Abstract
Purpose
We previously reported that 12 mo of resistance training (RT, 2×/wk, N= 19) or jump training (JUMP, 3×/wk, N= 19) increased whole body and lumbar spine BMD and increased serum bone formation markers relative to resorption in physically active (≥4 hr/wk) men (mean age: 44 ± 2 y; median: 44 y) with osteopenia of the hip or spine. The purpose of this secondary analysis was to examine the effects of the RT or JUMP intervention on potential endocrine mediators of the exercise effects on bone, specifically IGF-I, PTH and sclerostin.
Methods
Fasting blood samples were collected after a 24-h period of no exercise at baseline and after 12 months of RT or JUMP. IGF-I, PTH and sclerostin were measured in serum by ELISA. The effects of RT or JUMP on IGF-I, PTH and sclerostin were evaluated using 2×2 repeated measures ANOVA (time, group). This study was conducted in accordance with the Declaration of Helsinki and was approved by the University of Missouri IRB.
Results
Sclerostin concentrations in serum significantly decreased and IGF-I significantly increased after 12 mo of RT or JUMP; while PTH remained unchanged.
Conclusion
The beneficial effects of long-term, progressive-intensity RT or JUMP on BMD in moderately active men with low bone mass are associated with decreased sclerostin and increased IGF-I.
Keywords: exercise intervention, IGF-I, sclerostin, PTH, male osteopenia
1. Introduction
1.1. Mechanical loading, osteocytes and Wnt/β-catenin signaling
Even after achievement of peak bone mass, the adult skeleton adapts to mechanical loading to maintain bone strength sufficient to prevent fracture [1–3]. Bone adaptation occurs only at skeletal sites subjected to high strains [4]. With increased mechanical loading, bone strength is increased via changes in bone mass, shape, microarchitecture or material properties [2] that, ultimately, are orchestrated by osteocytes [5]. Osteocytes act as mechanosensors and transduce mechanical signals (e.g., strain, fluid shear stress) into biochemical signals detected by osteoblasts and osteoclasts [5]. There is no unique pathway linking mechanical loading to bone (re)modeling; rather, multiple pathways that are responsive to various signals, including mechanical strain, participate in the adaptive response [6]. Currently, the Wnt/β-catenin signaling pathway is viewed as a dominant pathway in adaptation to mechanical loading [5, 7–11]. Osteocytes regulate Wnt/β-catenin signaling via secretion of sclerostin, which antagonizes Wnt signaling by binding low-density lipoprotein receptor-related protein-5 and -6 [12]. In response to increased mechanical loading, osteocyte release of sclerostin decreases [2], enhancing Wnt/β-catenin signaling and its downstream effects [8, 12, 13].
1.2. Local mechanotransduction is sensitive to hormonal context
The hormonal milieu can influence local control of bone adaptation to mechanical loading [6]. That is, systemic and paracrine hormonal factors modulate osteocyte mechanotransduction and osteoblast response, thereby influencing local bone re(modeling) at the site of augmented mechanical strain. For example, insulin-like growth factor-I (IGF-I) plays an important role in the early stages of the osteoblast adaptive response to mechanical loading [14–17]. IGF-I is locally expressed in osteoblasts and osteocytes [18], but circulating IGF-I is increased by hepatic expression and IGF-I released from skeletal muscle has paracrine effects [19]. Systemic IGF-I stimulates bone formation in loaded bones, as evidenced by administration of exogenous IGF-I [20–22]. IGF-I produced by skeletal muscle localizes at the bone/muscle interface, and increases bone formation [23]. Likewise, parathyroid hormone (PTH) enhances the osteogenic response to mechanical loading [14]. Increased systemic PTH down-regulates sclerostin expression in bone [24, 25], thereby enhancing the reduction in sclerostin that occurs in response to mechanical loading and augmenting bone formation [6].
1.3. Mechansims by which exercise improves bone outcomes
Exercise increases bone mass and strength [26–28]. The increase mechanical strain exerted on the skeleton by gravitational- and joint-reaction forces (i.e., “impact” and “muscle-contraction” forces) down-regulates osteocyte sclerostin expression, increases Wnt/β-catenin signaling and, therefore, osteoblast activity [2, 5]. In addition to mechanical loading, exercise causes changes in systemic and local hormone concentrations, which might facilitate local skeletal adaptation to increased mechanical loading [14]. For example, PTH released during exercise controls osteocyte gene expression [29], and PTH signaling is associated with increased bone formation and improved structural and material properties [29]. Exercise also increases circulating IGF-I, the source of which is the liver or extrahepatic tissues. In particular, in response to exercise, skeletal muscle releases “myokines,” including IGF-I, which can have systemic and local effects on other tissues, including bone [19, 23, 30]. The osteoblast response to endogenous IGF-I is dependent on mechanical strain, thus, IGF-I is needed to optimize response to exercise [14, 31].
