Abstract
Objective
We propose that the fetal heart is highly resilient to hypoxic stress. Our objective was to elucidate the human fetal gene expression profile in response to simulated ischemia and reperfusion to identify molecular targets that account for the innate cardioprotection exhibited by the fetal phenotype.
Methods
Primary cultures of human fetal cardiac myocytes (gestational age, 15–20 weeks) were exposed to simulated ischemia and reperfusion in vitro by using a simulated ischemic buffer under anoxic conditions. Total RNA from treated and baseline cells were isolated, reverse transcribed, and labeled with Cy3 or Cy5 and hybridized to a human cDNA microarray for expression analysis. This analysis revealed a highly significant (false discovery rate, <3%) suppression of interleukin 6 transcript levels during the reperfusion phase confirmed by means of quantitative polymerase chain reaction (0.25 ± 0.11-fold). Interleukin 6 signaling during ischemia and reperfusion was assessed at the protein expression level by means of Western measurements of interleukin 6 receptor, the signaling subunit of the interleukin 6 receptor complex (gp130), and signal transducer of activated transcription 3. Posttranslational changes in the protein kinase B signaling pathway were determined on the basis of the phosphorylation status of protein kinase B, mitogen-activated protein kinase, and glycogen synthase kinase 3β. The effect of suppression of a prohypertrophic kinase, integrin-linked kinase, with short-interfering RNA was determined in an ischemia and reperfusion–stressed neonatal rat cardiac myocyte model. Endogenous secretion of interleukin 6 protein in culture supernatants was measured by enzyme-linked immunosorbent assay.
Results
Human fetal cardiac myocytes exhibited a significantly lower rate of apoptosis induction during ischemia and reperfusion and after exposure to staurosporine and recombinant interleukin 6 compared with that observed in neonatal rat cardiac myocytes (P < .05 for all comparisons, analysis of variance). Exposure to exogenously added recombinant interleukin 6 increased the apoptotic rate in both rat and human fetal cardiac myocytes (P < .05). Short-interfering RNA–mediated suppression of integrin-linked kinase, a prohypertrophy upstream kinase regulating protein kinase B and glycogen synthase kinase 3β phosphorylation, was cytoprotective against ischemia and reperfusion–induced apoptosis in neonatal rat cardiac myocytes (P < .05).
Conclusions
Human fetal cardiac myocytes exhibit a uniquely adaptive transcriptional response to ischemia and reperfusion that is associated with an apoptosis-resistant phenotype. The stress-inducible fetal cardiac myocyte gene repertoire is a useful platform for identification of targets relevant to the mitigation of cardiac ischemic injury and highlights a novel avenue involving interleukin 6 modulation for preventing the cardiac myocyte injury associated with ischemia and reperfusion.
The idea that the immature heart has an inherently greater capacity to resist stress associated with hypoxia is supported by several investigations,1,2 although contradictory interpretations have been made that appear to be model dependent.3,4 There is no PubMed-precedented information, however, regarding potential developmental changes in cardiomyocyte gene expression, which might reveal the molecular mechanisms accounting for the enhanced stress resistance in the immature human cardiac myocyte.
The idea that interleukin (IL) 6 pathway activation adversely affects cardiac function is solidly supported by clinical studies indicating that IL-6 and its specific receptor (IL-6Rα) and the 130-kd glycoprotein signaling subunit of the IL-6 receptor, gp130,5 are upregulated at the mRNA and protein levels in the myocardium in patients with advanced heart failure in comparison with a control group6 and by the large increases in IL-6 plasma concentration that occur during cardiopulmonary bypass.7 These studies, however, do not differentiate between the inference that cardiac stress engenders endogenous release of IL-6 as a protective response and the diametrically opposed viewpoint that IL-6 is per se causative of cardiac damage.
Cellular responses to IL-6 are elicited by binding of soluble IL-6 cytokine to IL-6Rα. The signaling capacity of the IL-6/IL-6Rα complex is governed by the recruitment of 2 gp130 subunits to the activated, multisubunit receptor complex. Activation of the IL-6 receptor-ligand complex is dependent on the recruitment and dimerization of gp130,8 which triggers activation of several collateral signaling pathways, including the Janus kinase/signal transducer and activator of transcription (JAK/STAT), Ras/mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3– kinase (PI3-K)– dependent pathway involving sequential phosphorylation of protein kinase B (PKB/Akt) and glycogen synthase kinase 3β (GSK-3β).9 Thus the IL-6 receptor complex consists of a ligand-binding molecule (IL-6Rα) and a signaling subunit, gp130, which provides a rapid membrane-to-nucleus signaling system regulating inflammatory gene expression.
We show here in expression-profiling experiments that human fetal cardiac myocytes (HFCMs) exposed to simulated ischemia with reperfusion (I/R) or without reperfusion exhibit a uniquely adaptive transcriptional response. The fetal response includes a limited number of functional clusters dominated by predicted anti-inflammatory properties, featuring repression of the IL-6 signaling evident at both the mRNA and protein expression levels during reperfusion-mediated stress. These data provide a plausible and therapeutically important explanation for the innately apoptosis-resistant HFCM phenotype.
