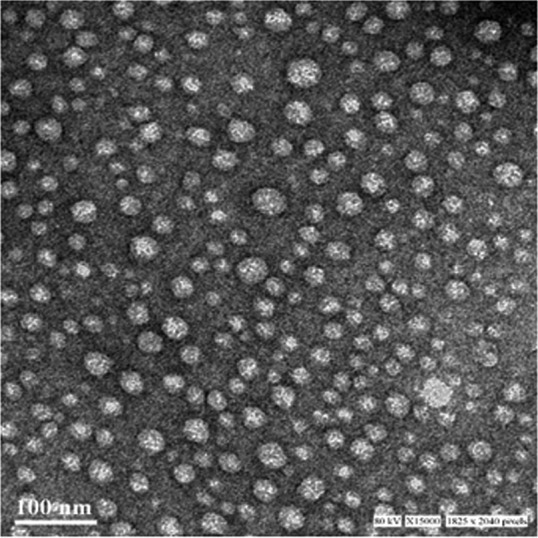
TEM image of CNPs. The nanoparticles were observed under a transmission electron microscope for their absolute diameter. The micrograph shows the round, nanosized NPs.
FIG 2.
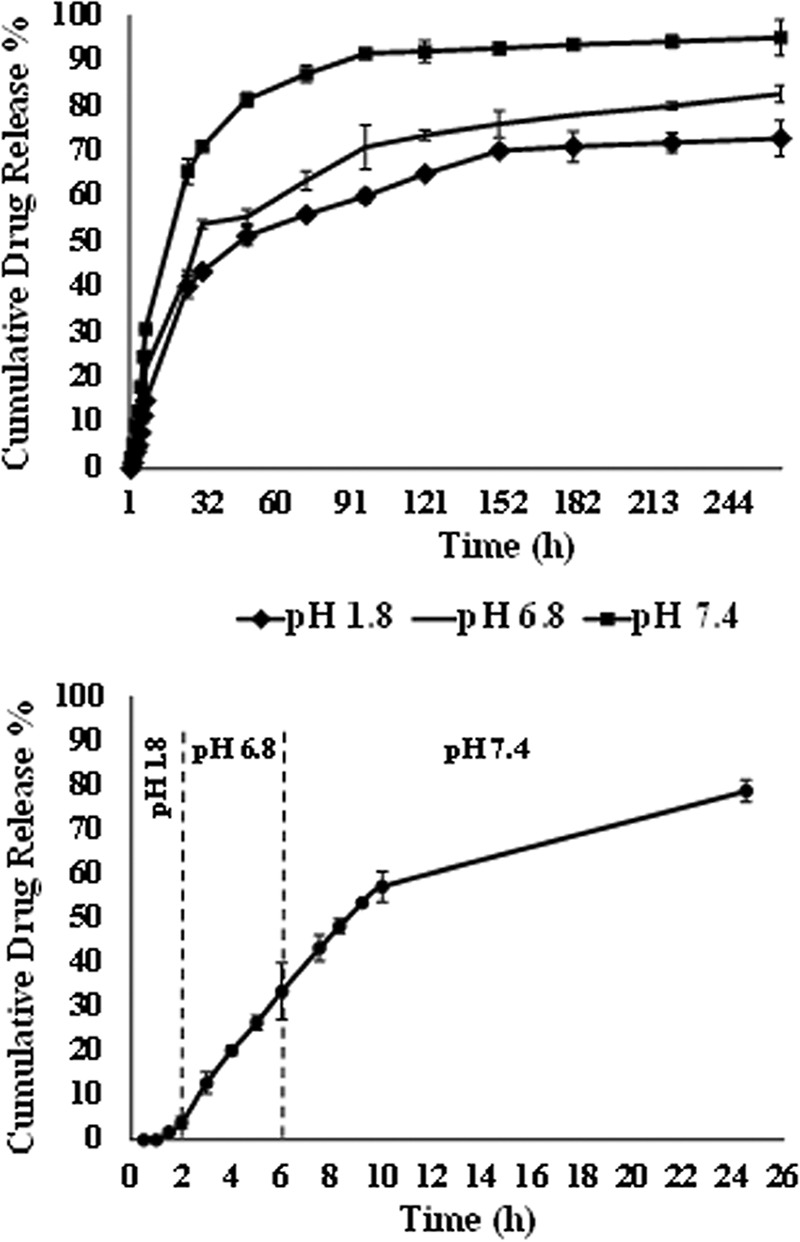
Cumulative percent release of curcumin from nanoparticles under different conditions. (Top) Data for pH 1.8, 6.8, and 7.4, separately. The experiment was set up by assessing the release of the drug from nanoparticles in buffers at different pH levels over a period of 12 days. (Bottom) Data for pH 1.8 for the first 2 h and pH 6.8 for the next 4 h, followed by pH 7.4 for 24 h. A release study was performed by dialyzing CNP against pH 1.8 buffer for the initial 2 h to simulate gut conditions. Further, the release was carried out in a pH 6.8 sink buffer for the next 4 h and then pH 7.4 for 24 h of the experiment (intestinal environment).
In vitro efficacy of curcumin nanoparticles, miltefosine, and combination.
Interestingly, CNP exhibited dose-dependent inhibition of both promastigotes and amastigotes, with 50% inhibitory concentrations (IC50s) of 1.34 ± 0.045 and 1.61 ± 0.032 μg/ml, respectively. In the presence of the drug combination, promastigotes displayed pronounced synergistic effect (Fig. 3, top panel). The combination indices (CIs) for 1.34 μg of CNP (IC50) with different miltefosine concentrations were 0.563 (0.125 μg), 0.426 (0.25 μg), 0.355 (0.5 μg), and 0.382 (1.0 μg), whereas the CIs for 0.125, 0.25, 0.5, and 1.0 μg of miltefosine with 0.67 μg of CNP were 0.470, 0.335, 0.271, and 0.309, respectively. All these doses illustrated significant synergism.
FIG 3.