1.4. Study objectives and hypotheses
Despite acceptance of Wnt/β-catenin signaling as the predominant pathway in bone adaptation to exercise, data supporting the role of osteocyte expression of sclerostin in the osteogenic response to exercise in humans are very limited. It is not feasible to examine exercise-induced changes in Wnt/β-catenin signaling in bone in humans. However, because osteocytes are the primary source of sclerostin, circulating sclerostin reflects bone sclerostin and is therefore a measurable component of Wnt/β-catenin signaling in human subjects. Measurement of circulating sclerostin, as well as hormones that might enhance the osteogenic response to exercise, would provide useful information on how bone adapts to exercise in humans. Thus, the objective of this secondary analysis was to examine changes in circulating sclerostin, IGF-I, and PTH after 12 months of resistance training (RT) or jump training (JUMP) that increased BMD in apparently healthy men with low bone mass [32]. Importantly, no study has determined if a long-term, progressive-intensity exercise intervention impacts sclerostin levels. We hypothesized that sclerostin would decrease and that IGF-I and PTH would increase after 12 months of either RT of JUMP.
2. Materials and Methods
2.1.Trial Design
This study was a secondary analysis of data collected from a 12-month randomized, parallel intervention clinical trial that examined the effects of either resistance training or high-intensity jump training on BMD [32]. This study was conducted in accordance with the Declaration of Helsinki and was approved by the University of Missouri IRB. Informed written consent was obtained from each study participant.
2.2. Participants
Apparently healthy, physically active (≥4 hours of leisure time physical activity/week for the past 24 months) men aged 25–60 years with low BMD of the lumbar spine or hip (>−2.5 SD T-score ≤ −1.0 SD) were eligible to participate in this study [32].
2.3. Study intervention
2.3.1. RT and JUMP exercise interventions
The RT and JUMP exercise interventions have been described in detail previously [32]. Briefly, the RT and JUMP interventions were designed to optimize the osteogenic response, and both used a progressive intensity design based on a 6-week cycle followed by a rest week; a total of 8 cycles were completed. Subjects randomized to the JUMP intervention were required to attend 3 training sessions per week with a minimum of 24 hours between sessions. The JUMP intervention included different jump exercises that varied in intensity, direction, single- or double-leg; 40–100 jumps were performed per session, depending on the session intensity. Subjects randomized to the resistance training (RT) intervention were required to complete 2 training sessions per week. The RT intervention included exercises that load the hip and spine: squats, bent-over-row, modified dead lift, military press, lunges, and calf raises. All training sessions were supervised by study personnel and were performed in McKee Gym Fitness Center. Participants were required to complete all training sessions. If a participant missed a scheduled training session (e.g., due to illness), he was required to make up the missed session. All participants were provided supplemental calcium (1200 mg calcium carbonate/d) and vitamin D (10 µg vitamin D3/d) (Nature Made, Mission Hills, CA, USA) to ensure adequate intake of these nutrients by all participants.
2.4. Outcomes
2.4.2. Blood collection and processing
Blood samples (15 mL) were collected from subjects at 0 and 12 months at the same time between 06:00 and 08:00 AM after an overnight fast and a 24-hour period of no exercise. All samples were allowed to clot at room temperature for 30 minutes as recommended [33] and then were centrifuged at 4°C for 15 min at 2000g in a Marathon 21000R centrifuge (Fisher Scientific, Pittsburgh, PA) for isolation of serum. The separated serum was transferred to cryogenic vials and stored at −80°C for subsequent analysis.