Methods
Cardiac Myocyte Cultures
Primary cultures of HFCMs were derived from structurally normal hearts after elective pregnancy termination (gestational age, 15–20 weeks) under an institutional review board–approved protocol. Primary cultures of cardiac myocytes were prepared from human fetal ventricles and from 2- to 3-day neonatal rat cardiac myocytes (NRCMs) by the following previously described protocol10 in compliance with Institutional Animal Care Guidelines. Cardiomyocytes were dissociated by means of repeated (3×) enzymatic digestion with 0.05% trypsin-collagenase solution (Life Technologies) at 37°C and the dissociated cells concentrated from the supernatant by means of centrifugation (2600 rpm × 7 minutes) and plated at a density of 1 × 105 cells/cm2 on 35-mm culture dishes with 2 mL of culture medium (Dulbeccco’s modified Eagle’s medium with 10% fetal bovine serum). Preplating of seeded cells onto 100-mm culture dishes to remove noncardiomyocytes yielded cultures containing approximately 80% β-myosin heavy chain–positive cardiac myocytes.
HFCMs and NRCMs were exposed to simulated I/R or ischemia without reperfusion in vitro for the indicated time intervals by using ischemic buffer and anoxic conditions, as previously described.11 To simulate ischemia, the cultures were washed twice with phosphate-buffered saline and transferred to a pH 6.5 simulated ischemia solution containing, in 100 mL of deionized water, 0.8 g of NaCl, 0.119 g of N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid, 0.164 g of 2-deoxyglucose, 0.89 mL of KCl, 0.472 mL of CaCl2, 0.238 mL of MgCl2, and 0.135 mL of DL-lactic acid saturated in 100% N2 for 1 hour, followed by addition of 0.1 g of bovine serum albumin. Cultures were placed in an airtight container at 37°C with 100% N2 inflow for periods ranging from 2 to 24 hours and subsequently washed twice with phosphate-buffered saline before harvesting. Cardiomyocyte cultures were re-exposed to the control normoxic serum-free medium to simulate reperfusion. Control cardiomyocyte cultures were maintained under normoxic conditions at 37°C until analysis. Thus ischemia is simulated by a combination of acidic pH, interference with glucose metabolism, and anoxia, and reperfusion is simulated by means of re-exposure to normoxia, which has been shown to provoke extensive apoptosis in similar models12 and is herein referred to as simulated I/R.
Microarray Gene Expression Analysis
RNA isolation, fluorescence labeling of cDNA, hybridization to spotted arrays containing 15,264 sequence-verified cDNA clones, and quantitative fluorescence scanning of gene expression intensity were performed at the University of Toronto Health Network Microarray Centre (www.microarray.ca), as previously reported by us13 and others (for a list of publications, see http://www.microarrays.ca/about/pub.html). Significance of changes in sequential gene expression in HFCMs exposed to I/R (at control, 4 hours of ischemia, and 4 hours of ischemia plus 4 hours of reperfusion) were determined by means of repeated permutation of MIAME-compliant (www.mged.org) data with Significance Analysis for Microarray.14 Results from the Significance Analysis for Microarray analysis were visualized as hierarchic clusters in Gene Traffic (www.iobion.com), and significant genes were classified by their differential response to ischemia, reperfusion, or both. The results shown in Table 1 and Figure 1 are based on 2 biologic and 2 technical (array) replicates at each indicated time point, with a false discovery rate indicative of the statistical risk of incorrect identification of differentially expressed genes set to less than 3%.15
TABLE 1.
Lists of differentially expressed genes during ischemia and reperfusion identified with SAM, as described in the text
| Unigene cluster ID | Alias | Fold change
|
|
|---|---|---|---|
| Ischemia | Reperfusion | ||
| Group A | Repression during ischemia (I) and reperfusion (R) | ||
| Hs.74615 | Platelet-derived growth factor receptor, alpha | 0.74 | 0.50 |
| Hs.433989 | Decorin | 0.63 | 0.60 |
| Hs.179573 | Collagen, type I, alpha 2 | 0.46 | 0.41 |
| Hs.119129 | Collagen, type IV, alpha 1 | 0.50 | 0.42 |
| Hs.87409 | Thrombospondin 1 | 0.70 | 0.52 |
| Hs.458104 | Mitogen-activated protein-kinase 1 | 0.62 | 0.62 |
| Hs.274464 | Diaphorase (NADH) (cytochrome b-5 reductase) | 0.73 | 0.60 |
| Hs.93913 | Interleukin 6 | 0.82 | 0.58 |
| Hs.75636 | Likely ortholog of mouse myosin light chain, regulatory A | 0.39 | 0.39 |
| Group B | Repression during ischemia | ||