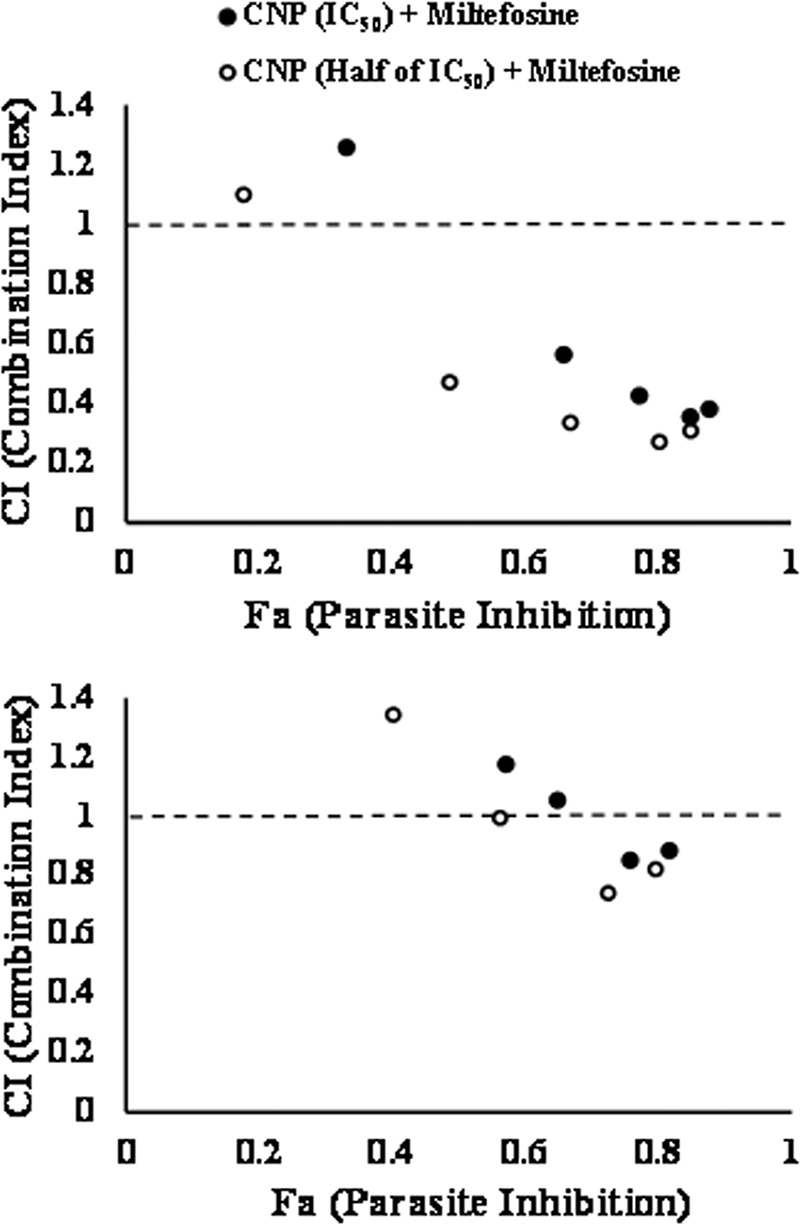
Fraction affected-combination index (Fa-CI) plots of combined treatment of CNP and miltefosine against promastigotes (top) and amastigotes (bottom). The Chou-Talalay method of synergy determination was used, along with CompuSyn software. The combined antileishmanial effect of miltefosine (at various concentrations) and CNP (at two concentrations: the IC50 and half the IC50) was determined and plotted.
Amastigotes in the presence of drug combination exhibited mixed effects (Fig. 3, bottom panel). In combination of IC50 of CNP (1.61 μg) for amastigotes, the CIs for 0.25, 0.5, and 1.0 μg of miltefosine were 1.055, 0.852, and 0.885, respectively, indicating a nearly additive effect at 0.25 μg and slight synergisms at 0.5 and 1 μg of miltefosine. At half the IC50 dose of CNP (0.8 μg), the combination exhibited moderate synergism at 0.5 (CI 0.741) and 1.0 (CI 0.821) μg of miltefosine, whereas the effect with 0.25 μg (CI 0.994) of miltefosine was nearly additive.
In vivo efficacy of curcumin and curcumin nanoparticles.
Both bulk curcumin- and Eudragit (Evonik Health Care)-coated CNPs were first evaluated for their in vivo antileishmanial efficacy at a 50-mg/kg dose. Bulk curcumin exhibited very low efficacy (only 34% inhibition of the parasite load), whereas CNPs exhibited almost 90% parasite inhibition. Therefore, for further experiments, only CNP was used.
Dose optimization of miltefosine and CNP in a hamster-L. donovani model.
Miltefosine was tested at doses ranging from 30 to 2 mg by the oral route for 5 days. Approximately 97% (96.99%) inhibition of parasitic load was observed at a 30-mg dose, followed by 92.35, 52.76, 30.83, and 12.34% parasite inhibition at doses of 20, 10, 5, and 2 mg, respectively. A subcurative miltefosine dose of 10 mg/kg was selected for combination therapy. CNP was also optimized for different doses: 50, 25, and 10 mg/kg for 5 days by the oral route. We observed 90.85% parasite inhibition at a 50-mg/kg dose and 19.57% inhibition at a 10-mg/kg dose. CNP at a 25-mg/kg dose yielded 70.26% parasitic inhibition. This dose was selected for the combination therapy study. The inhibition by CNP was consistent for up to 28 days after treatment.
Combination therapy.
Figure 4 depicts the results of antileishmanial efficacy of CNP or miltefosine alone or in combination. CNP alone exhibited 71% ± 1% parasitic load inhibition on day 28 of treatment, while miltefosine showed 55% ± 1% antileishmanial efficacy. Both of these drugs (nanocurcumin and miltefosine) upon administration in combination showed 85% ± 1% parasitic inhibition, which was significantly higher (P < 0.001) than the efficacy of miltefosine alone. Although the increase in inhibition of parasite burden by combination therapy compared to CNP monotherapy was modest (14% only), the difference was statistically significant. The data clearly indicate the adjunct effect of combination therapy of miltefosine with nanoparticles of curcumin.
FIG 4.

Mean percent parasite burden inhibition on day 28 posttreatment. L. donovani infection was given to each hamster intracardially (107 amastigotes/animal). Pretreatment biopsy was performed to examine the parasitic burden on day 20 postinfection. Animals with appropriate parasitic counts were treated with different drugs alone, as well as in combinations (adjunct). Posttreatment biopsies were performed, and the mean percent inhibition ± the SD was calculated by comparing the parasitic burdens of treated groups to those of control animals. Significance among different groups at day 28 posttreatment was calculated by using Bonferroni's multiple-comparison test. **, P < 0.01; ***, P < 0.001. CNP, Eudragit-coated nanoformulation of curcumin.
Biochemical assays.
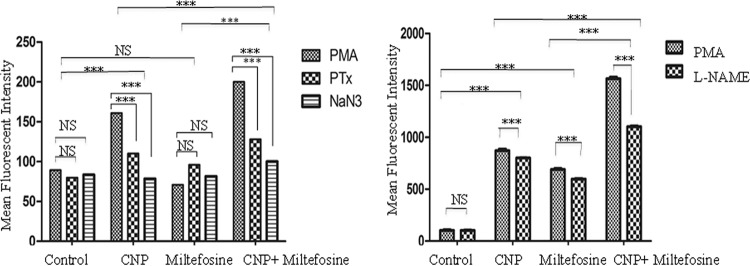
The changes in biochemical parameters are depicted in Fig. 5. The production of reactive oxygen species (ROS) in the treated group (CNP) was significantly higher than that of the untreated controls (P < 0.01), whereas no change was observed on treatment with miltefosine alone. The CNP-miltefosine-treated group showed much higher ROS production than did the control and other experimental group (P < 0.001) (Fig. 5, left panel). Animals treated with combination of CNP and miltefosine showed significantly increased levels of reactive nitrogen species (RNS) (P < 0.001) compared to animals treated with CNP or miltefosine alone (Fig. 5, right panel).
FIG 5.
ROS and NOS production. PECs from different treatment groups were collected after euthanasia of the animals. (Left) The production of ROS on PMA induction was studied by fluorescence-activated cell sorting (FACS) analysis and compared to the fluorescence obtained in the presence of respective inhibitors (PTX and NaN3). (Right) The production of NOS on PMA induction was studied by FACS analysis and compared to the fluorescence obtained in the presence of inhibitor (L-NAME). The significance of the activities of different treated groups was assessed against untreated (control) animals by using Dunnett's multiple-comparison tests. For O2˙/H2O2: PMA versus PTX/NaN3. ***, P < 0.001; NS, nonsignificant. CNP, Eudragit-coated nanoformulation of curcumin.