2.4.3. IGF-I, PTH, and sclerostin assays
All assays were performed in duplicate measurements and in a single run to eliminate inter-assay variability. Commercially available ELISA analysis kits were used to determine the serum concentrations of IGF-I, PTH, and sclerostin. The sclerostin and PTH ELISA kits were purchased from ALPCO Diagnostics (Salem, NH, USA) and had intra-assay CVs of 5.1 and 5.5%, respectively; the IGF-I ELISA kit was purchased from AssayPro (Saint Louis, MO, USA) and had an intra-assay CV of 4.1%. The normal ranges provided by manufacturers were as follows: sclerostin (median for healthy adults=24.14 pmol/l); IGF-I (30–300 ng/ml); PTH (9–94 pg/ml).
2.5. Statistical Analysis
2.5.1. Descriptive and hypothesis-testing statistics
Descriptive statistics were performed on demographic and anthropometric variables. Differences between RT and JUMP at baseline were evaluated using independent t-tests (2-tailed). A 2×2 repeated measures ANOVA (2 timepoints and 2 treatment groups) was employed to compare the effects of RT versus JUMP on serum concentrations of sclerostin, PTH and IGF-I. In the case of a significant interaction (p<0.1), a repeated measures one-way ANOVA within group was used to locate the interaction; within group changes over time were not examined unless the interaction was significant. Group means and least squared means were considered statistically different at p < 0.05, as determined by the protected least significant difference (LSD) technique. Pearson’s correlation was used to determine if there were significant associations between percent change in sclerostin, IGF-I, 25OH vitamin D, PTH, and previously reported changes in whole body, total hip and lumbar spine BMD. All statistical analyses were performed using the SPSS statistical package (SPSS/22.0, SPSS, Chicago, IL, USA). Data are presented as means (± SD).
3. Results
3.1. Participant baseline characteristics
Thirty-eight participants completed the 12-month intervention and were included in the statistical analysis (Table 1). Participants were apparently health men who ranged in age from 25–60 y (median: 43.5 y; mean ± SD: 43.7 ± 10.1 y). There were no differences in age, anthropometric characteristics, nutrient intakes or physical activity between RT and JUMP at baseline [32].
Table 1.
Baseline characteristics of participants in the RT and JUMP interventions [1]
| Group | RT (n=19) | JUMP (n=19) | p-value |
|---|---|---|---|
| Age (y) | 45.5 (9.6) | 42.1 (10.6) | 0.325 |
| Anthropometrics | |||
| Height (m) | 1.79 (0.08) | 1.76 (0.05) | 0.229 |
| Body mass (kg) | 82.6 (14.2) | 77.1 (9.7) | 0.252 |
| BMI (kg/m2) | 25.7 (4.0) | 24.0 (3.9) | 0.716 |
| LBM (kg) | 60.9 (8.8) | 58.9 (5.5) | 0.415 |
| Fat mass (kg) | 19.5 (7.3) | 16.0 (5.4) | 0.212 |
| % Body fat | 22.8 (6.1) | 20.2 (4.8) | 0.337 |
| BMD (g/cm2) | |||
| WB | 1.132 (0.081) | 1.114 (0.071) | 0.482 |
| TH | 0.898 (0.082) | 0.912 (0.116) | 0.675 |
| LS | 0.939 (0.069) | 0.919 (0.056) | 0.425 |
| Nutrient intake per day | |||
| Energy (kcal) | 2537 (693) | 2343 (616) | 0.158 |
| Calcium (mg) | 1151 (143) | 944 (459) | 0.070 |
| Vitamin D (µg) | 5.4 (5.2) | 3.9 (3.1) | 0.777 |
| Physical activity per day | |||
| Time (hr) | 0.6 (0.3) | 0.9 (1.7) | 0.449 |
| Energy (kcal) | 338 (243) | 439 (610) | 0.593 |
Data are means (SD). P-values are for independent t-test (2-tailed) comparison of RT and JUMP means.
3.2. Bone mineral density
As previously reported, whole body and LS BMD were significantly increased after six months of RT or JUMP relative to baseline and these increases were maintained at 12 months (Table 2). Total hip BMD was significantly increased at 6 and 12 months only by RT and not by JUMP [32].
Table 2.
Bone mineral density in apparently healthy men at baseline and after 6 and 12 months of RT or JUMP [1]
| Group | RT (n=19) | JUMP (n=19) | ||
|---|---|---|---|---|
| Time (mo) | 0 | 12 | 0 | 12 |
| BMD(g/cm2) | ||||
| WB* | 1.132 (0.081) | 1.137 (0.085) | 1.114 (0.071) | 1.119 (0.071) |
| TH** | 0.898 (0.082)b | 0.906 (0.089)a | 0.912 (0.116) | 0.907 (0.111) |
| LS* | 0.939 (0.069) | 0.955 (0.088) | 0.919 (0.056) | 0.928 (0.049) |
Data are means (S.D.)