| Hs.344080 | Chromosome 6 open reading frame 150 | 0.57 | 0.92 |
| Hs.185973 | Degenerative spermatocyte homolog, lipid desaturase (Drosophila) | 0.62 | 0.93 |
| Hs.348389 | Sideroflexin 1 | 0.56 | 0.78 |
| Hs.90998 | Septin 6 | 0.53 | 0.76 |
| Hs.194071 | Hypothetical protein FLJ36812 | 0.53 | 0.87 |
| Hs.168159 | Bifunctional apoptosis regulator | 0.57 | 0.89 |
| Hs.246381 | CD68 antigen | 0.62 | 0.90 |
| Hs.91299 | Guanine nucleotide binding protein (G protein), beta polypeptide 2 | 0.58 | 0.78 |
| Group C | Activation during I/R | ||
| Hs.3094 | KIAA0063 | 1.01 | 1.64 |
| Hs.433205 | Metallothionein 1E | 1.26 | 1.77 |
| Hs.457574 | Hypothetical gene supported by AK022428 | 1.42 | 1.91 |
| Hs.8364 | Pyruvate dehydrogenase kinase, isoenzyme 4 | 1.25 | 1.47 |
| Hs.405944 | Immunoglobulin lambda locus | 1.86 | 1.71 |
| Hs.117848 | Hemoglobin, epsilon 1 | 2.27 | 2.09 |
| Hs.76847 | Alpha glucosidase II alpha subunit | 2.05 | 1.54 |
| Hs.102950 | Immunoglobulin lambda joining 3 | 2.73 | 2.34 |
| Hs.194019 | Attractin | 1.73 | 1.38 |
| Hs.82646 | Hsp40 | 0.90 | 2.75 |
| Hs.108972 | Hypothetical protein LOC285705 | 0.91 | 3.24 |
| Hs.154078 | Lipopolysaccharide binding protein | 1.47 | 0.97 |
| Hs.372513 | Glycophorin B | 1.27 | 1.22 |
| Group D | Activation during I; repression during R | ||
| Hs.227656 | Polytropic retrovirus receptor | 1.33 | 0.84 |
| Hs.28805 | Hypothetical protein LOC255512 | 1.15 | 0.76 |
| Hs.85155 | Early response factor-1 (ERF-1) | 1.15 | 0.63 |
Unsupervised hierarchic clustering reveals 4 distinct expression strata, as shown in Figure 1. Fold changes are based on measurements at 4 hours of ischemia and 2 hours of reperfusion compared with control levels. The far left column contains the Unigene cluster IDs, the annotations for which are available at http://genome-www5.stanford.edu/cgi-bin/source/sourceSearch. SAM, Significance Analysis for Microarray.
Figure 1.

Heat map with hierarchic clustering of genes showing coherent expression patterns during ischemia (Isch) and reperfusion (R). Columns represent each of 2 different biologic replicates, each performed with 2 (dye-swapped) array replicates at each of the indicated time points. Red color indicates higher expression, and green color indicates lower expression. Genewise clustering reveals 4 temporally distinct expression strata: A, repression during ischemia and reperfusion; B, repression during ischemia; C, activation during ischemia, reperfusion, or both; and D, activation during ischemia and repression during reperfusion. The far right column contains the Unigene cluster ID, the annotation for which is available at http://genome-www5.stanford.edu/cgi-bin/source/sourceSearch. Genes with significantly different expression values from control are indicated in Table 1.
Validation With Quantitative Polymerase Chain Reaction
Independent confirmation of changes in IL-6 transcription levels was performed by using real-time quantitative polymerase chain reaction (qPCR), as previously described by us.13 Primers were constructed against the 3′ ends of IL-6, and amplicon abundance was determined in real time with SYBR Green Dye (Applied Biosystems) fluorescence measurement during the logarithmic phase and normalized to that of a control gene, cyclophilin.
Western Blot Analysis
Fetal cardiomyocyte extracts containing 20 μg of protein were subjected to sodium dodecylsulfate–polyacrylamide gel electrophoresis with 10% polyacrylamide gel and transferred onto Immobilon-P transfer membranes (Millipore). Analysis was performed with polyclonal PKB antibody (Transduction Laboratories), polyclonal serine 437 catalytically active, phosphorylation-specific PKB antibody (Cell Signaling Technology), polyclonal integrin-linked kinase (ILK) antibody (Upstate Biotechnology), and anti-IL-6 receptor (IL-6Rα) and anti-gp130 antibodies (Santa Cruz Biotech). Monoclonal antibodies used for the determination of total and phosphorylated GSK-3β protein levels were from Biosource; total and phosphorylated (Py705) STAT-3, (Thr202/Tyr204) MAPK42/44, and stress-activated protein kinase (SAPK-Thr183/Pyr185) monoclonal antibodies were from Cell Signaling.
IL-6 Measurements
IL-6 concentrations in the culture supernatants were determined by using an enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (Diaclone).16 The absorbance at 450 nm was measured, and concentrations were determined by means of interpolation of a standard calibration curve. The lower limit of detection of IL-6 was 0.78 pg/mL. Human recombinant IL-6 was from Sigma (1-1395).
