Abstract
Objective
Increasing evidence supports the efficacy of trauma-focused exposure therapy in the treatment of posttraumatic stress disorder (PTSD) and co-occurring substance use disorders. Little is known, however, about the mechanisms of change in treatment for patients with PTSD and co-occurring substance use disorders. The aim of the present study was to examine whether within- and between-session habituation of distress and substance craving during imaginal exposure relates to treatment outcomes among US military Veterans with PTSD and a co-occurring substance use disorder (N = 54).
Method
Veterans received Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE), a manualized integrated treatment combining PE with cognitive behavioral therapy for substance use disorders as part of a larger randomized clinical trial. Self-reported distress and craving ratings were collected during each imaginal exposure session.
Results
Data were analyzed using a series of random intercept and slope multilevel linear and generalized linear models. Results revealed that between-session habituation of distress and craving was associated with greater improvement in PTSD symptoms during treatment. Between-session habituation of craving was also associated with a marginally greater reduction in frequency of substance use among participants still reporting use during treatment. Within-session habituation of distress was unrelated to treatment outcome.
Conclusions
Together, these findings indicate that habituation in both distress and craving may be important in maximizing treatment outcome for patients with PTSD and comorbid substance use disorders.
Keywords: PTSD, posttraumatic stress disorder, substance use disorders, Veterans, prolonged exposure
Individuals with co-occurring PTSD and substance use disorders suffer a more complicated clinical course and less successful treatment outcome compared to those with either diagnosis alone (Flanagan, Korte, Killeen, & Back, 2016). Although Prolonged Exposure (PE; Foa, Hembree, & Rothbaum, 2007) is a well-established and effective treatment approach for PTSD (Institute of Medicine, 2008), the “sequential model”, wherein substance use is treated first and PTSD treatment is deferred to another venue and/or clinician, has been the standard of care for treating co-occurring PTSD and substance use disorders. (McCauley, Killeen, Gros, Brady, & Back, 2012). It was long assumed that distress associated with confronting traumatic memories during PE would lead to increased substance use, or relapse among those in recovery (Solomon, Gerrity, & Muff, 1992). However, a growing number of studies suggest that trauma-related exposure therapy yields greater improvement in PTSD symptoms compared to traditional substance use treatments; and there is no evidence of associated increases in substance use, risk of relapse, or treatment dropout (Roberts et al., 2015; Torchalla et al., 2012).
Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE; Back et al., 2014) is one such treatment that has received empirical support in both civilian and Veteran populations (Back et al., 2012, 2016; Brady, Dansky, Back, Foa, & Carroll, 2001, 2001; Mills et al., 2012). COPE is a 12-session cognitive-behavioral therapy (CBT), that integrates CBT for substance use disorders (e.g., Carroll, 1998) and PE therapy for PTSD (Foa et al., 2007). COPE is designed to reduce both PTSD and substance use disorder severity as well as to improve well-being and functioning.
Questions exist regarding the mechanisms of action underlying therapeutic change in integrated treatments such as COPE. Informed by the hypothesized mechanisms of exposure therapy outlined by emotional processing theory (EPT; Foa & Kozak, 1986), a robust empirical literature has examined predictors of change in PTSD symptoms during PE. EPT suggests that recovery from PTSD is achieved through a two-part process: 1) exposure to situations or memories that activate conditioned fear structures maintaining symptoms of PTSD, and 2) introduction of fear-incompatible information needed to develop a competing structure without pathological associations (Foa & McNally, 1996). Habituation of fear or distress during therapy occurring both within-session (W-S) and between-session (B-S) has been widely studied as one primary indicator of this change process. As reviewed by Foa & McLean (2016), B-S habituation (i.e., reduction in distress ratings from the first to the last imaginal exposure) predicts PTSD symptom improvement among patients receiving PE in most, but not all studies. In contrast, studies have generally failed to link degree of W-S habituation (i.e., reduction from peak distress ratings within individual imaginal exposure sessions) to PTSD symptom response.