WB, whole body. TH, total hip. LS, lumbar spine.
Significant time main effect for WB BMD [mean (SD), 95% CI. 0 mo: 1.123b (0.076), 1.098–1.148; 12 mo: 1.128a (0.078), 1.102–1.154 g/cm2; time main effect, p<0.05] and LS BMD [0 mo: 0.929b (0.069), 0.906–0.952; 12 mo: 0.941a (0.072), 0.918–0.965 g/cm2; time main effect, p<0.001]. Post hoc within group comparisons were not performed for WB or LS BMD as there were no significant time-by-group interactions.
Significant time-by-group interaction for TH BMD [mean (SD), 95% CI. RT 0 mo: 0.898b (0.082), 0.851–0.945; 12 mo: 0.906a (0.089), 0.860–0.953 g/cm2; time-by-group interaction, p<0.1].
Means with different letter superscripts are significantly different.
3.3. Pre- to post-intervention changes in sclerostin, PTH, IGF-I
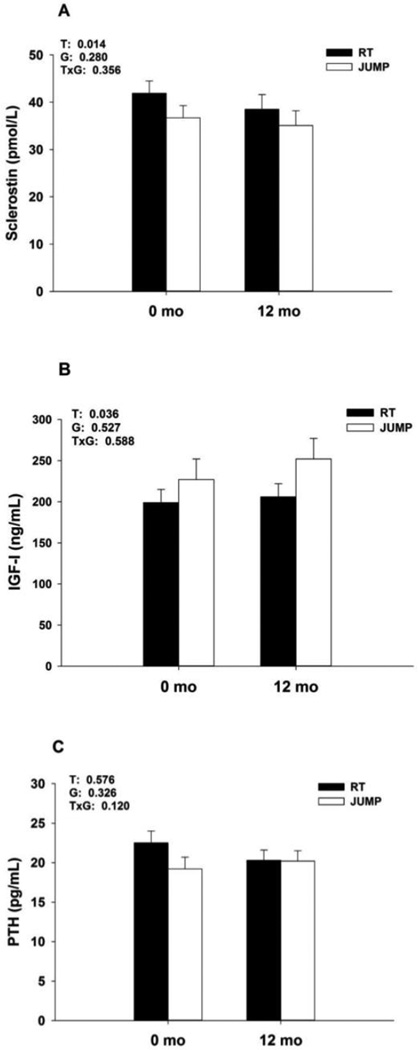
There was a significant time main effect for serum sclerostin (p=0.012), such that sclerostin decreased ~7% from 39.2 ± 11.6 pmol/L at baseline to 36.8 ± 13.3 pmol/L after 12 months of RT or JUMP (Figure 1). Mean percent change from baseline for sclerostin was −4.5 ± 3.6% for JUMP and −9.5 ± 3.5% for RT; these changes were significantly different from zero (p<0.05), but did not differ between groups. IGF-I increased ~26% from 203 ± 71 ng/mL to 239 ± 109 ng/mL after 12 months of RT or JUMP (time main effect, p=0.036; Figure 1). Mean percent change from baseline for IGF-I was 24.9 ± 11.6% for JUMP and 27.5 ± 13.3% for RT; these changes were significantly different from zero (p<0.01), but were not different between groups. PTH remained unchanged from baseline to 12 months. Sclerostin, IGF-I and PTH concentrations were within the normal range, as expected in apparently health men.
Figure 1.
Concentrations of sclerostin (A), IGF-I (B), and PTH (C) (means ± SEM) after 0 and 12 months of RT or JUMP. Significant time main effect for sclerostin and IGF-I; post hoc within group comparisons were not performed as there were no significant time-by-group interactions. Means with different letter superscripts are significantly different.
3.4. Associations between percent changes in hormonal outcomes and BMD
We previously reported that 25-OH vitamin D (25OHD) increased by 10.6 ± 4.0% after 12 months of daily supplementation with 10 µg vitamin D3. Changes in 25OHD were not significantly associated with changes in sclerostin or PTH, but there was a significant positive association between percent changes in 25OHD and IGF-I (r=0.502, p<0.001). Percent changes in whole body, total hip and lumbar spine BMD were not associated percent changes in sclerostin, IGF-I or PTH.