Measurement of Apoptosis
Apoptosis of variously treated cardiomyocytes was determined on the basis of nuclear condensation with Hoechst staining.17 Cardiomyocytes were stained with 1 μg/mL Hoescht 33342 trihydrochloride trihydrate (Molecular Probes) for detection of apoptotic nuclei. Dishes were analyzed at 20× magnification by using a Leica inverted deconvolution microscope with a coupled camera. Apoptotic cells were identified by their increased fluorescence caused by chromatin condensation and pyknotic morphology. A minimum of 300 nuclei were counted per field, and each data point consisted of 4 randomly selected fields. The measurement of cardiomyocyte apoptosis with Hoechst staining was found to correlate with, but was more sensitive than, that based on TdT-mediated dUTP nick-end labeling labeling with the APO-BRDU kit and enumeration by means of flow cytometry (FACScan/CELL Quest system, BD Biosciences) and that based on Western measurement of cleaved caspase-3 Asp175 antibody (Cell Signaling). The percentage of apoptosis was determined as the ratio of apoptotic nuclei/total Hoechst-positive nuclei, and statistical comparisons were made with Openlab 3.1.5 software. Statistical evaluation of intervention and cell type effects relied on a paired t test or 1-way analysis of variance. Data are expressed as ± standard error of the mean.
Synthesis and Transfection of ILK-Specific Short-Interfering RNA Molecules
Single-stranded short-interfering RNA (siRNA) were transcribed and annealed with a commercial kit, as outlined in the supplier’s manual (Silencer Kit, Ambion). The following sequences were used to construct ILK siRNA: ILK1, 50-AAGGGGACCACCCGCACTCGG-30; ILK2, 50-AAGGCACCAATTTCGTCGTGG-30; and ILK3, 50-AAGCTCAACGAGAATCACTCT-30.
The specificity of ILK siRNA targeting vector has been previously shown.18 GAPDH control siRNA was provided with the Silencer siRNA construction kit. Transient transfections of NRCMs were carried out using 6 μL of Lipofectin reagent (Invitrogen), according to the manufacturer’s instructions. To quantitate the extent of knockdown of ILK protein, horseradish peroxidase– conjugated immunoglobulin G was used as a secondary antibody, and ILK immunocomplexes were visualized with an enhanced chemiluminescence detection reagent (Amersham Pharmacia Biotech) and quantified by means of densitometry.
Results
Gene Expression Analysis Reveals an Antiinflammatory Transcriptional Response in HFCMs
Gene-wise clustering, as shown in Figure 1, reveals 4 temporally distinct expression strata: A, repression during I/R; B, repression during ischemia; C, activation during ischemia, reperfusion, or both; and D, activation during ischemia and repression during reperfusion. The annotation of significant genes and corresponding expression values are indicated in Table 1. Noteworthy was the significant repression of IL-6 transcription during ischemia and especially during reperfusion to 58% of preischemic levels by means of microarray analysis and to 25% ± 11% by means of qPCR measurement.
The Fetal Cardiomyocyte is Resistant to Apoptotic Stimuli
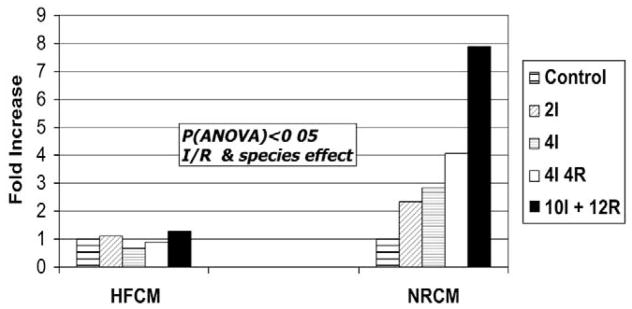
The rate of apoptosis measured with Hoechst staining, as shown in Figure 2, was significantly lower in the fetal cardiomyocytes (relative to that observed in neonatal rat–derived cardiomyocytes) in response to increasing duration of ischemia with or without of reperfusion (P < .05 for rat vs human cardiomyocytes, analysis of variance). Exogenous IL-6 (250 ng/mL) caused a similar approximately 3- to 4-fold increase in apoptosis that was maximal at 3 hours of exposure in both NRCMs (P = .012) and HFCMs (P = .034) during normoxia and resulted in a significant increase in the apoptotic rates in both cellular phenotypes after both 4 and 10 hours of ischemia (P < .05). IL-6–mediated fold increases in apoptotic rates were greater in NRCMSs compared with that in HFCMs (P = .035).
Figure 2.
Cardiomyocyte apoptotic rate during I/R. Primary cultures of human fetal cardiomyocytes (HFCM) and 2- to 3-day neonatal rat cardiomyocytes (NRCM) were exposed to simulated ischemia with or without reperfusion for the indicated time intervals. Cardiomyocytes were stained with 1 μg/mL Hoescht 33342 for detection of apoptotic nuclei on the basis of typical pyknotic nuclear morphology, and the results were expressed as a fold change in the ratio of apoptotic to normal nuclei relative to that of control levels. The rates of apoptosis increased significantly with increasing duration of ischemia and reperfusion and were higher in the neonatal rat cardiomyocytes compared with in the human fetal cardiomyocytes (P < .05, analysis of variance). I, Ischemia; R, reperfusion.