Integrated treatments such as COPE may have unique mechanisms of action as compared to treatments designed to address either PTSD or the substance use disorder alone. Thus, the present study sought to expand upon previous mechanistic investigations of PE by examining how W-S and B-S habituation of subjective distress and substance craving during COPE relates to symptom improvement among Veterans with co-occurring PTSD and substance use disorders. It is hypothesized that greater B-S habituation will be associated with more improvement in PTSD symptoms. In addition, exploratory hypotheses examined the association between habituation of distress and craving during PE and change in substance use outcomes as well as between habituation of craving and change in PTSD symptoms.
Method
Participants
Participants were 54 Veterans of the United States military (92.6% male; Mage = 39.72, SD = 10.98) with current PTSD and substance use disorders allocated to the COPE arm of a larger, randomized clinical trial (Back et al., 2016). Veterans were recruited via flyers posted in the local Veterans Affairs (VA) and community hospitals, newspaper advertisements, and advertisements placed online (e.g., Craigslist). Inclusion criteria were: 1) U.S. military veteran status, 2) 18-65 years old, 3) met DSM-IV diagnostic criteria for current PTSD and had a score of > 50 on the Clinician-Administered PTSD Scale; and 4) met DSM-IV diagnostic criteria for a current substance use disorder and had used substances in the past 90 days. Exclusion criteria were: 1) ongoing enrollment in another treatment for PTSD or substance use, 2) suicidal or homicidal ideation with intent, 3) psychiatric conditions that would likely require a higher level of care or could interfere with treatment (e.g., psychotic disorder, dissociative identity disorder), and 4) severe cognitive impairment. Psychotropic medication use (63.0%) had to be stable for four weeks prior to enrollment. Table 1 presents sample demographic and diagnostic information.
Table 1. Sample Demographic and Diagnostic Information.
| n | % | |
|---|---|---|
| Race | ||
| White | 37 | 68.5 |
| African American/Black | 16 | 29.6 |
| Other | 1 | 1.9 |
| Hispanic Ethnicity | 2 | 3.7 |
| Substance Use Disorder Diagnoses | ||
| Alcohol Use Disorder Only | 33 | 61.1 |
| Alcohol and Drug Use Disorders | 15 | 27.8 |
| Drug Use Disorder Only | 6 | 11.1 |
| Drug Use Disorders | ||
| Cocaine | 8 | 14.8 |
| Opioid | 9 | 16.7 |
| Marijuana | 5 | 9.3 |
| Sedative/Hypnotic/Anxiolytic | 1 | 1.9 |
| Other | 1 | 1.9 |
| Current Major Depressive Episode | 21 | 38.9 |
| Current Anxiety Disorder other than PTSD | 29 | 53.7 |
PTSD = posttraumatic stress disorder.
Procedure
Following informed consent, participants completed an interview and self-report assessments. Treatment consisted of 12 weekly, individual, 90-min sessions (Back et al., 2014) focused on goal-setting, psychoeducation, and methods for coping with cravings (sessions 1-3); in-vivo exposure (sessions 3-12); and imaginal exposure (sessions 4-11). Flexible application of the protocol allowed for up to two sessions to deviate from the intended procedure to address acute clinical issues. Participants were classified as treatment completers if they attended at least 8 of the 12 sessions and at least 3 imaginal exposure sessions (Brady et al., 2001).
Measures
PTSD symptoms
The CAPS (Blake et al., 1995), a semi-structured clinical interview considered the gold standard for PTSD assessment, was used to obtain a current diagnosis of PTSD and ensure a symptom severity score ≥ 50 at baseline. The PTSD Checklist-Military (PCL-M; Weathers, Litz, Huska, & Keane, 1994), a well-established 17-item self-report measure of PTSD symptoms based on DSM-IV criteria, was administered weekly to assess change in PTSD symptoms during treatment. Internal consistency of the PCL-M was good to excellent in the current sample (α = .86 - .96). Minimal missingness (< 1%) of individual items for available observations on the PCL-M was imputed using last observation carried forward (LOCF).
Substance use
The Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998), a structured interview with strong psychometric properties, was used to obtain a current diagnosis of a substance use disorder at baseline. Percent days use of any substances (PDU) was assessed for two months prior to baseline and weekly during treatment using the Timeline Follow-Back (TLFB; Sobell & Sobell, 1992). The TLFB uses a calendar and other memory prompts to stimulate recall of substance use. The TLFB yields consistently high test-retest correlations and correlates well with other self- and collateral-reports (Carey, 1997).