4. Discussion
4.1. Synopsis of study results
Serum sclerostin decreased significantly and IGF-I increased, while PTH was not changed after 12 months of RT or JUMP that increased BMD in apparently healthy men with low bone mass at baseline [32].
4.2. Sclerostin
The decrease in sclerostin observed following long-term RT or JUMP that increased BMD is consistent with the key role of osteocyte expression of sclerostin and the Wnt/β-catenin signaling pathway in the response to mechanical loading. A practical limitation of human studies is that it is not feasible to examine changes in osteocyte expression of sclerostin. However, because osteocytes are the primary source of sclerostin [5], the sclerostin in circulation is derived from osteocytes and should reflect bone levels. This was confirmed by Drake et al who reported a strong positive correlation between circulating serum sclerostin and bone marrow plasma sclerostin [24].
Very few studies have examined the relationship between exercise-associated mechanical loading and sclerostin in humans. Of the existing studies, most either made cross-sectional comparisons based on type or amount of physical activity [34–36] or evaluated acute changes in circulating sclerostin associated with intense, long-duration endurance exercise [37, 38]. Because osteocyte expression of sclerostin is responsive to multiple signals, conclusions about the role of sclerostin in the skeletal response to mechanical loading are often confounded by age, sex, and energy status [39] in cross-sectional comparisons. The acute sclerostin response to high-intensity, long-duration athletic competition, such as an ultra-marathon or cycling stage race, has similar limitations, as participation in these events can result in altered body mass, energy balance, and calcium status all of which might affect sclerostin release. Grasso reported that serum sclerostin increased after participation in a 3-week cycling stage race relative to baseline, and postulated that osteocytes increased sclerostin expression in response to high muscular workloads in the absence of gravitational load as occurs in cycling [37]. In contrast, sclerostin did not change from pre-ultra marathon to immediately post-race, but was significantly reduced 3 days after the race [38]. These acute changes in sclerostin reflect the osteocyte integration of multiple mechanical, hormonal, and metabolic inputs.
We could identify only two long-term, controlled exercise interventions studies that examined changes in circulating sclerostin from pre- to post-exercise in the literature. Twelve months of a physical activity intervention that increased hip, but not lumbar spine, BMD in postmenopausal women had no effect on serum sclerostin at completion of the intervention [40]. However, it is possible that sclerostin might have increased earlier during the training program. The training program provided a constant training stimulus for the duration of the 12-month intervention. Because bone responds to novel and unusual mechanical strain, after 12 months of the intervention, osteocytes might have no longer been responsive to the exercise. Armamento-Villareal examined the osteoprotective effects of exercise during 12 months of moderate weight loss (~10% of initial body weight) via caloric restriction in obese, older adults [41]. Weight reduction increased serum sclerostin by ~10% with concurrent reductions in hip BMD and geometry (e.g., cortical thickness, cross-sectional area) in obese older adults. Participants who exercised during caloric restriction also lost ~10% of their baseline body weight, but were protected against deleterious changes in hip geometry and did not increase serum sclerostin. Interestingly, participants who performed the same exercise, but maintained body weight, did not show any changes in hip outcomes or sclerostin [41].
In the present study, the first to examine sclerostin levels following a progressive intensity exercise intervention, we observed an average 7% decrease in serum sclerostin from pre-intervention to completion of the 12-month intervention. Data from the exercise intervention study by Armamento-Villareal [41] and a bed-rest study by Spatz [42] provide context for the biological significance of the 7% decrease we observed. After a 12-month weight loss intervention, serum sclerostin increased ~10% with a concurrent ~2–3% decrease in hip BMD [41]. In a 60-day bed rest study, sclerostin increased by ~20%, beginning ~2–3 weeks after the onset of bed rest, and BMD of the lower extremities (legs, hips) declined by ~2–4% [42]. Thus, the decrease in sclerostin that we observed after 12 months of osteogenic exercise was similar in magnitude to previously reported changes in sclerostin in response to increased or decreased mechanical loading.