IL-6 Signaling in HFCMs Is Uncoupled During I/R
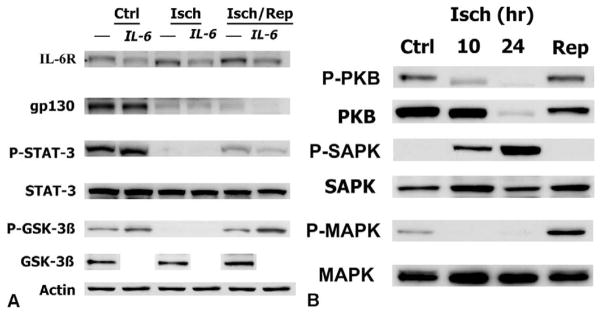
IL-6Rα in HFCMs is expressed at low levels under control conditions, increases during 4 hours of ischemia, and increases to still higher levels after 4 hours of reperfusion (Figure 3, A). STAT-3 is highly phosphorylated under control conditions, becomes almost completely dephosphorylated during ischemia, and is rephosphorylated to intermediate levels after reperfusion. Because STAT-3 is activated by the signal transducer gp130 subunit, the conspicuous decrease in gp130 levels during I/R is consistent with the correspondent dephosphorylation of STAT-3. Unexpectedly, the addition of IL-6 resulted in a decrease in the levels of IL-6Rα, gp130, and PY705-phosphorylated STAT-3 during ischemia and after reperfusion. This finding might reflect counter-regulatory degradation of IL-6Rα after ligation by exogenously added soluble IL-6. There was a coincident dephosphorylation of GSK-3β at Ser-9 during I/R, which represents an inhibitory modification for this classically antihypertrophic kinase.19 The addition of IL-6 increased the extent of GSK-3β phosphorylation under control conditions and after reperfusion in line with the prohypertrophic properties of this cytokine.5 Taken together, these data indicate posttranslational inhibition of IL-6 signaling during I/R and accords with the corresponding observed decrease in IL-6 message levels (Table 1).
Figure 3.
A, Deactivation of IL-6 pathway during ischemia-reperfusion. Western blot analysis was performed with lysates from human fetal cardiomyocytes at control (Ctrl), after 4 hours of simulated ischemia (Isch), and after 4 hours of reperfusion (Isch/Rep) with and without addition of recombinant IL-6 (250 ng/mL) at the onset of ischemia, as indicated at the top of each lane. Immunoblots were performed with total or phosphor-specific antibodies against components of the IL-6 signaling cascade as indicated, and taken together, the results reveal deactivation of IL-6 signaling during ischemia and reperfusion, as discussed in the text. Total STAT-3 and GSK-3β expression bands indicate equal protein loading in each lane, which was also confirmed with actin controls. The results shown here represent 3 experiments exhibiting similar effects. B, Kinase-specific dephosphorylation during ischemia-reperfusion. Western blot analysis was performed with lysates from human fetal cardiomyocytes at control (Ctrl), after 10 and 24 hours of simulated ischemia (Isch), and after 10 hours of reperfusion (Isch/Rep). Immunoblots were performed with both total and phosphor-specific antibodies against PKB/Akt, MAPK, and SAPK. The results indicate that deactivating dephosphorylation of PKB/Akt and MAPK occurs during ischemia, with rephosphorylation evident after reperfusion, whereas the opposite phosphorylation events occur with SAPK. The results shown here represent 3 experiments exhibiting similar effects. Abbreviations are defined in the text.
Deactivation of PKB/Akt and MAPK Signaling in HFCMs During I/R
The relay system that transmits signals from gp130 to the nucleus involves at least 3 distinct pathways of protein phosphorylation: the JAK/STAT,20 PI3-K,21 and Raf-1/MEK/MAPK20 pathways. Western analysis demonstrates dephosphorylation of PKB/Akt at Ser-473 at 10 hours of ischemia (Figure 3, B), although sequential measurements indicated an easily detectable loss of phosphorylation within 30 minutes of ischemia (data not shown). A decrease of similar magnitude in the phosphorylation of the p42/44 isoform of MAPK was evident during ischemia, with partial reperfusion-mediated rephosphorylation (Figure 3, B). In concert with the reduction in MAPK message levels by means of microarray analysis (Table 1), this posttranslational modification would predict deactivation of MAPK-mediated signaling during I/R. The finding that the stress-activated serine-threonine kinase/c-jun N-terminal kinase (SAPK/JNK), exhibited an increase in the (T183/Y185) phosphorylation signal during ischemia (Figure 3, B) indicates that the observed modifications in MAPK, PKB/Akt, and GSK-3β do not simply reflect nonspecific global protein dephosphorylation events. Because activation of SAPK/JNK has been linked to IL-6 gene expression on the basis of gene disruption in mouse embryonic fibroblasts,22 the lack of activation of this kinase during reperfusion is consistent with the generalized and concomitant repression of IL-6 signaling demonstrated in the HFCMs.