Subjective distress
Levels of subjective distress were monitored at the beginning and end of each imaginal exposure, as well as every 5 minutes during the imaginal exposure using the Subjective Units of Distress Scale (SUDS; Wolpe & Lazarus, 1966), a verbal rating of subjective distress from 0 (no distress) to 100 (the worst distress ever experienced). Consistent with previous work (Nacasch et al., 2015), W-S habituation for each imaginal exposure was computed by subtracting the peak SUDS rating from the post SUDS rating, yielding measures of average W-S habituation (across all imaginal exposures), early W-S habituation (during the first imaginal exposure), and late W-S habituation (during the final imaginal exposure). B-S habituation in SUDS was calculated by subtracting the peak SUDS from the first imaginal exposure from the peak SUDS of the final imaginal exposure session.
Substance craving
Subjective ratings of substance craving were collected at the beginning and end of each imaginal exposure session using a verbal rating scale ranging from 0 (no craving) to 100 (highest craving possible). B-S habituation in substance craving was calculated by subtracting the post-craving rating for the first imaginal exposure session from the post-craving rating for the final imaginal exposure session.
Data Analytic Approach
Slopes of change in PTSD symptoms and substance use from baseline through session 12 were examined using a series of unconditional and conditional random intercept and slope multilevel linear and generalized linear models with unstructured covariance matrices using robust maximum likelihood (MLR) estimation in Mplus version 7.4 (1998-2012). Session was coded as zero (intercept) at baseline for all participants. Due to the nature of substance use data (i.e., zero-inflation), substance use was analyzed using two-part modeling (Olsen & Schafer, 2001), in which separate parts are analyzed in the same model. One part of the model captures the likelihood of using (yes/no) and the other part captures frequency of use (i.e., percent days using any substances), conditional on using. W-S and B-S SUDS and craving habituation were mean-centered prior to entry into conditional models as level 2 predictors. Model fit was examined via -2 log likelihood comparisons. Significant interactions between habituation indices and PTSD/substance use change slopes were probed based on +/- 1 standard deviation from the mean on the habituation predictors (Shek & Ma, 2011).
Results
On average, participants attended 8.83 sessions (SD = 4.12; Range: 0 – 12). Fifty four percent of participants completed all 12 sessions (n = 29), and 66.7% (n = 36) completed 8 out of 12 sessions (with at least 3 imaginal exposures) and were considered treatment completers. Completers and non-completers (n = 18) did not differ significantly at baseline on any of the sociodemographic or clinical characteristics. Table 2 displays means and standard deviations for W-S and B-S habituation in SUDS and craving ratings.
Table 2. Mean (SDs) for the Within- and Between-Session SUDS Habituation and Between-Session Craving Habituation.
| n | Mean | SD | |
|---|---|---|---|
| Average within-session SUDS habituation | 46 | -16.74 | 12.37 |
| Average Peak SUDS | 46 | 60.59 | 20.41 |
| Average Post SUDS | 46 | 43.81 | 23.41 |
| Early within-session SUDS habituation | 46 | -22.13 | 22.83 |
| Early Peak SUDS (first imaginal exposure) | 46 | 80.44 | 18.37 |
| Early Post SUDS (first imaginal exposure) | 46 | 58.30 | 27.07 |
| Late within-session SUDS habituation | 36 | -8.86 | 13.08 |
| Late Peak SUDS (last imaginal exposure) | 36 | 38.33 | 23.72 |
| Late Post SUDS (last imaginal exposure) | 36 | 29.47 | 21.54 |
| Between-session SUDS habituation | 36 | -40.56 | 26.51 |
| Between-session craving habituation | 34 | -15.03 | 28.98 |
Note: SUDS = subjective units of distress. Eight participants withdrew from the study prior to session 4. Due to pressing clinical issues, the first imaginal exposure occurred during session 5 for 2 participants and during session 6 for 1 participant. Late within-session SUDS habituation and between-session SUDS habituation ratings were available for all treatment completers (n = 36). The final imaginal exposure session occurred prior to session 11 for 8 treatment completers. In these cases, ratings from the last completed imaginal exposure were used to calculate late within-session and between-session SUDS as well as between-session craving habituation. Craving ratings were not assessed for 2 participants.