4.3. IGF-I
In the present study, IGF-I increased by an average of 26% after 12 months of RT or JUMP in men with low bone mass, consistent with previous studies. Generally, resistance exercise training increases systemic IGF-I concentrations and this response is more robust and consistent in young, rather than aged, individuals [43]. The relative importance of bone-derived versus systemic IGF-I to bone health remains unresolved. Serum IGF-I is positively associated with BMD in humans and [44, 45] experimental animals [46, 47], and there is a negative association between circulating IGF-I and future fracture in adult women [48, 49]. Thus, the human data support the importance of serum IGF-I to bone health. Data from experimental animals provide stronger evidence for this relationship. Genetically modified animals that do not make IGF-I in the liver have significantly reduced cortical bone volume, periosteal circumference, and cross-sectional area [50, 51]. Thus, in vivo animal models demonstrate that systemic IGF-I is necessary for bone modeling (net gain) and mineralization [52].
4.4. PTH
Resting PTH concentrations were not affected by long-term RT or JUMP training in men with low bone mass. PTH increases during exercise, transiently decreases relative to pre-exercise, and returns to baseline values ~24 hours post-exercise [53]. Regular exercise that produces short-lived increases in PTH may stimulate bone formation, in a manner analogous to intermittent administration of exogenous PTH, which is the only approved bone-anabolic treatment for low bone mass [54]. We previously reported that the decrease in PTH following one session of RT or JUMP used in present study was positively correlated with decrease in TRAP5b [53]. Although we did not observe an increase in resting PTH after 12 months of osteogenic exercise, each session might have induced transient changes.
5. Conclusions
In summary, this is the first study to show that an exercise intervention that modulates intensity over time decreases circulating sclerostin along with increases in bone mineral density. Of note, either mode of exercise—resistance training or jump training—both increased bone mass and decreased sclerostin. These results are consistent with osteocyte expression of sclerostin in translating the mechanical stimulus of exercise into increased bone mass in humans. In addition, long-term exercise is associated with increased circulating IGF-I, which may enhance local skeletal response at sites of increase mechanical load. From a practical perspective, strategies, such as nutrition, that increase IGF-I or other bone anabolic hormones (e.g., estrogen), might enhance response to exercise.
Highlights.
Serum sclerostin decreased after 12 months of resistance training or jump training that increased whole body and lumbar spine BMD.
Serum IGF-I increased significantly after 12 months of resistance training or jump training.
Acknowledgments
The authors gratefully thank the study participants for their essential contribution to this project.
Authors’ roles: Study design: PH and JT. Study conduct: PH and PN. Data collection: PN. Data analysis: PH. Data interpretation: PH and JT. Drafting manuscript: PH. Revising manuscript content: PH, PN, and JT. Approving final version of manuscript: PH, PN, and JT.
Source of Funding
This study was funded by grants from the University of Missouri Research Board RB 07-44 (PSH) and the National Institutes of Health NIAMS R03AR055738 (PSH). JT was partially supported by R01DK088940.
Others with substantial contributions to the work reported in this manuscript: Study conduct and data collection: Robert Rogers, Andrew Dawson, Sarah Mobley, Melissa Carter, Tim Sinak, Adam Younkin, Zach Wehmeyer, Lynn Eaton, Jaccalyn Billeter, Jun Jiang, Nantian Lin, Matthew Strope, Blossom Nwaneri.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 2.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 3.Wolff J. Das gesetz der transformation der knochen (The law of bone remodeling) Berlin: Sprinter-Verlag; 1892. [Google Scholar]
- 4.Sugiyama T, Galea GL, Lanyon LE, Price JS. Mechanical loading-related bone gain is enhanced by tamoxifen but unaffected by fulvestrant in female mice. Endocrinology. 2010;151:5582–5590. doi: 10.1210/en.2010-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanyon LE. Regulation of bone mass: local control or systemic influence or both? IBMS BoneKEy. 2009;6:218–226. [Google Scholar]
- 7.A FL, Ahmed NU, Khan HM, Cevallos FG, Pekovic V. The Impact of Red Light Cameras on Crashes Within Miami-Dade County, Florida. Traffic Inj Prev. 2015;16:773–780. doi: 10.1080/15389588.2015.1023896. [DOI] [PubMed] [Google Scholar]