ILK Knockdown Protects Against Cardiomyocyte Stress-induced Apoptosis
Data on the effects of ILK knockdown in protection against cardiomyocyte stress-induced apoptosis are shown in Figure 4. ILK is a novel prohypertrophic kinase that causes phosphorylation of PKB/Akt and GSK-3β.18 We asked whether siRNA-mediated suppression of this proproliferative kinase, which should mimic the signaling effects observed in the fetal cardiomyocytes, could influence apoptotic threshold in the NRCMs during I/R. As shown in the insert of Figure 4, lipofectamine-mediated transfection of the ILK-specific siRNAs, but not the GAPDH siRNA, resulted in substantial (42%, P = .02) knockdown of ILK expression in NRCMs as determined by means of Western blot analysis at 72 hours after transduction. Exposure of ILK-silenced NRCMs to 4 hours of ischemia and 4 hours of reperfusion resulted in an approximate 50% decrease in the apoptosis rate in comparison with that seen in lipofectamine-only controls (P = .031).
Figure 4.
ILK suppression protects against I/R-induced apoptosis. Neonatal rat cardiomyocytes were transduced with lipofectamine only or lipofectamine containing ILK-targeted siRNA and exposed to 4 hours of ischemia and 4 hours of reperfusion (I/R), and the relative rates of apoptosis were quantitated with Hoescht 33342 staining, as described in the text. Exposure of ILK-silenced neonatal rat cardiomyocytes to 4 hours of ischemia and 4 hours of reperfusion resulted in an approximate 50% decrease (P = .031) in the apoptosis rate in comparison with lipofectamine-only controls (Ctrl). As shown in the insert, lipofectamine-mediated transfection of the ILK-specific siRNAs resulted in substantial (42%, P = .02) knockdown of ILK expression, as determined by means of Western blot analysis at 72 hours after transfection. Abbreviations are defined in the text.
Discussion
A major finding in the present study is that the HFCM exhibits resilience against proapoptotic stimuli. This was evident in the relative attenuation of cardiomyocyte apoptosis in comparison with that observed in a more mature cellular phenotype in response to simulated I/R and exogenous IL-6 exposure.
Genome expression profiling in the fetal cardiomyocyte revealed a conspicuous repression, or lack of induction, of IL-6 transcription during ischemia and especially during reperfusion (58% of preischemic levels by means of microarray, 25% by means of qPCR). IL-6 is a multifunctional cytokine with proproliferative and prohypertrophic properties in the heart.23 Upregulation of serum24 and myocardial levels of pro-inflammatory cytokines (tumor necrosis factor α, IL-1, and IL-6) have been reported in infants with tetralogy of Fallot,25 and increased IL-6 message is found in rat hearts undergoing I/R.26 However, the specific alterations in the IL-6 signaling pathway induced in the human cardiomyocyte during I/R are unknown, and it is unresolved in the literature as to whether a stress-induced increase in circulating IL-6 levels represents a cardiomyocyte-protective or cardiomyocyte-injurious response.
Our results indicate that the IL-6 pathway is inhibited at multiple levels of regulation in HFCMs in response to I/R, including gp130 receptor expression, dephosphorylation of cytoplasmic, IL-6–dependent kinases (STAT-3, PKB/Akt, and GSK-3β), and IL-6 transcription. Reduced STAT-3 phosphorylation and IL-6 transcription could result from downregulation of gp130 expression because this represents the proximal signaling module of the IL-6 receptor complex, 8,9 despite the finding of a concomitant increase in the IL-6Rα subunit during both the ischemic and reperfusion phases. The reason for downregulation of gp130 expression is unknown. Ischemia-induced disruption of membrane lipid rafts plausibly accounts for this finding because IL-6 receptor complex localization and STAT-3 signal transduction are raft dependent.27
The finding that IL-6 potentiates stress-induced cardiomyocyte apoptosis appears to conflict with previous reports demonstrating cardioprotective properties of this cytokine. 5,28 It might be speculated, however, that the mitogenic and prohypertrophic state associated with IL-6 stimulation could be energetically unfavorable under conditions of severe oxygen deprivation preceding reoxygenation, especially because IL-6 signaling has been linked to the generation of reactive oxygen species.29 The use of unbiased genome profiling provides further support for the idea that the HFCMs acquire a quiescent phenotype in response to oxygen restriction, including the finding of a significant reduction in the expression of MAPK 1 (Table 1). Activation of the MAPK cascade promotes an activating phosphorylation of the nuclear factor for IL-6, also termed C/EBPβ, a member of the CCAAT/enhancer-binding protein (c/EBP) family of transcription factors. Nuclear factor for IL-6 binds to a regulatory element in the IL-6 promoter that is essential for induction of IL-6 transcription after treatment with tumor necrosis factor α and IL-1.30 Deinduction of the MAPK signaling cascade, resulting from both decreased transcript and protein phosphorylation levels observed in the HFCM, might serve the energetically beneficial purpose of dampening agonist-induced, proinflammatory, and proproliferative signaling during I/R. IL-6 signaling might involve PI3-K–dependent, as well as STAT-3 and MAPK, pathways.21 Our data also indicate that deactivation of the PI3-K–dependent kinase PKB/Akt occurs in the HFCMs during I/R, thereby nullifying potential activation of IL-6 signaling through this collateral pathway.