PTSD Symptoms: PCL-M
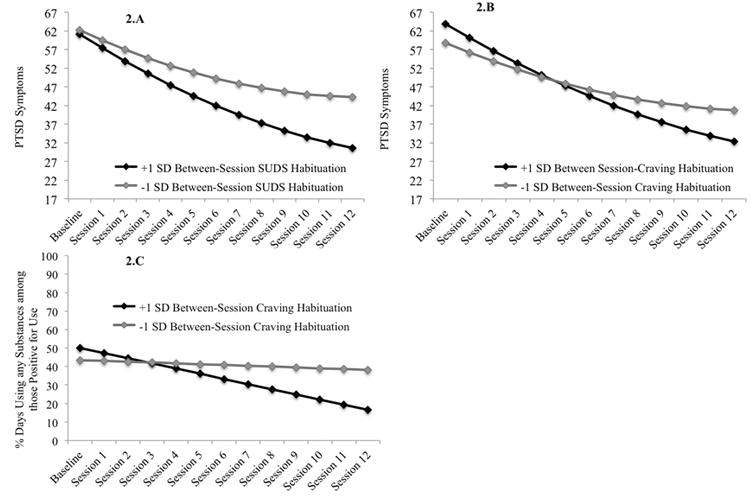
Table 3 displays results from the unconditional growth model as well as the conditional models with W-S and B-S habituation indices and their interactions with linear and quadratic session as predictors of PCL-M scores across treatment. There were significant linear and quadratic effects of session on PCL-M scores in the unconditional model, indicating that PTSD symptoms improved significantly across treatment, and the rate of change was not constant. Figure 1A displays the unconditional growth model for PCL-M scores across treatment, as well as raw means and corresponding standard errors at each session. In the conditional models W-S SUDS habituation (early, late, average) was unrelated to either initial PCL-M scores or rates of change. In contrast, B-S SUDS and craving habituation were both associated with more improvement in PCL-M scores. As displayed in Figure 2A participants scoring 1 SD above the mean, as compared to 1 SD below, in terms of B-S SUDS habituation scored an average of 13.58 points lower on the PCL-M at the end of treatment. Figure 2B demonstrates that participants scoring 1 SD above the mean, as compared to 1 SD below, on B-S craving habituation scored an average of 8.44 points lower on the PCL-M at the end of treatment.
Table 3. Results from Unconditional and Conditional Models with SUDS and Craving Habituation as Predictors of Change in PTSD Symptoms Across Treatment.
| Unconditional Model | B | SE | t | Average W-S SUDS | B | SE | t |
|---|---|---|---|---|---|---|---|
| Intercept | 61.95 | 1.59 | 39.02*** | Intercept | 62.26 | 1.74 | 35.89*** |
| Session | -2.88 | 0.58 | -4.96*** | Session | -2.93 | 0.61 | -4.81*** |
| Session2 | 0.10 | 0.04 | 2.34* | Session2 | 0.10 | 0.04 | 2.40* |
| W-S SUDS | 0.08 | 0.14 | 0.60 | ||||
| W-S SUDS*Session | 0.01 | 0.03 | 0.32 | ||||
|
| |||||||
| B-S SUDS | B | SE | t | Early W-S SUDS | B | SE | t |
|
| |||||||
| Intercept | 61.66 | 2.03 | 30.32*** | Intercept | 61.60 | 2.02 | 30.52*** |
| Session | -3.34 | 0.62 | -5.37*** | Session | -3.30 | 0.64 | -5.14*** |
| Session2 | 0.11 | 0.04 | 2.47* | Session2 | 0.11 | 0.04 | 2.45* |
| B-S SUDS | -0.02 | 0.09 | -0.18 | W-S SUDS | 0.04 | 0.07 | 0.59 |
| B-S SUDS*Session | 0.03 | 0.01 | 2.03* | W-S SUDS*Session | -0.01 | 0.01 | -0.49 |
| W-S SUDS*Session2 | -2.26 | 15.10 | -0.15 | ||||
|
| |||||||
| B-S Craving | B | SE | t | Late W-S SUDS | B | SE | t |
|
| |||||||
| Intercept | 61.37 | 1.95 | 31.46*** | Intercept | 61.62 | 2.06 | 29.94*** |
| Session | -3.27 | 0.39 | -8.35*** | Session | -3.31 | 0.64 | -5.15*** |
| Session2 | 0.10 | 0.02 | 4.22*** | Session2 | 0.11 | 0.04 | 2.46* |
| B-S Craving | -0.09 | 0.07 | -1.32 | W-S SUDS | -0.09 | 0.11 | -0.81 |
| B-S Craving*Session | 0.02 | 0.01 | 2.36* | W-S SUDS*Session | -0.01 | 0.02 | -0.35 |
Note: B-S = between-session, PTSD = posttraumatic stress disorder, SUDS = Subjective Units of Distress Scale, W-S = within-session.