- 8.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 9.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 10.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 11.Sapir-Koren R, Livshits G. Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles? Osteoporos Int. 2014;25:2685–2700. doi: 10.1007/s00198-014-2808-0. [DOI] [PubMed] [Google Scholar]
- 12.Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2010;25:1897–1904. doi: 10.1002/jbmr.161. [DOI] [PubMed] [Google Scholar]
- 13.Lara-Castillo N, Kim-Weroha NA, Kamel MA, Javaheri B, Ellies DL, Krumlauf RE, et al. In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone. 2015;76:58–66. doi: 10.1016/j.bone.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price JS, Sugiyama T, Galea GL, Meakin LB, Sunters A, Lanyon LE. Role of endocrine and paracrine factors in the adaptation of bone to mechanical loading. Curr Osteoporos Rep. 2011;9:76–82. doi: 10.1007/s11914-011-0050-7. [DOI] [PubMed] [Google Scholar]
- 15.Hu M, Qin YX. Dynamic fluid flow stimulation on cortical bone and alterations of the gene expressions of osteogenic growth factors and transcription factors in a rat functional disuse model. Arch Biochem Biophys. 2014;545:154–161. doi: 10.1016/j.abb.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesavan C, Wergedal JE, Lau KH, Mohan S. Conditional disruption of IGF-I gene in type 1alpha collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. Am J Physiol Endocrinol Metab. 2011;301:E1191–E1197. doi: 10.1152/ajpendo.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau KH, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li Z, et al. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab. 2013;305:E271–E281. doi: 10.1152/ajpendo.00092.2013. [DOI] [PubMed] [Google Scholar]
- 18.Lean JM, Mackay AG, Chow JW, Chambers TJ. Osteocytic expression of mRNA for c-fos and IGF-I: an immediate early gene response to an osteogenic stimulus. Am J Physiol. 1996;270:E937–E945. doi: 10.1152/ajpendo.1996.270.6.E937. [DOI] [PubMed] [Google Scholar]
- 19.Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res. 2004;19:436–446. doi: 10.1359/JBMR.0301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowlkes JL, Thrailkill KM, Liu L, Wahl EC, Bunn RC, Cockrell GE, et al. Effects of systemic and local administration of recombinant human IGF-I (rhIGF-I) on de novo bone formation in an aged mouse model. J Bone Miner Res. 2006;21:1359–1366. doi: 10.1359/JBMR.060618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakata T, Halloran BP, Elalieh HZ, Munson SJ, Rudner L, Venton L, et al. Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone. 2003;32:669–680. doi: 10.1016/s8756-3282(03)00088-7. [DOI] [PubMed] [Google Scholar]
- 23.Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10:64–70. [PMC free article] [PubMed] [Google Scholar]
- 24.Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, et al. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5056–5062. doi: 10.1210/jc.2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 26.Michaelsson K, Olofsson H, Jensevik K, Larsson S, Mallmin H, Berglund L, et al. Leisure physical activity and the risk of fracture in men. PLoS Med. 2007;4:e199. doi: 10.1371/journal.pmed.0040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izard RM, Fraser WD, Negus C, Sale C, Greeves JP. Increased density and periosteal expansion of the tibia in young adult men following short-term arduous training. Bone. 2016;88:13–19. doi: 10.1016/j.bone.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson M, Sundh D, Ohlsson C, Karlsson M, Mellstrom D, Lorentzon M. Exercise during growth and young adulthood is independently associated with cortical bone size and strength in old Swedish men. J Bone Miner Res. 2014;29:1795–1804. doi: 10.1002/jbmr.2212. [DOI] [PubMed] [Google Scholar]
- 29.Gardinier JD, Mohamed F, Kohn DH. PTH Signaling During Exercise Contributes to Bone Adaptation. J Bone Miner Res. 2015;30:1053–1063. doi: 10.1002/jbmr.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombardi G, Sanchis-Gomar F, Perego S, Sansoni V, Banfi G. Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine. 2015 doi: 10.1007/s12020-015-0834-0. [DOI] [PubMed] [Google Scholar]
- 31.Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE, et al. Mechanotransduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to Ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J Biol Chem. 2010;285:8743–8758. doi: 10.1074/jbc.M109.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinton PS, Nigh P, Thyfault J. Effectiveness of resistance training or jumping-exercise to increase bone mineral density in men with low bone mass: A 12-month randomized, clinical trial. Bone. 2015;79:203–212. doi: 10.1016/j.bone.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, et al. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab. 2012;97:148–154. doi: 10.1210/jc.2011-2152. [DOI] [PubMed] [Google Scholar]