Phagocytosis assay.
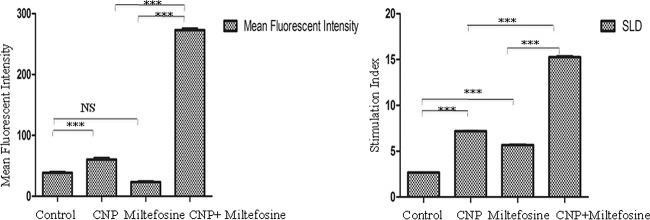
Figure 6, left panel, presents the phagocytosis indices of peritoneal exudate cells (PEC) after treatment with CNP, miltefosine, or a combination of both. Interestingly, miltefosine treatment did not increase the phagocytic index compared to untreated controls, whereas CNP exhibited a significantly increased (65.02 ± 0.04) phagocytic index compared to the control group (24 ± 1). The combination group exhibited a 5-fold-increased phagocytic index compared to the untreated control group.
FIG 6.
Phagocytosis and lymphocyte proliferation assay. PECs from different treatment groups were isolated after animal euthanasia. (Left) Phagocytosis assay. Cells were stimulated with FITC-labeled bacteria and incubated for 1 h. The mean fluorescence signals of stimulated and unstimulated cells of each group were compared. (Right) Lymphocytes were induced with specific SLA at 10 μg/ml each. Proliferation is represented as the ratio of the mean OD of the stimulated culture to that of the unstimulated control. Each bar represents pooled data (mean ± the SD) for six hamsters, and the data represent the means of triplicate wells ± the SD. The significance of the activities of different treated groups was assessed against untreated (control) animals by using Bonferroni's test. ***, P < 0001. CNP, Eudragit-coated nanoformulation of curcumin.
Lymphocyte proliferation assay.
Animals treated with CNPs (5.02), miltefosine (4.20) individually gave significant (P < 0.01), lymphocyte proliferation responses to soluble leishmania antigen (SLA) compared to untreated control (2.66) (Fig. 6, right panel). Further highly significant enhancement in proliferation was observed in animals dosed with CNP plus miltefosine (15.16) (P < 0.001).
Analysis of hepato- and nephrotoxicity.
Table 2 depicts the changes in biochemical parameters for nephro- and hepatotoxicity after treatment with CNP, miltefosine, or both in combination. No significant changes were observed in serum biomarkers of nephrotoxicity (urea, creatinine, or blood urea nitrogen) in infected animals compared to normal controls. Treatment with CNP monotherapy did not affect the levels compared to infected controls. However, an increase in creatinine levels was observed in miltefosine-treated animals, which were restored in combination therapy.
TABLE 2.
Changes in kidney and liver function biomarkers in animals (six animals per group) after treatment using CNP or miltefosine as a monotherapy or in combination
| Biochemical parameter | Mean ± SDa |
||||
|---|---|---|---|---|---|
| Normal | Infected | Treated |
|||
| CNP (25 mg/kg) | Miltefosine (10 mg/kg) | Miltefosine-CNP | |||
| Urea (mg/dl) | 38.81 ± 7.44 | 43.3 ± 10.70a,NS | 27.25 ± 8.45b,NS | 58.76 ± 13.10b,NS | 49.5 ± 11.87b,NS |
| Creatinine level (mg/dl) | 0.33 ± 0.10 | 0.35 ± 0.16a,NS | 0.32 ± 0.06b,NS | 0.95 ± 0.49b** | 0.55 ± 0.18b,NS |
| Blood urea nitrogen (mg/dl) | 18.26 ± 4.04 | 21.81 ± 6.28a,NS | 12.15 ± 3.74b,NS | 32.76 ± 11.84b,NS | 19.0 ± 9.25b,NS |
| Alanine aminotransferase (U/liter) | 138.35 ± 51.76 | 114.5 ± 22.35a,NS | 145.4 ± 55.50b,NS | 195.23 ± 26.46b,NS | 170 ± 52.96b,NS |
| Aspartate aminotransferase (U/liter) | 164.9 ± 47.26 | 91.82 ± 17.39a** | 76.26 ± 16.25b,NS | 128.89 ± 30.31b,NS | 133.33 ± 16.07b,NS |
| Alkaline phosphatase (U/liter) | 295.67 ± 16.34 | 545.72 ± 43.53a*** | 262.53 ± 51.55b*** | 431.97 ± 78.73b* | 289.56 ± 30.03b*** |
| Total bilirubin (mg/dl) | 0.14 ± 0.03 | 0.15 ± 0.04a,NS | 0.31 ± 0.09b,NS | 0.32 ± 0.16b,NS | 0.28 ± 0.06b,NS |
Significance of activities between groups was calculated using Bonferroni's multiple-comparison test. *, P > 0.05; **, P < 0.05; ***, P < 0.01; NS, not significant. CNP, curcumin nanoparticles. Superscripts: A, compared to normal group; B, compared to infected group.
No significant changes were observed in hepatotoxicity biomarkers (alanine aminotransferase [ALT] and total bilirubin) in infected untreated animals compared to control group. However, the levels of aspartate aminotransferase (AST) were significantly decreased and levels of alkaline phosphatase (ALP) were increased in infected animals. Treatment with CNP alone or in combination with miltefosine resulted in the recovery of both parameters to normal levels. The data suggest that treatment with CNP monotherapy or in combination with miltefosine does not exert any significant toxic effects in animals.
DISCUSSION
The development of resistance against first-line treatment, parenteral dosing, and the toxicity of second-line drugs have remained the major drawbacks of conventional leishmaniasis chemotherapy. Several successful reports on adjunct therapy of antimony with other drugs for treatment of leishmaniasis have been published (22–26). The antileishmanial efficacy of miltefosine, the only available oral drug, was also found to be enhanced when given in combination with paromomycin or an immunomodulator, picroliv (17, 18, 27).
Curcuminoids, isolated from the rhizome of Curcuma longa, have been in use for several centuries as therapeutic and health-promoting agents. Curcuminoids have a series of biological activities, including immunomodulation and anti-inflammatory activity (28). Recently, curcuminoids were reported to show in vitro antileishmanial activity against L. major (19), making them suitable candidates as antileishmanial agents. However, their poor water solubility at physiological pH, limited absorption, and hence poor bioavailability and chemical instability are major concerns, decreasing their efficacy under in vivo conditions. Biodegradable nanoparticles of curcumin have been developed for antimalarial activity (29). These studies encouraged us to synthesize a polymer-encapsulated formulation of curcumin coated with Eudragit (CNP) for the development of an effective combination oral antileishmanial therapy.