p < .05,
p < .01,
p < .001.
The interaction between Session2 and the level two predictor only improved model fit for the model with Early W-S SUDS. Reduced models without this fixed effect were retained for all other models.
Figure 1. Means, standard errors, and unconditional growth models for change in (A) PTSD symptoms, (B) percent of participants using any substance, and (C) percent days using any substances, conditional on use.
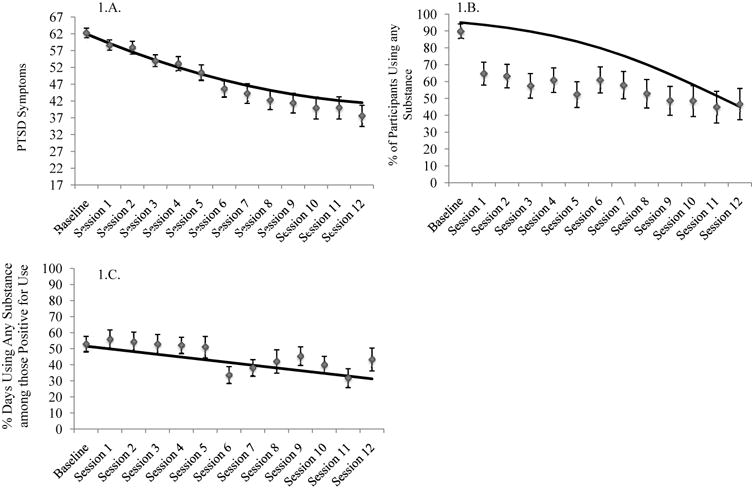
Figure 2. (A) Estimated change in PTSD symptoms across treatment as a function of between-session SUDS habituation, (B) Estimated change in PTSD symptoms across treatment as a function of between-session SUDS craving habituation, (C) Estimated change in percent days using any substances, conditional on use, as a function of between-session craving habituation.
Substance Use: TLFB
Unconditional model fit for predicting substance use was best when including the linear but not quadratic effect of session and restricting the covariance between the intercepts of use (any use vs. non-use) and frequency of use (PDU, conditional on use) to zero. Tables 4 (any use) and 5 (PDU) display results from the unconditional models as well as the conditional models with W-S and B-S habituation indices and their interactions with linear session as predictors of substance use across treatment. Figure 1B displays the probability of any use across sessions and Figure 1.C displays PDU across sessions, conditional on usage. There was a significant linear effect of session on probability of using and on PDU, indicating that both the likelihood of use and frequency of use declined significantly across treatment. W-S (early, late, average) and B-S SUDS habituation were unrelated to change in probability of using and PDU. However, higher late W-S SUDS habituation was linked to an increased probability of reporting use at baseline, while lower B-S SUDS habituation was linked to both an increased probability of using and a higher PDU at baseline. B-S craving habituation was marginally associated with a greater decrease in PDU across treatment for those who were using (p = .07). As displayed in Figure 2C, participants who were 1 SD above the mean, as compared to 1 SD below the mean, in terms of B-S craving habituation reported 21.54% fewer days of use at the end of treatment.