ILK is a prohypertrophic kinase that is regulated in a PI3-K–dependent manner after distinct signal inputs from integrins and growth factor receptor tyrosine kinases.18 ILK causes phosphorylation of PKB/Akt and GSK-3β,18 post-translational modifications that are diametrically opposite to those that were observed in HFCMs exposed to ischemic stress. Therefore we used siRNA-mediated silencing of ILK to recapitulate the fetal response in the more apoptosis-prone rat cardiomyocyte model. This manipulation revealed an antiapoptotic effect of ILK knockdown and provides additional indirect evidence that inhibition of prohypertrophic signaling might represent a cardioprotective strategy under conditions of severe oxygen deprivation.
The induction of the antioxidant gene metallothionein I observed in the fetal cardiomyocyte transcriptional profile during I/R suggests an additional explanation for the apoptosis resistance evident in this cellular phenotype because this antioxidant has been shown to directly potentiate antiapoptotic signal transduction, as well as to mitigate redox-mediated injury.31 The functional significance of other differentially expressed genes identified in this study (Table 1), either singly or in combinatorial permutations, will require further experimentation conducted in both vulnerable and apoptosis-resistant cardiomyocyte phenotypes involving gene-specific gain- and loss-of-function strategies.
Taken together, our data support the general conclusion that the HFCM represents a useful biosynthetic platform for the identification of innate cardioprotective responses at the molecular level. The adaptive stress response exemplified by the fetal cardiomyocyte implicates the IL-6 pathway as an important and therapeutically relevant arbiter of cellular survival.
Limitations to the Study
Our data indicate that the isolated HFCM is more resistant to apoptosis induction after exposure to simulated I/R than that of the neonatal rat. The difference in stress-induced apoptotic threshold is explainable on the basis of either species-related or development stage–related differences inherent in these distinct cellular phenotypes. The elucidation of developmental stage-specific aspects of the human cardiomyocyte stress response would require a comparative analysis of human neonatal or adult cardiomyocytes with that of the fetal phenotype. The fact that postnatal human cardiomyocytes have an inherently greater susceptibility to apoptosis during in vitro culture renders such studies difficult and implies that cardiomyocyte stress resistance degrades with increasing maturation. Nevertheless, our study is directed to the investigation of IL-6 signaling during redox-induced stress in the apoptosis-resistant HFCM phenotype and does not specifically address the developmental aspects of this response.
Footnotes
Read at the Eighty-fourth Annual Meeting of The American Association for Thoracic Surgery, Toronto, Ontario, Canada, April 25–28, 2004.
References
- 1.Shi Y, Baker JE, Zhang C, Tweddell JS, Su J, Pritchard KA., Jr Chronic hypoxia increases endothelial nitric oxide synthase generation of nitric oxide by increasing heat shock protein 90 association and serine phosphorylation. Circ Res. 2002;91:300–6. doi: 10.1161/01.res.0000031799.12850.1e. [DOI] [PubMed] [Google Scholar]
- 2.Madan A, Varma S, Cohen HJ. Developmental stage-specific expression of the alpha and beta subunits of the HIF-1 protein in the mouse and human fetus. Mol Genet Metab. 2002;75:244–9. doi: 10.1006/mgme.2001.3293. [DOI] [PubMed] [Google Scholar]
- 3.Friehs I, Cao-Danh H, Stamm C, Cowan DB, McGowan FX, del Nido PJ. Postnatal increase in insulin-sensitive glucose transporter expression is associated with improved recovery of postischemic myocardial function. J Thorac Cardiovasc Surg. 2003;126:263–71. doi: 10.1016/s0022-5223(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 4.Karimi M, Wang LX, Hammel JM, Mascio CE, Abdulhamid M, Barner EW, et al. Neonatal vulnerability to ischemia and reperfusion: cardioplegic arrest causes greater myocardial apoptosis in neonatal lambs than in mature lambs. J Thorac Cardiovasc Surg. 2004;127:490–7. doi: 10.1016/j.jtcvs.2003.07.052. [DOI] [PubMed] [Google Scholar]
- 5.Ancey C, Menet E, Corbi P, Fredj S, Garcia M, Rucker-Martin C, et al. Human cardiomyocyte hypertrophy induced in vitro by gp130 stimulation. Cardiovasc Res. 2003;59:78–85. doi: 10.1016/s0008-6363(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 6.Zolk O, Ng LL, O’Brien RJ, Weyand M, Eschenhagen T. Augmented expression of cardiotrophin-1 in failing human hearts is accompanied by diminished glycoprotein 130 receptor protein abundance. Circulation. 2002;106:1442–6. doi: 10.1161/01.cir.0000033117.39335.df. [DOI] [PubMed] [Google Scholar]