PLGA is a U.S. Food and Drug Administration (FDA)-approved polymer that degrades to lactic acid and glycolic acid in the biological system. These degradation products enter the Kreb's cycle to be eliminated as carbon dioxide and water; glycolic acid may also be excreted through kidneys. Its properties can be manipulated by varying the lactide/glycolide ratio and hence its degradation kinetics, which make it versatile and the preferred polymer of choice for biomedical applications (30–32). Curcumin is lipophilic in nature; its nanoparticles are prepared by a single-emulsion-solvent evaporation method (33). The synthesis parameters were optimized to generate nanosized particles with a high loading efficiency. The hydrophobicity of curcumin is also attributed to its high loading in the polymer. Polyvinyl alcohol was used as a stabilizer. To evade gut metabolism and degradation, an oral formulation of nanocurcumin was prepared using an enteric coating of Eudragit (34, 35). Eudragit offers a pH-dependent drug release and a much improved targeted action, thus elevating the drug's efficacy. We used Eudragit L30D in our method, which exhibits dissolution characteristics above pH 5.5, the duodenum being its site of action.
The particles were characterized for their size using two different techniques: DLS and TEM. Size is the most important characteristic of a nanoparticulate system since it is a major determinant of the uptake mechanism and intracellular route by which nanoparticles are internalized in cells (36). CNP displayed a nanorange size and a low polydispersity index. A low PDI indicates a low disparity in the particle sizes (Table 1). Nonuniformity in size can abrogate the efficacy of the drug by causing improper circulation, distribution, and clearance of the particles. The TEM size (Fig. 1) was observed to be much smaller than that measured by DLS. This is because the TEM measures the absolute size of nanoparticles in a dry state, whereas DLS gives the hydrodynamic diameter, as reported earlier (37).
Further, we examined the entrapment and loading efficiencies of curcumin in the polymeric formulation, which were found to be reasonably high owing to the hydrophobic nature of the drug. Loading efficiency gives the amount of drug present in a specified quantity of the nanoparticles, whereas the entrapment efficiency gives a comparative idea about how much drug has been entrapped out of the initial amount of drug used in synthesis.
An in vitro release study was performed to estimate the amount of drug released from the polymer at different time intervals (Fig. 2). As evident from the data, the nanoparticles exhibited a biphasic release pattern: an initial burst release attributed to surface desorption and diffusion of the drug, followed by a sustained release owing to the polymer degradation, as well as diffusion. This phenomenon has been fairly well explained in other reports also (38). The release study was set up at three different pH values (1.8, 6.8, and 7.4) (39), simulating the stomach and intestinal environments. Since the particles were coated with Eudragit, which degrades at the pH of duodenum, the release of curcumin increased with an increase in pH of the release medium. In the 12-day experimental setup, approximately 60, 75, and 90% of the encapsulated curcumin was released from the polymeric nanoparticles in pH 1.8, 6.8, and 7.4 release media, respectively (Fig. 2, top panel). Further, we followed an approach to imitate the biological conditions offered to the oral formulation, wherein the combined effect of all three pH values was considered by incubating the same particles in different pH buffers for different time periods. The release of curcumin from the nanospheres was highly hindered in pH 1.8 buffer, while 30% of the drug was released in the next 4 h (pH 6.8). In the 24 h study, almost 80% of the curcumin released from the polymer (Fig. 2, bottom panel). The dissolution of Eudragit coating at the raised pH enabled the rapid release of the drug. The slow release at gut pH and elevated release at intestinal pH helped to enhance the bioavailability of the drug and site-specific delivery.
First, the in vitro antileishmanial activity of CNP was assessed. CNP alone exhibited good antileishmanial activity with IC50s of 1.34 ± 0.045 and 1.61 ± 0.032 μg/ml for promastigotes and amastigotes, respectively. Interestingly, in combination with miltefosine, it exhibited a synergistic effect (Fig. 3) against both promastigotes and intracellular amastigotes.
We chose a subcurative dose (10 mg/kg) of miltefosine for combination therapy to reduce the toxicity of the drug. In accordance with earlier reports (20), bulk curcumin did not show any promising antileishmanial activity against L. donovani, the causative agent of human kala-azar. It has also been shown that long-term use of curcumin suppresses type 1 immunity and exacerbates visceral leishmaniasis in a chronic experimental model (40). However, nanotization of curcumin significantly enhanced the antileishmanial efficacy of free curcumin. Hence, Eudragit-coated nanocurcumin formulation (CNP) was used in the study with dose regime of 5 days. For combination therapy, CNP was also used in subcurative doses to avoid its toxicity, if any. This also helped to maintain the same route of administration for both drugs. The results of the present study indicate that nanocurcumin significantly enhanced the antileishmanial efficacy of miltefosine (Fig. 4). The leishmanicidal activity of the combination was maintained up to day 28 posttreatment.
Impairment of innate immune system is an indispensable event for L. donovani to establish a successful infection. The immunosuppressive effect associated with leishmaniasis is exerted by the alteration of Th1/Th2 cytokine balance due to deactivation of macrophages induced effector molecules (ROS and NO) (41). Therefore, treatment strategies that are based on modulation of the immune response, in addition to chemotherapy, result in improved efficacy during infection. Curcumin is considered to possess potent immunomodulatory properties. However, its influence on cells of innate immune system involved in defense against Leishmania still needs to be studied. Lymphocyte proliferation and the phagocytic index, in addition to the levels of ROS and NOS, are known indicators for the assessment of macrophage activation. A significant increase in the generation of ROS (Fig. 5, left panel) and RNS (Fig. 5, right panel) was observed in animals treated with a combination of miltefosine and CNP compared to animals treated using monotherapy, This combination also exhibited significant enhanced phagocytic (Fig. 6, left panel) and lymphocyte proliferation (Fig. 6, right panel) responses. Although enhanced phagocytosis by itself might enhance the parasite uptake/infection (42), it is possible that the observed increase in ROS and NOS, the activated macrophage effector molecules, which are critical for the elimination of intracellular pathogens, may counterbalance the increased number of parasites initially taken up by the host cells. Such immunotherapeutic effects have also been reported for conventional antileishmanial drugs such as antimonials (43), miltefosine (44), and amphotericin B (45) alone or in combination with immunomodulators (18, 27). Our findings here suggest that CNP helped miltefosine to exert a leishmanicidal effect at a subcurative dose through immunomodulation. Interestingly, CNP monotherapy or combination therapy with miltefosine did not exhibit any hepato- or nephrotoxicity effects (Table 2). Hence, the application of nanocurcumin in this combination appears to be a good strategy for the treatment of leishmaniasis.
MATERIALS AND METHODS
Parasite.
L. donovani promastigotes (WHO designation MHOM/IN/80/Dd8), originally obtained as a gift from the (late) P. C. C. Garnham and routinely maintained at the institute in golden hamsters, were used in the present study. Promastigotes were grown in medium 199 (Sigma) supplemented with 10% heat-inactivated fetal calf serum (GBL) and 1% penicillin (50 U/ml) and streptomycin (50 mg/ml) solution (Sigma) at 24°C (46). Luciferase-tagged promastigotes, maintained at 25 ± 1°C in medium 199 (Sigma) supplemented with 10% fetal calf serum (Gibco, Gaithersburg, MD) and G418 (20 μg/ml), were used for the in vitro evaluation of antileishmanial activity (47).
Compound.
Curcumin and miltefosine were purchased from Sigma-Aldrich India. Poly(lactic-co-glycolic) acid (PLGA) (50/50; 30 to 60 kDa) and poly(vinyl alcohol) (PVA; 30 kDa) were procured from Sigma, USA. Eudragit L30D was obtained from Corel Pharma Chemical Company, Pvt., Ltd. (Gujarat, India). The solvents used in the present study were of AR (analytical reagent) grade and purchased locally.