Table 4. Results from Unconditional and Conditional Models with SUDS and Craving Habituation as Predictors of Change in Likelihood of Substance Use Across Treatment.
| Unconditional Model | B | SE | t | Average W-S SUDS | B | SE | t |
|---|---|---|---|---|---|---|---|
| Threshold | -2.96 | 0.59 | -5.03*** | Threshold | -2.54 | 0.67 | -3.80*** |
| Session | -0.26 | 0.11 | -2.31* | Session | -0.26 | 0.11 | -2.29* |
| W-S SUDS | 0.05 | 0.05 | 1.09 | ||||
| W-S SUDS*Session | -0.01 | 0.01 | -0.80 | ||||
|
| |||||||
| B-S SUDS | B | SE | t | Early W-S SUDS | B | SE | t |
|
| |||||||
| Threshold | -3.19 | 0.43 | -7.50*** | Threshold | -2.54 | 0.65 | -3.91*** |
| Session | -0.31 | 0.10 | -3.03** | Session | -0.27 | 0.11 | -2.39* |
| B-S SUDS | -0.08 | 0.02 | -3.39*** | W-S SUDS | 0.01 | 0.02 | 0.26 |
| B-S SUDS*Session | 0.000 | 0.004 | 0.11 | W-S SUDS*Session | 0.00 | 0.004 | -0.02 |
|
| |||||||
| B-S Craving | B | SE | t | Late W-S SUDS | B | SE | t |
|
| |||||||
| Threshold | -3.13 | 1.03 | -3.05** | Threshold | -3.24 | 0.36 | -9.00*** |
| Session | -0.37 | 0.11 | -3.25*** | Session | -0.31 | 0.10 | -3.01** |
| B-S Craving | -0.01 | 0.03 | -0.41 | W-S SUDS | 0.16 | 0.06 | 2.80** |
| B-S Craving*Session | -0.004 | 0.004 | -0.99 | W-S SUDS*Session | -0.01 | 0.01 | -0.64 |
Note: B-S = between-session, PTSD = posttraumatic stress disorder, SUDS = Subjective Units of Distress Scale, W-S = within-session.
p < .05,
p < .01,
p < .001
Table 5. Results from Unconditional and Conditional Models with SUDS and Craving Habituation as Predictors of Change in Percent Days Use (PDU) of Any Substances for Participants Using Substances.
| Unconditional Model | B | SE | t | Average W-S SUDS | B | SE | t |
|---|---|---|---|---|---|---|---|
| Intercept | 51.57 | 4.52 | 11.42*** | Intercept | 50.10 | 4.81 | 10.41*** |
| Session | -1.69 | 0.61 | -2.76** | Session | -1.66 | 0.63 | -2.62** |
| W-S SUDS | 0.10 | 0.44 | 0.23 | ||||
| W-S SUDS*Session | -0.06 | 0.09 | -0.74 | ||||
|
| |||||||
| B-S SUDS | B | SE | t | Early W-S SUDS | B | SE | t |
|
| |||||||
| Intercept | 47.57 | 4.89 | 9.72*** | Intercept | 50.22 | 4.80 | 10.47*** |
| Session | -1.71 | 0.59 | -2.90** | Session | -1.69 | 0.62 | -2.75** |
| B-S SUDS | -0.35 | 0.16 | -2.17* | W-S SUDS | 0.03 | 0.19 | 0.16 |
| B-S SUDS*Session | 0.02 | 0.02 | 0.70 | W-S SUDS*Session | -0.01 | 0.03 | -0.46 |
|
| |||||||
| B-S Craving | B | SE | t | Late W-S SUDS | B | SE | t |
|
| |||||||
| Intercept | 46.72 | 5.09 | 9.19*** | Intercept | 48.85 | 5.15 | 9.48*** |
| Session | -1.61 | 0.65 | -2.49* | Session | -1.76 | 0.65 | -2.73** |
| B-S Craving | -0.12 | 0.18 | -0.67 | W-S SUDS | -0.04 | 0.42 | -0.09 |
| B-S Craving*Session | 0.04 | 0.02 | 1.80 | W-S SUDS*Session | -0.01 | 0.06 | -0.17 |
Note: B-S = between-session, PTSD = posttraumatic stress disorder, SUDS = Subjective Units of Distress Scale, W-S = within-session.