- 35.Lombardi G, Lanteri P, Colombini A, Mariotti M, Banfi G. Sclerostin concentrations in athletes: role of load and gender. J Biol Regul Homeost Agents. 2012;26:157–163. [PubMed] [Google Scholar]
- 36.Jurimae J, Tillmann V, Cicchella A, Stefanelli C, Vosoberg K, Tamm AL, et al. Increased sclerostin and preadipocyte factor-1 levels in prepubertal rhythmic gymnasts: associations with bone mineral density, body composition, and adipocytokine values. Osteoporos Int. 2016;27:1239–1243. doi: 10.1007/s00198-015-3301-0. [DOI] [PubMed] [Google Scholar]
- 37.Grasso D, Corsetti R, Lanteri P, Di Bernardo C, Colombini A, Graziani R, et al. Bone-muscle unit activity, salivary steroid hormones profile, and physical effort over a 3-week stage race. Scand J Med Sci Sports. 2015;25:70–80. doi: 10.1111/sms.12147. [DOI] [PubMed] [Google Scholar]
- 38.Kerschan-Schindl K, Thalmann MM, Weiss E, Tsironi M, Foger-Samwald U, Meinhart J, et al. Changes in Serum Levels of Myokines and Wnt-Antagonists after an Ultramarathon Race. PLoS One. 2015;10:e0132478. doi: 10.1371/journal.pone.0132478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falk B, Haddad F, Klentrou P, Ward W, Kish K, Mezil Y, et al. Differential sclerostin and parathyroid hormone response to exercise in boys and men. Osteoporos Int. 2016;27:1245–1249. doi: 10.1007/s00198-015-3310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergstrom I, Parini P, Gustafsson SA, Andersson G, Brinck J. Physical training increases osteoprotegerin in postmenopausal women. J Bone Miner Metab. 2012;30:202–207. doi: 10.1007/s00774-011-0304-6. [DOI] [PubMed] [Google Scholar]
- 41.Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27:1215–1221. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, et al. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97:E1736–E1740. doi: 10.1210/jc.2012-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkhouse WS, Coupland DC, Li C, Vanderhoek KJ. IGF-1 bioavailability is increased by resistance training in older women with low bone mineral density. Mech Ageing Dev. 2000;113:75–83. doi: 10.1016/s0047-6374(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 44.Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, et al. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–4262. doi: 10.1210/jcem.83.12.5308. [DOI] [PubMed] [Google Scholar]
- 45.Patel MB, Arden NK, Masterson LM, Phillips DI, Swaminathan R, Syddall HE, et al. Investigating the role of the growth hormone-insulin-like growth factor (GH-IGF) axis as a determinant of male bone mineral density (BMD) Bone. 2005;37:833–841. doi: 10.1016/j.bone.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Rosen CJ, Dimai HP, Vereault D, Donahue LR, Beamer WG, Farley J, et al. Circulating and skeletal insulin-like growth factor-I (IGF-I) concentrations in two inbred strains of mice with different bone mineral densities. Bone. 1997;21:217–223. doi: 10.1016/s8756-3282(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 47.Rosen CJ. IGF-I and osteoporosis. Clin Lab Med. 2000;20:591–602. [PubMed] [Google Scholar]
- 48.Bauer DC, R C, Cauley J, Cummings SR. Low serum IGF-1 but not IGFBP-3 predicts hip and spine fracture: The study of osteoporotic fracture. 1998;23:561–562. [Google Scholar]
- 49.Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int. 2000;11(Suppl 6):S2–S17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 50.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Courtland HW, DeMambro V, Maynard J, Sun H, Elis S, Rosen C, et al. Sex-specific regulation of body size and bone slenderness by the acid labile subunit. J Bone Miner Res. 2010;25:2059–2068. doi: 10.1002/jbmr.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosen CJ. Insulin-like growth factor I and bone mineral density: experience from animal models and human observational studies. Best Pract Res Clin Endocrinol Metab. 2004;18:423–435. doi: 10.1016/j.beem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Rogers RS, Dawson AW, Wang Z, Thyfault JP, Hinton PS. Acute response of plasma markers of bone turnover to a single bout of resistance training or plyometrics. J Appl Physiol (1985) 2011;111:1353–1360. doi: 10.1152/japplphysiol.00333.2011. [DOI] [PubMed] [Google Scholar]
- 54.Mosekilde L, Torring O, Rejnmark L. Emerging anabolic treatments in osteoporosis. Curr Drug Saf. 2011;6:62–74. doi: 10.2174/157488611795684712. [DOI] [PubMed] [Google Scholar]