Animal.
Male hamsters weighing 40 to 45 g were used in the study. All the experiments were conducted in consent with the Institutional Animal Ethics Committee (IAEC) guidelines. The hamsters were housed at 23 ± 2°C, with humidity at 60 to 63%, and fed standard rodent pellet and fresh drinking water.
Synthesis of CNPs.
The nanoparticles were prepared by using an emulsion-solvent evaporation method with slight modifications (48, 49). For the synthesis, 50 mg of curcumin dissolved in 1 ml of acetone was added slowly to 200 mg of PLGA solution in 5 ml of dichloromethane and stirred for 2 h at room temperature. The organic phase was then emulsified by its dropwise addition to 50 ml of 1% (wt/vol) PVA solution containing 0.1% Eudragit, with stirring at 1,500 rpm, followed by sonication for 5 min (30% amplitude, 45 s:15 s pulse on/pulse off) to yield the oil/water emulsion. Stirring was continued for 10 to 12 h for the complete removal of organic solvents and nanoparticle hardening. The reaction mixture was then centrifuged at 10,000 rpm for 15 min at 4°C. The pellet obtained was resuspended in double-distilled water and washed three times with water (20 ml each time). The suspension was freeze-dried to obtain the nanoformulation (CNP) and stored at 4°C under anhydrous conditions.
Characterization of CNPs.
For the dynamic light scattering (DLS) analyses, the mean particle size and zeta potential of CNPs were determined using Zetasizer Nano-ZS (Malvern Instruments, Malvern, United Kingdom) employing a nominal 5-mW He-Ne laser operating at 633-nm wavelength in accordance with the established method (50). For the assessment, the formulation (0.5 mg/ml) was dispersed in water and sonicated for 5 min (30% amplitude, 45 s:15 s pulse on/pulse off). The measurements were made in triplicate, as an average of 20 to 30 runs per measurement. The data were analyzed from the electrophoretic mobility by the application of Smoluchowski approximation.
TEM.
For TEM analyses, the polymeric nanoparticles were dispersed in water by sonication for 5 min (30% amplitude, 50:10 pulse on/pulse off). A drop of the suspension was placed over a carbon-coated grid, negatively stained using uranyl acetate, and allowed to dry at room temperature. The grid was observed by using an transmission electron microscope (Tecnai G2 Spirit; FEI, Eindhoven, The Netherlands) at a working voltage of 80 kV using Gatan digital micrograph software (37).
Drug loading and entrapment efficiency.
The drug loading and entrapment efficiencies of the CNP were determined spectrophotometrically (Cary 300 Bio, UV-visible spectrophotometer). Approximately 2-mg particles were dissolved in 1 ml of dimethyl sulfoxide. Curcumin exhibited absorption at around 427 nm, so the absorbance of the solution was measured at this wavelength. A standard curve was constructed by analyzing serially diluted curcumin in the same solvent. The loading efficiency (LE) and entrapment efficiency (EE) were then calculated using the following equations:
In vitro release study.
The release of curcumin from the nanoparticles was assessed by dialysis method. Since our polymeric formulation was enteric coated, the drug release was studied at three different pH values, i.e., 1.8 (gastric juice) and 6.8 and 7.4 (intestinal fluids), to simulate biological fluid conditions (51). A weighed amount of CNP (∼10 mg) was dispersed in 1 ml of phosphate-buffered saline (PBS; pH 7.4) in a dialysis tubing (molecular mass cutoff, 12 kDa) and transferred to vials containing 10 ml of 30% ethanolic PBS (vol/vol) maintained at three different pH values. The vials were placed in an incubator shaker at 37°C and stirred at 50 rpm. At predetermined intervals, 1-ml aliquots were withdrawn and replaced with the same amount of fresh PBS maintained at the requisite pH. The samples were then analyzed spectrophotometrically for the amount of curcumin. To closely mimic the biological environment faced by the drug after oral consumption, another experiment was set up wherein the nanoparticles (∼10 mg) were dispersed in 1 ml of PBS (pH 7.4) in dialysis tubing, which was placed first in a vial with 10 ml of ethanol-PBS (pH 1.8) for 2 h. Ethanol-PBS (pH 1.8) was then replaced by ethanol-PBS (pH 6.8), and dialysis was continued for 4 h, which was then followed by further dialysis in ethanol-PBS (pH 7.4) for 24 h. During the dialysis, aliquots were withdrawn at 30- to 60-min intervals and analyzed spectrophotometrically (52).
Antipromastigote activity.
The in vitro effect of miltefosine and the nanoformulation of curcumin on the growth of promastigotes was assessed by monitoring the luciferase activity of viable cells after treatment (47). The transgenic late-log-phase promastigotes were seeded at 5 × 105 cells/well in 96-well flat-bottomed microtiter plates (Cellstar; Greiner Bio-One GmbH, Monroe, NC), followed by incubation for 72 h in medium in the absence (control) or presence of serial dilutions of antileishmanial agents alone (10 ng to 10 μg) or in combination (IC50 or half of IC50 concentration of CNP and different concentrations of miltefosine). Nanoformulations (CNP) were suspended in water and sonicated for 3 min (30% amplitude, 50:10 pulse on/pulse off). After incubation, an aliquot (50 μl) of promastigote suspension was aspirated from each well of a 96-well plate and then mixed with an equal volume of Steady Glo reagent (Promega, Madison, WI), and the luminescence was measured using a luminometer. Values were expressed as relative luminescence units.
Anti-amastigote activity.
To assess the activity of compounds against the amastigote stage of the parasite, mouse macrophage cell line (J-774A.1), infected with promastigotes expressing the firefly luciferase reporter gene, was used. Macrophage cells were seeded in a 96-well plate (5 × 104 cells/200 μl/well) in RPMI 1640 containing 10% fetal calf serum. The plates were incubated at 37°C in a CO2 incubator. After 24 h, the medium was replaced with fresh medium containing stationary-phase promastigotes (2.5 × 105/200 μl/well). Promastigotes invade the macrophage and are transformed into amastigotes. The test agents(s) alone or in combination was added after replacing the previous medium, and the plates were incubated at 37°C in a CO2 incubator for 48 h. After incubation, the drug-containing medium was decanted and 50 μl of PBS was added to each well and mixed with an equal volume of the Steady-Glo reagent. After gentle shaking for 1 to 2 min, the reading was taken using a luminometer (47).
Synergy determination.
The interactions between CNP (at the IC50 and at half the IC50) and miltefosine (various concentrations) were assessed by using the combination index (CI) calculated using CompuSyn software (ComboSyn, Inc., Paramus, NJ), which is based on the Chou-Talalay method (53). Parasite inhibition was expressed as the fraction affected (Fa). The combination doses lying below CI = 1 in the Fa-CI plot attributed to synergism. CI values greater than 1 indicated antagonism, whereas a CI = 1 indicated an additive effect.
In vivo antileishmanial activity in hamster model.