p < .05,
p < .01,
p < .001
Discussion
This study tested whether W-S and B-S SUDS and B-S craving habituation predicted change in PTSD symptoms and substance use during COPE. The hypothesis that B-S SUDS habituation would predict improvement in PTSD symptoms was supported. This finding replicates prior work demonstrating that B-S, and not W-S, SUDS habituation predicts PTSD symptom improvement (Foa & McLean, 2016). Although B-S SUDS habituation was unrelated to change in substance use, participants reporting less B-S SUDS habituation were more likely to have been using (or using more frequently) at the start of treatment. It will be important for future studies to examine how baseline substance use patterns affect PTSD treatment response as well as purported mechanisms of change during integrated treatments.
Results further demonstrated that B-S craving habituation was linked to greater improvement in PTSD symptoms and marginally greater improvement in frequency of substance use. Although there is some evidence that repeated exposure to alcohol-related cues may lead to decreased use and risk of relapse among individuals with alcohol use disorders (e.g., Drummond, & Glautier, 1994), this is the first study to document an association between exposure-related changes in craving in response to trauma cues, and improvement in PTSD and substance use. Although further research and replication are needed, the influence of B-S craving habituation may be important in advancing our understanding of integrated treatments.
Several limitations of the study warrant consideration. The relatively small sample consisted of primarily male military Veterans, which may limit the generalizability of the results. The limited measure of craving precluded examination of how W-S craving habituation may relate to treatment outcome. Future studies should consider incorporating more frequent assessments of craving, perhaps in parallel with SUDS ratings. Finally, this study focused on habituation as a hypothesized mechanism of change. Alternative mechanisms including reductions in trauma-related negative cognitions (e.g., “The world is dangerous”; Foa & McLean, 2016) and expectancy violation (e.g., “If I confront my traumatic memory, I will lose control”; Craske, Treanor, Conway, Zbozinek, & Vervliet, 2014) are being increasingly recognized as critical mediators of PTSD symptom change during exposure therapy. Future studies should examine habituation along with these factors in order to build a more complete picture of relevant change mechanisms.
Despite these limitations, this study adds substantially to the literature as the first to examine how SUDS and craving habituation relate to treatment outcome in a sample receiving an integrated, exposure-based treatment for PTSD and co-occurring substance use disorders. These findings converge with those of previous studies suggesting that B-S SUDS habituation, rather than W-S SUDS habituation, should be a focus of treatment during exposure. This work further indicates that greater attention needs to be paid to changes in craving across treatment, as this may be an important and under recognized mechanism of change in patients receiving treatment for comorbid PTSD and substance use disorders.
Acknowledgments
The authors would like to acknowledge support from NIDA grant number DA030143 (Back, S.E.), the Department of Veteran Affairs Clinical Sciences Research and Development Career Development Award CX000845 (PI: Gros), and resources at the Ralph H. Johnson VAMC. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of NIDA, the Department of Veterans Affairs, or the United States government.
Footnotes
There are no conflicts of interest.