- 7.Corbi P, Rahmati M, Delwail A, Potreau D, Menu P, Wijdenes J, et al. Circulating soluble gp130, soluble IL-6R, and IL-6 in patients undergoing cardiac surgery, with or without extracorporeal circulation. Eur J Cardiothorac Surg. 2000;18:98–103. doi: 10.1016/s1010-7940(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 8.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–7. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komuro I, Kaida T, Shibazaki Y, Kurabayashi M, Katoh Y, Hoh E, et al. Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem. 1990;265:3595–8. [PubMed] [Google Scholar]
- 11.Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabo R, et al. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003;17:923–5. doi: 10.1096/fj.02-0644fje. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, McPherson BC, Yao Z. Preconditioning attenuates apoptosis and necrosis: role of protein kinase Cε and -δ isoforms. Am J Physiol Heart Circ Physiol. 2001;281:H404–10. doi: 10.1152/ajpheart.2001.281.1.H404. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinov IE, Coles JG, Boscarino C, Takahashi M, Goncalves J, Ritter J, et al. Gene expression profiles in children undergoing cardiac surgery for right heart obstructive lesions. J Thorac Cardiovasc Surg. 2004;127:746–54. doi: 10.1016/j.jtcvs.2003.08.056. [DOI] [PubMed] [Google Scholar]
- 14.Tusher VG, Tibshiran R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holloway AJ, vanLaar RK, Tothill RW, Bowtell DDL. Options available— from start to finish—for obtaining data from DNA microarrays II. Nat Genet. 2002;32:481–9. doi: 10.1038/ng1030. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Ikeda U, Shimpo M, Ohki R, Takahashi M, Yamamoto K, et al. HMG-CoA reductase inhibitors reduce interleukin-6 synthesis in human vascular smooth muscle cells. Cardiovasc Drugs Ther. 2002;16:121–6. doi: 10.1023/a:1015701415588. [DOI] [PubMed] [Google Scholar]
- 17.Craig R, Wagner M, McCardle T, Craig AG, Glembotski CC. The cytoprotective effects of the glycoprotein 130 receptor-coupled cytokine, cardiotrophin-1, require activation of NF-kappa B. J Biol Chem. 2001;276:37621–9. doi: 10.1074/jbc.M103276200. [DOI] [PubMed] [Google Scholar]
- 18.Kumar AS, Naruszewicz I, Wang P, Leung-Hagesteijn C, Hannigan GE. ILKAP regulates ILK signaling and inhibits anchorage-independent growth. Oncogene. 2004;23:3454–61. doi: 10.1038/sj.onc.1207473. [DOI] [PubMed] [Google Scholar]
- 19.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, et al. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2002;99:907–12. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunisada K, Hirota H, Fujio Y, Matsui H, Tani Y, Yamauchi-Takihara K, et al. Activation of JAK-STAT and MAP kinases by leukemia inhibitory factor through gp130 in cardiac myocytes. Circulation. 1996;94:2626–32. doi: 10.1161/01.cir.94.10.2626. [DOI] [PubMed] [Google Scholar]
- 21.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, et al. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem. 1998;273:9703–10. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- 22.Nishitai G, Shimizu N, Negishi T, Kishimoto H, Nakagawa K, Kitagawa D, et al. Stress induces mitochondria-mediated apoptosis independent of SAPK/JNK activation in embryonic stem cells. J Biol Chem. 2004;279:1621–6. doi: 10.1074/jbc.M310335200. [DOI] [PubMed] [Google Scholar]
- 23.Hunter JJ, Chien KR. Signaling pathway for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–83. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 24.Hovels-Gurich HH, Schumacher K, Vazquez-Jimenez JF, Qing M, Huffmeier U, Buding B, et al. Cytokine balance in infants undergoing cardiac operation. Ann Thorac Surg. 2002;73:601–8. doi: 10.1016/s0003-4975(01)03391-4. [DOI] [PubMed] [Google Scholar]
- 25.Qing M, Schumacher K, Heise R, Woltje M, Vazquez-Jimenez JF, Richter T, et al. Intramyocardial synthesis of pro- and anti-inflammatory cytokines in infants with congenital cardiac defects. J Am Coll Cardiol. 2003;41:2266–74. doi: 10.1016/s0735-1097(03)00477-7. [DOI] [PubMed] [Google Scholar]
- 26.Onody A, Zvara A, Hackler L, Jr, Vigh L, Ferdinandy P, Puskas LG. Effect of classic preconditioning on the gene expression pattern of rat hearts: a DNA microarray study. FEBS Lett. 2003;536:35–40. doi: 10.1016/s0014-5793(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Adam RM, Solomon KR, Freeman MR. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology. 2004;145:613–9. doi: 10.1210/en.2003-0772. [DOI] [PubMed] [Google Scholar]
- 28.Pennica D, King KL, Shaw KJ, Luis E, Rullamas J, Luoh SM, et al. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci U S A. 1995;92:1142–6. doi: 10.1073/pnas.92.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 30.Hattori T, Ohoka N, Hayashi H, Onozaki K. C/EBP homologous protein (CHOP) up-regulates IL-6 transcription by trapping negative regulating NF-IL6 isoform. FEBS Lett. 2003;541:33–9. doi: 10.1016/s0014-5793(03)00283-7. [DOI] [PubMed] [Google Scholar]
- 31.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–98. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]