The modified method (54) was used for in vivo screening. Golden male hamsters (inbred strain) weighing 40 to 45g were infected intracardially with 107 amastigotes per animal. Pretreatment spleen biopsy of all the animals was carried out to assess the degree of infection on day 20 of the infection. The animals with +1 infection (5 to 15 amastigotes/100 spleen cell nuclei) were included in the experiment. The infected animals were randomized into several groups of six animals each. Drug treatment by the oral route was initiated 2 days after the biopsy and continued for 5 consecutive days. Negative-control-group hamsters were administered 0.2 ml of saline solution. Posttreatment biopsies were performed on day 7 and on day 28 of the last drug administration, and amastigote counts were assessed after Giemsa staining. The intensity of infection in both treated and untreated animals and also the initial count in treated animals was compared, and the efficacy was expressed in terms of the percent inhibition (PI) according to the following formula:
where PI is the percent inhibition of amastigotes multiplication, ANAT is the actual number of amastigotes in treated animals, INAT is the initial number of amastigotes in treated animals, and TIUC is the fold increase in parasites in untreated control animals.
For the in vivo evaluation, an aqueous solution of the bulk compound (curcumin) was prepared by suspending the accurately weighed sample in a standard suspension vehicle of 10% Tween 80–ethanol (70:30) in double-distilled water. The final volume contains 10% of the vehicle for inoculation by the intraperitoneal route. For oral treatment, the nanoformulation was suspended in water and sonicated for 5 min (30% amplitude, 50:10 pulse on/pulse off).
Biochemical analysis to measure the production of ROS and RNS.
Fluorescent probe, dichlorofluorescein diacetate (DCFDA; Invitrogen) was used to measure ROS, and DAF2-FM (Invitrogen) was used to measure RNS. PMA (phorbol 12-myristate 13-acetate; Sigma) was used as an inducer for the generation of ROS. Pentoxifylline (PTX) was used to inhibit the NADP-oxidase that is necessary for superoxide radical production. Sodium azide (NaN3) is a known inhibitor of catalase that is required for H2O2 production, and L-NAME (Nω-Nitrol-arginine methyl ester hydrochloride) inhibits NO synthase, required for production of NO2. The decrease in fluorescence of inhibitor-treated cells compared to untreated ones was taken as a measure of the ROS or NOS. PECs were extracted from both control and all experimental group animals. The cells (106/ml/well) were layered in 24-well tissue culture plate (Nunclon Delta Surface; Thermo Scientific), followed by incubation in a CO2 incubator at 37°C and 5% CO2 for 24 h. The nonadherent cells were removed by washing them with incomplete RPMI medium. The cells were treated with inhibitors—10 μM PTX, 10 mM NaN3, and 1 mM L-NAME—for 1 h at 37°C and 5% CO2 in a CO2 incubator. The cells were induced with PMA (30 nM) for 30 min at 37°C and 5% CO2, followed by the addition of a suitable dye, DCFDA-ROS or DAF-FM-RNS, at a concentration of 10 or 5 μM, respectively. The cells were incubated further for 15 to 20 min. Each step was followed by washing. Free radicals generated from peritoneal macrophages oxidized nonfluorescent forms of dyes to fluorescent forms. Fluorescent signals from the dyes were read using a CellQuest FACSCalibur (Becton Dickinson) with an FL1 UV band-pass filter (excitation at 488 nm and emission at 510 ± 30 to 513 ± 30 nm).
Phagocytosis assay.
A flow cytometry-based method was used to study the phagocytic activity of macrophages (55). The PECs of all experimental and control groups were seeded (106/ml/well) in 24-well tissue culture plates, followed by incubation in CO2 incubator at 37°C and 5% CO2 for 24 h. Nonadherent cells were removed by washing them with RPMI medium. Fluorescein isothiocyanate (FITC)-labeled bacteria in 1:10 ratio were added to each well, except controls, followed by incubation for 1 h in CO2 incubator. Nonphagocytized bacteria were removed by washing them, and the cells were collected in tubes. Phagocytosis of FITC-labeled bacteria by macrophages was observed by FACSCalibur (Becton Dickinson) with an FL1 UV band-pass filter (excitation at 488 nm and emission at 510 ± 30 nm to 513 ± 30 nm). The results are presented as the phagocytic index, which is the ratio of mean optical density (OD) of stimulated cells to the mean OD of unstimulated cells.
Lymphocyte proliferation assay {XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] cell viability assay in a hamster-L. donovani model.
A cell suspension of lymphocytes was prepared from the mesenteric lymph nodes of hamsters after killing them with an overdose of anesthetic ether. The cells were plated in 96-well flat-bottom tissue culture plates (Nunc, Denmark), followed by incubation with SLA at 10 μg/ml (56). Concanavalin A (5 μg/ml) was used as a standard stimulant. The result is expressed as the stimulation index, the ratio of the mean OD of the stimulated culture to the mean OD of the unstimulated culture.
Assessment of hepato- and nephrotoxicity.
After day 28 of treatment, blood samples were collected from the animals of each group (uninfected controls, infected controls, and infected animals treated with CNP, miltefosine, or both in combination). Samples were allowed to stand at room temperature for half an hour to get the serum separated and then centrifuged at 3,000 rpm for 10 min. Sera were collected and kept at 4°C until further analyses.
Urea, creatinine, BUN (blood urea nitrogen), AST (aspartate aminotransferase), ALP (alkaline phosphatase), ALT (alanine aminotransferase), and total bilirubin were estimated in the samples by using the semiautomated blood chemistry analyzer (Selectra Junior) according to a standard protocol described by the manufacturer. Values were analyzed, tabulated, and compared.
Statistical analysis.
The results are presented as the means ± the standard deviations (SD) of two experiments, and analysis of the data was carried out using Bonferroni's multiple-comparison tests and Dunnett's multiple-comparison tests. Differences with a P of <0.05 were considered significant.
ACKNOWLEDGMENTS
This study was supported by CSIR-Network Project HOPE (BSC0114). The Council of Scientific and Industrial Research is gratefully acknowledged for financial support to B.T. (CSIR-Network Project HOPE BSC0114) and R.P. (CSIR-Network Project NanoSHE BSC0112). K.C.G. acknowledges the Indian Council of Medical Research, New Delhi, India, for awarding a Distinguished Scientist Chair at the CSIR-Institute of Genomics and Integrative Biology, Delhi, India. This paper is CDRI communication no. 9398.
We thank A. L. Vishwakarma for technical assistance in flow cytometry, Karthik R. for technical assistance in animal work, Sudhakar Yadav and Anil Kumar Meena for help in blood collection and autopsy, and Anurag Kumar Srivastava for technical help in blood biochemistry.
Footnotes
This is CDRI communication no. 9398.