References
- Back S, Foa E, Killeen T, Mills K, Teesson M, Carroll K, et al. Brady K. Concurrent Treatment of PTSD and Substance Use Disorders using Prolonged Exposure (COPE) Therapist Manual. New York, New York: Oxford University Press; 2014. [Google Scholar]
- Back S, Killeen T, Badour C, Flanagan J, Allen N, Beylotte F, Korte K, Foa E, Brady K. A randomized controlled trial of integrated exposure-based treatment of PTSD and co-occurring substance use disorders among veterans. Manuscript in preparation 2016 [Google Scholar]
- Back S, Killeen T, Foa E, Santa Ana E, Gros D, Brady K. Use of an integrated therapy with prolonged exposure to treat PTSD and comorbid alcohol dependence in an Iraq veteran. American Journal of Psychiatry. 2012;7:688–691. doi: 10.1176/appi.ajp.2011.11091433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Gusman F, Charney D, Keane T. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brady K, Dansky B, Back S, Foa E, Carroll K. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Preliminary findings. Journal of Substance Abuse Treatment. 2001;21:47–54. doi: 10.1016/s0740-5472(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Carey K. Reliability and validity of the time-line follow-back interview among psychiatric outpatients. A preliminary report. Psychology of Addictive Behaviors. 1997;11:26–33. [Google Scholar]
- Carroll K. A cognitive behavioral approach: treating cocaine addiction. Vol. 1. National Institute on Drug Abuse; Rockville, MD: 1998. [Google Scholar]
- Craske M, Treanor M, Conway C, Zbozinek T, Vervliet B. Maximizing exposure therapy: An inhibitory learning approach. Behaviour Research and Therapy. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond D, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. Journal of Consulting and Clinical Psychology. 1994;62:809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Flanagan J, Korte K, Killeen T, Back S. Concurrent treatment of substance use and PTSD. Current Psychiatry Reportes. 2016;18:70. doi: 10.1007/s11920-016-0709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E, Kozak M. Emotional processing of fear: Exposure to corrective information. Psycholgoical Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Foa E, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. Oxford University Press; 2007. [Google Scholar]
- Foa E, McLean C. The efficacy of exposure therapy for anxiety-related disorders and its underlying mechanisms: The case of OCD and PTSD. Annual Review of Clinical Psychology. 2016;12:1–28. doi: 10.1146/annurev-clinpsy-021815-093533. [DOI] [PubMed] [Google Scholar]
- Foa E, McNally R. Mechanisms of change in exposure therapy. In: Rapee R, editor. Current controversies in the anxiety disorders. Ney York: Guilford; 1996. pp. 329–343. [Google Scholar]
- Institute of Medicine. Treatment of posttraumatic stress disorder: An assessment of the evidence. Washington, DC: The National Academies Press; 2008. [Google Scholar]
- McCauley J, Killeen T, Gros D, Brady K, Back S. Posttraumatic stress disorder and co-occurring substance use disorders: Advances in assessment and treatment. Clinical psychology : a publication of the Division of Clinical Psychology of the American Psychological Association. 2012;19 doi: 10.1111/cpsp.12006. 10.1111/cpsp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K, Teesson M, Back S, Brady K, Baker A, Hopwood S, et al. Rosenfeld J. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: A randomized controlled trial. JAMA: the Journal of the American Medical Association. 2012;308:690–699. doi: 10.1001/jama.2012.9071. [DOI] [PubMed] [Google Scholar]
- Nacasch N, Huppert J, Su YJ, Kivity Y, Dinshtein Y, Yeh R, Foa E. Are 60-minute prolonged exposure sessions with 20-minute imaginal exposure to traumatic memories sufficient to successfully treat PTSD? A randomized noninferiority clinical trial. Behavior Therapy. 2015;46:328–341. doi: 10.1016/j.beth.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Olsen M, Schafer J. A two-part random-effects model for semicontinuous longitudinal data. Journal of the American Statistical Association. 2001;96:730–745. [Google Scholar]
- Roberts N, Roberts P, Jones N, Bisson J. Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: A systematic review and meta-analysis. Clinical Psychology Review. 2015;38:25–38. doi: 10.1016/j.cpr.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, et al. Dunbar G. The M.I.N.I. International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview. Journal of Clinical Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- Shek D, Ma C. Longitudinal data analyses using linear mixed models in SPSS: Concepts, procedures and illustrations. ScientificWorldJournal. 2011;5:42–76. doi: 10.1100/tsw.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Solomon S, Gerrity E, Muff A. Efficacy of treatments for posttraumatic stress disorder. JAMA: the Journal of the American Medical Association. 1992;268:633–638. [PubMed] [Google Scholar]
- Torchalla I, Nosen L, Rostam H, Allen P. Integrated treatment programs for individuals with concurrent substance use disorders and trauma experiences: A systematic review and meta-analysis. Journal of Substance Abuse Treatment. 2012;42:65–77. doi: 10.1016/j.jsat.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Weathers F, Litz B, Huska J, Keane T. In: PTSD Checklist-Military version. PTSD NCf, editor, editor. Behavioral Sciences Division; Boston: 1994. [Google Scholar]
- Wolpe J, Lazarus A. Behavior therapy techniques: A guide to the treatment of neuroses. New York, NY: Pergamon Press; 1966. [Google Scholar]