REFERENCES
- 1.Alvar J, Yactayo S, Bern C. 2006. Leishmaniasis and poverty. Trends Parasitol 22:552–557. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M. 2010. Combination therapy for visceral leishmaniasis. Lancet Infect Dis 10:184–194. doi: 10.1016/S1473-3099(10)70011-6. [DOI] [PubMed] [Google Scholar]
- 3.Maltezou HC. 2010. Drug resistance in visceral leishmaniasis. J Biomed Biotechnol 2010:617521. doi: 10.1155/2010/617521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kedzierski L, Sakthianandeswaren A, Curtis JM, Andrews PC, Junk PC, Kedzierska K. 2009. Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr Med Chem 16:599–614. doi: 10.2174/092986709787458489. [DOI] [PubMed] [Google Scholar]
- 5.Seifert K, Croft SL. 2006. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob Agents Chemother 50:73–79. doi: 10.1128/AAC.50.1.73-79.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunder S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health 11:849–854. [DOI] [PubMed] [Google Scholar]
- 7.Bern C, Moore JL, Berenguer J, Boelaert M, Boer M, Davidson R, Figueras C, Gardoni L, Kafetzis AD. 2006. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis 43:917–924. doi: 10.1086/507530. [DOI] [PubMed] [Google Scholar]
- 8.Croft S, Neal R, Pendergast W, Chan J. 1987. The activity of alkyl phosphocholines and related derivates against Leishmania donovani. Biochem Pharmacol 36:2633–2636. doi: 10.1016/0006-2952(87)90543-0. [DOI] [PubMed] [Google Scholar]
- 9.Sundar S, Murray HW. 2005. Availability of miltefosine for the treatment of kala-azar in India. Bull World Health Organ 83:394–395. [PMC free article] [PubMed] [Google Scholar]
- 10.White NJ. 1999. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41:301–308. [PubMed] [Google Scholar]
- 11.Seaman J, Pryce D, Sondorp HE, Moody A, Bryceson AD, Davidson RN. 1993. Epidemic visceral leishmaniasis in Sudan: a randomized trial of aminosidine plus sodium stibogluconate versus sodium stibogluconate alone. J Infect Dis 168:715–720. doi: 10.1093/infdis/168.3.715. [DOI] [PubMed] [Google Scholar]
- 12.Chunge CN, Gachihi G, Muigai R, Wasunna K, Rashid JR, Chulay JD, Anabwani G, Oster CN, Bryceson AD. 1985. Visceral leishmaniasis unresponsive to antimonial drugs. III. Successful treatment using a combination of sodium stibogluconate plus allopurinol. Trans R Soc Trop Med Hyg 79:715–718. [DOI] [PubMed] [Google Scholar]
- 13.Musa A, Khalil E, Hailu A, Olobo J, Balasegaram M, Omollo R, Edwards T, Rashid J, Mbui J, Musa B, Abuzaid AA, Ahmed O, Fadlalla A, El-Hassan A, Mueller M, Mucee G, Njoroge S, Manduku V, Mutuma G, Apadet L, Lodenyo H, Mutea D, Kirigi G, Yifru S, Mengistu G, Hurissa Z, Hailu W, Weldegebreal T, Tafes H, Mekonnen Y, Makonnen E, Ndegwa S, Sagaki P, Kimutai R, Kesusu J, Owiti R, Ellis S, Wasunna M. 2012. Sodium stibogluconate (SSG) and paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl Trop Dis 6:e1674. doi: 10.1371/journal.pntd.0001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakur CP, Sinha PK, Singh RK, Hassan SM, Narain S. 2000. Miltefosine in a case of visceral leishmaniasis with HIV coinfection; and rising incidence of this disease in India. Ans R Soc Trop Med Hyg 94:696–697. doi: 10.1016/S0035-9203(00)90238-4. [DOI] [PubMed] [Google Scholar]
- 15.Chunge CN, Owate J, Pamba HO, Donno L. 1990. Treatment of visceral leishmaniasis in Kenya by aminosidine alone or combined with sodium stibogluconate. Trans R Soc Trop Med Hyg 84:221–225. doi: 10.1016/0035-9203(90)90263-E. [DOI] [PubMed] [Google Scholar]
- 16.Wolday D, Berhe N, Akuffo H, Britton S. 1999. Leishmania-HIV interaction: immunopathogenic mechanisms. Parasitol Today 15:182–187. doi: 10.1016/S0169-4758(99)01431-3. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Ramesh-Sharma SC, Shrivastava VML. 2005. Efficacy of Picroliv combination with miltefosine, an orally effective antileishmanial drug against experimental visceral leishmaniasis. Acta Trop 94:41–47. doi: 10.1016/j.actatropica.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Sane S, Shakya N, Gupta S. 2011. Immunomodulatory effect of Picroliv on the efficacy of paromomycin and miltefosine in combination in experimental visceral leishmaniasis. Exp Parasitol 127:376–381. doi: 10.1016/j.exppara.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Fouladvand M, Barazesh A, Tahmasebi R. 2013. Evaluation of in vitro antileishmanial activity of curcumin and its derivatives “gallium curcumin, indium curcumin, and diacethyl-curcumin. Eur Rev Med Pharmacol Sci 24:3306–3308. [PubMed] [Google Scholar]
- 20.Alves LV, Temporal RM, Cysne Finkelstein L, Leon LL. 2003. Efficacy of a diarylheptanoid derivative against Leishmania amazonensis. Mem Inst Oswaldo Cruz 4:553–555. [DOI] [PubMed] [Google Scholar]
- 21.Ali Cheikh ZI, Caron J, Cojean S, Bories C, Couvreur P, Loiseau PM, Desmaële D, Poupon E, Champy P. 2015. “Squalenoylcurcumin” nanoassemblies as water-dispersible drug candidates with antileishmanial activity. Chem Med Chem 10:411–418. doi: 10.1002/cmdc.201402449. [DOI] [PubMed] [Google Scholar]
- 22.Solgi G, Kariminia A, Abdi K, Darabi M, Behnaz Ghareghozloo. 2006. Effects of combined therapy with thalidomide and glucantime on leishmaniasis induced by Leishmania major in BALB/c mice. J Parasitol 1:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olliaro PL. 2010. Drug combinations for visceral leishmaniasis. Curr Opin Infect Dis 23:595–602. doi: 10.1097/QCO.0b013e32833fca9d. [DOI] [PubMed] [Google Scholar]
- 24.Omollo R, Alexander N, Edwards T, Khalil EA, Younis BM, Abuzaid AA, Wasunna M, Njoroge N, Kinoti D, Kirigi G, Dorlo TP, Ellis S, Balasegaram M, Musa AM. 2011. Safety and efficacy of miltefosine alone and in combination with sodium stibogluconate and liposomal amphotericin B for the treatment of primary visceral leishmaniasis in East Africa: study protocol for a randomized controlled trial. Trials 12:166. doi: 10.1186/1745-6215-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dastgheib L, Naseri M, Mirashe Z. 2012. Both combined oral azithromycin plus allopurinol and intramuscular glucantime yield low efficacy in the treatment of Old World cutaneous leishmaniasis: a randomized controlled clinical trial. Int J Dermatol 51:1508–1511. doi: 10.1111/j.1365-4632.2012.05610.x. [DOI] [PubMed] [Google Scholar]
- 26.Llanos-Cuentas A, Echevarría J, Cruz M, La Rosa A, Campos P, Campos M, Franke E, Berman J, Modabber F, Marr J. 1997. Efficacy of sodium stibogluconate alone and in combination with allopurinol for treatment of mucocutaneous leishmaniasis. Clin Infect Dis 25:677–684. doi: 10.1086/513776. [DOI] [PubMed] [Google Scholar]
- 27.Puri A, Sahai R, Haq W, Zaidi A, Guru PY, Tripathi LM, Srivastava VM. 2005. Immunomodulatory activity of analog of muramyl dipeptide and their use as adjunct to chemotherapy of Leishmania donovani in hamster. Int Immunopharmacol 6:937–946. [DOI] [PubMed] [Google Scholar]
- 28.Chandrasekaran CV, Sundarajan K, Edwin JR, Gururaja GM, Mundkinajeddu D, Agarwal A. 2013. Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. Pharmacognosy Res 5:71–79. doi: 10.4103/0974-8490.110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh A, Banerjee T, Bhandary S, Surolia A. 2014. Formulation of nanotized curcumin and demonstration of its antimalarial efficacy. Int J Nanomed 9:5373–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crotts G, Park TG. 1998. Protein delivery from poly (lactic-co-glycolic acid) biodegradable microspheres release kinetics and stability issues. J Microencapsul 15:699–713. doi: 10.3109/02652049809008253. [DOI] [PubMed] [Google Scholar]
- 31.Makadia HK, Siegel SJ. 2011. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier polymer. Polymers (Basel) 3:1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh G, Kaur T, Kaur R, Kaur A. 2014. Recent biomedical applications and patents on biodegradable polymer: PLGA. Int J Pharm Sci 1:30–42. [Google Scholar]
- 33.Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel T. 2007. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm 334:137–148. doi: 10.1016/j.ijpharm.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 34.Tummala S, Kumar MNS, Prakash A. 2014. Formulation and characterization of 5-fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharmaceut J 23:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang J, Xu N, Ji H, Liu H, Wang Z, Wu L. 2011. Eudragit nanoparticles containing genistein: formulation, development, and bioavailability assessment. Int J Nanomed 6:2429–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. 2001. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 70:1–2. doi: 10.1016/S0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 37.Pahuja R, Seth K, Shukla K, Shukla RK, Bhatnagar P, Chauhan L, Saxena PN, Arun J, Chaudhari BP, Patel DK, Singh SP, Shukla R, Khanna VK, Kumar P, Chaturvedi RK, Gupta KC. 2015. Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in Parkinsonian rats. ACS Nano 9:4850–4871. doi: 10.1021/nn506408v. [DOI] [PubMed] [Google Scholar]
- 38.Bala I, Hariharan S, Kumar MNVR. 2004. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Therapeutic Drug Carrier Systems 21:387–422. doi: 10.1615/CritRevTherDrugCarrierSyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 39.Gupta V, Shukla SK, Shrivastava SM, Shukla S, Kumar K, Saxena DP, Shrivastava B, Chaudhary M. 2010. Studies of in vitro evaluation and dormulation of aceclofenac loaded PLGA microspheres. Int J Pharm 6:726–731. doi: 10.3923/ijp.2010.726.731. [DOI] [Google Scholar]
- 40.Adapala N, Chan MM. 2008. Long-term use of an anti-inflammatory, curcumin, suppressed type 1 immunity and exacerbated visceral leishmaniasis in a chronic experimental model. Lab Invest 12:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali N, Bhattacharya P. 2013. Translating immune cell cross-talk into a treatment opportunity for visceral leishmaniasis. Immunotherapy 5:1025–1032. doi: 10.2217/imt.13.104. [DOI] [PubMed] [Google Scholar]
- 42.Lodge R, Ouellet M, Barat C, Andreani G, Kumar P, Tremblay MJ. 2012. HIV-1 promotes intake of Leishmania parasites by enhancing phosphatidylserine-mediated, CD91/LRP-1-dependent phagocytosis in human macrophages. PLoS One 7:e32761. doi: 10.1371/journal.pone.0032761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Saldanha RR, Martins-Papa MC, Sampaio RN, Muniz-Junqueira MI. 2012. Meglumine antimonate treatment enhances phagocytosis and TNF-α production by monocytes in human cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 106:596–603. doi: 10.1016/j.trstmh.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Murray HW, Delph-Etienne S. 2000. Visceral leishmanicidal activity of hexadecylphosphocholine (miltefosine) in mice deficient in T cells and activated macrophage microbicidal mechanisms. J Infect Dis 181:795–799. doi: 10.1086/315268. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee AK, Gupta G, Bhattacharjee S, Guha SK, Majumder S, Adhikari A, Bhattachrya P, Majumdar SB, Majumdar S. 2010. Amphotericin B regulates the host immune response in visceral leishmaniasis: reciprocal regulation of protein kinase C isoforms. J Infect 61:173–184. doi: 10.1016/j.jinf.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Debrabant A, Gottilieb M, Dwyer DM. 1995. Isolation and characterization of gene encoding the surface membrane 3-nucleotidase/nuclease of Leishmania donovani. Mol Biochem Parasitol 71:51–63. doi: 10.1016/0166-6851(95)00035-Y. [DOI] [PubMed] [Google Scholar]
- 47.Gupta A, Ramesh S, Sundar S, Goyal N. 2005. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrob Agents Chemother 49:3776–3783. doi: 10.1128/AAC.49.9.3776-3783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiwari S, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, Karmakar M, Kumar IM, Lalit C, Patel DK, Srivastava V, Singh D, Gupta SK, Tripathi A, Chaturvedi RK, Gupta KC. 2014. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer's disease model via canonical Wnt/β-catenin pathway. ACS Nano 8:76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 49.Bhatnagar P, Patnaik S, Srivastava AK, Mudiam MK, Shukla Y, Panda AK, Pant AB, Kumar P, Gupta KC. 2014. Anti-cancer activity of bromelain nanoparticles by oral administration. J Biomed Nanotechnol 10:3558–3575. doi: 10.1166/jbn.2014.1997. [DOI] [PubMed] [Google Scholar]
- 50.Swami AI, Aggarwal A, Pathak A, Patnaik S, Kumar P, Singh Y, Gupta KC. 2007. Imidazolyl-PEI modified nanoparticles for enhanced gene delivery. Int J Pharm 335:180–192. doi: 10.1016/j.ijpharm.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 51.Cetin M, Aktas MS, Vural I, Ozturk M. 2012. Salmon calcitonin-loaded Eudragit- and Eudragit-PLGA nanoparticles: in vitro and in vivo evaluation. J Microencapsulation 29:156–166. doi: 10.3109/02652048.2011.635426. [DOI] [PubMed] [Google Scholar]
- 52.Mundargi RC, Rangaswamy V, Aminabhavi TM. 2011. pH-Sensitive oral insulin delivery systems using Eudragit microspheres. Drug Dev Ind Pharm 37:977–985. doi: 10.3109/03639045.2011.552908. [DOI] [PubMed] [Google Scholar]
- 53.Chou TC, Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 54.Bhatnagar S, Guru PY, Katiyar JC, Srivastava R, Mukherjee A, Akhtar MS, Seth M, Bhaduri AP. 1989. Exploration of antileishmanial activity in heterocycles; results of their in vivo and in vitro bioevaluations. Indian J Med Res 89:439–444. [PubMed] [Google Scholar]
- 55.Sharma P, Raghavan SA, Saini R, Dikshit M. 2004. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocyte: modulatory effect of nitric oxide. J Leukoc Biol 75:1070–1078. doi: 10.1189/jlb.0903415. [DOI] [PubMed] [Google Scholar]
- 56.Shrivastava JK, Misra A, Sharma P, Shrivastava B, Naik S, Dube A. 2003. Prophylactic potential of autoclaved Leishmania donovani with BCG against experimental visceral leishmaniasis. Parasitology 127:107–114. doi: 10.1017/S0031182003003457. [DOI] [PubMed] [Google Scholar]